Thu, Apr 18, 2024
[Archive]
Volume 18, Issue 6 (June 2020)
IJRM 2020, 18(6): 471-476 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ghaffari F, Miralaie S, Chekini Z, Faridi M. Pregnancy after frozen embryo transfer in mycobacterium tuberculous salpingitis: A case report and literature review. IJRM 2020; 18 (6) :471-476
URL: http://ijrm.ir/article-1-1308-en.html
URL: http://ijrm.ir/article-1-1308-en.html
1- Department of Endocrinology and Female Infertility, Reproductive Biomedicine Research Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran. , ghafaryf@yahoo.com
2- Department of Endocrinology and Female Infertility, Reproductive Biomedicine Research Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran.
3- Department of Surgery, Iranmehr Hospital, Tehran, Iran.
2- Department of Endocrinology and Female Infertility, Reproductive Biomedicine Research Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran.
3- Department of Surgery, Iranmehr Hospital, Tehran, Iran.
Full-Text [PDF 793 kb]
(796 Downloads)
| Abstract (HTML) (2132 Views)
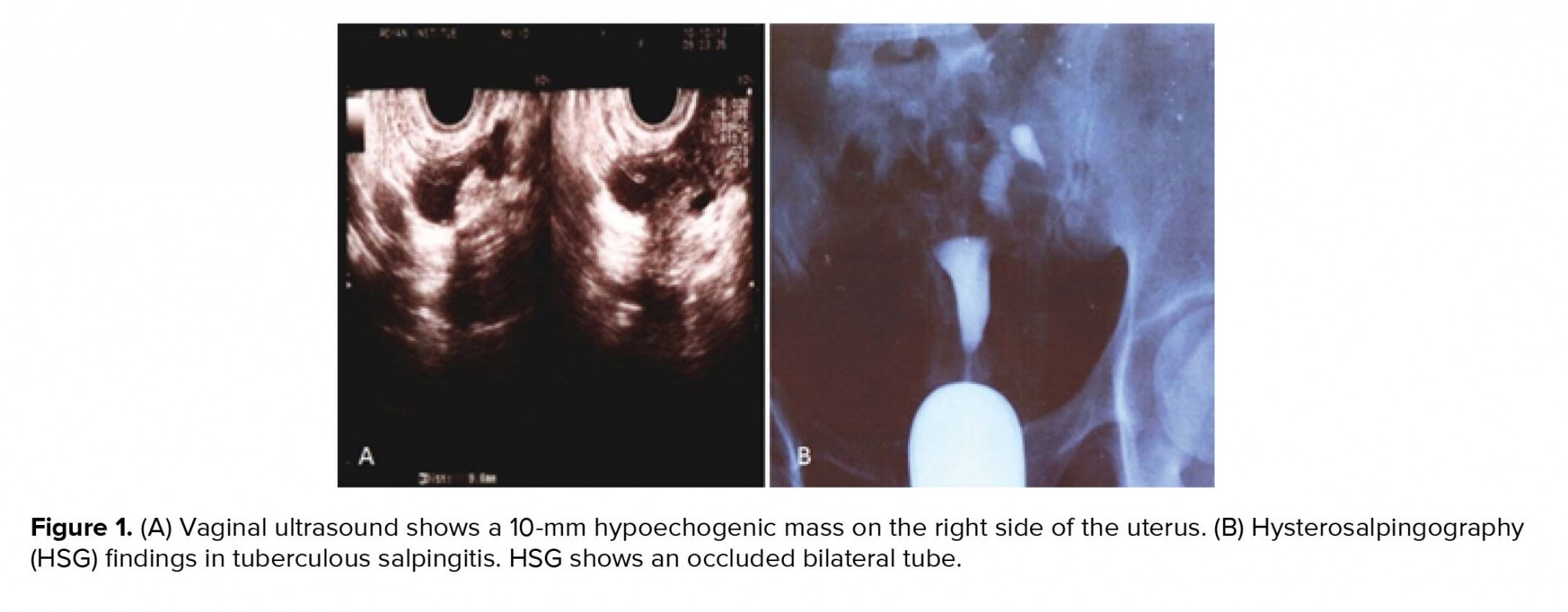
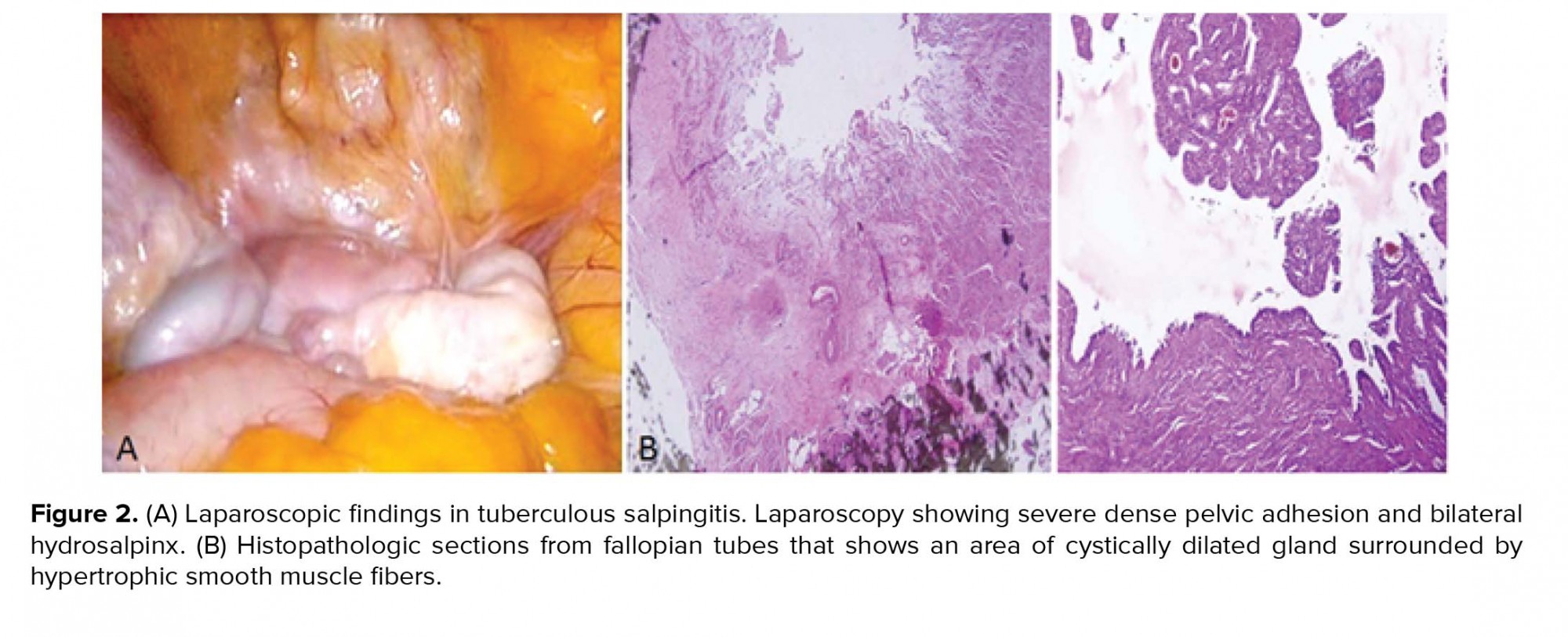
At the first admission in our institute, she had a negative tuberculin test (PPD) and a normal chest X-ray with no clinical evidence of TB. Hormonal evaluation on day 3 of the menstrual cycle showed a follicle-stimulating hormone (FSH) level of 6.02 mIU/ml and luteinizing hormone (LH) level of 7.2 mIU/ml. Vaginal ultrasound results revealed a 10-mm hypoechogenic structure in the right adnexa that agreed with a right hydrosalpinx (Figure 1A). Hysterosalpingography (HSG) results showed a bilateral hydrosalpinx (Figure 1B). Infertility workup for the husband indicated that he had a normal sperm analysis according to the World Health Organization criteria (5). The patient underwent ovarian stimulation according to the standard long protocol where she received 0.1 mg/day Decapeptyl (Ipsen Pharma Biotech, France) from day 20 of the pre-stimulation cycle until the day of the hCG injection. Once the down-regulation was confirmed, from day 3 of the new menstrual cycle, she received subcutaneous injections of 2 ampules/day of Gonal-F (Merck Serono, Darmstadt, Germany). Follicular growth was monitored by serial transvaginal ultrasound and repeated until the detection of at least three follicles > 18 mm in diameter. At that time, the patient received an intramuscular injection of hCG (10000 IU). After 36 hours, 21 oocytes were retrieved. Of these, 15 oocytes were fertilized and cleaved. Because of the risk for ovarian hyperstimulation syndrome, all embryos were frozen on the third day. After three months, the patient elected to undergo a laparoscopy/hysteroscopy due to hydrosalpinx (es). Laparoscopic findings included dense adhesions of the pelvic organs and a frozen pelvis. The surgeon could not perform a salpingectomy or proximal tubal occlusion so both ostium of the uterine tube were cauterized by hysteroscopy according to a previously reported procedure (6). However, direct smear and bacterial culture of endometrial tissue and evaluation of tissue by polymerase chain reaction (PCR) were negative for Mycobacterium TB. After eight months, the patient underwent FET. After GnRh agonist down-regulation, she received estradiol valerate (Aburaihan Co., Iran) as hormone replacement therapy in the following doses: 4 mg/day for the first six days, followed by 6 mg/day for another six days, until endometrial thickness reached 9 mm. When the optimal endometrial thickness was obtained, intramuscular progesterone (100 mg; Aburaihan Co, Iran) was administered and three days later, two good-quality embryos were transferred. The luteal phase was supported with estradiol and progesterone. However, she did not achieve pregnancy. One year later the patient underwent a laparoscopy. A skillful laparoscopist carried out the operation. There was a severe dense pelvic adhesion and frozen pelvis (Figure 2A). After adhesiolysis, she had a bilateral hydrosalpinx and both tubes were removed. The next day, the patient was released without any problem. Surgical specimens were collected and stained with hematoxylin and eosin and evaluated with light microscopy. We observed cystically dilated glands surrounded by hypertrophic smooth muscle fibers, which demonstrated bilateral hydrosalpingitis isthmica nodosa (Figure 2B). After one year, she underwent a second artificial FET, which was similar to the previous transfer. When the endometrial thickness reached 9.5 mm, we transferred three high-quality embryos. The luteal phase was supported by 100 mg of daily progesterone injections (Aburaihan Co., Tehran, Iran). Biochemical pregnancy was detected by a β-hCG test 2 wk after the embryo transfer and clinical pregnancy was confirmed by the presence of a gestational sac with fetal echoes at 6 wk after embryo transfer. The patient was followed and had a cesarean delivery at 38 wk of gestation. Her child was a healthy girl who weighed 3,100 g.
