Thu, Apr 25, 2024
[Archive]
Volume 18, Issue 6 (June 2020)
IJRM 2020, 18(6): 407-414 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Pooransari P, Ebrahimi A, Nazemi N, Yaminifar F, Abediasl Z. Is gross morphology of placenta, umbilical cord, and neonatal outcome in well-controlled gestational diabetes mellitus pregnancy different? A case-control study. IJRM 2020; 18 (6) :407-414
URL: http://ijrm.ir/article-1-1438-en.html
URL: http://ijrm.ir/article-1-1438-en.html
1- Shohada Tajrish Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Shohada Tajrish Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran. , Natasha.nazemi@gmail.com
3- IVF Department, Bahman Hospital, Tehran, Iran.
2- Shohada Tajrish Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran. , Natasha.nazemi@gmail.com
3- IVF Department, Bahman Hospital, Tehran, Iran.
Full-Text [PDF 278 kb]
(710 Downloads)
| Abstract (HTML) (2062 Views)
Considering the possibility of the relation between the placenta morphology, umbilical cord, and newborn parameters with diabetes, this study was aimed to evaluate the relationship between placental morphological parameters such as weight, diameter, number of lobes, thickness, umbilical cord insertion, length, coiling and diameter of the umbilical cord with the outcome of pregnancies complicated with DM.
Our inclusion criteria for selecting the case group were gestational age ≥ 37 weeks at delivery and diagnosed gestational diabetes mellitus.
All women with delivery at < 37 weeks, pregnant women with other maternal medical diseases (PIH, chronic hypertension, DM type I and II started before pregnancy, other systemic diseases), multiple pregnancy, tumors of placenta (angioma etc.), two vessels umbilical cord were excluded from the study.
GDM was diagnosed either by 75 gr or by 100 gr oral glucose tolerance test.
Placenta parameters, umbilical cord features, and newborn outcomes were compared between the two groups.
The aim of glycemic control was HbA1C < %6, without significant hypoglycemia, Fasting blood sugar < 95 mg/dL and either, 1-hr postprandial < 140 mg/dL or 2-hr postprandial < 120 mg/dL according to American diabetes association (7). The control group had normal response to oral glucose tolerance test.
Data including maternal parameters (age, Body mass index (BMI), gestational age, parity, gravity), placental morphological parameters (after they were tagged and washed thoroughly to remove blood and mucus) such as weight, diameter from the widest part, number of lobes, thickness (that was taken from cord insertion area), umbilical cord insertion, length from placental end to the fetal end, coiling, diameter of umbilical cord in transverse cut, and newborn parameters (NICU admission, weight of newborn, ABG, Apgar score, presence of meconium in amniotic fluid) were collected.
Umbilical cord was considered vellamentous if it was inserted in the membranes before reaching the chorionic plate. Umbilical cord was considered marginal when ≤ 1 cm is left to placental margin, central when cord insertion place was ≤ 1 cm away from center, other types of cord insertion we named paracentral. The coiling index is the amount of coils divided by length of umbilical cord in cm. Coiling index was considered hypocoiled if it was below 0.1 coils/cm and was considered hypercoiled if it was above 0.3 coils/cm (8).
Thirty-eight women in the case group and twenty-two women in the control group underwent cesarean delivery, the difference between the two groups was significant (p= 0.03).
Blood sugar in all mothers with GDM were well controlled; 55 women were controlled only by diet and 5 mothers consumed insulin.
