Thu, Apr 25, 2024
[Archive]
Volume 18, Issue 8 (August 2020)
IJRM 2020, 18(8): 637-650 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ashkar F, Eftekhari M H, Tanideh N, Koohpeyma F, Mokhtari M, Irajie K et al . Effect of hydroalcoholic extract of Berberis integerrima and resveratrol on ovarian morphology and biochemical parameters in Letrozole-induced polycystic ovary syndrome rat model: An experimental study. IJRM 2020; 18 (8) :637-650
URL: http://ijrm.ir/article-1-1486-en.html
URL: http://ijrm.ir/article-1-1486-en.html
Fatemeh Ashkar1 

 , Mohammad Hassan Eftekhari1
, Mohammad Hassan Eftekhari1 

 , Nader Tanideh *
, Nader Tanideh * 

 2, Farhad Koohpeyma3
2, Farhad Koohpeyma3 

 , Maral Mokhtari4
, Maral Mokhtari4 

 , Kambyz Irajie5
, Kambyz Irajie5 

 , Aida Iraji6
, Aida Iraji6 




 , Mohammad Hassan Eftekhari1
, Mohammad Hassan Eftekhari1 

 , Nader Tanideh *
, Nader Tanideh * 

 2, Farhad Koohpeyma3
2, Farhad Koohpeyma3 

 , Maral Mokhtari4
, Maral Mokhtari4 

 , Kambyz Irajie5
, Kambyz Irajie5 

 , Aida Iraji6
, Aida Iraji6 


1- Department of Clinical Nutrition, School of Nutrition and Food Sciences, Shiraz University of Medical Sciences, Shiraz, Iran
2- Stem cells Technology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran , tanidehn@gmail.com
3- Department of Endocrinology, Endocrinology and Metabolism Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
4- Department of Pathology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
5- Department of Medical Biotechnology, School of Advanced Medical Sciences and Technologies, Shiraz University of Medical Sciences, Shiraz, Iran
6- Central Research Laboratory, Shiraz University of Medical Sciences, Shiraz, Iran
2- Stem cells Technology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran , tanidehn@gmail.com
3- Department of Endocrinology, Endocrinology and Metabolism Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
4- Department of Pathology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
5- Department of Medical Biotechnology, School of Advanced Medical Sciences and Technologies, Shiraz University of Medical Sciences, Shiraz, Iran
6- Central Research Laboratory, Shiraz University of Medical Sciences, Shiraz, Iran
Full-Text [PDF 2193 kb]
(839 Downloads)
| Abstract (HTML) (2371 Views)
This syndrome is associated with a range of reproductive, endocrine, and metabolic specifications, including obesity, being overweight, hyperandrogenism, anovulation, and infertility that results in hyperinsulinism and type II diabetes.
There are numerous researches about inflammation in chronic diseases such as PCOS, and the inflammatory markers include C-reactive (CRP) protein, interleukin (IL) 6, and tumor necrosis factor alpha (TNF-α). As a result of inflammation, the risk of developing type 2 diabetes, cardiovascular disease, ovarian dysfunction, and hyperandrogenism increases because of the free radicals. Furthermore, oxidative damage is aggravated by the decrease in the body's antioxidant defense mechanisms such as superoxide dismutase (SOD) and catalase (CAT) activities that act as free radicals’ scavengers. Total antioxidant capacity (TAC) is sensitive to changes in plasma antioxidant levels and degree of insulin resistance. Therefore, a wide range of oxidative stress biomarkers, including malondialdehyde (MDA), protein carbonyl, TAC, SOD, glutathione peroxidase (GPx), and glutathione (GSH) have been tested in the patients (2-4).
Due to the low efficacy and safety of the synthetic drugs and the increased risk of insulin resistance and hyperlipidemia, there is a growing tendency toward the consumption of medicinal plants instead of synthetic compounds (5).
B. integerrima is a regular ingredient of Iranian cuisine (6). The high antioxidant capacity of this plant is because of the presence of natural flavonoids and phenolic compounds such as anthocyanins and carotenoids in Berberis which are known to be antidiabetic, anticancer, and antimicrobial natural agents. Research on the cytotoxicity of B. integerrima extract demonstrated no cellular toxicity (7-9).
Resveratrol (3, 5, 4’-trihydroxy-trans-stilbene) is commonly found in grapes, peanuts, and some types of berries as a natural polyphenolic compound. It is recognized as a powerful antioxidant with antiplatelet, anticancer, anti-inflammation, and antiaging effects. Several studies have demonstrated direct antioxidant effects of resveratrol, and its protective effects may be due to the enhancement of antioxidant capacity in both humans and rodents (10, 11). As a result, antioxidants are extremely important because they inactivate the reactive species and increase the protection of biological sites (9).
In this study, the effectiveness of resveratrol, B. integerrima, and their combination were evaluated in the PCOS rat models for the first time. Moreover, the biochemical parameters, including oxidative stress, inflammatory and lipid profiles, as well as ovarian pathology were determined.
The DPPH scavenging effect was calculated as follows:
Scavenging effect (%) =

Where A0 is the absorbance of the control, and A1 is the absorbance in the presence of the sample. All determinations were performed in triplicate.
Lipid profiles [total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL)] were estimated via the enzymatic colorimetric method using a biochemical Auto-analyzer (BT-1500, Italy) and the kits (Pars Azmoon Co., Iran).
SOD and TAC were determined via the enzymatic colorimetric method with the microplate reader using enzymatic kits (ZV-TAC-A96) purchased from Zellbio Co., Germany. Additionally, MDA activity was determined via spectrophotometric analysis using thiobarbituric acid reactive substances (TBARS) method (19).
Plasma insulin level was measured via enzyme-linked immunosorbent assay (ELISA) method using insulin kits (Rat Insulin Mercodia 10-1250-01, Sweden) (2).
The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the following formula (20):

TNF-α was measured by the ELISA method using TNF-α kits (865.000.96 Diaclone, France) (21).

