Fri, Apr 26, 2024
[Archive]
Volume 16, Issue 12 (December 2018)
IJRM 2018, 16(12): 791-800 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Karimipour M, Dibayi Z, Ahmadi A, Zirak Javanmard M, Hosseinalipour E. The protective effect of vitamin C on phenylhydrazine-induced hemolytic anemia on sperm quality and in-vitro embryo development in mice. IJRM 2018; 16 (12) :791-800
URL: http://ijrm.ir/article-1-1335-en.html
URL: http://ijrm.ir/article-1-1335-en.html
Mojtaba Karimipour1 

 , Zahra Dibayi1
, Zahra Dibayi1 

 , Abass Ahmadi *
, Abass Ahmadi * 

 2, Masoumeh Zirak Javanmard1
2, Masoumeh Zirak Javanmard1 

 , Elnaz Hosseinalipour1
, Elnaz Hosseinalipour1 




 , Zahra Dibayi1
, Zahra Dibayi1 

 , Abass Ahmadi *
, Abass Ahmadi * 

 2, Masoumeh Zirak Javanmard1
2, Masoumeh Zirak Javanmard1 

 , Elnaz Hosseinalipour1
, Elnaz Hosseinalipour1 


1- Department of Anatomy and Histology, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran
2- Department of Basic Sciences, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran , ahmadiabbas36@yahoo.com
2- Department of Basic Sciences, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran , ahmadiabbas36@yahoo.com
Full-Text [PDF 4670 kb]
(798 Downloads)
| Abstract (HTML) (3039 Views)
Full-Text: (448 Views)
Anemia is a common and serious world pub- lic health affecting people of all ages and is related to a high risk of morbidity and mortality, especially in developing countries (1). Exposure to some chemicals and drugs have been associated with red blood cell (RBC) destruction and causing hemolytic anemia. Phenylhydrazine (PHZ), due to its toxic effects on RBC, is a useful agent in experimental model study of hemolytic anemia (2). This compound was first prescribed at the end of the nineteenth century as an antipyretic drug and is now well-known for its ability to RBC hemolysis in animals and humans inducing hemolytic anemia (3).
Results of a previous study indicated that PHZ adversely affected the male reproductive system in mice that suggested to induce oxidative damage. It was indicated that administration of PHZ in mice for 35 days caused decrease in the epididymal sperm parameters quality (motility, count, and mor- phology). It was also shown that PHZ through the formation of lipid peroxidation causes DNA damage in sperms (4). Amer and colleagues reported that PHZ elevates reactive oxygen species (ROS) and lipid peroxidation and alleviates glutathione (5). These effects may have been reversed following antioxidant administration.
Oxidative stress is a major cause of reproductive failure, and it has been proven that male germ cells may be prone to oxidative damage due to the high amount of polyunsaturated fatty acids and low capacity for DNA repair (6). Other investigators have indicated that damage in testicular tissue is associated with lipid peroxidation and they suggested that high production of ROS may play a major role in inducing testicular degeneration, resulting in infertility (7). Thus, administration of compounds with antioxidant properties can help the body to combat against pathological status caused by ROS or free radicals.
Further studies have shown that antioxidants scavenge ROS are produced by leukocytes and protect sperm DNA from fragmentation and can
also improve semen quality in persons exposed to oxidative stress (4, 8). Vitamin C (Vit C) or ascorbic acid is one of the major antioxidant compounds in biological systems (9). Supplementation of Vit C can protect the blood-testis barrier destruction induced by cadmium through the inhibition of oxidative stress (10). Talebi and colleagues indi- cated that Vit C as a potent antioxidant allevi- ates adverse effects of diabetes on the sperm parameters, chromatin maturity and apoptosis in mice (11). Vit C deficiency in guinea pigs caused great degeneration of the seminiferous tubules and reduced the weight of the reproductive system, including testis and accessory sex organs, and Vit C supplementation reversed this reduction (12).
Considering the positive effects of Vit C on the male reproductive system, the current study was conducted to evaluate the protective effects of Vit C on sperm parameters and IVF potential (embryonic development) in male mice exposed to PHZ-induced hemolytic anemia.
(temperature 23 ± 2∘C and 12 hr light/dark cycles).
The animals were randomly assigned into four
groups (n = 8): group I – control group, received intraperitoneal (IP) normal saline (0.1 mL, daily); group II – PHZ group, received PHZ (Sigma-Aldrich, St. Louis, USA) at the dose of 8 mg/100 g/body weight/IP at first, followed later at the dose of 6 mg/100 g every 48 hr; group III – Vit C group, received Vit C (Shahre Darou Company, Tehran, Iran) daily at the dose of 10mg/kg/IP; and group IV
– PHZ + Vit C group, received PHZ and Vit C at the same dose. The treatment time for all the groups was 35 days.
This duration of time of experiment was selected according to the timing of mouse spermatogenesis in previous studies (13, 14). The doses of PHZ and Vit C were selected according to previous studies (4, 11). During the study, the animals were accessed to food and water ad libitum. After 35 days, the mice were euthanized with an overdose of ketamine (Alfasan, Woerden, the Netherlands). Then, the caudal portion of both epididymides of each mouse was transferred and minced in a petri dish containing 1 mL of human tubal fluid medium (HTF; Sigma-Aldrich, St. Louis, USA) preheated at
37∘C and then incubated for 30 min to release
sperm from the epididymis.
100× magnification, at least 200 spermatozoa were
counted in each slide and the data were expressed
as a percentage (4).
AO is a fluorescent staining that is used to evaluate chromatin integrity and determine dam- age to DNA. Indeed, this staining detects double- and single-stranded regions in sperm chromatin. Briefly, air-dried sperm smear slides were fixed for 2 h in Carnoy’s fixative (methanol/acetic acid 1:3). Then, the slides dried at room temperature were stained with AO solution for 7 min and dried again. The slides were examined using a fluorescent
microscope with 100× magnification and at least
200 spermatozoa per mouse were evaluated, and
the percentage of normal sperms (green color) and abnormal ones (red color) were determined (4).