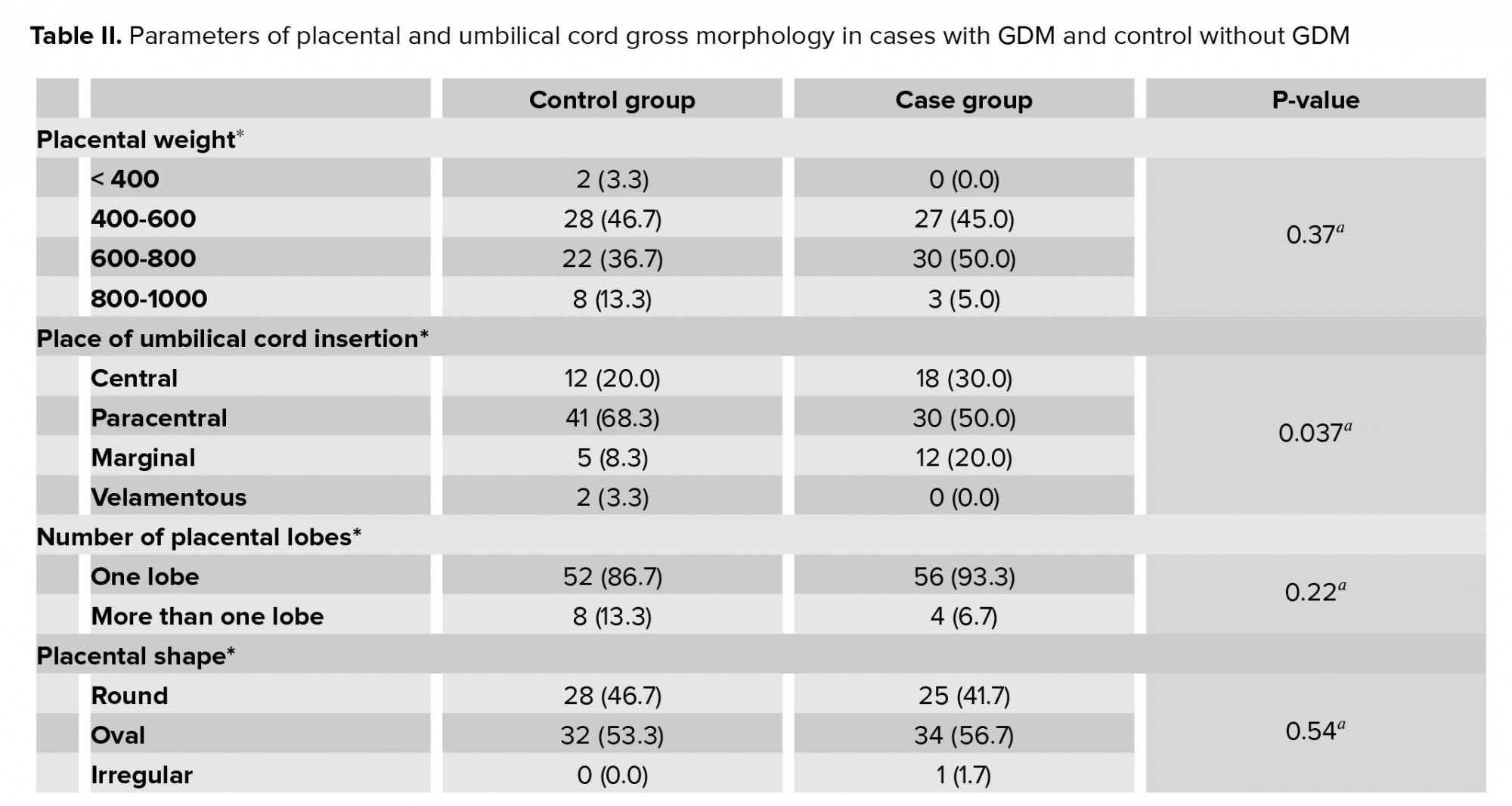
The placental and umbilical cord gross morphology including placental weight, diameter, number of lobes, thickness, place of umbilical cord insertion, cord length, coiling, the diameter of umbilical cord, and placental weight-to-newborn weight ratio had no significant differences between the two groups (Table II).
The mean newborn weight in case group was 3380.8 ± 404.1 gr and in control group 3320 ± 432.1 gr (p = 0.427, with no significant differences). All newborn in case and control groups had Apgar score of 10 at the 5th min and normal ABG parameters. About 10% of the newborns in the case group and 8.3% newborns in the control group were hospitalized to NICU (p = 0.67). Four newborns (6.7%) in the control group and none in the case group had meconium excretion in amniotic fluid (p = 0.042, significant).


