Sun, Jul 13, 2025
[Archive]
Volume 2, Issue 1 (7-2004)
IJRM 2004, 2(1): 15-22 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hashemitabar M, Ghavamizadeh B, Javadnia F, Sadain E. The Impact of Ovarian Stimulation and Luteal Phase Support on. IJRM 2004; 2 (1) :15-22
URL: http://ijrm.ir/article-1-13-en.html
URL: http://ijrm.ir/article-1-13-en.html
Full-Text [PDF 347 kb]
(786 Downloads)
| Abstract (HTML) (3377 Views)
Full-Text: (462 Views)
Introduction
The process of implantation depends on the quality of embryo and receptivity of the uterus. Both quality of embryo (Ertzeid and Storeng 2001) and receptivity of the uterus (Tavaniotou et al., 2002) reduced significantly following the controlled ovarian hyperstimulation (COH) by gonadotropin hormone.
However, the Gonadotropins have been widely used for COH during in vitro fertilization (IVF) procedures. It must be born in mind the negative role
of ovarian stimulation on the quality of embryo which reduce the number of blastomers and increase the fragmentation of embryo in preimplantation stage (Van der Auwera and Hooghe 2001). On the other hand, using hMG in an IVF protocols may exert the release of a high level of estrogen in vivo. The estrogen with a positive feedback lead to the early surge of the LH and early luteal phase induction and so proceeding of implantation window to open in an earlier time than in natural cycle. This may be reflected to a defective asynchrony in embryo-endometrial interaction and low level of pregnancy rate (Valbuena et al., 1999). It is not exactly clear whether the COH will cause the unreceptivity of uterus directly, or it is distrupted the estrogen/ progesterone balance indirectly (Zayed et al., 2003). The embryo transfer to uterus in an IVF cycle (which is often carried out on the second day after insemination at 4 to 8 cells stage) is usually accompanied by closing of implantation window; therefore, it fails to implant successfully (Paulson et al., 1990).
In order to improve the implantation process, it is strongly suggested to use the progestrone in hormone replacement protocols. So, the progesterone improves receptivity of uterus by increasing the proliferation of endometrial stroma cells and coil the endometrial gland consequently to induction of secretary phase to uterus (Ben–Nun et al., 1990). Therefore, the study of preparing uterus by progesterone is a critical step in the assisted reproductive technology (ART) to acquire better result of implantation following the embryo transfer. The LH and the hCG act on the corpus luteum by binding to LH receptor on the granolosa cells; thereby, enhancing the progesterone secretion which has a high potential to improve the implantation rate indirectly (Garcia et al., 1988).
The positive effect of progesterone and hCG for preparation of uterus has been pointed out in replacement hormone therapy and in the natural cycle. We used progesterone and hCG by the same function as the LH to evaluate the possible positive role of either of these regimens in improving the implentation process following the COH protocol
Materials and Methods
The total number of 60 mice (NMRI) aged 6-8 weeks were superovulated by hMG (7.5 IU) intraperitonaly (IP), and 48 hr later with hCG (10 IU) IP. The mice divided to 3 groups (no=20 for each groups): 1 -control; 2- treated by hCG; 3- treated by progesterone. Then, the mice were mated by a male (NMRI) and checked by vaginal plug on the next day to determine the positive plug for confirmation of pregnancy in each group.
The mice in each group was subdivided equaly into two subgroups: first subgroup were studied on day 5 of pregnancy (n=30) and the second subgroup were studied on day 13 of pregnancy (n=30). The study of luteal phase support on day 5 of pregnancy was carried out as follows: 1- No adminstration in group one, using only from COH regimen as a control group (n=10). 2- Using hCG IP (5 IU/day) from day 1-4 of pregnancy in group two (n=10). 3- Using progesterone administration (sc) (1 mg/day) from day 1-4 in group three (n=10).
The positive plug mice were killed by cervical dislocation on day 5 of pregnancy and the uterine horns were flushed by M2 media to count the number of blastocysts and their quality. The quality of embryos was evaluated according to routine IVF procedures (Trounson and Gardner 2000). To study the luteal phase support on day 13 of pregnancy; the above treatment was continued for another 8 days. So, no adminstration in group one (n=10), hCG adminstration in group two (n=10) and progesterone in group three (n=10), as the same manner of first half of each groups. The positive plug mice were killed on day 13 and the implantated embryos were counted.