At the end of the study (35 days), the male mice were prepared for IVF. The authors used
60 adult female (10 wk. old) mice to obtain enough oocytes for IVF assay. To induce superovu- lation and collect mature oocytes from oviducts, each female was intraperitoneal injected with 10 IU pregnant mare‘s serum gonadotropin hormone (PMSG, Folligon, The Netherland) and followed after 48 hr with IP injecting of 10 IU human chorionic gonadotropin hormone (hCG). About 12– 14 hr after hCG injection, female mice were sacri- ficed and then the fallopian tubes were removed and transferred to a drop of HTF-BSA medium
previously equilibrated in an incubator (5% CO2, 37∘C). The oviducts were dissected and the oocytes
were removed, and after washing, were placed in droplets under mineral oil for fertilization.
Then, 1×106 capacitated sperms from each male
mouse were added to oocytes in the fertilization
droplets.
24 hr, the number of two cell embryos were counted, and finally after 120 hr, the percentage of blastocyst development and arrested embryos were determined. The flowchart for the experiment design has been shown in Figure 1.
software (USA). All results were shown as means ±
SD and a p < 0.05 was determined as statistically
significant.
+ Vit C (26.12 × 106± 2.83) had lower epididymis sperm counts (p < 0.001 and p = 0.002, respec-
in group IV (PHZ + Vit C) were significantly (p < tively). The results also showed that sperm counts
0.001) higher than group II (PHZ) (Table I). There was no significant difference between group III (Vit
C) and group I. The percentage of sperm motility in studying groups are also shown in Table I. In comparison with group I (control), sperm motility showed a significant decrease in groups II (PHZ)
and IV (PHZ + Vit C) (p < 0.001 and p = 0.008,
respectively). In addition, the sperm motility of the
mice receiving PHZ + Vit C was significantly (p = 0.007) greater than those of the mice receiving only PHZ.
that it was significantly (p < 0.001) reduced. This decrease of sperm viability was significantly (p <
0.001) improved in group IV (PHZ + Vit C), but it was still significantly (p = 0.023) lower than group I (control). However, sperm viability in group IV (PHZ
+ Vit C) was significantly (p =0.006) greater than group II (PHZ) (Table I).
cantly low (p < 0.001). However, supplementation
with Vit C in group IV (PHZ + Vit C) improved it
significantly (p = 0.02) compared to group II (PHZ).
I (control) and III (Vit C) (p < 0.001). A statistically significant reduction (p < 0.001) in the percentage
of sperm with DNA damage was observed in group IV (PHZ + Vit C) when compared to group II (PHZ), but in comparison with group I, it was still higher (p
< 0.001) (Table II).
sperm in groups II (21.5 ± 4.79) and IV (9 ± 3.9) were significantly (p < 0.001) higher than groups I and III (2 ± 0.81 and 1.5 ± 0.57, respectively). However, in group IV (PHZ + Vit C), it was significantly (p <
0.001) reduced in comparison with group II (PHZ). Therefore, treatment with Vit C could not restore the mean percentage of immature sperms to control and Vit C groups levels.
and IV (PHZ + Vit C), (p < 0.001 and p = 0.002,
respectively). The percentage of two cell embryos formation in group II compared to group I was
significantly reduced (p <0.001) but, in comparison
with group IV, it was not significant. Table III also
The results showed a significant (p < 0.001) shows the percentage of blastocyst production.
reduction in group II compared to group I, but in comparison with group IV, the reduction was not significant. Overall, the percentage of arrested embryos before the blastocyst formation in group II (PHZ) was significantly higher than groups I (control) and III (Vit C). This result in group IV (PHZ
+ Vit C) was lower than group II (PHZ), but it was not significant.
Table I: The results of the sperm parameters analysis in different
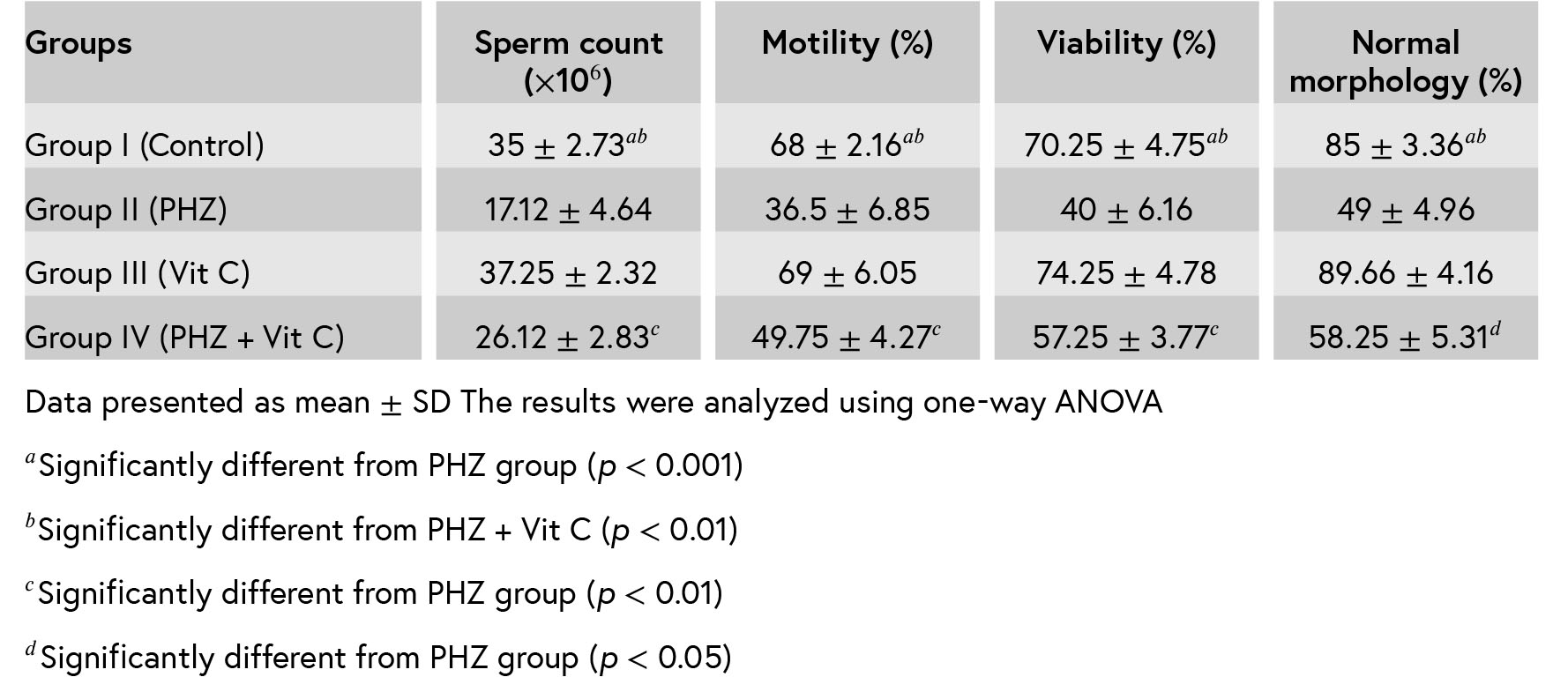
Table II: The results of sperm DNA damage and chromatin immature in different groups.