4. Discussion
This investigation assessed the differences between placental gross morphology and pregnancy outcome in GDM and healthy pregnancy. The study suggests that there is no difference in the aforementioned parameters between a healthy pregnancy and a good controlled GDM pregnancy. Good controlled GDM group and healthy pregnancy had almost same parameters of placental gross morphology and pregnancy outcome. This shows the effect of well GDM treatment.
So, ultrasound investigation of the placenta and umbilical cord antepartum in pregnancy with well-controlled GDM will not help to predict bad pregnancy outcome in these patients. In our research four newborns in the control group had meconium in amniotic fluid but none in the case group had meconium excreted in the amniotic fluid. These results might be due to immaturity of the gastrointestinal tract in newborns of mothers with GDM. But further evaluation is required because all four cases in the control group were born by normal vaginal delivery, and more cesarean section was performed in the GDM group; this can cause differences because in elective cesarean section we rarely see meconium in amniotic fluid compared to normal vaginal delivery.
It is found that umbilical coiling index (UCI) can be a marker of adverse pregnancy outcomes, for example; hypocoiling (< 0.12) is associated with placental abruption, decreased amniotic fluid, preeclampsia, abnormal fetal heart rate.
Hypercoiling (> 0.36) has relation with increased amniotic fluid, congenital disorders, delivery by cesarean section, and respiratory distress of the newborn (9). This finding is in some ways consistent with a study conducted in 2019 which concluded that GDM is connected to increase of hospital admission, congenital abnormalities, emergent delivery by cesarean section, PROM, preterm birth. Increase in the UCI is connected to macrosomia and meconium-stained amniotic fluid in patients with GDM (10). Further, in some investigations, the evaluations showed that antenatal UCI that was performed by ultrasound at 18-23 wk of gestation could predict postnatal UCI with strong diagnostic accuracy in GDM group (11, 12).
In a study assessing the possible adverse effects of uncontrolled DM on morphometric of the umbilical cord and its vessels, the investigation showed that single umbilical artery (SUA) was much more frequently seen in mothers with gestational diabetes compared to normal pregnancy. It was shown that lean cord and SUA were connected to GDM and has association with unfavorable fetal outcome (13). Our study shows that proper glycemic control has managed these adverse effects in our patients with GDM.
It was shown in another investigation that the circumference and the mean diameter of umbilical cord is larger in GDM mothers than non-diabetics (p= 0.0001). But difference in type of insertion, coiling index, false knots, and length of umbilical cord were insignificant between GDM and healthy pregnant women.
Also, the number of umbilical cord vessels in both groups was the same and true knots were absent (14).
Contrary to the results of our study in the study by Rabia Arshad and coworkers the group of GDM on diet had heavier placenta compared to the control’s. They also demonstrated that villous immaturity, chorangiosis, infarction, and syncytial knots in light microscopy were present in the GDM group versus the control group (15).
Limitation
The limitations to this study are that all patients of case group had well-controlled GDM, so, we suggest studying poor controlled GDM next time and comparing it with healthy pregnancies. Most women in GDM group had elective cesarean sections, but in control group the mothers delivered through normal vaginal delivery NVD. Although it can’t change placental morphological parameters, it may influence the pregnancy outcome. Most women in this study controlled their GDM by diet, it is recommended to study mothers controlling GDM with insulin and compare the results.
5. Conclusion
This study identifies that good controlled GDM at pregnancy makes the outcome of the pregnancy and gross structure of placenta equal to healthy pregnancies without GDM. It shows the significant role of prenatal care and early GDM diagnosis and treatment in newborns’ health.
Acknowledgments
This article has been extracted from the thesis written by Mrs Nazemi at the School of Medicine, this article was financially supported by Shahid Beheshti University of Medical Sciences (Registration No: 203).