2.8. Ethical consideration
The study was conducted in accordance with the recommendations of the European Council Directive (86/609/EEC) on November 24, 1986, regarding the protection of animals used for experimental purposes (http://data.europa.eu/eli/dir/1986/609/oj). The animal protocols were also approved by the Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran (Code: IR. SUMS. REC.1394.S1208).
2.9. Statistical analysis
The results have been reported as mean ± standard deviation. Additionally, data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s test. P < 0.05 was considered to be statistically significant. All analyses were done using the SPSS statistical software, version 22 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Biochemical assessment
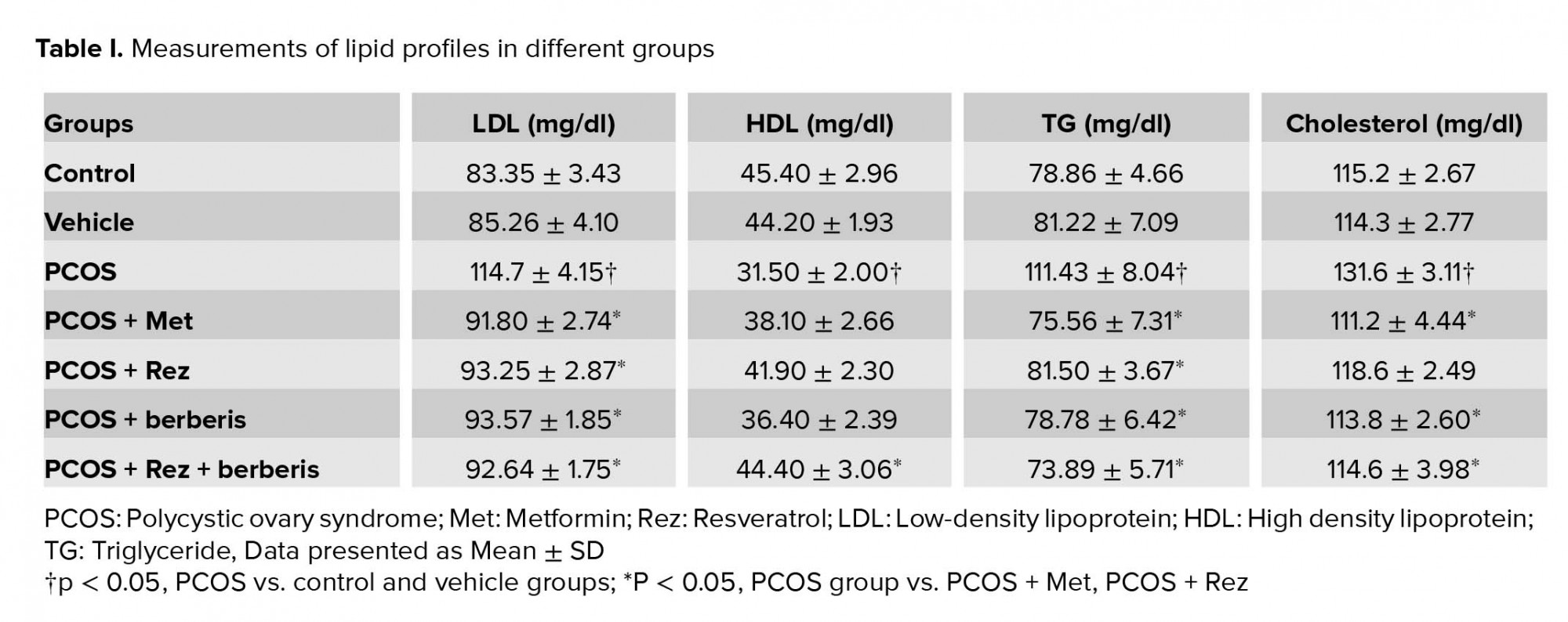
According to Table I, the PCOS-induced group caused significant changes in serum lipid levels compared to the controls. Additionally, TG, TC, and LDL levels meaningfully increased (p = 0.02, p = 0.01, and p = 0.01, respectively), while the HDL level decreased significantly (p = 0.01) compared to the controls. However, metformin, barberry, and resveratrol significantly reduced TG (p = 0.01), TC (p = 0.01), and LDL (p = 0.01) levels compared to the group with PCOS. Nevertheless, the results showed no significant changes between the barberry + resveratrol rats and resveratrol, and barberry groups related to the levels of HDL, LDL, TC, and TG (p ≤ 0.05). There was no significant difference between the treatment groups.
The effects of barberry and resveratrol on antioxidant activity and inflammatory factors are presented in Figure 2. Accordingly, the PCOS-induced group (without treatment) showed a significant decrease in SOD (p = 0.01) and TAC (p = 0.04) levels, but an increase in TNFα (p = 0.01) and MDA (p = 0.01) concentrations. Treatment with metformin, resveratrol, and barberry could restore SOD (p = 0.01) and TAC (p = 0.01) levels close to those in the control groups. However, MDA (p = 0.01) and TNF-α (p = 0.02) levels reduced significantly in metformin-, resveratrol-, and barberry-treated groups in comparison to the control groups. Nonetheless, no significant differences between the barberry + resveratrol rats and those in the resveratrol and barberry groups related to MDA, SOD, TNF-α, and TAC concentrations (p = 0.99) were observed. There was no significant difference between the treatment groups.
Table II shows the ovarian weights of rats in the control and experimental groups. The ovarian weight increased significantly in the PCOS rats in comparison to the control rats (p = 0.01). Treatment with metformin, barberry, resveratrol, and barberry + resveratrol significantly decreased the ovarian weight in PCOS rats after 42 days (p = 0.01). The ovarian weights were not significant between the barberry + resveratrol rats and those in the resveratrol and barberry groups (p = 0.99).
3.2. Insulin resistance