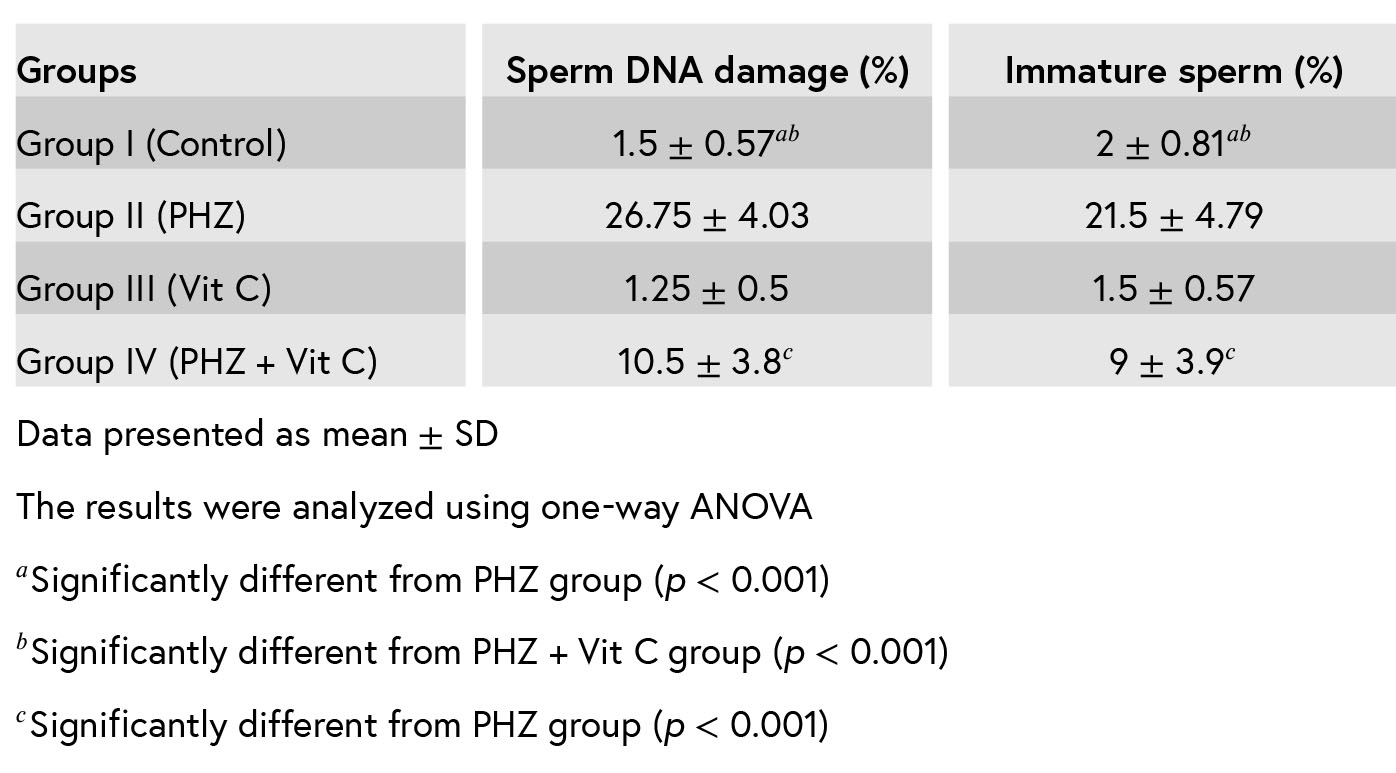
Table III: The outcomes of IVF in mice.

Figure 1: Flowchart for the experiment design.
Recent reports have indicated that infertility is considered to be a major clinical problem, and it has been reported in one in four couples (17, 18). Oxidative stress is one of the main causes of infertility in men (19). Overproduction of ROS in sperm induces nuclear DNA fragmentation, lipid peroxidation that leads to cell death (20, 21). Due to the high amount of polyunsaturated fatty acids in the spermatozoa and also the lack of ability for DNA repair, they are sensitive to ROS elevation. Thus, the increased ROS amount has been associated with declined sperm parameters quality and quantity (6, 20).
In agreement with our findings, Mozafari and col- leagues showed that sperm parameters’ quality and quantity significantly decreased in mice treated with PHZ compared to control mice. They showed that PHZ increased the level of malondialdehyde (MDA) in testicular tissue in mice. MDA is an indicator of the degree of lipid peroxidation and causes testis tissue damages (4).
Prior studies also demonstrated that short-term administration of rats with PHZ resulted in hemol- ysis anemia with decreased RBC and hemoglobin (Hb) (22, 23). Hemolysis or declined levels of RBC induced by PHZ leads to overproduction of ROS and lipid peroxidation. Thus, oxidative damage plays a major role in the pathogenesis of PHZ-induced testicular damage. To protect sperm from ROS, it is important to use antioxidative defense mechanisms
in the testis (24). Considering the effect of PHZ on the antioxidant state, the authors hypothesized that Vit C as a potent antioxidant could prevent the PHZ-dependent damages in mice sperm parameters and fertility potential.
These findings revealed that PHZ significantly reduced the sperm count and motility. The decrease of sperm count is largely related to the onset of testicular damage, and reduction in sperm motility is mainly dependent on oxidative stress damage. Thus, a decrease in sperm motility is the first sign of elevated ROS level (25, 26). This increase in ROS level affects sperm enzymatic content and enhances phospholipids peroxidation, which even- tually leads to a decrease in cell membrane fluidity and sperm motility (27). However, supplementation with Vit C resulted in a notable improvement in sperm count and motility in mice received PHZ. Observations have indicated that PHZ-treated mice showed a significant increase in the percentage of sperm with abnormal morphology. In contrast to our findings, in an experimental study in diabetic rats, it was indicated that treatment with Vit C reverses only testicular damage but is not able to restore sperm motility and fertility (28).