Conflict of Interest
There is no conflict of interest to be declared.
Full-Text: (690 Views)
- Introduction
Considering the possibility of the relation between the placenta morphology, umbilical cord, and newborn parameters with diabetes, this study was aimed to evaluate the relationship between placental morphological parameters such as weight, diameter, number of lobes, thickness, umbilical cord insertion, length, coiling and diameter of the umbilical cord with the outcome of pregnancies complicated with DM.
- Materials and Methods
Our inclusion criteria for selecting the case group were gestational age ≥ 37 weeks at delivery and diagnosed gestational diabetes mellitus.
All women with delivery at < 37 weeks, pregnant women with other maternal medical diseases (PIH, chronic hypertension, DM type I and II started before pregnancy, other systemic diseases), multiple pregnancy, tumors of placenta (angioma etc.), two vessels umbilical cord were excluded from the study.
GDM was diagnosed either by 75 gr or by 100 gr oral glucose tolerance test.
Placenta parameters, umbilical cord features, and newborn outcomes were compared between the two groups.
The aim of glycemic control was HbA1C < %6, without significant hypoglycemia, Fasting blood sugar < 95 mg/dL and either, 1-hr postprandial < 140 mg/dL or 2-hr postprandial < 120 mg/dL according to American diabetes association (7). The control group had normal response to oral glucose tolerance test.
Data including maternal parameters (age, Body mass index (BMI), gestational age, parity, gravity), placental morphological parameters (after they were tagged and washed thoroughly to remove blood and mucus) such as weight, diameter from the widest part, number of lobes, thickness (that was taken from cord insertion area), umbilical cord insertion, length from placental end to the fetal end, coiling, diameter of umbilical cord in transverse cut, and newborn parameters (NICU admission, weight of newborn, ABG, Apgar score, presence of meconium in amniotic fluid) were collected.
Umbilical cord was considered vellamentous if it was inserted in the membranes before reaching the chorionic plate. Umbilical cord was considered marginal when ≤ 1 cm is left to placental margin, central when cord insertion place was ≤ 1 cm away from center, other types of cord insertion we named paracentral. The coiling index is the amount of coils divided by length of umbilical cord in cm. Coiling index was considered hypocoiled if it was below 0.1 coils/cm and was considered hypercoiled if it was above 0.3 coils/cm (8).
- 1. Ethical consideration
- 2. Statistical analysis
- Results
Thirty-eight women in the case group and twenty-two women in the control group underwent cesarean delivery, the difference between the two groups was significant (p= 0.03).
Blood sugar in all mothers with GDM were well controlled; 55 women were controlled only by diet and 5 mothers consumed insulin.
The placental and umbilical cord gross morphology including placental weight, diameter, number of lobes, thickness, place of umbilical cord insertion, cord length, coiling, the diameter of umbilical cord, and placental weight-to-newborn weight ratio had no significant differences between the two groups (Table II).
The mean newborn weight in case group was 3380.8 ± 404.1 gr and in control group 3320 ± 432.1 gr (p = 0.427, with no significant differences). All newborn in case and control groups had Apgar score of 10 at the 5th min and normal ABG parameters. About 10% of the newborns in the case group and 8.3% newborns in the control group were hospitalized to NICU (p = 0.67). Four newborns (6.7%) in the control group and none in the case group had meconium excretion in amniotic fluid (p = 0.042, significant).



4. Discussion
This investigation assessed the differences between placental gross morphology and pregnancy outcome in GDM and healthy pregnancy. The study suggests that there is no difference in the aforementioned parameters between a healthy pregnancy and a good controlled GDM pregnancy. Good controlled GDM group and healthy pregnancy had almost same parameters of placental gross morphology and pregnancy outcome. This shows the effect of well GDM treatment.