2.1. Ethical consideration
The patient signed the written informed consent for reporting this case.
3. Discussion
GTB is a relatively rare chronic disease with unspecific symptoms of menstrual disturbance, vaginal discharge, pelvic pain, and a considerable cause of PID (4). Conversely, GTB occasionally remains undetected on clinical examination and is often diagnosed during an infertility evaluation (1).
The potential risk factors for a TB infection include a past history of TB, low socioeconomic status, HIV-seropositive persons (the highest risk among TB-infected individuals is clearly HIV co-infection, which suppresses cellular immunity), immigrants from countries with a high prevalence of TB, and Mycobacteriology laboratory personnel (7). In the current report, the patient had reported a history of TB 10 years ago, but did not have other risk factors, such as AIDS or drug addiction, that predisposed her to GTB.
HSG, laparoscopy, endometrial tissue biopsy, PCR, and histopathologic examination are useful instruments for the diagnosis of pelvic TB (3). TB has various appearances on HSG that include specific changes such as beaded tube, golf club tube, pipestem tube, cobble stone tube, leopard skin tube in addition to nonspecific changes of hydrosalpinx, tubal occlusion, hydroconvoluted tube, and tubal fixity. The effects of GTB on the endometrium may be seen as specific features and include a T-shaped uterine appearance, pseudounicornuate uterus, and collar stud abscess (3, 6). However, tubal obstruction is a common HSG finding in the majority of GTB cases (1). The HSG finding in this case was bilateral hydrosalpinx that was confirmed by laparoscopy. However, bacterial culture of the endometrial tissue and PCR were negative. In this case, the first attempt laparoscopy was not successful due to severe destruction of the tubes and pelvic adhesions. In the second laparoscopy, performed by a more experienced laparoscopist, a salpingectomy was performed with difficulty but had a favorable outcome.
