Fri, Apr 26, 2024
[Archive]
Volume 18, Issue 9 (September 2020)
IJRM 2020, 18(9): 765-776 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mehdikhani H, Agababa H, Sadeghi L. Effect of Zirconium oxide nanoparticle on serum level of testosterone and spermatogenesis in the rat: An experimental study. IJRM 2020; 18 (9) :765-776
URL: http://ijrm.ir/article-1-1068-en.html
URL: http://ijrm.ir/article-1-1068-en.html
1- Department of Biology, Shiraz Branch, Islamic Azad University, Shiraz, Iran.
2- Department of Biology, Arsanjan Branch, Islamic Azad University, Arsanjan, Iran. , heydar2001@yahoo.com
3- Department of Biology, Arsanjan Branch, Islamic Azad University, Arsanjan, Iran.
2- Department of Biology, Arsanjan Branch, Islamic Azad University, Arsanjan, Iran. , heydar2001@yahoo.com
3- Department of Biology, Arsanjan Branch, Islamic Azad University, Arsanjan, Iran.
Full-Text [PDF 4339 kb]
(745 Downloads)
| Abstract (HTML) (1587 Views)
1. Introduction
Nanotechnology refers to the technology of materials that measure about 1-100 nanometers (1). The nanoparticles have unique physical and chemical properties that cannot be seen even in the materials from which they are derived. The small size and high surface area of the nanoparticles increase their chemical activity and allow them to act as a high performance catalyst (2).
Since 1960, Zirconium nanoparticles have been introduced as biomaterials for use in orthopedics in the form of artificial joints and in dentistry as coatings, implants, and composites (3). Zirconium oxide ceramic nanostructures have also been widely used in drug transducers as drug carriers for several drugs such as Myconazol, Pennyceline, Alendronit, and Zolendronitthat (4, 5). Zirconium nanoparticles have been shown to cause significant DNA damage (6) and mesenchymal stem cell death (7) and inhibit cell proliferation in rodent fibroblast cell lineages (8).
While a study by Demir and co-worker showed that Zirconium oxide nanoparticles lead to gene toxicity in peripheral blood lymphocytes and cultured renal embryonic cells (9), a study by Ye and co-worker found that high concentrations of Zirconium oxide nanoparticles induce cell death and morphological changes in cultured 3T3-E1 cells. In addition, zirconium oxide nanoparticles induced osteogenic differentiation (10). A study by Gad and co-worker showed that Zirconium oxide nanoparticles have antifungal activity against Candida albicans (11).
Moreover, in a study by Banerjee and co-worker, it was found that Zirconium oxide nanoparticles have antimicrobial activity (12), and Atalay and co-worker in 2018 reported that Zirconium oxide nanoparticles induce DNA damage and cell death in L929 mouse fibroblast cell liners (13). While Arefian and co-worker reported in their study that Zirconium oxide nanoparticles had strong toxic effects on liver and kidney factors (14) Omidi and co-worker showed that zirconium oxide nanoparticles at a dose of 400 ppm resulted in a significant decrease in Luteinizing hormone (LH), Follicle-stimulating hormone (FSH), and testosterone in female rats (15).
Recent reports indicate that many nanoparticles have harmful or toxic effects on spermatogenesis. In contrast, some nanoparticles have a non-toxic effect on spermatogenesis. These reports indicate that animal species, drug use, nanoparticle dosage and its characteristics including size, shape, chemical composition, surface area, and surface charge play an important role in determining the effect of nanoparticles on spermatogenesis. How nanoparticles penetrate into the blood-testicular barrier plays a crucial role in explaining the toxicity of nanoparticles on spermatogenesis (16).
According to our best knowledge, there is no study that investigate the effect of Zirconium oxide nanoparticles on spermatogenesis and serum levels of testosterone in rats. Similarly, there has been no study to evaluate the effect of Zirconium oxide nanoparticles on testicular germ cells and seminal tubular epithelium in rats for 30 days. Due to the role of Zirconium oxide nanoparticles in the production and growth of free radicals, it is likely that these nanoparticles may have pathological effects on the structure of seminal vesicles and consequently decrease sperm quality and fertility. In this study, we investigated the possible effect of Zirconium oxide nanoparticles prepared by the Tecnan company manufactured in Spain (CAS#1314-23-4) on serum levels of testosterone and spermatogenesis in adult rat.
2. Materials and Methods
2.1. Animals
In this experimental study, 32 adult (2.5-3 month) male Wistar rats weighing approximately 150-200 gr were used. Animals were kept under the same conditions at temperatures of 20-22ºC for 12 hr light/dark cycle. The animals were provided with adequate water and food and ethical considerations were observed.
2.2. Animal treatment
The animals were divided into four groups of eight animals. Control group: received 0.5 ml of sterile distilled water. Experimental group (I): received 50 ppm Zrconium oxide nanoparticlessolution.
Experimental group (II): received 200 ppm Zirconium oxide nanoparticles solution. Experimental group (III): received 400 ppm Zirconium oxide nanoparticles solution. All solutions were given daily for 30 days orally (15). Animals body weight was measured using digital scales at the end of the experiment and were anesthetized with ether.
Blood samples were taken from the left ventricle of the heart, and the samples were kept in laboratory conditions for 20 min and centrifuged at 3000 rpm for 10 min. Then, the serum of each tube was collected. Serum concentrations of testosterone were determined by enzyme-linked immunosorbent assay.
2.3. Histological tests
After the animals were sacrificed, their left testicles were removed. At the fixation stage, the tissues were fixed in 10% buffered formalin. The dehydration step was performed with the help of alcohol at different concentrations (from low to high). The clearing step was performed by placing the tissues in two containers containing xylene. In the replacement phase, tissues were placed in three containers of molten paraffin (65ºC) for 1 hr. In the molding phase, the parts of Salto Kohart were used. At the cross-section, tissue sections were cut to 4-5 μm in thickness and hematoxylin-eosin dye for staining. All tissue studies were performed under the supervision of a pathologist. Tissue sections were morphometrically examined and imaged by a Dino-Eye-AM 423 camera. In each slide, four points were randomly selected and the walls of seminiferous tubules and the diameter of seminiferous tubules were morphometrically measured. At least five seminiferous tubules were selected from each slide. Numbers of spermatogonia, spermatocytes, spermatids, Sertoli and Leydig cells present in lumen were compared between different groups (17).
In this study, 14 nm zirconium nanoparticles (Figure 1) were used which were determined by X-ray diffraction methods and Transmission Electron Microscopy (TEM) and determination of extraction impurities by ICP-MS technique was 99/993% (Figure 2) (18, 19).