According to the results presented in Table II, the insulin level and HOMA-IR score significant changes in the metformin-, resveratrol-, and barberry-treated groups compared to the control groups (p = 0.007, p = 0.004, respectively) demonstrated significant differences. The results also demonstrated no significant differences between the barberry + resveratrol rats and those in resveratrol and barberry groups with respect to insulin level, glucose level, and HOMA-IR score (p = 0.99).
3.3. Histomorphological changes
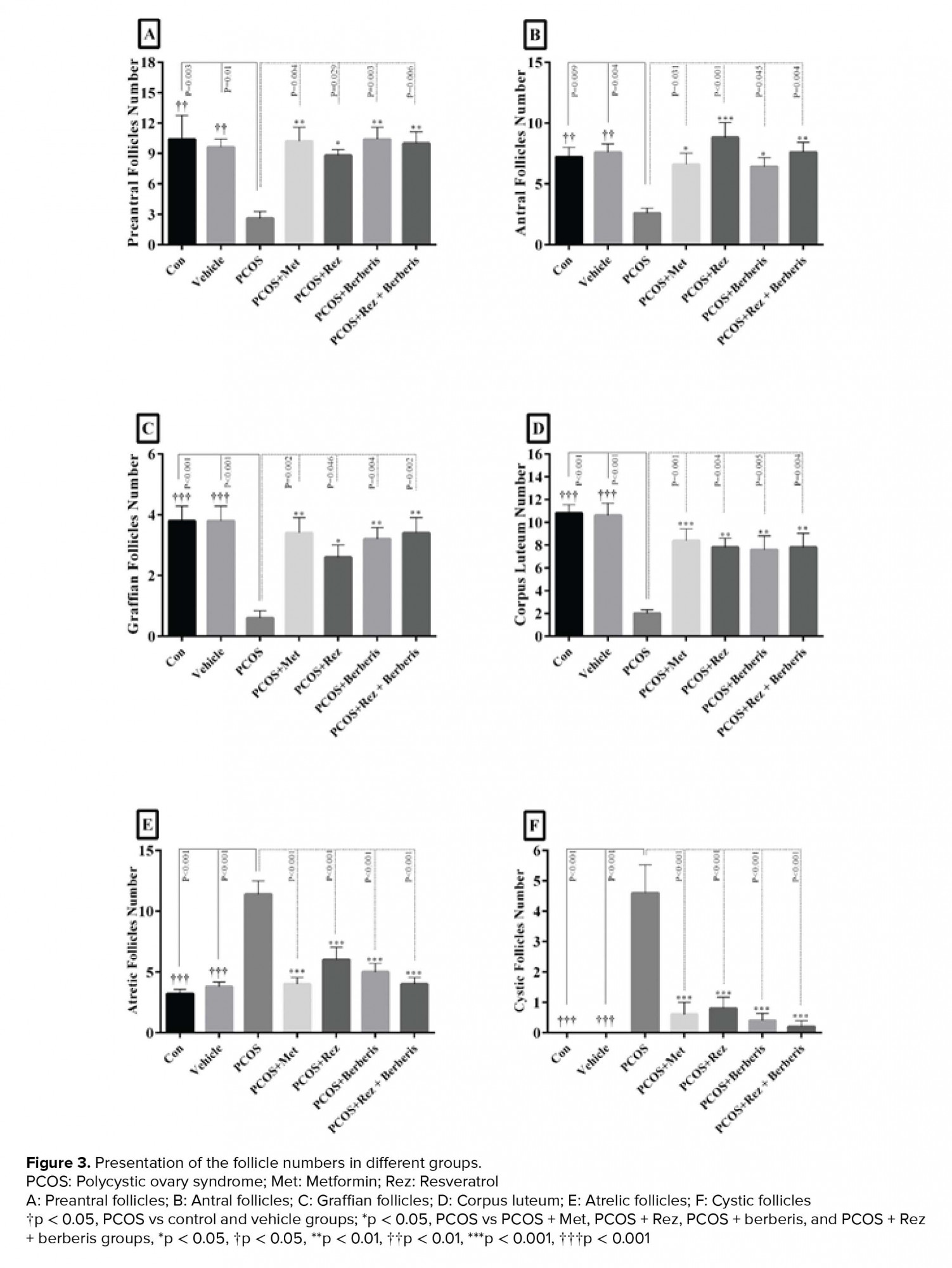
In this study, a significant decrease in the number of preantral follicles, antral follicles, graafian follicles, and corpus luteum in PCOS group compared to the control and vehicle (p = 0.01) groups is observed (Figure 3A-D). On the other hand, the number of follicles increased significantly in comparison with PCOS (p = 0.01). The number of atretic and cystic follicles increased significantly in the PCOS group in contrast with the control and vehicle groups; while, on the other hand, they decreased significantly in barberry, resveratrol and their combination groups (p= 0.01) (Figure 3E-F). Ovarian sections of control group animals exhibited healthy follicles with oocytes at different development stages (Figure 4A). PCOS- induced group exhibited numerous subcapsular cysts with very thin or no granulosa layers. Additionally, complete absence of corpora lutea were noticed, representing anovulation. Few follicles were observed at their early stages of development. In addition, they were accompanied by atretic follicles containing fluid-filled antrum and a larger number of pyknotic granulosa cells (Figure 4B). On the other hand, metformin treatment group led to the removal of the cysts and appearance of healthy follicles and corpora lutea (Figure 4C). Sections of the resveratrol (20 mg/kg) group exhibited larger follicles and few corpora lutea. Indeed, the cysts were absent and normal-sized healthy follicles were found at different development stages (Figure 4D). Photomicrograph from the barberry group revealed an increase in this group’s corpus luteum (Figure 4E). Treatment with barberry and resveratrol also resulted in the appearance of many corpora lutea and antral follicles with clearly differentiated oocytes, granulosa cell layers, corona radiates, cumulus oophorus, and theca cells (Figure 4F).
3.4. Determination of TPC and antioxidant activity
The DPPH antioxidant assay was performed to determine the ability of the sample to inactivate DPPH radical. The IC50 of Berberis hydroalcholic extract and Quercetin as positive control were 33.1 ± 1.07 μg/mL and 9.43 ±2.26 μM, respectively.
The TPC of the extract as determined by Folin-Ciocalteu method are reported as gallic acid equivalent. As expected, the amount of total phenolic compounds was found to be rich in Berberis hydroalcoholic extract (214.12 ± 6.95 mg GAE/gr dry extract). Values were expressed as mean ± standard error of mean (SEM) of triplicate experiments.