The most common causes of PID are Chlamydia trachomatis, Neisseria gonorrhea, and anaerobic organisms. However, GTB may cause PID and distal tubal occlusion from salpingitis or peritubal adhesions, and subsequently hydrosalpingus (1, 3, 4). TB salpingitis can be either unilateral or bilateral, and manifested by thickened and enlarged tubal walls that turn into fibrosis and scar tissue (3). Hydrosalpinx fluid potentially has embryotoxic components and growth-inhibiting factors. The hydrosalpinx fluid may also decrease endometrial receptivity and embryo implantation. The possibility of the mechanical effect of fluid and wash-out of embryos through leakage of fluid through the endometrial cavity should be considered (8). Salpingectomy can lead to improved clinical pregnancy rate and live birth rates in patients whose infertility is the result of tuberculous salpingitis, in particular those exposed to toxic hydrosalpingeal fluid (2, 6). Dense pelvic adhesions due to salpingitis disturbances in ovarian function (2), and a higher level of FSH and LH, lower mean ovarian volume, and number of antral follicles demonstrate poor ovarian reserve in GTB (9). Possible explanations for the poor results could be endometrial insufficiency, poor response to gonadotropins, and low-quality oocytes and embryos (2, 9).
IVF procedures after anti-TB treatment have been recommended by several researchers and particularly for patients with damaged tubes and undamaged endometria (3). The pregnancy rate in such GTB cases was similar to women with infertility due to tubal factor; however, the pregnancy rate in unilateral hydrosalpinx was higher compared to bilateral hydrosalpinx (3).
Caliskan and co-workers describe GTB cases who underwent IVF cycles after antitubercular therapy and salpingectomy resulted in 15.5% clinical pregnancy per ET and 9% live birth. However, seven (33.3%) of them had hydrosalpinx and only two (9.5%) of which had severe pelvic adhesions (8). Salpingectomy before IVF cycles were reported in the underlying indication of hydrosalpinx such as ectopic pregnancy, pelvic adhesions, pyosalpinx, hematosalpinx, and hydrosalpinx (10).
Since salpingectomy has a potential risk in the frozen pelvis due to severe salpingitis, an alternative approach is cauterized ostium. In the current case, this method resulted in failed implantation. We suggested the only definitive treatment for this patient was a salpingectomy, which leads to success in implantation and lives birth.
4. Conclusion
Tuberculous salpingitis is a tubal cause for infertility, especially in high prevalence countries. IVF-ET provides a treatment for tubal TB with receptive endometrium. Laparoscopic salpingectomy prior to embryo transfer plays a critical role in predicting the occurrence of a pregnancy in a patient with hydrosalpingitis especially attributed to TB. GTB is a granulomatous disease where inflammation distorts the pelvic tissues, causing severe adhesions and fibrosis. An experienced laparoscopist should perform the surgery in order to prevent trauma to the pelvis and abdomen.
Conflict of Interest
The authors declare that they have no conflict of interest.
Full-Text: (516 Views)
- Introduction
- Case Report
At the first admission in our institute, she had a negative tuberculin test (PPD) and a normal chest X-ray with no clinical evidence of TB. Hormonal evaluation on day 3 of the menstrual cycle showed a follicle-stimulating hormone (FSH) level of 6.02 mIU/ml and luteinizing hormone (LH) level of 7.2 mIU/ml. Vaginal ultrasound results revealed a 10-mm hypoechogenic structure in the right adnexa that agreed with a right hydrosalpinx (Figure 1A). Hysterosalpingography (HSG) results showed a bilateral hydrosalpinx (Figure 1B). Infertility workup for the husband indicated that he had a normal sperm analysis according to the World Health Organization criteria (5). The patient underwent ovarian stimulation according to the standard long protocol where she received 0.1 mg/day Decapeptyl (Ipsen Pharma Biotech, France) from day 20 of the pre-stimulation cycle until the day of the hCG injection. Once the down-regulation was confirmed, from day 3 of the new menstrual cycle, she received subcutaneous injections of 2 ampules/day of Gonal-F (Merck Serono, Darmstadt, Germany). Follicular growth was monitored by serial transvaginal ultrasound and repeated until the detection of at least three follicles > 18 mm in diameter. At that time, the patient received an intramuscular injection of hCG (10000 IU). After 36 hours, 21 oocytes were retrieved. Of these, 15 oocytes were fertilized and cleaved. Because of the risk for ovarian hyperstimulation syndrome, all embryos were frozen on the third day. After three months, the patient elected to undergo a laparoscopy/hysteroscopy due to hydrosalpinx (es). Laparoscopic findings included dense adhesions of the pelvic organs and a frozen pelvis. The surgeon could not perform a salpingectomy or proximal tubal occlusion so both ostium of the uterine tube were cauterized by hysteroscopy according to a previously reported procedure (6). However, direct smear and bacterial culture of endometrial tissue and evaluation of tissue by polymerase chain