2.4. Ethical consideration
All experiments were conducted according to the protocol provided by the ethics committee of Islamic Azad University Arsanjan Branch (IR.IAU.A.REC.1397.001).
2.5. Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS software, Chicago, Illinois), version 18.0., analysis of variance (ANOVA) and Tukey Test. Statistical inference boundary was statistically significant for the experimental groups receiving different amounts of zirconium oxide nanoparticles at P ≤ 0.05 level compared to the control group. In this study, the results of experiments performed along with the relevant statistical calculations are presented in the form of graphs.
3. Results
While the mean body weights in the experimental groups (I) and (II) did not change significantly compared to control group, the mean body weight in the experimental group (III) significantly decreased compared to the control group (p ≤ 0.05, Figure 3).
The mean testicular weight in all experimental groups receiving different amounts of zirconium oxide nanoparticles did not change significantly compared to the control group (Figure 4).
Interestingly, while the average number of spermatogonial cells in the experimental groups (I) and (II) did not change significantly compared to the control group, the average number of spermatogonial cells in the experimental group (III) decreased significantly compared to the control group (p ≤ 0.01) (Figure 5).
Further, although the average number of spermatocyte cells in the experimental groups (I) and (II) did not change significantly compared to the control group, the average number of spermatocyte cells in the experimental group (III) decreased significantly compared to the control group (p ≤ 0.01) (Figure 5).
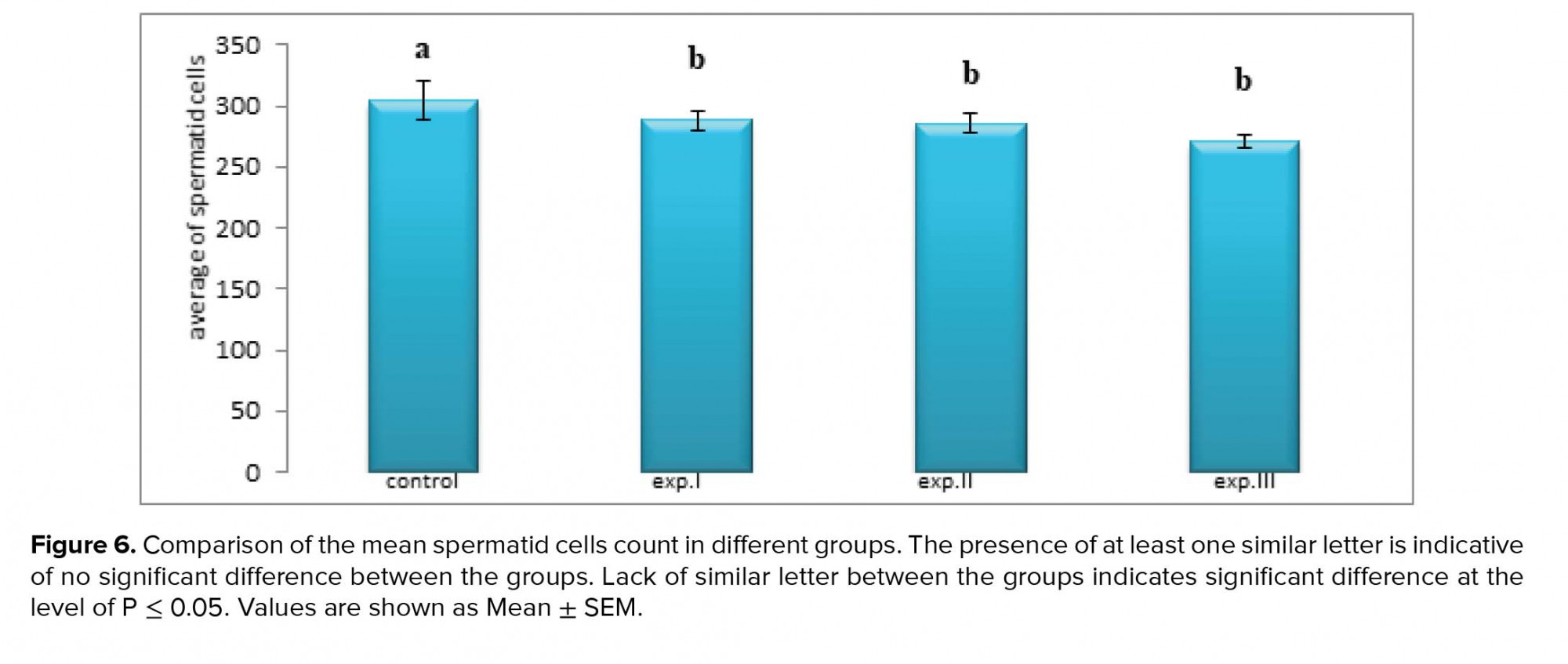
However, the average number of spermatid cells in all experimental groups (I), (II) and (III) significantly decreased compared to the control group (p ≤ 0.0001, respectively) (Figure 6).