4. Discussion
Generally, the animal models are used to gain clinical insight and high degree of evolutionary conservation into human reproductive disorders, especially PCOS (23). PCOS induced by letrozole is histologically and biochemically similar to PCOS in humans that increases testosterone but decreases progesterone and estrogen levels, eventually leading to the appearance of cysts that inhibit the aromatase enzyme (24). Based on Webber and colleagues study in 2003, PCOS could result in an increase in the number of antral follicles, ovarian stroma, hyperplasia theca cells, and ovarian cortical thickness (25). Moreover, it can be concluded that the number of primordial and primary forms of prenatal follicles is increased in PCOS compared with normal ovaries. Indeed, the number of primary follicles (early growing) is increased significantly in both ovulatory and anovulatory PCOS, with a reciprocal decrease in the proportion of primordial follicles compared to normal ovaries. These results are in line with those of the present investigation.
The histomorphological slides in the resveratrol group showed fewer cysts and the presence of corpus luteum in the ovaries, indicating follicle maturation and ovulation. This was in the same line with the results of a previous study by Ergenoglu and co-workers In that study, the treatment of PCOS-induced rats with 10 mg/kg resveratrol led to the development of antral follicles to the normal state (11). These favorable changes indicate that barberry, resveratrol, and their combination may have positive effects on PCOS symptoms. Interestingly, the histomorphological changes in the barberry, resveratrol, and their combination groups were totally comparable to those in the metformin-treated group.
In this study, it was found that B. integerrima is a predominant source of antioxidant (DPPH, assay, IC50 = 33.1 + 1.07 µg/mL). Our data are supported by a few investigations on antioxidant activity and phenolic content amounts of Berberis species (26-28). High amounts of antioxidant and polyphenol present in Berberis act as free radical scavengers, making it a necessary aid to human health.
Our results revealed a significant increase in body weight among the rats suffering from letrozole-induced PCOS in comparison with the control and vehicle groups, which was consistent with the results obtained by Maharjan (29). On the other hand, our findings confirmed that there is a significant decrease in body weight in the groups treated with barberry, metformin, and resveratrol. These effects are consistent with the previously published data.
Evidence indicate that there is an excessive occurrence of alterations in lipid profiles and lipedema in women with PCOS (30). The current results demonstrated that barberry extract and resveratrol could reduce TC, LDL, and TG levels following an increase in HDL concentrations. Besides, the simultaneous use of barberry and resveratrol provides better results in comparison with metformin in terms of HDL and TG concentration.
Recent studies have shown that Peroxisome proliferation-activated receptor-α (PPAR-α) is predominantly expressed in tissues that have fatty acids metabolizing function such as liver, heart, kidney, and muscles. Activation of PPAR-α could reduce serum TG and also raise HDL level. Barberry is able to activate PPAR-α and AMP-activated protein kinase (AMPK) which is accompanied by reduction of lipid accumulation in adipocytes (31). Similarly, Derosa and colleagues examined the potential application of Berberine in the regulation of plasma lipids in patients with cardiovascular diseases and found that Berberine present in barberry could decrease TC, LDL, and TG levels following an increase in HDL concentration (32).
Another study found that Berberine might improve the liver function and secretion of bile acids. It seems that the high levels of polyphenolic compounds might inhibit intestinal cholesterol absorption, inactivate 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCoA) enzyme, and reduce the intestinal production of chylomicrons in berberine which in turn results in constructive effects of this substance on lipid profiles (33).
The hypocholesterolemia-inducing effect of resveratrol may be explained by its phenolic hydroxyls compounds that result in oxidation of unsaturated fatty acids, as well as a decrease in circulating cholesterol, downregulation of HMG-CoA reductase, and an increase in the expression of the LDL receptors (LDL-R) in hepatocytes. In addition, it is reported that the alternations in medium and long-chain triglyceride concentrations, decreased apolipoprotein C-III, reduced activity of hepatic acyl-CoA cholesterol acyltransferase, and the upregulation of genes involved in lipid metabolism might be linked to the hypotriglyceridemic effect of resveratrol which is in line with our findings (34-36).
A clinical trial demonstrated that 250-1000 mg daily consumption of resveratrol declined LDL in patients with type II diabetes. In the same way, treatment with resveratrol (150 mg daily) lowered plasma TG in healthy obese men (37).
It was previously reported by Victor and colleagues that changes in the mitochondrial membrane potentially resulted in the activation of kappa B nuclear factor ( a pro-inflammatory transcription factor) that induces reactive oxygen species (ROS) , TNF-α, and endothelial dysfunction in patients with PCOS (38). Although, TNF-α could increase the inflammatory factors such as IL-1, IL-6, and inducible nitric oxide synthase, resveratrol might play an essential role in inhibiting the expression of inflammatory factors (39). A number of studies showed that resveratrol could decrease TNF-α concentration and improve SOD and glutathione peroxidase 1 levels, in addition to disabling superoxide and hydrogen peroxide which is in correlation with the findings of the present study (40, 41). A number of studies suggested that Berberine exerts its anti-inflammatory effects by inhibiting cyclooxygenase-2, which is an enzyme that is responsible for inflammation, as well as nuclear factor kappa-light-chain-enhancer of activated B cells. In this way, it directly scavenges superoxide-free radicals in the system, increases the expression of sirtuin 1, and decreases oxidative stress by reducing expression of nicotinamide and adenine dinucleotide phosphate oxidase which are critical sources for ROS production in cells. In line with the present research, the review study performed by Imenshahidi and co-workers showed that barberry and resveratrol might suppress TNF-α, IL-1, nitric oxide (NO), and MDA through the aforementioned mechanism (9, 19, 42, 43). According to this mechanism, resveratrol and barberry increase SOD and TAC levels. As a result, the simultaneous consumption of barberry and resveratrol increased the antioxidant activity and significantly reduced the inflammation in the PCOS group compared with the metformin group at TAC level. In other words, the simultaneous consumption of barberry and resveratrol led to more desirable results possibly due to their synergistic effects.