The histological sections were carried out on both positive and negative plug mice on days 5 and 13 of pregnancy. The samples were fixed by formalin buffer. Then, samples were process by the routine procedures of dehydration, and clearing, followed by impregnation and paraffin blocks. Finally, the 5µm sections were stained using Hematoxilline and Eosin (H&E).
Statistical Analysis was performed by student t-test to compare the mean of embryos among the different groups by using SPSS software.
Results
Embryo Assessment
Table I presents the results generated from three groups of control, hCG treated and progesterone. The hCG and the progesterone adminstration revealed that a significantly lower number of the blastocysts, were obtained when compared to control group. It also showed a significantly decrease in the quality of embryos in hCG group, but not in progesterone group; whereas, there was a retardation in cleavage rate. The mean number of embryos that reached the blastocyst stage (54%) was significantly lower in progestron group when compared to control group (75% P< 0.05).
The total number of implanted embryos were 29/2 mice in hCG treated group, which was 13 living fetus in one and 16 resorptions fetus in another mouse.No embryo implantation was observed in the other groups.
Morphological assessment of utrian horn
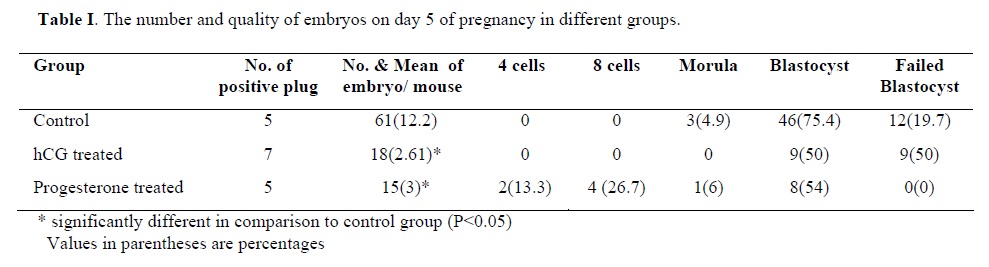
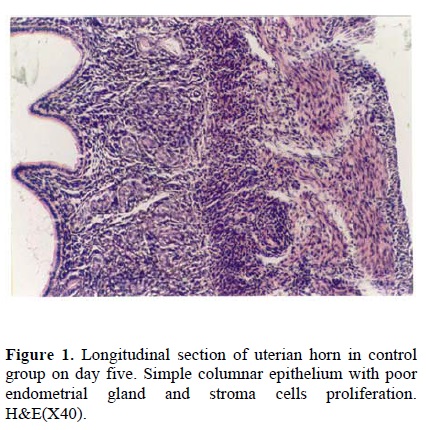
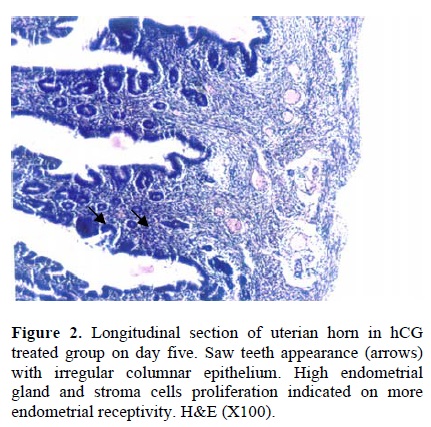
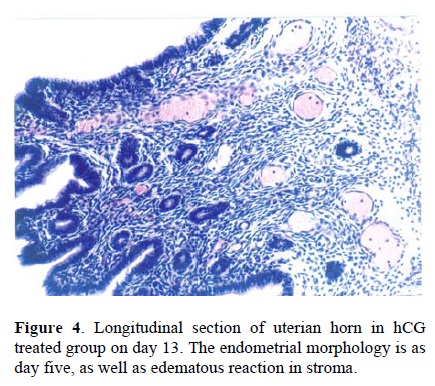
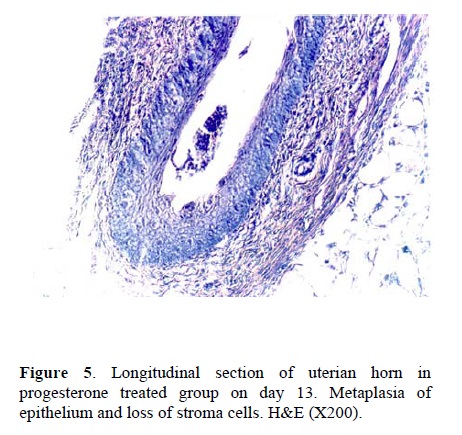
Following the COH with hMG in control group, the morphology of the endometrium changed to undeveloped state as; a poor storma, poor endometrial glands and a regular simple columnar epithelium (Fig. 1). Treatment with hCG following COH in group two changed the endometrial morphology to a developed state as; increasing the stormal cells proliferation, increasing the number of endometrial glands and changing the epithelium to irregular simple columnar. The most important index in improvement endometrium was establishment of saw-teeth appearance (Fig. 2). The progesterone treatment following the COH in group three improved all the indexes such as: stroma and glandular cells proliferation, but there was no saw teeth appearance (Fig 3). The morphological changes of endometrium on day 13 was as same as day 5 in groups one and two. The continued treatment with hCG improved the receptivity of uterus showed by observation of more arterial profiles in lamina propria layer (Fig. 4). However, continued adminstration of progesterone strongly affected the morphology of endometrium by metaplasia induction and changing the epithelium to stratified layer as well as elimination of stroma cells and lamina propria layer (Fig. 5).