So, ultrasound investigation of the placenta and umbilical cord antepartum in pregnancy with well-controlled GDM will not help to predict bad pregnancy outcome in these patients. In our research four newborns in the control group had meconium in amniotic fluid but none in the case group had meconium excreted in the amniotic fluid. These results might be due to immaturity of the gastrointestinal tract in newborns of mothers with GDM. But further evaluation is required because all four cases in the control group were born by normal vaginal delivery, and more cesarean section was performed in the GDM group; this can cause differences because in elective cesarean section we rarely see meconium in amniotic fluid compared to normal vaginal delivery.
It is found that umbilical coiling index (UCI) can be a marker of adverse pregnancy outcomes, for example; hypocoiling (< 0.12) is associated with placental abruption, decreased amniotic fluid, preeclampsia, abnormal fetal heart rate.
Hypercoiling (> 0.36) has relation with increased amniotic fluid, congenital disorders, delivery by cesarean section, and respiratory distress of the newborn (9). This finding is in some ways consistent with a study conducted in 2019 which concluded that GDM is connected to increase of hospital admission, congenital abnormalities, emergent delivery by cesarean section, PROM, preterm birth. Increase in the UCI is connected to macrosomia and meconium-stained amniotic fluid in patients with GDM (10). Further, in some investigations, the evaluations showed that antenatal UCI that was performed by ultrasound at 18-23 wk of gestation could predict postnatal UCI with strong diagnostic accuracy in GDM group (11, 12).
In a study assessing the possible adverse effects of uncontrolled DM on morphometric of the umbilical cord and its vessels, the investigation showed that single umbilical artery (SUA) was much more frequently seen in mothers with gestational diabetes compared to normal pregnancy. It was shown that lean cord and SUA were connected to GDM and has association with unfavorable fetal outcome (13). Our study shows that proper glycemic control has managed these adverse effects in our patients with GDM.
It was shown in another investigation that the circumference and the mean diameter of umbilical cord is larger in GDM mothers than non-diabetics (p= 0.0001). But difference in type of insertion, coiling index, false knots, and length of umbilical cord were insignificant between GDM and healthy pregnant women.
Also, the number of umbilical cord vessels in both groups was the same and true knots were absent (14).
Contrary to the results of our study in the study by Rabia Arshad and coworkers the group of GDM on diet had heavier placenta compared to the control’s. They also demonstrated that villous immaturity, chorangiosis, infarction, and syncytial knots in light microscopy were present in the GDM group versus the control group (15).
Limitation
The limitations to this study are that all patients of case group had well-controlled GDM, so, we suggest studying poor controlled GDM next time and comparing it with healthy pregnancies. Most women in GDM group had elective cesarean sections, but in control group the mothers delivered through normal vaginal delivery NVD. Although it can’t change placental morphological parameters, it may influence the pregnancy outcome. Most women in this study controlled their GDM by diet, it is recommended to study mothers controlling GDM with insulin and compare the results.
5. Conclusion
This study identifies that good controlled GDM at pregnancy makes the outcome of the pregnancy and gross structure of placenta equal to healthy pregnancies without GDM. It shows the significant role of prenatal care and early GDM diagnosis and treatment in newborns’ health.
Acknowledgments
This article has been extracted from the thesis written by Mrs Nazemi at the School of Medicine, this article was financially supported by Shahid Beheshti University of Medical Sciences (Registration No: 203).
Conflict of Interest
There is no conflict of interest to be declared.