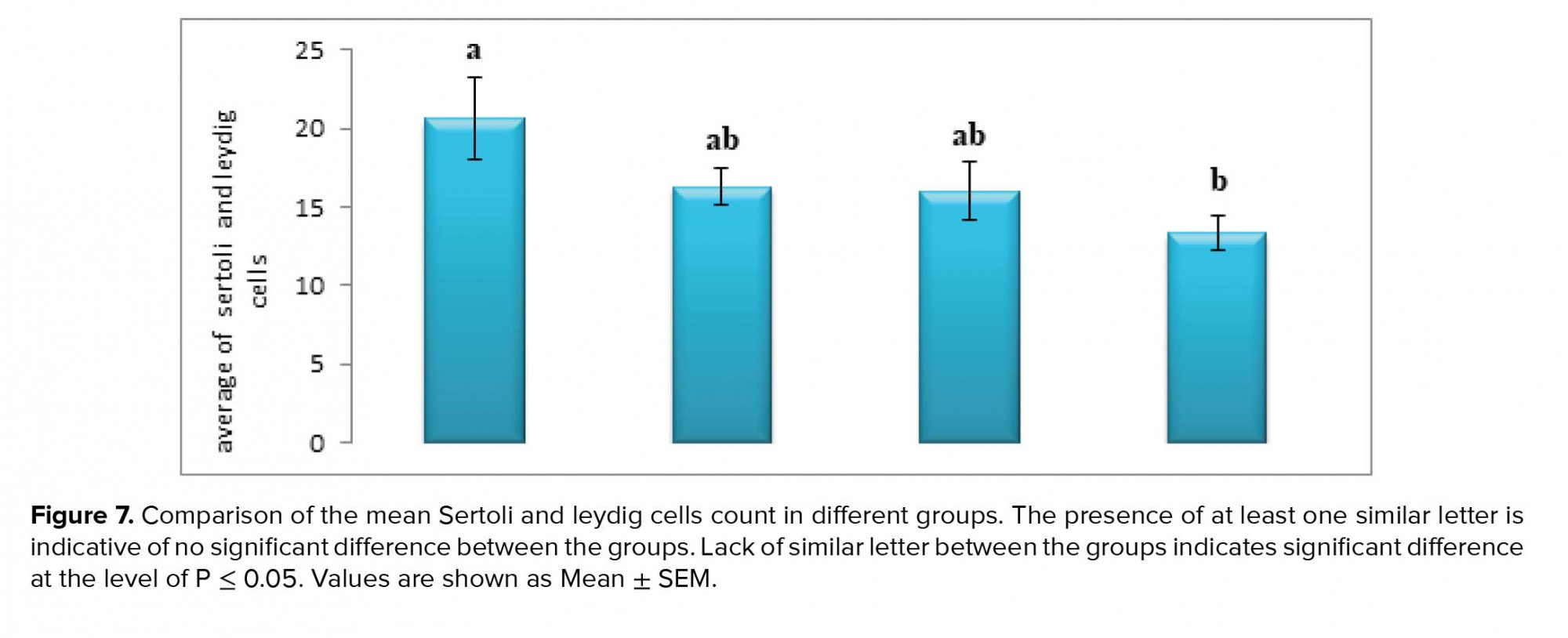
As for the Leydig cells, the average number of Leydig cells in the experimental groups (I) and (II) did not change significantly compared to the control group. However, the average number of Leydig cells in experimental group (III) decreased significantly compared to the control group (p ≤ 0.05, Figure 7).
Further, the average number of Sertoli cells in the experimental groups (I) and (II) also did not change significantly compared to the control group. However, the average number of Sertoli cells in the experimental group (III) decreased significantly compared to the control group (p ≤ 0.05, Figure 7).
The mean serum testosterone concentration in all experimental groups receiving different amounts of zirconium oxide nanoparticles did not change significantly compared to the control group (Figure 8).
Results of testicular tissue studies
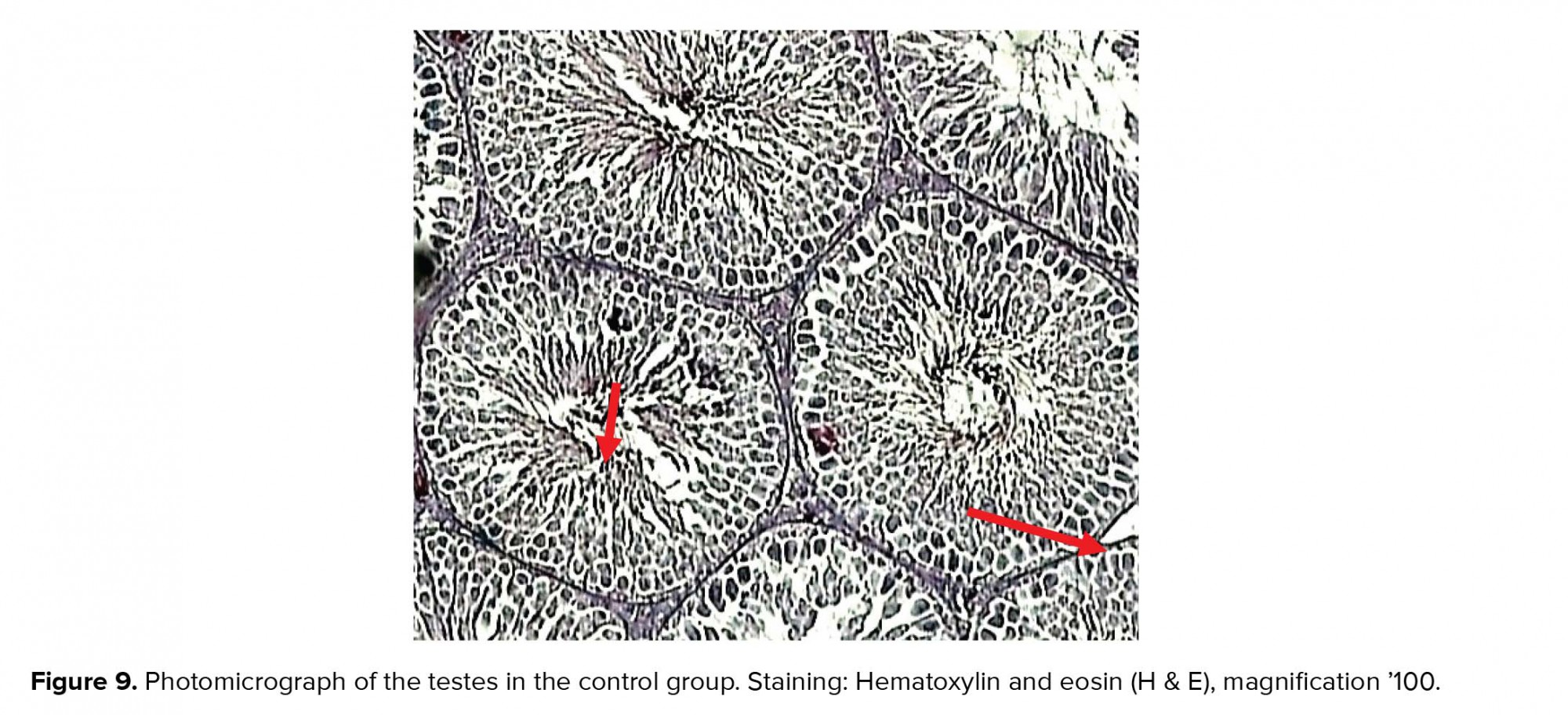
There were no pathological changes in testicular photomicrographs of the control group and testicular tubules, and germ cells appeared normal (Figure 9).
In the experimental group (I), recipients of zirconium oxide nanoparticles (50 ppm) showed a decrease in the density of different types of germ cells compared to the control group (Figure 10).