Morphological assessment of ovary
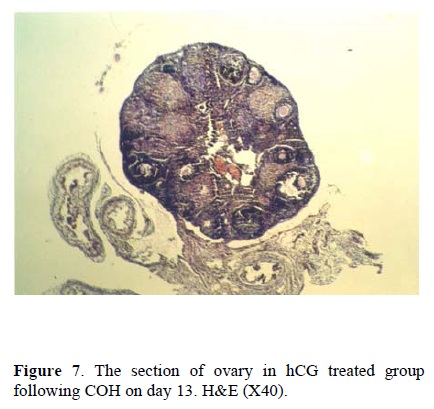
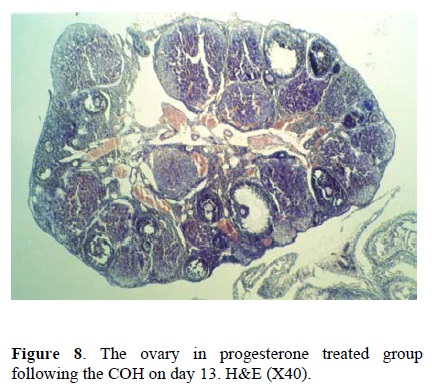
Considering the size of corpus luteum, the morphological study of the ovary on day 5 showed no differences between the three groups. This suggest that using the hCG and progesterone treatment in groups two and three has no considerable effects on the size of corpus luteum in comparison to control group. The morphological changes on day 13 showed that the number of immature follicles increased in control group (fig. 6), while the treatment by hCG and progesterone have no such consequences. The continued adminstration of hCG caused the hypotrophy of ovary and decreased the size of corpus luteum (fig. 7). Continued injection of progesterone has no effect on the size of corpus luteum and the number of primary or secondary follicles (fig. 8).

Discussion
The purpose of embryo evaluation our study was to investigate whether the oversecration of esteroid hormones orhCG may affect the quality of the embryo in oviduct and uterine horn. Ertzeid and Storeng (2001) previously reported that in natural cycle, the number of mice embryo in one cell stage reaching to blastocyst stage was 60% versus 41% in an COH cycle. Auwera Vander et al., (1999) also reported a similar result in a case of COH cycle and showed that implantation rate improves following embryo retrieval and in vitro culture until blastocyst stage for transfer to pseudopregnant mice. The results of this study also indicate that manipulation of uterus for superovulation by progestrone or hCG for inhancing the implantation rate may impair the oviduct microenvironment. As in our study, the hCG and progestrone decreased the number of embryo significantly from 12.2% in control group to 2.6% and 3% in hCG and progesterone group respectively. In addition, the number of failed blastocysts increased from 19.7% in control to 50% in hCG treated group. Although, the main goal of using hCG treatment is to improve the implantation rate, but our study showed that it impaired the quality of embryo as well this effect could be due to alteration of oviduct milieu, following the superovulation and hCG therapy which may exerts an abnormal effect on oviductal secretion (growth factor and proteins) necessary for early embryo development (Boatman 1997).