In order to find out how PHZ induce sperm parameters impairment, it should be consid- ered that during spermatogenesis process a part of sperm cytoplasm must be phagocytosis and removed by Sertoli cells (29, 30). Remaining of these cytoplasmic droplets in the middle piece of sperm impair sperm maturation. Therefore, it is logical to hypothesize that the adverse effects of PHZ on sperm maturation, during spermatogenesis, exert partly through impairing the normal physiological function of Sertoli cells, which may happen due to PHZ-induced oxidative stress damage. On the other hand, Vit C-treated mice revealed a significant reduction in the percentage of abnormal sperm. Therefore, it can be postulated that Vit C recovers Sertoli cells physiologic activity by improving the antioxidant state. These findings in PHZ-induced low sperm count were in accordance with a previous study (4). This reduction in sperm count may also be associated with a decrease in production of testosterone hormone due to damage to Leydig and Sertoli cells following PHZ supplementation (4).
Regarding the AO staining test, which can detect abnormal single-stranded DNA from normal double-stranded sperms, it can be assumed that PHZ elevates denaturation of sperm DNA strands and co-treatment with Vit C reduces sperm DNA damage induced by PHZ, probably through a reduc- tion in ROS production. In accordance with the current results, in a prior study, it was shown that the addition of Vit C to semen and prepared spermatozoa improved sperm parameters quality and DNA integrity following verification (31).
About AB test, it should be considered that there are some different reports on the use of vitamins to improve sperm chromatin abnormalities. Silver and colleagues demonstrated that the administration of Vit C and E did not have any positive effects on sperm chromatin maturity or condensation (32). This controversy may be due to the different mechanisms of infertility in the studies and the administered doses.
In vitro fertilization and embryonic development in the PHZ-treated group was significantly lower than the control group and the percentage of embryonic arresting was also higher. Studies indi- cated that there is a positive correlation between sperm parameters quality and fertilization rate, embryonic growth and high incidence of birth defects both in vivo and in vitro (8, 33, 34). In the present study, Vit C was only able to ameliorate the side effect of PHZ on fertilization rate. Thus, the findings of the present study showed that Vit C supplementation could not improve the percent- age of two cell embryos, blastocyst and embryo arresting in mice co-treated with PHZ. Considering these findings, it can be postulated that Vit C significantly but not completely protects against the PHZ-induced testicular damage. The present study is the first report on the relationship between sperm parameters and embryonic development
impairments and Vit C supplementation in PHZ- induced male reproductive system disorders. Thus, to compare these findings with other studies, the authors did not find a similar study. However, they were not able to explain why Vit C can only improve the fertilization rate. This may be because the Vit C dose used in this study was not sufficient to recover the embryonic development completely.
The testis is sensitive to different stressors including hyperthermia, inflammation, radiation and also exposure to toxic agents that induce apoptosis of germ cells. Sertoli cells in testicular tissue have the main role in supporting normal spermatogenesis by creating a special environment that induces germ cells differentiation and also controls the entry of nutrients, hormones and other agents into seminiferous tubules (35). Thus, Sertoli cells are important in creating blood-testis-barrier and nor- mal physiologic function of the male reproductive system is dependent on the maintenance of this selective physiologic barrier (35).
The mechanism by which Vit C enters the adluminal compartment of seminiferous tubules to enter the developing germ cells was an unsolved problem for many years. So it is obvious that for Vit C to reach the adluminal part of seminiferous tubules, it must pass through Sertoli cells by passive diffusion or facilitative transporters (36). Angulo and colleagues explained this problem by showing that Sertoli cells express two functional active Vit C transporters that give the ability to Sertoli cells to metabolize and regulate the delivery of Vit C to germ cells. Thus, Sertoli cells have a major role in controlling Vit C concentration in the adluminal part of seminiferous tubules (37).
Results of a previous study indicated that PHZ adversely affected the male reproductive system in mice that suggested to induce oxidative damage. It was indicated that administration of PHZ in mice for 35 days caused decrease in the epididymal sperm parameters quality (motility, count, and mor- phology). It was also shown that PHZ through the formation of lipid peroxidation causes DNA damage in sperms (4). Amer and colleagues reported that PHZ elevates reactive oxygen species (ROS) and lipid peroxidation and alleviates glutathione (5). These effects may have been reversed following antioxidant administration.
Oxidative stress is a major cause of reproductive failure, and it has been proven that male germ cells may be prone to oxidative damage due to the high amount of polyunsaturated fatty acids and low capacity for DNA repair (6). Other investigators have indicated that damage in testicular tissue is associated with lipid peroxidation and they suggested that high production of ROS may play a major role in inducing testicular degeneration, resulting in infertility (7). Thus, administration of compounds with antioxidant properties can help the body to combat against pathological status caused by ROS or free radicals.
Further studies have shown that antioxidants scavenge ROS are produced by leukocytes and protect sperm DNA from fragmentation and can
also improve semen quality in persons exposed to oxidative stress (4, 8). Vitamin C (Vit C) or ascorbic acid is one of the major antioxidant compounds in biological systems (9). Supplementation of Vit C can protect the blood-testis barrier destruction induced by cadmium through the inhibition of oxidative stress (10). Talebi and colleagues indi- cated that Vit C as a potent antioxidant allevi- ates adverse effects of diabetes on the sperm parameters, chromatin maturity and apoptosis in mice (11). Vit C deficiency in guinea pigs caused great degeneration of the seminiferous tubules and reduced the weight of the reproductive system, including testis and accessory sex organs, and Vit C supplementation reversed this reduction (12).
Considering the positive effects of Vit C on the male reproductive system, the current study was conducted to evaluate the protective effects of Vit C on sperm parameters and IVF potential (embryonic development) in male mice exposed to PHZ-induced hemolytic anemia.
1.Materials and Methods
1.1.Animals and treatment
Thirty-two adult male NMRI mice (25–27 gr) were maintained in standard animal house conditions(temperature 23 ± 2∘C and 12 hr light/dark cycles).