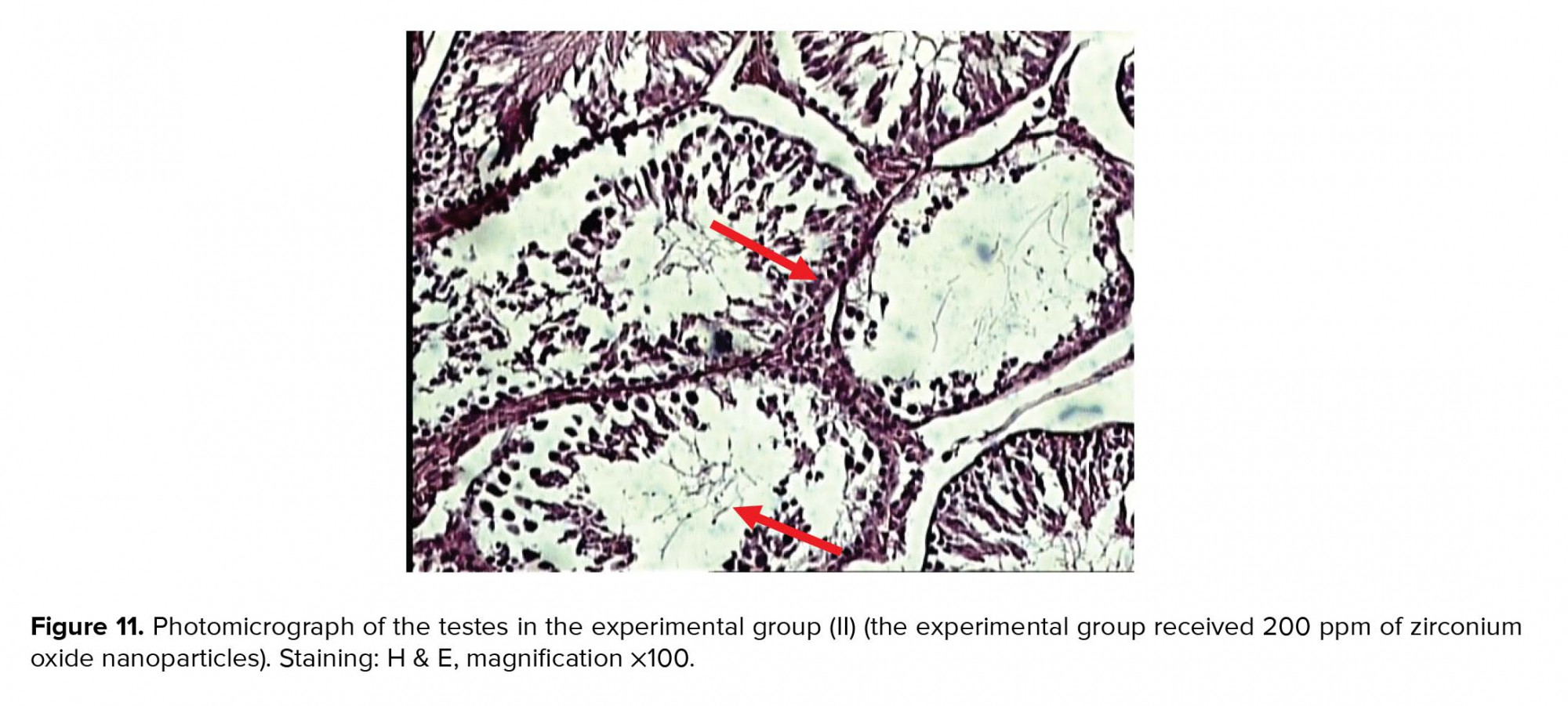
In the experimental group (II), recipients of zirconium oxide nanoparticles (200 ppm) showed better shrinkage of seminiferous tubules and decreased number of germ cells, especially spermatid cells, compared to the control group (Figure 11).
In the experimental group (III) showed a sharp decrease in the number of seminiferous tubules. The irregularity and disruption of the germinal epithelium and shrinkage of the tubules were visible. In addition, Spermatogonia, Spermatocytes, Spermatids, Sertoli and Leydig cells were noticeable in the lumen. Therefore, tissue degradation is clearly observed in this experimental group (Figure 12).
In the experimental groups receiving different amounts of Zirconium oxide nanoparticles, the number of spermatogonia, primary spermatocytes, and spermatids decreased compared to the control group. These effects are dose dependent and in the receiving group the maximum amount of Zirconium oxide nanoparticles (400 ppm) is higher than the other experimental groups.






4. Discussion
The mean body weight in the experimental group receiving 400 ppm zirconium oxide nanoparticles decreased significantly compared to the control group (p ≤ 0.05). These results are in line with a 2010 study by Kim and co-worker, where it was shown that the administration of 500 mg/kg of silver nanoparticles orally for 13 weeks in male mice resulted in lower body weight and increased blood cholesterol levels (20).
The mean testicular weight in all experimental groups receiving different amounts of zirconium oxide nanoparticles did not change significantly compared to the control group. A study by Fan and co-worker showed that exposure to mice with silicon oxide nanoparticles resulted in a significant decrease in testicular weight. Silicon oxide nanoparticles are likely to reduce the testicular weight of rat by oxidative damage to the testes by increasing ROS (reactive oxygen species) (21).
The average number of Sertoli cells in the experimental group receiving 400 ppm zirconium oxide nanoparticles decreased significantly compared to the control group (p ≤ 0.05). A 2013 study by Talebi and co-worker showed that zinc oxide nanoparticles damage Sertoli cells. The mechanism of the effects of nanoparticles on the testes of mice is not known. Zinc oxide nanoparticles are likely to have deleterious effects on Sertoli cell function through the formation of multinuclear giant cells, increasing vacuoles in Sertoli cells. The results of this study were consistent with the results of the study (22). Also, Braydich-Stolle and co-worker showed that silver nanoparticles decrease ATP levels in Sertoli cells by reducing FYN/Kinase enzyme levels in Sertoli cells. Silver nanoparticles appear to damage the Sertoli cells and the germ cells by reducing the amount of FYN/Kinase enzyme followed by ATP depletion. Silver nanoparticles can disrupt Sertoli cell damage by inhibiting Glial cell-derived neurotrophic factor (GDNF) signaling pathway, which is secreted from Sertoli cells, resulting in a proliferation of spermatogonial cells (23).
Further, the average number of Leydig cells in the experimental group receiving 400 ppm zirconium oxide nanoparticles decreased significantly compared to the control group (p ≤ 0.05). These results are consistent with the study of Talebi and co-worker (22). The results in their study showed that silver nanoparticles can affect Leydig cells and testosterone inhibitor synapses, and the number of Leydig cells was significantly reduced in the groups treated with silver nanoparticles (20).