In the case of progestrone treated group, there was a retardation in cleavage division as well as smaller number of embryos compared to controls. Juneja (1995) explained that the blocking of pregestrone with monoclonal antibody RU486 in preimplant stage of embryos will cause the cleavage retardation in vivo and in vitro. Certainly, there is a receptor for progestrone transport in the embryo cells which activate the cleavage division, but it is not clear why both the Progestrone (in our study) and Antiprogestrone drugs (in the above study) act with the same manner and cause the retardation in cleavage division. In addition, the results of our study for implanted embryos in hCG treated group indicated that from 5 positive plug mice, 29 embryos implanted per 2 mice. This is a promising result for implantation rate. So, to determine the positive effect of hCG on uterus, the morphological assessment designed on all the positive and negative plug mice. This positive effect on days 5 and 13 was defected as the acceleration of stroma and glandular cells proliferation and consequently the improvement of receptivity of uterus by means of more secretion of endogenous progesterone from theca cells of corpus luteum. But, more recently Ku et al., (2002) and Zhou et al., (1999) have shown that presence of the LH/hCG receptors on human endometrial cells means that the endometrial cells can directly respond to hCG by increasing the vasodilation action of uterian arteries. Also, by more differentiation of stroma cells to react as decidualization as well as edeomatous reaction and by stimulation of epithelial cells to interact with blastocyst and continuation of pregnancy. Therefore, both direct or indirect effect of hCG in maintenance of corpus luteum confirmed the efficiency of hCG in progression of implantation. This was in contrast to exogenous progesterone which in the short time usage, despite of its positive effect on morphology of uterus, is insufficient to support a successful pregnancy. Also, in long term adminstration there is a metaplesia in epithelium and elimination of stroma cells instead of implantation improvement.
Therefore, in this study the progesterone has no advantages in optimization of implantation process; although, it is a conventional method in preparation of uterus in routine IVF cycle and in hormone replacement method for embryo transfer (Navot et al., 1989). Therefore, we suggest more safety effects for hCG instead of progesterone in maintenance of implantation event. Of course, we have to be aware of the serious effect of hCG on the quality of early embryo. Therefore, more investigations are nessessary to solve this controversy between the hCG effect on quality of embryo and its effect on implantation improvement.
Acknowledgement
We would like to thank the Medical Research Council of Ahwaz Medical University for the financial support.
The process of implantation depends on the quality of embryo and receptivity of the uterus. Both quality of embryo (Ertzeid and Storeng 2001) and receptivity of the uterus (Tavaniotou et al., 2002) reduced significantly following the controlled ovarian hyperstimulation (COH) by gonadotropin hormone.
However, the Gonadotropins have been widely used for COH during in vitro fertilization (IVF) procedures. It must be born in mind the negative role
of ovarian stimulation on the quality of embryo which reduce the number of blastomers and increase the fragmentation of embryo in preimplantation stage (Van der Auwera and Hooghe 2001). On the other hand, using hMG in an IVF protocols may exert the release of a high level of estrogen in vivo. The estrogen with a positive feedback lead to the early surge of the LH and early luteal phase induction and so proceeding of implantation window to open in an earlier time than in natural cycle. This may be reflected to a defective asynchrony in embryo-endometrial interaction and low level of pregnancy rate (Valbuena et al., 1999). It is not exactly clear whether the COH will cause the unreceptivity of uterus directly, or it is distrupted the estrogen/ progesterone balance indirectly (Zayed et al., 2003). The embryo transfer to uterus in an IVF cycle (which is often carried out on the second day after insemination at 4 to 8 cells stage) is usually accompanied by closing of implantation window; therefore, it fails to implant successfully (Paulson et al., 1990).