The high prevalence of abnormal blood sugar levels in PCOS patients indicates the presence of deficiencies in insulin secretion as well as its function. One study found that androgens could cause insulin resistance and change insulin action in the target tissues in PCOS patients, which may eventually increase visceral adiposity and reduce the secretion of adiponectin, which is the major insulin-sensitizing adipokine (44).
According to the studies, resveratrol and barberry might reduce insulin resistance by regulating insulin signaling pathway through increasing protein kinase B (PKB) expression. This is following the increase in the activity of peroxisome proliferator-activated receptor-gamma (PPAR-γ) and expression of glucose transporter type 4, sirtuin 1, and enhancing glucose uptake in the absence of insulin. In addition, the inhibition of insulin secretion from new island cells, reducing intestinal glucose absorption by inhibiting α-glucosidase activity, and stimulating healthy pancreatic beta cells are other results of this pathway. A study has discovered that berberine reduces insulin resistance in metabolic syndrome (45-47).
The results of this study revealed that the administration of barberry extract and resveratrol in adult female rats with PCOs can lead to significant changes in their insulin resistance.
However, there were no significant changes in the serum glucose levels. This might be a result of low antioxidant dosages and short study duration.
5. Conclusion
In conclusion, it can be stated that the combination of Resveratrol and B. integerrima (as natural products) can decrease biochemical factors related to the pathogenesis of PCOS. They have the promising antioxidant capacity and anti-inflammatory activities that might ameliorate the complications, and they might be able to regenerate the ovarian morphology to the normal state. Future studies in larger groups, as well as randomized clinical trials on resveratrol and barberry, will help researchers in introducing new treatments for PCOS patients.
Acknowledgments
The present article has been extracted from project no. IR.sums.REC. 1394.S1208 approved by the Shiraz University of Medical Sciences, Shiraz, Iran. The authors hereby thank the Center of Comparative and Experimental Medicine and the Pathology Laboratory of Shahid Faghihi Hospital for their cooperation in the research. They also wish to thank Ms. A. Keivanshekouh at the Research Improvement Center of Shiraz University of Medical Sciences for improving the language of the manuscript. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
None declared.
Full-Text: (427 Views)
- Introduction
This syndrome is associated with a range of reproductive, endocrine, and metabolic specifications, including obesity, being overweight, hyperandrogenism, anovulation, and infertility that results in hyperinsulinism and type II diabetes.
There are numerous researches about inflammation in chronic diseases such as PCOS, and the inflammatory markers include C-reactive (CRP) protein, interleukin (IL) 6, and tumor necrosis factor alpha (TNF-α). As a result of inflammation, the risk of developing type 2 diabetes, cardiovascular disease, ovarian dysfunction, and hyperandrogenism increases because of the free radicals. Furthermore, oxidative damage is aggravated by the decrease in the body's antioxidant defense mechanisms such as superoxide dismutase (SOD) and catalase (CAT) activities that act as free radicals’ scavengers. Total antioxidant capacity (TAC) is sensitive to changes in plasma antioxidant levels and degree of insulin resistance. Therefore, a wide range of oxidative stress biomarkers, including malondialdehyde (MDA), protein carbonyl, TAC, SOD, glutathione peroxidase (GPx), and glutathione (GSH) have been tested in the patients (2-4).
Due to the low efficacy and safety of the synthetic drugs and the increased risk of insulin resistance and hyperlipidemia, there is a growing tendency toward the consumption of medicinal plants instead of synthetic compounds (5).
B. integerrima is a regular ingredient of Iranian cuisine (6). The high antioxidant capacity of this plant is because of the presence of natural flavonoids and phenolic compounds such as anthocyanins and carotenoids in Berberis which are known to be antidiabetic, anticancer, and antimicrobial natural agents. Research on the cytotoxicity of B. integerrima extract demonstrated no cellular toxicity (7-9).
Resveratrol (3, 5, 4’-trihydroxy-trans-stilbene) is commonly found in grapes, peanuts, and some types of berries as a natural polyphenolic compound. It is recognized as a powerful antioxidant with antiplatelet, anticancer, anti-inflammation, and antiaging effects. Several studies have demonstrated direct antioxidant effects of resveratrol, and its protective effects may be due to the enhancement of antioxidant capacity in both humans and rodents (10, 11). As a result, antioxidants are extremely important because they inactivate the reactive species and increase the protection of biological sites (9).
In this study, the effectiveness of resveratrol, B. integerrima, and their combination were evaluated in the PCOS rat models for the first time. Moreover, the biochemical parameters, including oxidative stress, inflammatory and lipid profiles, as well as ovarian pathology were determined.
- Materials and Methods
- 1. Induction of PCOS
- 2. Experimental design
- Group (I): control, healthy rats not receiving any interventions;
- Group (II): vehicle, healthy rats receiving 1 cc normal saline for 63 days;
- Group (III): letrozole-induced PCOS (Aburaihan Pharma.co., Tehran, Iran), rats receiving 1 cc normal saline for 42 days orally;
- Group (IV): PCOS + receiving 150 mg/kg metformin (Shafa Pharma.co., Tehran, Iran) dissolved in 1 cc normal saline for 42 days orally, after letrozole-induced PCOS (13);
- Group (V): PCOS + receiving 20 mg/kg resveratrol (Nutrivit Co., USA) dissolved in 1 cc normal saline for 42 days orally, after letrozole-induced PCOS (11);
- Group (VI): PCOS + 3 gr/kg barberry dissolved in 1 cc normal saline for 42 days orally, after letrozole-induced PCOS (14);
- Group (VII): PCOS + receiving 3 gr/kg barberry and 20 mg/kg resveratrol dissolved in 1 cc normal saline for 42 days orally, after letrozole-induced PCOS.
- 3. Barberry extract preparation
- 4. Antioxidant activity
The DPPH scavenging effect was calculated as follows:
Scavenging effect (%) =