Omidi and co-worker investigated the effect of zirconium nanoparticles on testosterone, LH, and FSH in female rat. Zirconium nanoparticles led to a significant decrease in plasma levels of testosterone in female rat (15). Testosterone is higher in the male than in the female. In a recent study, the dose of zirconium oxide nanoparticles used was not sufficient to influence testosterone levels in male rats. Therefore, in all groups the level of testosterone decreased but this decrease was not significant (p ≤ 0.05).
Jia and co-worker reported that titanium dioxide nanoparticles significantly reduced serum testosterone levels and expression in rat testes. Titanium dioxide nanoparticles decreased the expression of 17 beta-hydroxy steroids dehydrogenase and P 450 17 alpha-hydroxysteroid dehydrogenase and increased the expression of cytochrome P 450-19 (key enzyme for translating testosterone to estradiol) in mice. Titanium dioxide nanoparticles at the translational level cause changes in testosterone. In addition, titanium dioxide nanoparticles may reduce spermatogenesis by reducing serum testosterone synthesis (24). Due to similarities in zirconium and titanium nanoparticles in their properties, a decrease in serum testosterone level was observed in male rats treated with zirconium nanoparticle oxide, but this decrease was not significant.
Other researchers have shown that nanoparticles lead to the decline of germ cells. Miresmaeili et al reported that silver nanoparticles led to a significant decrease in the number of spermatogonia, primary spermatocytes, spermatids, and spermatozoa (25).
Lan and Yang showed that nanoparticles are capable of crossing the blood-testicular barrier, thereby exerting inflammatory effects on testicular tissue. The nanoparticles do not directly destroy the nucleus membrane. Nanoparticles first affect the cytoskeleton. They then destroy the nucleus membrane. In addition, the nanoparticles may be endocytosed by sex cells or Leydig cells, causing changes in inflammation in the cell and then exocytosis (16).
Cellular and tissue damage can be caused by the extensive production of ROS in inflammatory diseases (26). In addition, increasing ROS production can lead to increased production of external cytokines and further damage (27).
Various studies have shown that zirconium nanoparticles can lead to increased production of ROS and increased caspase-3, mitochondrial membrane potential, and DNA strand breaks. Zirconium nanoparticles can cause cellular toxicity by causing mitochondrial damage (28, 13).
Studies have shown that exposure to zirconium oxide nanoparticles resulted in a significant decrease in Glutathione peroxidase (GPx) in rat. In fact, the imbalance between the production of free radicals and their ability to detoxify by GPx in the molecular mechanism of toxicity causes cell damage (29, 30, 31). Concerning the results, it seems that irconium oxide nanoparticles decrease spermatogenesis through the mechanisms described. It is also possible that zirconium oxide nanoparticles may decrease spermatogenesis by reducing serum testosterone synthesis.
5. Conclusion
Studies have shown that zirconium oxide nanoparticles can damage testicular tissue, which is dose-dependent. In addition, the doses of zirconium oxide nanoparticles in this study did not lead to a significant change in the serum concentration of testosterone.
Acknowledgments
The authors hereby express their deep appreciation for the sincere cooperation of the research deputy of Arsanjan Azad University to conduct this research.
Conflicts of interest
The authors declare that there is no conflict of interest.
Full-Text: (572 Views)
1. Introduction
Nanotechnology refers to the technology of materials that measure about 1-100 nanometers (1). The nanoparticles have unique physical and chemical properties that cannot be seen even in the materials from which they are derived. The small size and high surface area of the nanoparticles increase their chemical activity and allow them to act as a high performance catalyst (2).
Since 1960, Zirconium nanoparticles have been introduced as biomaterials for use in orthopedics in the form of artificial joints and in dentistry as coatings, implants, and composites (3). Zirconium oxide ceramic nanostructures have also been widely used in drug transducers as drug carriers for several drugs such as Myconazol, Pennyceline, Alendronit, and Zolendronitthat (4, 5). Zirconium nanoparticles have been shown to cause significant DNA damage (6) and mesenchymal stem cell death (7) and inhibit cell proliferation in rodent fibroblast cell lineages (8).
While a study by Demir and co-worker showed that Zirconium oxide nanoparticles lead to gene toxicity in peripheral blood lymphocytes and cultured renal embryonic cells (9), a study by Ye and co-worker found that high concentrations of Zirconium oxide nanoparticles induce cell death and morphological changes in cultured 3T3-E1 cells. In addition, zirconium oxide nanoparticles induced osteogenic differentiation (10). A study by Gad and co-worker showed that Zirconium oxide nanoparticles have antifungal activity against Candida albicans (11).
Moreover, in a study by Banerjee and co-worker, it was found that Zirconium oxide nanoparticles have antimicrobial activity (12), and Atalay and co-worker in 2018 reported that Zirconium oxide nanoparticles induce DNA damage and cell death in L929 mouse fibroblast cell liners (13). While Arefian and co-worker reported in their study that Zirconium oxide nanoparticles had strong toxic effects on liver and kidney factors (14) Omidi and co-worker showed that zirconium oxide nanoparticles at a dose of 400 ppm resulted in a significant decrease in Luteinizing hormone (LH), Follicle-stimulating hormone (FSH), and testosterone in female rats (15).
Recent reports indicate that many nanoparticles have harmful or toxic effects on spermatogenesis. In contrast, some nanoparticles have a non-toxic effect on spermatogenesis. These reports indicate that animal species, drug use, nanoparticle dosage and its characteristics including size, shape, chemical composition, surface area, and surface charge play an important role in determining the effect of nanoparticles on spermatogenesis. How nanoparticles penetrate into the blood-testicular barrier plays a crucial role in explaining the toxicity of nanoparticles on spermatogenesis (16).
According to our best knowledge, there is no study that investigate the effect of Zirconium oxide nanoparticles on spermatogenesis and serum levels of testosterone in rats. Similarly, there has been no study to evaluate the effect of Zirconium oxide nanoparticles on testicular germ cells and seminal tubular epithelium in rats for 30 days. Due to the role of Zirconium oxide nanoparticles in the production and growth of free radicals, it is likely that these nanoparticles may have pathological effects on the structure of seminal vesicles and consequently decrease sperm quality and fertility. In this study, we investigated the possible effect of Zirconium oxide nanoparticles prepared by the Tecnan company manufactured in Spain (CAS#1314-23-4) on serum levels of testosterone and spermatogenesis in adult rat.
2. Materials and Methods
2.1. Animals
In this experimental study, 32 adult (2.5-3 month) male Wistar rats weighing approximately 150-200 gr were used. Animals were kept under the same conditions at temperatures of 20-22ºC for 12 hr light/dark cycle. The animals were provided with adequate water and food and ethical considerations were observed.