In order to improve the implantation process, it is strongly suggested to use the progestrone in hormone replacement protocols. So, the progesterone improves receptivity of uterus by increasing the proliferation of endometrial stroma cells and coil the endometrial gland consequently to induction of secretary phase to uterus (Ben–Nun et al., 1990). Therefore, the study of preparing uterus by progesterone is a critical step in the assisted reproductive technology (ART) to acquire better result of implantation following the embryo transfer. The LH and the hCG act on the corpus luteum by binding to LH receptor on the granolosa cells; thereby, enhancing the progesterone secretion which has a high potential to improve the implantation rate indirectly (Garcia et al., 1988).
The positive effect of progesterone and hCG for preparation of uterus has been pointed out in replacement hormone therapy and in the natural cycle. We used progesterone and hCG by the same function as the LH to evaluate the possible positive role of either of these regimens in improving the implentation process following the COH protocol
Materials and Methods
The total number of 60 mice (NMRI) aged 6-8 weeks were superovulated by hMG (7.5 IU) intraperitonaly (IP), and 48 hr later with hCG (10 IU) IP. The mice divided to 3 groups (no=20 for each groups): 1 -control; 2- treated by hCG; 3- treated by progesterone. Then, the mice were mated by a male (NMRI) and checked by vaginal plug on the next day to determine the positive plug for confirmation of pregnancy in each group.
The mice in each group was subdivided equaly into two subgroups: first subgroup were studied on day 5 of pregnancy (n=30) and the second subgroup were studied on day 13 of pregnancy (n=30). The study of luteal phase support on day 5 of pregnancy was carried out as follows: 1- No adminstration in group one, using only from COH regimen as a control group (n=10). 2- Using hCG IP (5 IU/day) from day 1-4 of pregnancy in group two (n=10). 3- Using progesterone administration (sc) (1 mg/day) from day 1-4 in group three (n=10).
The positive plug mice were killed by cervical dislocation on day 5 of pregnancy and the uterine horns were flushed by M2 media to count the number of blastocysts and their quality. The quality of embryos was evaluated according to routine IVF procedures (Trounson and Gardner 2000). To study the luteal phase support on day 13 of pregnancy; the above treatment was continued for another 8 days. So, no adminstration in group one (n=10), hCG adminstration in group two (n=10) and progesterone in group three (n=10), as the same manner of first half of each groups. The positive plug mice were killed on day 13 and the implantated embryos were counted.
The histological sections were carried out on both positive and negative plug mice on days 5 and 13 of pregnancy. The samples were fixed by formalin buffer. Then, samples were process by the routine procedures of dehydration, and clearing, followed by impregnation and paraffin blocks. Finally, the 5µm sections were stained using Hematoxilline and Eosin (H&E).
Statistical Analysis was performed by student t-test to compare the mean of embryos among the different groups by using SPSS software.
Results
Embryo Assessment
Table I presents the results generated from three groups of control, hCG treated and progesterone. The hCG and the progesterone adminstration revealed that a significantly lower number of the blastocysts, were obtained when compared to control group. It also showed a significantly decrease in the quality of embryos in hCG group, but not in progesterone group; whereas, there was a retardation in cleavage rate. The mean number of embryos that reached the blastocyst stage (54%) was significantly lower in progestron group when compared to control group (75% P< 0.05).
The total number of implanted embryos were 29/2 mice in hCG treated group, which was 13 living fetus in one and 16 resorptions fetus in another mouse.No embryo implantation was observed in the other groups.




Morphological assessment of utrian horn
Following the COH with hMG in control group, the morphology of the endometrium changed to undeveloped state as; a poor storma, poor endometrial glands and a regular simple columnar epithelium (Fig. 1). Treatment with hCG following COH in group two changed the endometrial morphology to a developed state as; increasing the stormal cells proliferation, increasing the number of endometrial glands and changing the epithelium to irregular simple columnar. The most important index in improvement endometrium was establishment of saw-teeth appearance (Fig. 2). The progesterone treatment following the COH in group three improved all the indexes such as: stroma and glandular cells proliferation, but there was no saw teeth appearance (Fig 3). The morphological changes of endometrium on day 13 was as same as day 5 in groups one and two. The continued treatment with hCG improved the receptivity of uterus showed by observation of more arterial profiles in lamina propria layer (Fig. 4). However, continued adminstration of progesterone strongly affected the morphology of endometrium by metaplasia induction and changing the epithelium to stratified layer as well as elimination of stroma cells and lamina propria layer (Fig. 5).