The animals were randomly assigned into four
groups (n = 8): group I – control group, received intraperitoneal (IP) normal saline (0.1 mL, daily); group II – PHZ group, received PHZ (Sigma-Aldrich, St. Louis, USA) at the dose of 8 mg/100 g/body weight/IP at first, followed later at the dose of 6 mg/100 g every 48 hr; group III – Vit C group, received Vit C (Shahre Darou Company, Tehran, Iran) daily at the dose of 10mg/kg/IP; and group IV
– PHZ + Vit C group, received PHZ and Vit C at the same dose. The treatment time for all the groups was 35 days.
This duration of time of experiment was selected according to the timing of mouse spermatogenesis in previous studies (13, 14). The doses of PHZ and Vit C were selected according to previous studies (4, 11). During the study, the animals were accessed to food and water ad libitum. After 35 days, the mice were euthanized with an overdose of ketamine (Alfasan, Woerden, the Netherlands). Then, the caudal portion of both epididymides of each mouse was transferred and minced in a petri dish containing 1 mL of human tubal fluid medium (HTF; Sigma-Aldrich, St. Louis, USA) preheated at
37∘C and then incubated for 30 min to release
sperm from the epididymis.
1.1.Sperm parameters analysis (Sperm count, motility, viability, and morphology)
After diluting, sperms were counted using a Neubauer slide under a light microscope. The motility of sperm was expressed by the percentage of motile sperm of the total sperm counted (15). For sperm viability, 20 µL of eosin solution was added into the same value of sperm sample and then 20 µL of nigrosine solution was added and smear prepared; after drying the slides, the percentage of unstained colorless alive sperms and red stained dead sperms were determined. The percentage of sperm morphology was evaluated using the slides stained by aniline blue (4). To determine all percentages (motility, viability, and morphology), 200 sperms were counted per slide.1.2.Aniline blue (AB) staining
AB stains lysine-rich histones and is considered as a marker of sperm chromatin evaluation used to detect sperm nucleus maturity. Briefly, dried smears from sperm solution were fixed for 30 min in 3% glutaraldehyde. The smears were stained with 5% AB for 7 min. In this test, normal mature sperms were pale and abnormal immature sperms were dark blue in color. Under a light microscope using100× magnification, at least 200 spermatozoa were
counted in each slide and the data were expressed
as a percentage (4).
1.1.Acridine orange (AO) staining
AO is a fluorescent staining that is used to evaluate chromatin integrity and determine dam- age to DNA. Indeed, this staining detects double- and single-stranded regions in sperm chromatin. Briefly, air-dried sperm smear slides were fixed for 2 h in Carnoy’s fixative (methanol/acetic acid 1:3). Then, the slides dried at room temperature were stained with AO solution for 7 min and dried again. The slides were examined using a fluorescent
microscope with 100× magnification and at least
200 spermatozoa per mouse were evaluated, and
the percentage of normal sperms (green color) and abnormal ones (red color) were determined (4).
1.2.In Vitro Fertilization (IVF)
- Animals, oocytes collection and fertilization
At the end of the study (35 days), the male mice were prepared for IVF. The authors used
60 adult female (10 wk. old) mice to obtain enough oocytes for IVF assay. To induce superovu- lation and collect mature oocytes from oviducts, each female was intraperitoneal injected with 10 IU pregnant mare‘s serum gonadotropin hormone (PMSG, Folligon, The Netherland) and followed after 48 hr with IP injecting of 10 IU human chorionic gonadotropin hormone (hCG). About 12– 14 hr after hCG injection, female mice were sacri- ficed and then the fallopian tubes were removed and transferred to a drop of HTF-BSA medium
previously equilibrated in an incubator (5% CO2, 37∘C). The oviducts were dissected and the oocytes
were removed, and after washing, were placed in droplets under mineral oil for fertilization.
Then, 1×106 capacitated sperms from each male
mouse were added to oocytes in the fertilization
droplets.
1.1.Assessment of fertilization and embryonic development
Under an inverted microscope, the fertilization process was evaluated after 3–5 hr by observing two pronuclei. After culturing these zygotes for24 hr, the number of two cell embryos were counted, and finally after 120 hr, the percentage of blastocyst development and arrested embryos were determined. The flowchart for the experiment design has been shown in Figure 1.
1.2.Ethical consideration
This study was approved by the Ethics com- mittee of Urmia University of Medical Sciences (IR.umsu.rec.1394.153).1.3.Statistical analysis
The data of IVF assay were analyzed by two proportional test using Minitab software (version 15.1, Minitab Inc., PA, USA). Other results were examined by One-Way ANOVA using SPSS 16software (USA). All results were shown as means ±
SD and a p < 0.05 was determined as statistically
significant.
2.Results
2.1.Sperm count and motility
Compared to group I (control) (35 × 106± 2.73), mice that received PHZ (17.12 × 106± 4.64) and PHZ+ Vit C (26.12 × 106± 2.83) had lower epididymis sperm counts (p < 0.001 and p = 0.002, respec-
in group IV (PHZ + Vit C) were significantly (p < tively). The results also showed that sperm counts
0.001) higher than group II (PHZ) (Table I). There was no significant difference between group III (Vit
C) and group I. The percentage of sperm motility in studying groups are also shown in Table I. In comparison with group I (control), sperm motility showed a significant decrease in groups II (PHZ)
and IV (PHZ + Vit C) (p < 0.001 and p = 0.008,
respectively). In addition, the sperm motility of the
mice receiving PHZ + Vit C was significantly (p = 0.007) greater than those of the mice receiving only PHZ.
2.2.Sperm viability
The comparison of the percentage of alive sperm in group II (PHZ) with group I (control) indicatedthat it was significantly (p < 0.001) reduced. This decrease of sperm viability was significantly (p <
0.001) improved in group IV (PHZ + Vit C), but it was still significantly (p = 0.023) lower than group I (control). However, sperm viability in group IV (PHZ
+ Vit C) was significantly (p =0.006) greater than group II (PHZ) (Table I).
2.3.Sperm morphology
Table I also shows the analysis of sperm mor- phology data. Compared to group I (control), the percentages of sperm with normal morphology in groups II (PHZ) and IV (PHZ + Vit C) were signifi-cantly low (p < 0.001). However, supplementation
with Vit C in group IV (PHZ + Vit C) improved it
significantly (p = 0.02) compared to group II (PHZ).