2.2. Animal treatment
The animals were divided into four groups of eight animals. Control group: received 0.5 ml of sterile distilled water. Experimental group (I): received 50 ppm Zrconium oxide nanoparticlessolution.
Experimental group (II): received 200 ppm Zirconium oxide nanoparticles solution. Experimental group (III): received 400 ppm Zirconium oxide nanoparticles solution. All solutions were given daily for 30 days orally (15). Animals body weight was measured using digital scales at the end of the experiment and were anesthetized with ether.
Blood samples were taken from the left ventricle of the heart, and the samples were kept in laboratory conditions for 20 min and centrifuged at 3000 rpm for 10 min. Then, the serum of each tube was collected. Serum concentrations of testosterone were determined by enzyme-linked immunosorbent assay.
2.3. Histological tests
After the animals were sacrificed, their left testicles were removed. At the fixation stage, the tissues were fixed in 10% buffered formalin. The dehydration step was performed with the help of alcohol at different concentrations (from low to high). The clearing step was performed by placing the tissues in two containers containing xylene. In the replacement phase, tissues were placed in three containers of molten paraffin (65ºC) for 1 hr. In the molding phase, the parts of Salto Kohart were used. At the cross-section, tissue sections were cut to 4-5 μm in thickness and hematoxylin-eosin dye for staining. All tissue studies were performed under the supervision of a pathologist. Tissue sections were morphometrically examined and imaged by a Dino-Eye-AM 423 camera. In each slide, four points were randomly selected and the walls of seminiferous tubules and the diameter of seminiferous tubules were morphometrically measured. At least five seminiferous tubules were selected from each slide. Numbers of spermatogonia, spermatocytes, spermatids, Sertoli and Leydig cells present in lumen were compared between different groups (17).
In this study, 14 nm zirconium nanoparticles (Figure 1) were used which were determined by X-ray diffraction methods and Transmission Electron Microscopy (TEM) and determination of extraction impurities by ICP-MS technique was 99/993% (Figure 2) (18, 19).


2.4. Ethical consideration
All experiments were conducted according to the protocol provided by the ethics committee of Islamic Azad University Arsanjan Branch (IR.IAU.A.REC.1397.001).
2.5. Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS software, Chicago, Illinois), version 18.0., analysis of variance (ANOVA) and Tukey Test. Statistical inference boundary was statistically significant for the experimental groups receiving different amounts of zirconium oxide nanoparticles at P ≤ 0.05 level compared to the control group. In this study, the results of experiments performed along with the relevant statistical calculations are presented in the form of graphs.
3. Results
While the mean body weights in the experimental groups (I) and (II) did not change significantly compared to control group, the mean body weight in the experimental group (III) significantly decreased compared to the control group (p ≤ 0.05, Figure 3).
The mean testicular weight in all experimental groups receiving different amounts of zirconium oxide nanoparticles did not change significantly compared to the control group (Figure 4).
Interestingly, while the average number of spermatogonial cells in the experimental groups (I) and (II) did not change significantly compared to the control group, the average number of spermatogonial cells in the experimental group (III) decreased significantly compared to the control group (p ≤ 0.01) (Figure 5).
Further, although the average number of spermatocyte cells in the experimental groups (I) and (II) did not change significantly compared to the control group, the average number of spermatocyte cells in the experimental group (III) decreased significantly compared to the control group (p ≤ 0.01) (Figure 5).
However, the average number of spermatid cells in all experimental groups (I), (II) and (III) significantly decreased compared to the control group (p ≤ 0.0001, respectively) (Figure 6).
As for the Leydig cells, the average number of Leydig cells in the experimental groups (I) and (II) did not change significantly compared to the control group. However, the average number of Leydig cells in experimental group (III) decreased significantly compared to the control group (p ≤ 0.05, Figure 7).
Further, the average number of Sertoli cells in the experimental groups (I) and (II) also did not change significantly compared to the control group. However, the average number of Sertoli cells in the experimental group (III) decreased significantly compared to the control group (p ≤ 0.05, Figure 7).
The mean serum testosterone concentration in all experimental groups receiving different amounts of zirconium oxide nanoparticles did not change significantly compared to the control group (Figure 8).
Results of testicular tissue studies
There were no pathological changes in testicular photomicrographs of the control group and testicular tubules, and germ cells appeared normal (Figure 9).
In the experimental group (I), recipients of zirconium oxide nanoparticles (50 ppm) showed a decrease in the density of different types of germ cells compared to the control group (Figure 10).
In the experimental group (II), recipients of zirconium oxide nanoparticles (200 ppm) showed better shrinkage of seminiferous tubules and decreased number of germ cells, especially spermatid cells, compared to the control group (Figure 11).
In the experimental group (III) showed a sharp decrease in the number of seminiferous tubules. The irregularity and disruption of the germinal epithelium and shrinkage of the tubules were visible. In addition, Spermatogonia, Spermatocytes, Spermatids, Sertoli and Leydig cells were noticeable in the lumen. Therefore, tissue degradation is clearly observed in this experimental group (Figure 12).
In the experimental groups receiving different amounts of Zirconium oxide nanoparticles, the number of spermatogonia, primary spermatocytes, and spermatids decreased compared to the control group. These effects are dose dependent and in the receiving group the maximum amount of Zirconium oxide nanoparticles (400 ppm) is higher than the other experimental groups.










4. Discussion
The mean body weight in the experimental group receiving 400 ppm zirconium oxide nanoparticles decreased significantly compared to the control group (p ≤ 0.05). These results are in line with a 2010 study by Kim and co-worker, where it was shown that the administration of 500 mg/kg of silver nanoparticles orally for 13 weeks in male mice resulted in lower body weight and increased blood cholesterol levels (20).
The mean testicular weight in all experimental groups receiving different amounts of zirconium oxide nanoparticles did not change significantly compared to the control group. A study by Fan and co-worker showed that exposure to mice with silicon oxide nanoparticles resulted in a significant decrease in testicular weight. Silicon oxide nanoparticles are likely to reduce the testicular weight of rat by oxidative damage to the testes by increasing ROS (reactive oxygen species) (21).