Morphological assessment of ovary
Considering the size of corpus luteum, the morphological study of the ovary on day 5 showed no differences between the three groups. This suggest that using the hCG and progesterone treatment in groups two and three has no considerable effects on the size of corpus luteum in comparison to control group. The morphological changes on day 13 showed that the number of immature follicles increased in control group (fig. 6), while the treatment by hCG and progesterone have no such consequences. The continued adminstration of hCG caused the hypotrophy of ovary and decreased the size of corpus luteum (fig. 7). Continued injection of progesterone has no effect on the size of corpus luteum and the number of primary or secondary follicles (fig. 8).




Discussion
The purpose of embryo evaluation our study was to investigate whether the oversecration of esteroid hormones orhCG may affect the quality of the embryo in oviduct and uterine horn. Ertzeid and Storeng (2001) previously reported that in natural cycle, the number of mice embryo in one cell stage reaching to blastocyst stage was 60% versus 41% in an COH cycle. Auwera Vander et al., (1999) also reported a similar result in a case of COH cycle and showed that implantation rate improves following embryo retrieval and in vitro culture until blastocyst stage for transfer to pseudopregnant mice. The results of this study also indicate that manipulation of uterus for superovulation by progestrone or hCG for inhancing the implantation rate may impair the oviduct microenvironment. As in our study, the hCG and progestrone decreased the number of embryo significantly from 12.2% in control group to 2.6% and 3% in hCG and progesterone group respectively. In addition, the number of failed blastocysts increased from 19.7% in control to 50% in hCG treated group. Although, the main goal of using hCG treatment is to improve the implantation rate, but our study showed that it impaired the quality of embryo as well this effect could be due to alteration of oviduct milieu, following the superovulation and hCG therapy which may exerts an abnormal effect on oviductal secretion (growth factor and proteins) necessary for early embryo development (Boatman 1997).
In the case of progestrone treated group, there was a retardation in cleavage division as well as smaller number of embryos compared to controls. Juneja (1995) explained that the blocking of pregestrone with monoclonal antibody RU486 in preimplant stage of embryos will cause the cleavage retardation in vivo and in vitro. Certainly, there is a receptor for progestrone transport in the embryo cells which activate the cleavage division, but it is not clear why both the Progestrone (in our study) and Antiprogestrone drugs (in the above study) act with the same manner and cause the retardation in cleavage division. In addition, the results of our study for implanted embryos in hCG treated group indicated that from 5 positive plug mice, 29 embryos implanted per 2 mice. This is a promising result for implantation rate. So, to determine the positive effect of hCG on uterus, the morphological assessment designed on all the positive and negative plug mice. This positive effect on days 5 and 13 was defected as the acceleration of stroma and glandular cells proliferation and consequently the improvement of receptivity of uterus by means of more secretion of endogenous progesterone from theca cells of corpus luteum. But, more recently Ku et al., (2002) and Zhou et al., (1999) have shown that presence of the LH/hCG receptors on human endometrial cells means that the endometrial cells can directly respond to hCG by increasing the vasodilation action of uterian arteries. Also, by more differentiation of stroma cells to react as decidualization as well as edeomatous reaction and by stimulation of epithelial cells to interact with blastocyst and continuation of pregnancy. Therefore, both direct or indirect effect of hCG in maintenance of corpus luteum confirmed the efficiency of hCG in progression of implantation. This was in contrast to exogenous progesterone which in the short time usage, despite of its positive effect on morphology of uterus, is insufficient to support a successful pregnancy. Also, in long term adminstration there is a metaplesia in epithelium and elimination of stroma cells instead of implantation improvement.
Therefore, in this study the progesterone has no advantages in optimization of implantation process; although, it is a conventional method in preparation of uterus in routine IVF cycle and in hormone replacement method for embryo transfer (Navot et al., 1989). Therefore, we suggest more safety effects for hCG instead of progesterone in maintenance of implantation event. Of course, we have to be aware of the serious effect of hCG on the quality of early embryo. Therefore, more investigations are nessessary to solve this controversy between the hCG effect on quality of embryo and its effect on implantation improvement.