2.4.Sperm DNA damage
The percentage of sperm with DNA damage in group II (PHZ) was significantly higher than groupsI (control) and III (Vit C) (p < 0.001). A statistically significant reduction (p < 0.001) in the percentage
of sperm with DNA damage was observed in group IV (PHZ + Vit C) when compared to group II (PHZ), but in comparison with group I, it was still higher (p
< 0.001) (Table II).
2.5.Sperm nucleus immaturity
Table II shows the results of analysis of sperm nucleus immaturity. The percentages of immaturesperm in groups II (21.5 ± 4.79) and IV (9 ± 3.9) were significantly (p < 0.001) higher than groups I and III (2 ± 0.81 and 1.5 ± 0.57, respectively). However, in group IV (PHZ + Vit C), it was significantly (p <
0.001) reduced in comparison with group II (PHZ). Therefore, treatment with Vit C could not restore the mean percentage of immature sperms to control and Vit C groups levels.
1.1.IVF assessments
Table III shows IVF and embryo development outcomes in different groups. The results revealed that the percentage of fertilization in group II (PHZ) was significantly lower than groups I (control)and IV (PHZ + Vit C), (p < 0.001 and p = 0.002,
respectively). The percentage of two cell embryos formation in group II compared to group I was
significantly reduced (p <0.001) but, in comparison
with group IV, it was not significant. Table III also
The results showed a significant (p < 0.001) shows the percentage of blastocyst production.
reduction in group II compared to group I, but in comparison with group IV, the reduction was not significant. Overall, the percentage of arrested embryos before the blastocyst formation in group II (PHZ) was significantly higher than groups I (control) and III (Vit C). This result in group IV (PHZ
+ Vit C) was lower than group II (PHZ), but it was not significant.
Table I: The results of the sperm parameters analysis in different

Table II: The results of sperm DNA damage and chromatin immature in different groups.

Table III: The outcomes of IVF in mice.


Figure 1: Flowchart for the experiment design.
1.Discussion
The data of this study showed that almost all sperm parameters, including count, motility and abnormal morphology, had a significant decline following PHZ administration, and that Vit C as an important antioxidant compound for male repro- duction (16) improved them. The authors’ find- ings also revealed that PHZ administration had a detrimental effect on IVF outcomes and embryonic development, and treatment with Vit C reverses only fertilization rate but is insufficient to restore in vitro embryonic growth.Recent reports have indicated that infertility is considered to be a major clinical problem, and it has been reported in one in four couples (17, 18). Oxidative stress is one of the main causes of infertility in men (19). Overproduction of ROS in sperm induces nuclear DNA fragmentation, lipid peroxidation that leads to cell death (20, 21). Due to the high amount of polyunsaturated fatty acids in the spermatozoa and also the lack of ability for DNA repair, they are sensitive to ROS elevation. Thus, the increased ROS amount has been associated with declined sperm parameters quality and quantity (6, 20).
In agreement with our findings, Mozafari and col- leagues showed that sperm parameters’ quality and quantity significantly decreased in mice treated with PHZ compared to control mice. They showed that PHZ increased the level of malondialdehyde (MDA) in testicular tissue in mice. MDA is an indicator of the degree of lipid peroxidation and causes testis tissue damages (4).
Prior studies also demonstrated that short-term administration of rats with PHZ resulted in hemol- ysis anemia with decreased RBC and hemoglobin (Hb) (22, 23). Hemolysis or declined levels of RBC induced by PHZ leads to overproduction of ROS and lipid peroxidation. Thus, oxidative damage plays a major role in the pathogenesis of PHZ-induced testicular damage. To protect sperm from ROS, it is important to use antioxidative defense mechanisms
in the testis (24). Considering the effect of PHZ on the antioxidant state, the authors hypothesized that Vit C as a potent antioxidant could prevent the PHZ-dependent damages in mice sperm parameters and fertility potential.
These findings revealed that PHZ significantly reduced the sperm count and motility. The decrease of sperm count is largely related to the onset of testicular damage, and reduction in sperm motility is mainly dependent on oxidative stress damage. Thus, a decrease in sperm motility is the first sign of elevated ROS level (25, 26). This increase in ROS level affects sperm enzymatic content and enhances phospholipids peroxidation, which even- tually leads to a decrease in cell membrane fluidity and sperm motility (27). However, supplementation with Vit C resulted in a notable improvement in sperm count and motility in mice received PHZ. Observations have indicated that PHZ-treated mice showed a significant increase in the percentage of sperm with abnormal morphology. In contrast to our findings, in an experimental study in diabetic rats, it was indicated that treatment with Vit C reverses only testicular damage but is not able to restore sperm motility and fertility (28).
In order to find out how PHZ induce sperm parameters impairment, it should be consid- ered that during spermatogenesis process a part of sperm cytoplasm must be phagocytosis and removed by Sertoli cells (29, 30). Remaining of these cytoplasmic droplets in the middle piece of sperm impair sperm maturation. Therefore, it is logical to hypothesize that the adverse effects of PHZ on sperm maturation, during spermatogenesis, exert partly through impairing the normal physiological function of Sertoli cells, which may happen due to PHZ-induced oxidative stress damage. On the other hand, Vit C-treated mice revealed a significant reduction in the percentage of abnormal sperm. Therefore, it can be postulated that Vit C recovers Sertoli cells physiologic activity by improving the antioxidant state. These findings in PHZ-induced low sperm count were in accordance with a previous study (4). This reduction in sperm count may also be associated with a decrease in production of testosterone hormone due to damage to Leydig and Sertoli cells following PHZ supplementation (4).
Regarding the AO staining test, which can detect abnormal single-stranded DNA from normal double-stranded sperms, it can be assumed that PHZ elevates denaturation of sperm DNA strands and co-treatment with Vit C reduces sperm DNA damage induced by PHZ, probably through a reduc- tion in ROS production. In accordance with the current results, in a prior study, it was shown that the addition of Vit C to semen and prepared spermatozoa improved sperm parameters quality and DNA integrity following verification (31).
About AB test, it should be considered that there are some different reports on the use of vitamins to improve sperm chromatin abnormalities. Silver and colleagues demonstrated that the administration of Vit C and E did not have any positive effects on sperm chromatin maturity or condensation (32). This controversy may be due to the different mechanisms of infertility in the studies and the administered doses.