The average number of Sertoli cells in the experimental group receiving 400 ppm zirconium oxide nanoparticles decreased significantly compared to the control group (p ≤ 0.05). A 2013 study by Talebi and co-worker showed that zinc oxide nanoparticles damage Sertoli cells. The mechanism of the effects of nanoparticles on the testes of mice is not known. Zinc oxide nanoparticles are likely to have deleterious effects on Sertoli cell function through the formation of multinuclear giant cells, increasing vacuoles in Sertoli cells. The results of this study were consistent with the results of the study (22). Also, Braydich-Stolle and co-worker showed that silver nanoparticles decrease ATP levels in Sertoli cells by reducing FYN/Kinase enzyme levels in Sertoli cells. Silver nanoparticles appear to damage the Sertoli cells and the germ cells by reducing the amount of FYN/Kinase enzyme followed by ATP depletion. Silver nanoparticles can disrupt Sertoli cell damage by inhibiting Glial cell-derived neurotrophic factor (GDNF) signaling pathway, which is secreted from Sertoli cells, resulting in a proliferation of spermatogonial cells (23).
Further, the average number of Leydig cells in the experimental group receiving 400 ppm zirconium oxide nanoparticles decreased significantly compared to the control group (p ≤ 0.05). These results are consistent with the study of Talebi and co-worker (22). The results in their study showed that silver nanoparticles can affect Leydig cells and testosterone inhibitor synapses, and the number of Leydig cells was significantly reduced in the groups treated with silver nanoparticles (20).
Omidi and co-worker investigated the effect of zirconium nanoparticles on testosterone, LH, and FSH in female rat. Zirconium nanoparticles led to a significant decrease in plasma levels of testosterone in female rat (15). Testosterone is higher in the male than in the female. In a recent study, the dose of zirconium oxide nanoparticles used was not sufficient to influence testosterone levels in male rats. Therefore, in all groups the level of testosterone decreased but this decrease was not significant (p ≤ 0.05).
Jia and co-worker reported that titanium dioxide nanoparticles significantly reduced serum testosterone levels and expression in rat testes. Titanium dioxide nanoparticles decreased the expression of 17 beta-hydroxy steroids dehydrogenase and P 450 17 alpha-hydroxysteroid dehydrogenase and increased the expression of cytochrome P 450-19 (key enzyme for translating testosterone to estradiol) in mice. Titanium dioxide nanoparticles at the translational level cause changes in testosterone. In addition, titanium dioxide nanoparticles may reduce spermatogenesis by reducing serum testosterone synthesis (24). Due to similarities in zirconium and titanium nanoparticles in their properties, a decrease in serum testosterone level was observed in male rats treated with zirconium nanoparticle oxide, but this decrease was not significant.
Other researchers have shown that nanoparticles lead to the decline of germ cells. Miresmaeili et al reported that silver nanoparticles led to a significant decrease in the number of spermatogonia, primary spermatocytes, spermatids, and spermatozoa (25).
Lan and Yang showed that nanoparticles are capable of crossing the blood-testicular barrier, thereby exerting inflammatory effects on testicular tissue. The nanoparticles do not directly destroy the nucleus membrane. Nanoparticles first affect the cytoskeleton. They then destroy the nucleus membrane. In addition, the nanoparticles may be endocytosed by sex cells or Leydig cells, causing changes in inflammation in the cell and then exocytosis (16).
Cellular and tissue damage can be caused by the extensive production of ROS in inflammatory diseases (26). In addition, increasing ROS production can lead to increased production of external cytokines and further damage (27).
Various studies have shown that zirconium nanoparticles can lead to increased production of ROS and increased caspase-3, mitochondrial membrane potential, and DNA strand breaks. Zirconium nanoparticles can cause cellular toxicity by causing mitochondrial damage (28, 13).
Studies have shown that exposure to zirconium oxide nanoparticles resulted in a significant decrease in Glutathione peroxidase (GPx) in rat. In fact, the imbalance between the production of free radicals and their ability to detoxify by GPx in the molecular mechanism of toxicity causes cell damage (29, 30, 31). Concerning the results, it seems that irconium oxide nanoparticles decrease spermatogenesis through the mechanisms described. It is also possible that zirconium oxide nanoparticles may decrease spermatogenesis by reducing serum testosterone synthesis.
5. Conclusion
Studies have shown that zirconium oxide nanoparticles can damage testicular tissue, which is dose-dependent. In addition, the doses of zirconium oxide nanoparticles in this study did not lead to a significant change in the serum concentration of testosterone.
Acknowledgments
The authors hereby express their deep appreciation for the sincere cooperation of the research deputy of Arsanjan Azad University to conduct this research.
Conflicts of interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Andrology
References
1. Masciangioli T, Zhang WX. Environmental technologies at the nanoscale. Environ Sci Technol 2003; 37: 102-108. [DOI:10.1021/es0323998] [PMID]
2. Erb U, Aust KT, Palumbo G. Nanostructured materials: processing, properties and potential applications. New York, Noyes Publications; 2002: 179-222.
3. Aitken RJ, Chaudhry MQ, Boxall AB, Hull M. Manufacture and use of nanomaterials: current status in the UK and global trends. Occup Med 2006; 56: 300-306. [DOI:10.1093/occmed/kql051] [PMID]
4. Nakarani M, Misra AK, Patel JK, Vaghani SS. Itraconazole nanosuspension for oral delivery: formulation, characterization and in vitro comparison with marketed formulation. Daru 2010; 18: 84-90.
5. Tan K, Cheang P, Ho IAW, Lam PYP, Hui KM. Nanosized bioceramic particles could function as efficient gene delivery vehicles with target specificity for the spleen. Gene Ther 2007; 14: 828-835. [DOI:10.1038/sj.gt.3302937] [PMID]
6. Caicedo M, Jacobs JJ, Reddy A, Hallab NJ. Analysis of metal ion‐induced DNA damage, apoptosis, and necrosis in human (Jurkat) t‐cells demonstrates Ni2+ and V3+ are more toxic than other metals: Al3+, Be2+, Co2+, Cr3+, Cu2+, Fe3+, Mo5+, Nb5+, Zr2+. J Biomed Mater Res Part A 2008; 86: 905-913. [DOI:10.1002/jbm.a.31789] [PMID]
7. Wang ML, Tuli R, Manner PA, Sharkey PF, Hall DJ, Tuan RS. Direct and indirect induction of apoptosis in human mesenchymal stem cells in response to titanium particles. J Orthop Res 2003; 21: 697-707. [DOI:10.1016/S0736-0266(02)00241-3]
8. Brunner TJ, Wick P, Manser P, Spohn P, Grass RN, Limbach LK, et al. In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environ Sci Technol 2006; 40: 4374-4381. [DOI:10.1021/es052069i] [PMID]
9. Demir E, Burgucu D, Turna F, Aksakal S, Kaya B. Determination of TiO2, ZrO2, and Al2O3 nanoparticles on genotoxic responses in human peripheral blood lymphocytes and cultured embyronic kidney cells. J Toxicol Environ Health A 2013; 76: 990-1002. [DOI:10.1080/15287394.2013.830584] [PMID]
10. Ye M, Shi B. Zirconia nanoparticles-induced toxic effects in osteoblast-like 3T3-E1 cells. Nanoscale Res Lett 2018; 13: 353-364. [DOI:10.1186/s11671-018-2747-3] [PMID] [PMCID]
11. Gad MM, Al-Thobity AM, Shahin SY, Alsaqer BT, Ali AA. Inhibitory effect of zirconium oxide nanoparticles on Candida albicans adhesion to repaired polymethyl methacrylate denture bases and interim removable prostheses: a new approach for denture stomatitis prevention. Int J Nanomedicine 2017; 12: 5409-5419. [DOI:10.2147/IJN.S142857] [PMID] [PMCID]
12. Banerjee K, Prithviraj M, Augustine N, Pradeep SP, Thiagarajan P. Analytical characterization and antimicrobial activity of nano zirconia particles. J Chem Pharm Sci 2016; 9: 1186-1190.