reaction (PCR) were negative for Mycobacterium TB. After eight months, the patient underwent FET. After GnRh agonist down-regulation, she received estradiol valerate (Aburaihan Co., Iran) as hormone replacement therapy in the following doses: 4 mg/day for the first six days, followed by 6 mg/day for another six days, until endometrial thickness reached 9 mm. When the optimal endometrial thickness was obtained, intramuscular progesterone (100 mg; Aburaihan Co, Iran) was administered and three days later, two good-quality embryos were transferred. The luteal phase was supported with estradiol and progesterone. However, she did not achieve pregnancy. One year later the patient underwent a laparoscopy. A skillful laparoscopist carried out the operation. There was a severe dense pelvic adhesion and frozen pelvis (Figure 2A). After adhesiolysis, she had a bilateral hydrosalpinx and both tubes were removed. The next day, the patient was released without any problem. Surgical specimens were collected and stained with hematoxylin and eosin and evaluated with light microscopy. We observed cystically dilated glands surrounded by hypertrophic smooth muscle fibers, which demonstrated bilateral hydrosalpingitis isthmica nodosa (Figure 2B). After one year, she underwent a second artificial FET, which was similar to the previous transfer. When the endometrial thickness reached 9.5 mm, we transferred three high-quality embryos. The luteal phase was supported by 100 mg of daily progesterone injections (Aburaihan Co., Tehran, Iran). Biochemical pregnancy was detected by a β-hCG test 2 wk after the embryo transfer and clinical pregnancy was confirmed by the presence of a gestational sac with fetal echoes at 6 wk after embryo transfer. The patient was followed and had a cesarean delivery at 38 wk of gestation. Her child was a healthy girl who weighed 3,100 g.


2.1. Ethical consideration
The patient signed the written informed consent for reporting this case.
3. Discussion
GTB is a relatively rare chronic disease with unspecific symptoms of menstrual disturbance, vaginal discharge, pelvic pain, and a considerable cause of PID (4). Conversely, GTB occasionally remains undetected on clinical examination and is often diagnosed during an infertility evaluation (1).
The potential risk factors for a TB infection include a past history of TB, low socioeconomic status, HIV-seropositive persons (the highest risk among TB-infected individuals is clearly HIV co-infection, which suppresses cellular immunity), immigrants from countries with a high prevalence of TB, and Mycobacteriology laboratory personnel (7). In the current report, the patient had reported a history of TB 10 years ago, but did not have other risk factors, such as AIDS or drug addiction, that predisposed her to GTB.
HSG, laparoscopy, endometrial tissue biopsy, PCR, and histopathologic examination are useful instruments for the diagnosis of pelvic TB (3). TB has various appearances on HSG that include specific changes such as beaded tube, golf club tube, pipestem tube, cobble stone tube, leopard skin tube in addition to nonspecific changes of hydrosalpinx, tubal occlusion, hydroconvoluted tube, and tubal fixity. The effects of GTB on the endometrium may be seen as specific features and include a T-shaped uterine appearance, pseudounicornuate uterus, and collar stud abscess (3, 6). However, tubal obstruction is a common HSG finding in the majority of GTB cases (1). The HSG finding in this case was bilateral hydrosalpinx that was confirmed by laparoscopy. However, bacterial culture of the endometrial tissue and PCR were negative. In this case, the first attempt laparoscopy was not successful due to severe destruction of the tubes and pelvic adhesions. In the second laparoscopy, performed by a more experienced laparoscopist, a salpingectomy was performed with difficulty but had a favorable outcome.
The most common causes of PID are Chlamydia trachomatis, Neisseria gonorrhea, and anaerobic organisms. However, GTB may cause PID and distal tubal occlusion from salpingitis or peritubal adhesions, and subsequently hydrosalpingus (1, 3, 4). TB salpingitis can be either unilateral or bilateral, and manifested by thickened and enlarged tubal walls that turn into fibrosis and scar tissue (3). Hydrosalpinx fluid potentially has embryotoxic components and growth-inhibiting factors. The hydrosalpinx fluid may also decrease endometrial receptivity and embryo implantation. The possibility of the mechanical effect of fluid and wash-out of embryos through leakage of fluid through the endometrial cavity should be considered (8). Salpingectomy can lead to improved clinical pregnancy rate and live birth rates in patients whose infertility is the result of tuberculous salpingitis, in particular those exposed to toxic hydrosalpingeal fluid (2, 6). Dense pelvic adhesions due to salpingitis disturbances in ovarian function (2), and a higher level of FSH and LH, lower mean ovarian volume, and number of antral follicles demonstrate poor ovarian reserve in GTB (9). Possible explanations for the poor results could be endometrial insufficiency, poor response to gonadotropins, and low-quality oocytes and embryos (2, 9).