In vitro fertilization and embryonic development in the PHZ-treated group was significantly lower than the control group and the percentage of embryonic arresting was also higher. Studies indi- cated that there is a positive correlation between sperm parameters quality and fertilization rate, embryonic growth and high incidence of birth defects both in vivo and in vitro (8, 33, 34). In the present study, Vit C was only able to ameliorate the side effect of PHZ on fertilization rate. Thus, the findings of the present study showed that Vit C supplementation could not improve the percent- age of two cell embryos, blastocyst and embryo arresting in mice co-treated with PHZ. Considering these findings, it can be postulated that Vit C significantly but not completely protects against the PHZ-induced testicular damage. The present study is the first report on the relationship between sperm parameters and embryonic development
impairments and Vit C supplementation in PHZ- induced male reproductive system disorders. Thus, to compare these findings with other studies, the authors did not find a similar study. However, they were not able to explain why Vit C can only improve the fertilization rate. This may be because the Vit C dose used in this study was not sufficient to recover the embryonic development completely.
The testis is sensitive to different stressors including hyperthermia, inflammation, radiation and also exposure to toxic agents that induce apoptosis of germ cells. Sertoli cells in testicular tissue have the main role in supporting normal spermatogenesis by creating a special environment that induces germ cells differentiation and also controls the entry of nutrients, hormones and other agents into seminiferous tubules (35). Thus, Sertoli cells are important in creating blood-testis-barrier and nor- mal physiologic function of the male reproductive system is dependent on the maintenance of this selective physiologic barrier (35).
The mechanism by which Vit C enters the adluminal compartment of seminiferous tubules to enter the developing germ cells was an unsolved problem for many years. So it is obvious that for Vit C to reach the adluminal part of seminiferous tubules, it must pass through Sertoli cells by passive diffusion or facilitative transporters (36). Angulo and colleagues explained this problem by showing that Sertoli cells express two functional active Vit C transporters that give the ability to Sertoli cells to metabolize and regulate the delivery of Vit C to germ cells. Thus, Sertoli cells have a major role in controlling Vit C concentration in the adluminal part of seminiferous tubules (37).
1.Conclusion
The data obtained from this study suggest that Vit C as a potent antioxidant can attenuate the detrimental effects of PHZ on sperm parameters and DNA integrity and improve in vitro fertilization rate but is insufficient to restore the in vitro embryonic development and fertility potential.Acknowledgments
This study was financially supported by Urmia University of Medical Sciences.Conflict of Interest
There are no conflicts of interest to declare.
Type of Study: Original Article |
References
1. [1] Onyeabo C, Achi NK, Ekeleme-Egedigwe CA, Ebere CU, Okoro CK. Haematological and biochemical studies on Justicia carnea leaves extract in phenylhydrazine induced- anemia in albino rats. Acta Sci Pol Technol Aliment 2017; 16: 217–230.
2. [2] Berger J. Phenylhydrazine haematotoxicity. J Appl Biomed 2007; 5: 125–130.
3. [3] Ashour TH. Hematinic and anti-anemic effect of thy- moquinone against phenylhydrazine-induced hemolytic anemia in rats. Res J Med Sci 2014; 8: 67–72.
4. [4] Mozafari AA, Shahrooz R, Ahmadi A, Malekinjad H, Mardani K. Protective effect of ethyl pyruvate on mice sperm parameters in phenylhydrazine induced hemolytic anemia. Vet Res Forum 2016; 7: 63–68.
5. [5] Amer J, Goldfarb A, Fibach E. Flow cytometric analysis of the oxidative status of normal and thalassemic red blood cells. Cytometry A 2004; 60: 73–80. [DOI:10.1002/cyto.a.20017]
6. [6] Aitken RJ, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil 1987; 81: 459–469. [DOI:10.1530/jrf.0.0810459]
7. [7] Köksal IT, Tefekli A, Usta M, Erol H, Abbasoglu S, Kadioglu A.The role of reactive oxygen species in testicular dysfunction associated with varicocele. BJU Int 2000; 86: 549–552. [DOI:10.1046/j.1464-410X.2000.00755.x]
8. [8] Fraga CG, Motchnik PA, Shigenaga MK, Helbock HJ, Jacob RA, Ames BN. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc Natl Acad Sci USA 1991; 88: 11003–11006. [DOI:10.1073/pnas.88.24.11003]
9. [9] Fernandes GS, Fernandez CD, Campos KE, Damasceno DC, Anselmo-Franci JA, Kempinas WD. Vitamin C partially attenuates male reproductive deficits in hyperglycemic rats. Reprod Biol Endocrinol 2011; 9: 100. [DOI:10.1186/1477-7827-9-100]
10. [10] Chen N, Su P, Wang M, Li YM. Ascorbic acid inhibits cadmium-induced disruption of the blood-testis barrier by regulating oxidative stress-mediated p38 MAPK pathways. Environ Sci Pollut Res Int 2018; 25: 21713–21720. [DOI:10.1007/s11356-018-2138-4]
11. [11] Talebi AR, Mangoli E, Nahangi H, Anvari M, Pourentezari M, Halvaei I. Vitamin C attenuates detrimental effects of diabetes mellitus on sperm parameters, chromatin quality and rate of apoptosis in mice. Eur J Obstet Gynecol Reprod Biol 2014; 181: 32–36. [DOI:10.1016/j.ejogrb.2014.07.007]
12. [12] Sapra M, Sharma P, Kothari LK. Effect of vitamin C deficiency on testicular structure in the guinea pig. J Postgrad Med 1987; 33: 69–73.
13. [13] Hess RA, Chen P. Computer tracking of germ cells in the cycle of the seminiferous epithelium and prediction of changes in cycle duration in animals commonly used in reproductive biology and toxicology. J Androl 1992; 13: 185–190.
14. [14] Orazizadeh M, Daneshi E, Hashemitmar M, Absalan F, Khorsandi L. Protective effect of beta-carotene against titanium dioxide nanoparticles induced apoptosis in mouse testicular tissue. Andrologia 2015; 47: 816–825. [DOI:10.1111/and.12336]
15. [15] Badkoobeh P, Parivar K, Kalantar SM, Hosseini SD, Salabat A. Effect of nano-zinc oxide on doxorubicin- induced oxidative stress and sperm disorders in adult male Wistar rats. Iran J Reprod Med 2013; 11: 355–364.