Where A0 is the absorbance of the control, and A1 is the absorbance in the presence of the sample. All determinations were performed in triplicate.
- 5. Determination of total phenolic content (TPC) by Folin-Ciocalteu assay
- 6. Biochemical markers
Lipid profiles [total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL)] were estimated via the enzymatic colorimetric method using a biochemical Auto-analyzer (BT-1500, Italy) and the kits (Pars Azmoon Co., Iran).
SOD and TAC were determined via the enzymatic colorimetric method with the microplate reader using enzymatic kits (ZV-TAC-A96) purchased from Zellbio Co., Germany. Additionally, MDA activity was determined via spectrophotometric analysis using thiobarbituric acid reactive substances (TBARS) method (19).
Plasma insulin level was measured via enzyme-linked immunosorbent assay (ELISA) method using insulin kits (Rat Insulin Mercodia 10-1250-01, Sweden) (2).
The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the following formula (20):

TNF-α was measured by the ELISA method using TNF-α kits (865.000.96 Diaclone, France) (21).
- 7. Ovarian histopathology

2.8. Ethical consideration
The study was conducted in accordance with the recommendations of the European Council Directive (86/609/EEC) on November 24, 1986, regarding the protection of animals used for experimental purposes (http://data.europa.eu/eli/dir/1986/609/oj). The animal protocols were also approved by the Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran (Code: IR. SUMS. REC.1394.S1208).
2.9. Statistical analysis
The results have been reported as mean ± standard deviation. Additionally, data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s test. P < 0.05 was considered to be statistically significant. All analyses were done using the SPSS statistical software, version 22 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Biochemical assessment
According to Table I, the PCOS-induced group caused significant changes in serum lipid levels compared to the controls. Additionally, TG, TC, and LDL levels meaningfully increased (p = 0.02, p = 0.01, and p = 0.01, respectively), while the HDL level decreased significantly (p = 0.01) compared to the controls. However, metformin, barberry, and resveratrol significantly reduced TG (p = 0.01), TC (p = 0.01), and LDL (p = 0.01) levels compared to the group with PCOS. Nevertheless, the results showed no significant changes between the barberry + resveratrol rats and resveratrol, and barberry groups related to the levels of HDL, LDL, TC, and TG (p ≤ 0.05). There was no significant difference between the treatment groups.
The effects of barberry and resveratrol on antioxidant activity and inflammatory factors are presented in Figure 2. Accordingly, the PCOS-induced group (without treatment) showed a significant decrease in SOD (p = 0.01) and TAC (p = 0.04) levels, but an increase in TNFα (p = 0.01) and MDA (p = 0.01) concentrations. Treatment with metformin, resveratrol, and barberry could restore SOD (p = 0.01) and TAC (p = 0.01) levels close to those in the control groups. However, MDA (p = 0.01) and TNF-α (p = 0.02) levels reduced significantly in metformin-, resveratrol-, and barberry-treated groups in comparison to the control groups. Nonetheless, no significant differences between the barberry + resveratrol rats and those in the resveratrol and barberry groups related to MDA, SOD, TNF-α, and TAC concentrations (p = 0.99) were observed. There was no significant difference between the treatment groups.
Table II shows the ovarian weights of rats in the control and experimental groups. The ovarian weight increased significantly in the PCOS rats in comparison to the control rats (p = 0.01). Treatment with metformin, barberry, resveratrol, and barberry + resveratrol significantly decreased the ovarian weight in PCOS rats after 42 days (p = 0.01). The ovarian weights were not significant between the barberry + resveratrol rats and those in the resveratrol and barberry groups (p = 0.99).
3.2. Insulin resistance
According to the results presented in Table II, the insulin level and HOMA-IR score significant changes in the metformin-, resveratrol-, and barberry-treated groups compared to the control groups (p = 0.007, p = 0.004, respectively) demonstrated significant differences. The results also demonstrated no significant differences between the barberry + resveratrol rats and those in resveratrol and barberry groups with respect to insulin level, glucose level, and HOMA-IR score (p = 0.99).
3.3. Histomorphological changes
In this study, a significant decrease in the number of preantral follicles, antral follicles, graafian follicles, and corpus luteum in PCOS group compared to the control and vehicle (p = 0.01) groups is observed (Figure 3A-D). On the other hand, the number of follicles increased significantly in comparison with PCOS (p = 0.01). The number of atretic and cystic follicles increased significantly in the PCOS group in contrast with the control and vehicle groups; while, on the other hand, they decreased significantly in barberry, resveratrol and their combination groups (p= 0.01) (Figure 3E-F). Ovarian sections of control group animals exhibited healthy follicles with oocytes at different development stages (Figure 4A). PCOS- induced group exhibited numerous subcapsular cysts with very thin or no granulosa layers. Additionally, complete absence of corpora lutea were noticed, representing anovulation. Few follicles were observed at their early stages of development. In addition, they were accompanied by atretic follicles containing fluid-filled antrum and a larger number of pyknotic granulosa cells (Figure 4B). On the other hand, metformin treatment group led to the removal of the cysts and appearance of healthy follicles and corpora lutea (Figure 4C). Sections of the resveratrol (20 mg/kg) group exhibited larger follicles and few corpora lutea. Indeed, the cysts were absent and normal-sized healthy follicles were found at different development stages (Figure 4D). Photomicrograph from the barberry group revealed an increase in this group’s corpus luteum (Figure 4E). Treatment with barberry and resveratrol also resulted in the appearance of many corpora lutea and antral follicles with clearly differentiated oocytes, granulosa cell layers, corona radiates, cumulus oophorus, and theca cells (Figure 4F).
3.4. Determination of TPC and antioxidant activity
The DPPH antioxidant assay was performed to determine the ability of the sample to inactivate DPPH radical. The IC50 of Berberis hydroalcholic extract and Quercetin as positive control were 33.1 ± 1.07 μg/mL and 9.43 ±2.26 μM, respectively.
The TPC of the extract as determined by Folin-Ciocalteu method are reported as gallic acid equivalent. As expected, the amount of total phenolic compounds was found to be rich in Berberis hydroalcoholic extract (214.12 ± 6.95 mg GAE/gr dry extract). Values were expressed as mean ± standard error of mean (SEM) of triplicate experiments.