13. Atalay H, Çeli̇k A, Ayaz F. Investigation of genotoxic and apoptotic effects of zirconium oxide nanoparticles (20 nm) on L929 mouse fibroblast cell line. Chem Biol Interact 2018; 296: 98-104. [DOI:10.1016/j.cbi.2018.09.017] [PMID]
14. Arefian Z, Pishbin F, Negahdary M, Ajdary M. Potential toxic effects of Zirconia Oxide nanoparticles on liver and kidney factors. Biomedical Research 2015; 26: 89-97.
15. Omidi S, Negahdary M, Aghababa H. The effects of zirconium oxide nanoparticles on FSH, LH and testosterone hormones in female Wistar rats. Electron J Biol 2015; 11: 46-51.
16. Lan Z, Yang WX. Nanoparticles and spermatogenesis: how do nanoparticles affect spermatogenesis and penetrate the blood-testis barrier. Nanomedicine 2012; 7: 579-596. [DOI:10.2217/nnm.12.20] [PMID]
17. Tripathi UK, Chhillar S, Kumaresan A, Aslam MM, Rajak SK, Nayak S, et al. Morphometric evaluation of seminiferous tubule and proportionate numerical analysis of Sertoli and spermatogenic cells indicate differences between crossbred and purebred bulls. Vet World 2015; 8: 645-650. [DOI:10.14202/vetworld.2015.645-650] [PMID] [PMCID]
18. Pabisch S, Feichtenschlager B, Kickelbick G, Peterlik H. Effect of interparticle interactions on size determination of zirconia and silica based systems-A comparison of SAXS, DLS, BET, XRD and TEM. Chem Phys Lett 2012; 521: 91-97. [DOI:10.1016/j.cplett.2011.11.049] [PMID] [PMCID]
19. Chu M, Nguyen LTH, Mai XT, Thuan DV, Bach LG, Nguyen DC, et al. Nano ZrO2 synthesis by extraction of Zr (IV) from ZrO (NO3) 2 by PC88A, and determination of extraction impurities by ICP-MS. Metals 2018; 8: 851-865. [DOI:10.3390/met8100851]
20. Kim YS, Song MY, Park JD, Song KS, Ryu HR, Chung YH, et al. Subchronic oral toxicity of silver nanoparticles. Part Fibre Toxicol 2010; 7: 20-30. [DOI:10.1186/1743-8977-7-20] [PMID] [PMCID]
21. Fan YO, Zhang YH, Zhang XP, Liu B, Ma YX, Jin YH. Comparative study of nanosized and microsized silicon dioxide on spermatogenesis function of male rats. Wei Sheng Yan Jiu 2006; 35: 549-553.
22. Talebi AR, Khorsandi L, Moridian M. The effect of zinc oxide nanoparticles on mouse spermatogenesis. J Assist Reprod Genet 2013; 30: 1203-1209. [DOI:10.1007/s10815-013-0078-y] [PMID] [PMCID]
23. Braydich-Stolle LK, Lucas B, Schrand A, Murdock RC, Lee T, Schlager JJ, et al. Silver nanoparticles disrupt GDNF/Fyn kinase signaling in spermatogonial stem cells. Toxicol Sci 2010; 116: 577-589. [DOI:10.1093/toxsci/kfq148] [PMID] [PMCID]
24. Jia F, Sun Z, Yan X, Zhou B, Wang J. Effect of pubertal nano-TiO2 exposure on testosterone synthesis and spermatogenesis in mice. Arch Toxicol 2014; 88: 781-788. [DOI:10.1007/s00204-013-1167-5]
25. Miresmaeili SM, Halvaei I, Fesahat F, Fallah A, Nikonahad N, Taherinejad M. Evaluating the role of silver nanoparticles on acrosomal reaction and spermatogenic cells in rat. Iran J Reprod Med 2013; 11: 423-430.
26. Greenwald RA. Oxygen radicals, inflammation, and arthritis: pathophysiological considerations and implications for treatment. Semin Arthritis Rheum 1991; 20: 219-240. [DOI:10.1016/0049-0172(91)90018-U]
27. Tucci M, Baker R, Benghuzzi H, Hughes J. Levels of hydrogen peroxide in tissues adjacent to failing implantable devices may play an active role in cytokine production. Biomed Sci Instrum 2000; 36: 215-220.
28. Alzahrani FM, Mohammed Saleh Katubi K, Ali D, Alarifi S. Apoptotic and DNA-damaging effects of yttria-stabilized zirconia nanoparticles on human skin epithelial cells. Int J Nanomedicine 2019; 14: 7003-7016. [DOI:10.2147/IJN.S212255] [PMID] [PMCID]
29. Asadpour E, Sadeghnia HR, Ghorbani A, Boroushaki MT. Effect of zirconium dioxide nanoparticles on glutathione peroxidase enzyme in PC12 and N2a cell lines. Iran J Pharm Res 2014; 13: 1141-1148.
30. Mauricio MD, Guerra-Ojeda S, Marchio P, Valles SL, Aldasoro M, Escribano-Lopez I, et al. Nanoparticles in medicine: a focus on vascular oxidative stress. Oxid Med Cell Longev 2018; 2018: 1-20. [DOI:10.1155/2018/6231482] [PMID] [PMCID]
31. Liu Y, Wang S, Wang Z, Ye N, Fang H, Wang D. TiO2, SiO2 and ZrO2 nanoparticles synergistically provoke cellular oxidative damage in freshwater microalgae. Nanomaterials 2018; 8: 95-106. [DOI:10.3390/nano8020095] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