Acknowledgement
We would like to thank the Medical Research Council of Ahwaz Medical University for the financial support.
Type of Study: Original Article |
References
1. Auwera Van der I., Pijhenborg, and Koninckx P.R. (1999) The influence of in vitro culture versus stimulated and untreated oviductal environment on mouse embryo development and implantation. Hum Reprod 14 (10): 2570-2574. [DOI:10.1093/humrep/14.10.2570] [PMID]
2. Auwera van der I., and Hooghe T.D. (2001) Superovulation of female mice delays embryonic and fetal development. Hum Reprod 16(6): 1237-1243. [DOI:10.1093/humrep/16.6.1237] [PMID]
3. Ben-Nun I., Ghetler Y., Jaffe R., Siegal A., Kaeti H., and Fejgin M. (1990) Effect of preovulatory progesterone administration on the endometrial maturation and implantation rate after in vivo fertilization and embryo transfer. Fertil Steril 53(2): 276-281. [DOI:10.1016/S0015-0282(16)53281-6]
4. Boatman D.E. (1997) Responses of gametes to oviductal environment. Human reproductive, 12 Natl. Suppl. JBFS 2(2): 133-149.
5. Ertzeid G., and Storeng R. (2001) The impact of ovarian stimulation on implantation and fetal development in mice. Hum reprod 16(2):221-225. [DOI:10.1093/humrep/16.2.221] [PMID]
6. Garcia E., Bouchard P., Debrux J., Berdah J., Frydman R., Schaison G., Milgrom E., and Perrot Applant (1988) Use of immunocytochemistry of progesterone and Estrogen Receptors for Endometrial dating. J of Clin Endocrin and Metabol 67:80-87. [DOI:10.1210/jcem-67-1-80] [PMID]
7. Juneja S., and Dodson M. G. (1995) Ova recovery and in vitro fertilization of ova from Ru-486 treated PMSG/hCG primed mice. Reprod. Fertil Dev. 7: 1243-1248. [DOI:10.1071/RD9951243] [PMID]
8. Ku S.Y., Choi Y.M., Suh C.S., Kim S.H., Kim J.G.., Moon S.Y., and Lee L.Y. (2002). Effect of gonadotropins on human endometrial stromal Cell proliferation in vitro. Arch Gynecol. Obstet. 266(4): 223-228. [DOI:10.1007/s00404-002-0292-9] [PMID]
9. Navot D. , Anderson Ted l., Droesch K., Scott T.R., Kreiner D., and Rosenwaks Z. (1989) Hormonal manipulation of clinical Endocrinology and Metabolism, 68(4): 801-807. [DOI:10.1210/jcem-68-4-801] [PMID]
10. Paulson R.S., Sauer M.V., and Lobo R.A. (1990) Embryo implantation after human in vitro fertilization: Importance of endometrial receptivity. Ferti Steril 53(5), 870 -874. [DOI:10.1016/S0015-0282(16)53524-9]
11. Tavaniotou A., Albanoe C., Smitz J., and Devroey P. (2002) Impact of ovarian stimulation corpus luteum function and embryonic implantation. J. Reprod. Immunol. 55(1-2):123-130. [DOI:10.1016/S0165-0378(01)00134-6]
12. Trounson Alan O., and Gardner David R. (2000) Handbook of in vitro fertilization. CRC press. 174 and 248.
13. Valbuena D., Jasper M., Remohi J., Pellieer A., and Simon C. (1999) Ovarian Stimulation and endometrial receptivity. Hum Reprod 14(Suppl 2)107-111. [DOI:10.1093/humrep/14.suppl_2.107] [PMID]
14. Zayed F.F., El-Jallad M.F., and Al-Chalabi H.A. (2003) Luteal phase support in ovarian induction cycles using human chorionic gonadotropin or oral progestagens. Saudi Med. J., 24(1) 134-136.
15. Zhou X.L. , Lei Z.M., and Rod Ch.V. (1999) Treatment of human endometrial gland epithelial cells with choronic gonadotropin / Luteinizing Hormone increases the expression of the cyclooxygenase -2 Gene. J of Clin Endocrin and Metabol 48 (9): 3364-3377. [DOI:10.1210/jcem.84.9.5943] [PMID]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