4. Discussion
Generally, the animal models are used to gain clinical insight and high degree of evolutionary conservation into human reproductive disorders, especially PCOS (23). PCOS induced by letrozole is histologically and biochemically similar to PCOS in humans that increases testosterone but decreases progesterone and estrogen levels, eventually leading to the appearance of cysts that inhibit the aromatase enzyme (24). Based on Webber and colleagues study in 2003, PCOS could result in an increase in the number of antral follicles, ovarian stroma, hyperplasia theca cells, and ovarian cortical thickness (25). Moreover, it can be concluded that the number of primordial and primary forms of prenatal follicles is increased in PCOS compared with normal ovaries. Indeed, the number of primary follicles (early growing) is increased significantly in both ovulatory and anovulatory PCOS, with a reciprocal decrease in the proportion of primordial follicles compared to normal ovaries. These results are in line with those of the present investigation.
The histomorphological slides in the resveratrol group showed fewer cysts and the presence of corpus luteum in the ovaries, indicating follicle maturation and ovulation. This was in the same line with the results of a previous study by Ergenoglu and co-workers In that study, the treatment of PCOS-induced rats with 10 mg/kg resveratrol led to the development of antral follicles to the normal state (11). These favorable changes indicate that barberry, resveratrol, and their combination may have positive effects on PCOS symptoms. Interestingly, the histomorphological changes in the barberry, resveratrol, and their combination groups were totally comparable to those in the metformin-treated group.
In this study, it was found that B. integerrima is a predominant source of antioxidant (DPPH, assay, IC50 = 33.1 + 1.07 µg/mL). Our data are supported by a few investigations on antioxidant activity and phenolic content amounts of Berberis species (26-28). High amounts of antioxidant and polyphenol present in Berberis act as free radical scavengers, making it a necessary aid to human health.
Our results revealed a significant increase in body weight among the rats suffering from letrozole-induced PCOS in comparison with the control and vehicle groups, which was consistent with the results obtained by Maharjan (29). On the other hand, our findings confirmed that there is a significant decrease in body weight in the groups treated with barberry, metformin, and resveratrol. These effects are consistent with the previously published data.
Evidence indicate that there is an excessive occurrence of alterations in lipid profiles and lipedema in women with PCOS (30). The current results demonstrated that barberry extract and resveratrol could reduce TC, LDL, and TG levels following an increase in HDL concentrations. Besides, the simultaneous use of barberry and resveratrol provides better results in comparison with metformin in terms of HDL and TG concentration.
Recent studies have shown that Peroxisome proliferation-activated receptor-α (PPAR-α) is predominantly expressed in tissues that have fatty acids metabolizing function such as liver, heart, kidney, and muscles. Activation of PPAR-α could reduce serum TG and also raise HDL level. Barberry is able to activate PPAR-α and AMP-activated protein kinase (AMPK) which is accompanied by reduction of lipid accumulation in adipocytes (31). Similarly, Derosa and colleagues examined the potential application of Berberine in the regulation of plasma lipids in patients with cardiovascular diseases and found that Berberine present in barberry could decrease TC, LDL, and TG levels following an increase in HDL concentration (32).
Another study found that Berberine might improve the liver function and secretion of bile acids. It seems that the high levels of polyphenolic compounds might inhibit intestinal cholesterol absorption, inactivate 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCoA) enzyme, and reduce the intestinal production of chylomicrons in berberine which in turn results in constructive effects of this substance on lipid profiles (33).
The hypocholesterolemia-inducing effect of resveratrol may be explained by its phenolic hydroxyls compounds that result in oxidation of unsaturated fatty acids, as well as a decrease in circulating cholesterol, downregulation of HMG-CoA reductase, and an increase in the expression of the LDL receptors (LDL-R) in hepatocytes. In addition, it is reported that the alternations in medium and long-chain triglyceride concentrations, decreased apolipoprotein C-III, reduced activity of hepatic acyl-CoA cholesterol acyltransferase, and the upregulation of genes involved in lipid metabolism might be linked to the hypotriglyceridemic effect of resveratrol which is in line with our findings (34-36).
A clinical trial demonstrated that 250-1000 mg daily consumption of resveratrol declined LDL in patients with type II diabetes. In the same way, treatment with resveratrol (150 mg daily) lowered plasma TG in healthy obese men (37).
It was previously reported by Victor and colleagues that changes in the mitochondrial membrane potentially resulted in the activation of kappa B nuclear factor ( a pro-inflammatory transcription factor) that induces reactive oxygen species (ROS) , TNF-α, and endothelial dysfunction in patients with PCOS (38). Although, TNF-α could increase the inflammatory factors such as IL-1, IL-6, and inducible nitric oxide synthase, resveratrol might play an essential role in inhibiting the expression of inflammatory factors (39). A number of studies showed that resveratrol could decrease TNF-α concentration and improve SOD and glutathione peroxidase 1 levels, in addition to disabling superoxide and hydrogen peroxide which is in correlation with the findings of the present study (40, 41). A number of studies suggested that Berberine exerts its anti-inflammatory effects by inhibiting cyclooxygenase-2, which is an enzyme that is responsible for inflammation, as well as nuclear factor kappa-light-chain-enhancer of activated B cells. In this way, it directly scavenges superoxide-free radicals in the system, increases the expression of sirtuin 1, and decreases oxidative stress by reducing expression of nicotinamide and adenine dinucleotide phosphate oxidase which are critical sources for ROS production in cells. In line with the present research, the review study performed by Imenshahidi and co-workers showed that barberry and resveratrol might suppress TNF-α, IL-1, nitric oxide (NO), and MDA through the aforementioned mechanism (9, 19, 42, 43). According to this mechanism, resveratrol and barberry increase SOD and TAC levels. As a result, the simultaneous consumption of barberry and resveratrol increased the antioxidant activity and significantly reduced the inflammation in the PCOS group compared with the metformin group at TAC level. In other words, the simultaneous consumption of barberry and resveratrol led to more desirable results possibly due to their synergistic effects.
The high prevalence of abnormal blood sugar levels in PCOS patients indicates the presence of deficiencies in insulin secretion as well as its function. One study found that androgens could cause insulin resistance and change insulin action in the target tissues in PCOS patients, which may eventually increase visceral adiposity and reduce the secretion of adiponectin, which is the major insulin-sensitizing adipokine (44).
According to the studies, resveratrol and barberry might reduce insulin resistance by regulating insulin signaling pathway through increasing protein kinase B (PKB) expression. This is following the increase in the activity of peroxisome proliferator-activated receptor-gamma (PPAR-γ) and expression of glucose transporter type 4, sirtuin 1, and enhancing glucose uptake in the absence of insulin. In addition, the inhibition of insulin secretion from new island cells, reducing intestinal glucose absorption by inhibiting α-glucosidase activity, and stimulating healthy pancreatic beta cells are other results of this pathway. A study has discovered that berberine reduces insulin resistance in metabolic syndrome (45-47).
The results of this study revealed that the administration of barberry extract and resveratrol in adult female rats with PCOs can lead to significant changes in their insulin resistance.