IVF procedures after anti-TB treatment have been recommended by several researchers and particularly for patients with damaged tubes and undamaged endometria (3). The pregnancy rate in such GTB cases was similar to women with infertility due to tubal factor; however, the pregnancy rate in unilateral hydrosalpinx was higher compared to bilateral hydrosalpinx (3).
Caliskan and co-workers describe GTB cases who underwent IVF cycles after antitubercular therapy and salpingectomy resulted in 15.5% clinical pregnancy per ET and 9% live birth. However, seven (33.3%) of them had hydrosalpinx and only two (9.5%) of which had severe pelvic adhesions (8). Salpingectomy before IVF cycles were reported in the underlying indication of hydrosalpinx such as ectopic pregnancy, pelvic adhesions, pyosalpinx, hematosalpinx, and hydrosalpinx (10).
Since salpingectomy has a potential risk in the frozen pelvis due to severe salpingitis, an alternative approach is cauterized ostium. In the current case, this method resulted in failed implantation. We suggested the only definitive treatment for this patient was a salpingectomy, which leads to success in implantation and lives birth.
4. Conclusion
Tuberculous salpingitis is a tubal cause for infertility, especially in high prevalence countries. IVF-ET provides a treatment for tubal TB with receptive endometrium. Laparoscopic salpingectomy prior to embryo transfer plays a critical role in predicting the occurrence of a pregnancy in a patient with hydrosalpingitis especially attributed to TB. GTB is a granulomatous disease where inflammation distorts the pelvic tissues, causing severe adhesions and fibrosis. An experienced laparoscopist should perform the surgery in order to prevent trauma to the pelvis and abdomen.
Conflict of Interest
The authors declare that they have no conflict of interest.
Type of Study: Case Report |
Subject:
Assisted Reproductive Technologies
References
1. Gatongi DK, Gitau G, Kay V, Ngwenya S, Lafong C, Hasan A. Female genital tuberculosis. Obstet Gynaecol 2005; 7: 75-79. [DOI:10.1576/toag.7.2.075.27000]
2. Shahzad S. Investigation of the prevalence of female genital tract tuberculosis and its relation to female infertility: An observational analytical study. Iran J Reprod Med 2012; 10: 581-588.
3. Sharma JB. Current Diagnosis and Management of Female Genital Tuberculosis. J Obstet Gynecol India 2015; 65: 362-371. [DOI:10.1007/s13224-015-0780-z] [PMID] [PMCID]
4. Bapna N, Swarankar M, Kotia N. Genital tuberculosis and its consequences on subsequent fertility. J Obstet Gynaecol India 2005; 55: 534-537.
5. Organization WH. WHO laboratory manual for the examination and processing of human semen. 5th Ed. World Health Organization Presss, Switzerland; 2010.
6. Darwish AM, El Saman AM. Is there a role for hysteroscopic tubal occlusion of functionless hydrosalpinges prior to IVF/ICSI in modern practice? Acta Obstet Gynecol Scand 2007; 86: 1484-1489. [DOI:10.1080/00016340701714893] [PMID]
7. Jameson LJ, Fauci A, Kasper D, Hauser S, Longo D, Loscalzo J. Harrison's principles of internal medicine. 20th Ed. Mcgraw Hill, New York; 2015.
8. Caliskan E, Cakiroglu Y, Sofuoglu K, Doger E, Akar ME, Ozkan SO. Effects of salpingectomy and antituberculosis treatments on fertility results in patients with genital tuberculosis. J Obstet Gynaecol Res 2014; 40: 2104-2109. [DOI:10.1111/jog.12450] [PMID]
9. Malhotra N, Sharma V, Bahadur A, Sharma JB, Roy KK, Kumar S. The effect of tuberculosis on ovarian reserve among women undergoing IVF in India. Int J Gynecol Obstet 2012; 117: 40-44. [DOI:10.1016/j.ijgo.2011.10.034] [PMID]
10. Pereira N, Pryor KP, Voskuilen-Gonzalez A, Lekovich JP, Elias RT, Spandorfer SD, et al. Ovarian response and in vitro fertilization outcomes after salpingectomy: does salpingectomy indication matter? J Minim Invasive Gynecol 2017; 24: 446-454. [DOI:10.1016/j.jmig.2016.12.023] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