16. [16] Leite GAA, Figueiredo TM, Guerra MT, Borges CDS, Fernandes FH, Anselmo-Franci JA, et al. Ascorbic acid co-administered with rosuvastatin reduces reproductive impairment in the male offspring from male rats exposed to the statin at pre-puberty. Food Chem Toxicol 2018; 118: 416–429. [DOI:10.1016/j.fct.2018.05.043]
17. [17] Arabi M. Nicotinic infertility: assessing DNA and plasma membrane integrity of human spermatozoa. Andrologia 2004; 36: 305–310. [DOI:10.1111/j.1439-0272.2004.00623.x]
18. [18] Auger J, Eustache F, Andersen AG, Irvine DS, Jørgensen N, Skakkebaek NE, et al. Sperm morphological defects related to environment, lifestyle and medical history of 1001 male partners of pregnant women from four European cities. Hum Reprod 2001; 16: 2710–2717. [DOI:10.1093/humrep/16.12.2710]
19. [19] Kaur P, Bansal MP. Effect of experimental oxidative stress on steroidogenesis and DNA damage in mouse testis. J Biomed Sci 2004; 11: 391–397. [DOI:10.1007/BF02254444]
20. [20] Alvarez JG, Touchstone JC, Blasco L, Storey BT. Sponta- neous lipid peroxidation and production of hydrogen per- oxide and superoxide in human spermatozoa Superoxide dismutase as major enzyme protectant against oxygen toxicity. J Androl 1987; 8: 338–348. [DOI:10.1002/j.1939-4640.1987.tb00973.x]
21. [21] Liu W, Gong F, Luo K, Lu G. Comparing the pregnancy rates of one versus two intrauterine inseminations (IUIs) in male factor and idiopathic infertility. J Assist Reprod Genet 2006; 23: 75–79. [DOI:10.1007/s10815-005-9017-x]
22. [22] Chauhan SP, Sheth NR, Suhagia BN. Hematinic effect of fruits of Opuntia elatior Mill. on phenylhydrazine-induced anemia in rats. Ayu 2015; 36: 208–213. [DOI:10.4103/0974-8520.175549]
23. [23] Unami A, Nishina N, Terai T, Sato S, Tamura T, Noda K, et al. Effects of cisplatin on erythropoietin production in rats. J Toxicol Sci 1996; 21: 157–165. [DOI:10.2131/jts.21.3_157]
24. [24] Zarei L, Shahrooz R, Sadrkhanlou R, Malekinejad H, Ahmadi A, Bakhtiary Z. Protective effects of cornus mas extract on in vitro fertilization potential in methotrexate treated male mice. Vet Res Forum 2015; 6: 55–61.
25. [25] Agarwal A, Ikemoto I, Loughlin KR. Relationship of sperm parameters with levels of reactive oxygen species in semen specimens. J Urol 1994; 152: 107–110. [DOI:10.1016/S0022-5347(17)32829-X]
26. [26] Bakhtiary Z, Shahrooz R, Ahmadi A, Zarei L. Evaluation of antioxidant effects of crocin on sperm quality in cyclophosphamide treated adult mice. Vet Res Forum 2014; 5: 213–218.
27. [27] Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril 2003; 79: 829–843. [DOI:10.1016/S0015-0282(02)04948-8]
28. [28] Aguirre-Arias MV, Velarde V, Moreno RD. Effects of ascorbic acid on spermatogenesis and sperm parameters in diabetic rats. Cell Tissue Res 2017; 370: 305–317. [DOI:10.1007/s00441-017-2660-6]
29. [29] Gil-Guzman E, Ollero M, Lopez MC, Sharma RK, Alvarez JG, Thomas AJ Jr, et al. Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum Reprod 2001; 16: 1922–1930. [DOI:10.1093/humrep/16.9.1922]
30. [30] Huszar G, Sbracia M, Vigue L, Miller DJ, Shur BD. Sperm plasma membrane remodeling during spermiogenetic mat- uration in men: relationship among plasma Membrane 1, 4-galactosyltransferase, cytoplasmic creatine phospho- kinase, and creatine phosphokinase isoform ratios. Biol Reprod 1997; 56: 1020–1024. [DOI:10.1095/biolreprod56.4.1020]
31. [31] Mangoli E, Talebi AR, Anvari M, Taheri F, Vatanparast M, Rahiminia T, et al. Vitamin C attenuates negative effects of vitrification on sperm parameters, chromatin quality, apoptosis and acrosome reaction in neat and prepared normozoospermic samples. Taiwan J Obstet Gynecol 2018; 57: 200–204. [DOI:10.1016/j.tjog.2018.02.006]
32. [32] Silver EW, Eskenazi B, Evenson DP, Block G, Young S, Wyrobek AJ. Effect of antioxidant intake on sperm chromatin stability in healthy nonsmoking men. J Androl 2005; 26: 550–556. [DOI:10.2164/jandrol.04165]
33. [33] Agarwal A, Makker K, Sharma R. Clinical relevance of oxidative stress in male factor infertility: an update. Am J Reprod Immunol 2008; 59: 2–11. [DOI:10.1111/j.1600-0897.2007.00559.x]
34. [34] Lim CC, Lewis SE, Kennedy M, Donnelly ET, Thompson W. Human sperm morphology and in vitro fertilization: sperm tail defects are prognostic for fertilization failure. Andrologia 1998; 30: 43–47. [DOI:10.1111/j.1439-0272.1998.tb01381.x]
35. [35] Angulo C, Maldonado R, Pulgar E, Mancilla H, Córdova A, Villarroel F, et al. Vitamin C and oxidative stress in the seminiferous epithelium. Biol Res 2011; 44: 169–180. [DOI:10.4067/S0716-97602011000200009]
36. [36] Augustine LM, Markelewicz RJ Jr, Boekelheide K, Cher- rington NJ. Xenobiotic and endobiotic transporter mRNA expression in the blood-testis barrier. Drug Metab Dispos 2005; 33: 182–189. [DOI:10.1124/dmd.104.001024]
37. [37] Angulo C, Castro MA, Rivas CI, Segretain D, Maldonado R, Ya-ez AJ, et al. Molecular identification and functional characterization of the vitamin C transporters expressed by Sertoli cells. J Cell Physiol 2008; 217: 708–716. [DOI:10.1002/jcp.21545]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