Type of Study: Original Article |
Subject:
Perinatology
References
1. Albrecht SS, Kuklina EV, Bansil P, Jamieson DJ, Whiteman MK, Kourtis AP, et al. Diabetes trends among delivery hospitalizations in the US, 1994-2004. Diabetes Care 2010; 33: 768-773. [DOI:10.2337/dc09-1801] [PMID] [PMCID]
2. Gagnon AJ, McDermott S, Rigol‐Chachamovich J, Bandyopadhyay M, Stray‐Pedersen B, Stewart D, et al. International migration and gestational diabetes mellitus: a systematic review of the literature and meta‐analysis. Paediatr Perinat Epidemiol 2011; 25: 575-592. [DOI:10.1111/j.1365-3016.2011.01230.x] [PMID]
3. Desoye G, Hauguel-de Mouzon S. The human placenta in gestational diabetes mellitus: the insulin and cytokine network. Diabetes care. 2007 Jul 1;30(Supplement 2):S120-6. [DOI:10.2337/dc07-s203] [PMID]
4. Berceanu C, Tetileanu AV, Ofiţeru AM, Brătilă E, Mehedinţu C, Voicu NL, et al. Morphological and ultrasound findings in the placenta of diabetic pregnancy. Rom J Morphol Embryol 2018; 59: 175-186.
5. Huynh J, Dawson D, Roberts D, Bentley-Lewis R. A systematic review of placental pathology in maternal diabetes mellitus. Placenta 2015; 36: 101-114. [DOI:10.1016/j.placenta.2014.11.021] [PMID] [PMCID]
6. Hiden U, Desoye G. The placenta in a diabetic pregnancy. J Reproduktionsmed Endokrinol 2010; 7: 27-33. [DOI:10.1002/9781444315196.ch3]
7. American Diabetes Association. Management of diabetes in pregnancy: Standards of medical care in diabetes-2019. Diabetes Care 2019; 42 (Suppl.): S165-S172. [DOI:10.2337/dc19-S014] [PMID]
8. Strong Jr TH, Jarles DL, Vega JS, Feldman DB. The umbilical coiling index. American journal of obstetrics and gynecology 1994; 170: 29-32.
https://doi.org/10.1016/S0002-9378(94)70378-7 [DOI:10.1016/S0002-9378(13)70274-6]
9. Chitra T, Sushanth YS, Raghavan S. Umbilical coiling index as a marker of perinatal outcome: an analytical study. Obstet Gynecol Int 2012; 2012: 13689. [DOI:10.1155/2012/213689] [PMID] [PMCID]
10. Najafi L, Abedini A, Kadivar M, Khajavi A, Bordbar A, Noohi AH, et al. Gestational diabetes mellitus: the correlation between umbilical coiling index, and intrapartum as well as neonatal outcomes. J Diabetes Metab Disord 2019; 18: 51-57. [DOI:10.1007/s40200-019-00389-z] [PMID] [PMCID]
11. Najafi L, Malek M, Abedini A, Kadivar M, Ebrahim Valojerdi A, Zahmatkesh E, et al. Prediction of postnatal abnormal coiling of the umbilical cord in gestational diabetes mellitus: a diagnostic accuracy study. J Matern Fetal Neonatal Med 2020; 33: 1107-1113. [DOI:10.1080/14767058.2018.1514596] [PMID]
12. Najafi L, Khamseh ME, Kashanian M, Younesi L, Abedini A, Valojerdi AE, et al. Antenatal umbilical coiling index in gestational diabetes mellitus and non-gestational diabetes pregnancy. Taiwan J Obstet Gynecol 2018; 57: 487-492. [DOI:10.1016/j.tjog.2018.04.033] [PMID]
13. Lateef RH. Adverse effects of gestational diabetes mellitus (GDM) on measurements of the umbilical cord and its vessels. Pak J Biol Sci 2015; 18: 346-351. [DOI:10.3923/pjbs.2015.346.351]
14. Ennazhiyil SV, Ramakrishnan PK, Akshara VR, Premlal KS, Chitra S, Benjamin W, et al. Effects of gestational diabetes mellitus on umbilical cord morphology: A comparative study. Journal of Clinical and Diagnostic Research 2019; 13: AC06-AC09. [DOI:10.7860/JCDR/2019/40085.12543]
15. Arshad R, Kanpurwala MA, Karim N, Hassan JA. Effects of diet and metformin on placental morphology in gestational diabetes mellitus. Pak J Med Sci 2016; 32: 1522-1527. [DOI:10.12669/pjms.326.10872] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