However, there were no significant changes in the serum glucose levels. This might be a result of low antioxidant dosages and short study duration.
5. Conclusion
In conclusion, it can be stated that the combination of Resveratrol and B. integerrima (as natural products) can decrease biochemical factors related to the pathogenesis of PCOS. They have the promising antioxidant capacity and anti-inflammatory activities that might ameliorate the complications, and they might be able to regenerate the ovarian morphology to the normal state. Future studies in larger groups, as well as randomized clinical trials on resveratrol and barberry, will help researchers in introducing new treatments for PCOS patients.
Acknowledgments
The present article has been extracted from project no. IR.sums.REC. 1394.S1208 approved by the Shiraz University of Medical Sciences, Shiraz, Iran. The authors hereby thank the Center of Comparative and Experimental Medicine and the Pathology Laboratory of Shahid Faghihi Hospital for their cooperation in the research. They also wish to thank Ms. A. Keivanshekouh at the Research Improvement Center of Shiraz University of Medical Sciences for improving the language of the manuscript. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
None declared.
Type of Study: Original Article |
Subject:
Fertility & Infertility
References
1. Zahiri Z, Sharami SH, Milani F, Mohammadi F, Kazemnejad E, Ebrahimi H, et al. Metabolic syndrome in patients with polycystic ovary syndrome in Iran. Int J Fertil Steril 2016; 9: 490-496.
2. Ghowsi M, Khazali H, Sisakhtnezhad S. The effect of resveratrol on oxidative stress in the liver and serum of a rat model of polycystic ovary syndrome: An experimental study. Int J Reprod Biomed 2018; 16: 149-158. [DOI:10.29252/ijrm.16.3.149]
3. Bailey B, Phinney S, Volek J. Polycystic Ovarian Syndrome, Insulin Resistance and Inflammation. Retrieved (2019, February 2) from https://blog virtahealth com/pcos-polycystic-ovarian-syndrome. 2019.
4. Ziaee A, Oveisi S, Abedini A, Hashemipour S, Karimzadeh T, Ghorbani A. Effect of metformin and pioglitazone treatment on cardiovascular risk profile in polycystic ovary syndrome. Acta Med Indones 2012; 44: 16-22.
5. Kwon CY, Lee B, Park KS. Oriental herbal medicine and moxibustion for polycystic ovary syndrome: A meta-analysis. Medicine 2018; 97: e12942-e12954. [DOI:10.1097/MD.0000000000012942] [PMID] [PMCID]
6. Minaiyan M, Ghannadi A, Mahzouni P, Jaffari-Shirazi E. Comparative study of Berberis vulgaris fruit extract and berberine chloride effects on acetic acid-induced colitis in rats. Iran J Pharm Res 2011; 10: 97-104.
7. El-Wahab AEA, Ghareeb DA, Sarhan EEM, Abu-Serie MM, El Demellawy MA. In vitro biological assessment of berberis vulgaris and its active constituent, berberine: antioxidants, anti-acetylcholinesterase, anti-diabetic and anticancer effects. BMC Complement Altern Med 2013; 13: 218-229. [DOI:10.1186/1472-6882-13-218] [PMID] [PMCID]
8. Lazavi F, Mirmiran P, Sohrab G, Nikpayam O, Angoorani P, Hedayati M. The barberry juice effects on metabolic factors and oxidative stress in patients with type 2 diabetes: a randomized clinical trial. Complement Ther Clin Pract 2018; 31: 170-174. [DOI:10.1016/j.ctcp.2018.01.009] [PMID]
9. Kumar A, Chopra K, Mukherjee M, Pottabathini R, Dhull DK. Current knowledge and pharmacological profile of berberine: An update. Eur J Pharmacol 2015; 761: 288-297. [DOI:10.1016/j.ejphar.2015.05.068] [PMID]
10. Tomé-Carneiro J, Larrosa M, González-Sarrías A, Tomas-Barberan FA, Teresa Garcia-Conesa M, Carlos Espin J. Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. Curr Pharm Des 2013; 19: 6064-6093. [DOI:10.2174/13816128113199990407] [PMID] [PMCID]
11. Ergenoglu M, Yildirim N, Sahingoz Yildirim AG, Yeniel O, Erbas O, Yavasoglu A, et al. Effects of resveratrol on ovarian morphology, plasma anti-mullerian hormone, IGF-1 levels, and oxidative stress parameters in a rat model of polycystic ovary syndrome. Reprod Sci 2015: 22: 942-947. [DOI:10.1177/1933719115570900] [PMID]
12. Baravalle C, Salvetti NR, Mira GA, Pezzone N, Ortega HH. Microscopic characterization of follicular structures in letrozole-induced polycystic ovarian syndrome in the rat. Arch Med Res 2006; 37: 830-839. [DOI:10.1016/j.arcmed.2006.04.006] [PMID]
13. Di Pietro M, Parborell F, Irusta G, Pascuali N, Bas D, Bianchi MS, et al. Metformin regulates ovarian angiogenesis and follicular development in a female polycystic ovary syndrome rat model. Endocrinology 2015; 156: 1453-1463. [DOI:10.1210/en.2014-1765] [PMID]
14. Tanideh N, Afaridi E, Mehrabani D, Azarpira N, Hosseinzadeh M, Amini M, et al. The healing effect of berberis vulgaris in acetic acid-induced ulcerative colitis in rat. Middle-East J Sci Res 2014; 21: 1288-1294.
15. Iraji A, Firuzi O, Khoshneviszadeh M, Nadri H, Edraki N, Miri R. Synthesis and structure-activity relationship study of multi-target triazine derivatives as innovative candidates for treatment of Alzheimer's disease. Bioorg Chem 2018; 77: 223-235. [DOI:10.1016/j.bioorg.2018.01.017] [PMID]
16. Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food science and Technology 1995; 28: 25-30. [DOI:10.1016/S0023-6438(95)80008-5]
17. Koohi-Hosseinabadi O, Ranjbar Z, Sepehrimanesh M, AndisheTadbir A, Poorbaghi SL, Bahranifard H, et al. Biochemical, hematological, and pathological related healing effects of Elaeagnus angustifolia hydroalcoholic extract in 5-fluorouracil-induced oral mucositis in male golden hamster. Enviro Sci Pollut Res Int 2017; 24: 24447-24453. [DOI:10.1007/s11356-017-0137-5] [PMID]
18. Tanideh N, Bahrani M, Khoshnood-Mansoorkhani MJ, Mehrabani D, Firoozi D, Koohi-Hosseinabadi O, et al. Evaluating the effect of melillotus officinalis L. Aqueous extracts on healing of acetic acid-induced ulcerative colitis in male rats. Ann Colorectal Res 2016; 4: e42856-e42862. [DOI:10.17795/acr-42856]
19. Valavi M, Mezginejad F, Haghighi F, Hemmati M, Zarban A, Rabiei Gask E. Effectiveness of aqueous and alcoholic extracts of barberry, jujube, and saffron against oxidative stress in streptozotocin-induced diabetic rats. Mod Care J 2016; 13: e11162-e11167. [DOI:10.5812/modernc.11162]
20. Fonseca ÉJNdC, Rocha TPO, Nogueira IAL, Melo JBd, Silva BLE, Lopes EJ, et al. Metabolic syndrome and insulin resistance by HOMA-IR in menopause. Int J Cardiovasc Sci 2018; 31: 201-208. [DOI:10.5935/2359-4802.20180009]
21. Ihsan I, Tehreem A, Rasool S. Significance of TNF-Alpha and insulin resistance in women with polycystic ovarian syndrome. Pakistan J Med Health Sci 2018; 12: 459-463.
22. Manneras L, Cajander S, Holmang A, Seleskovic Z, Lystig T, Lönn M, et al. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology 2007; 148: 3781-3791. [DOI:10.1210/en.2007-0168] [PMID]
23. Walters KA, Bertoldo MJ, Handelsman DJ. Evidence from animal models on the pathogenesis of PCOS. Best Pract Res Clin Endocrinol Metab 2018; 32: 271-281. [DOI:10.1016/j.beem.2018.03.008] [PMID]
24. Noorafshan A, Ahmadi M, Mesbah SF, Karbalay-Doust S. Stereological study of the effects of letrozole and estradiol valerate treatment on the ovary of rats. Clin Exp Reprod Med 2013; 40: 115-121. [DOI:10.5653/cerm.2013.40.3.115] [PMID] [PMCID]
25. Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K, et al. Formation and early development of follicles in the polycystic ovary. Lancet 2003; 362: 1017-1021. [DOI:10.1016/S0140-6736(03)14410-8]
26. Charehsaz M, Sipahi H, Celep E, Üstündağ A, Ülker ÖC, Duydu Y, et al. The fruit extract of Berberis crataegina DC: exerts potent antioxidant activity and protects DNA integrity. DARU J Pharm Sci 2015; 23: 24-30. [DOI:10.1186/s40199-015-0108-7] [PMID] [PMCID]
27. Sabahi Z, Farmani F, Soltani F, Moein M. DNA protection, antioxidant and xanthine oxidase inhibition activities of polyphenol-enriched fraction of Berberis integerrima Bunge fruits. Iran J Basic Med Sci 2018; 21: 411-416.
28. Sharifi F, Poorakbar L. The survey of antioxidant properties of phenolic compounds in fresh and dry hybrid Barberry fruits (Berberis integerrima× vulgaris). Cumhuriyet Üniversitesi Fen-Edebiyat Fakültesi Fen Bilimleri Dergisi 2015; 36: 1609-1617.
29. Maharjan R, Nagar PS, Nampoothiri L. Effect of Aloe barbadensis Mill. formulation on Letrozole induced polycystic ovarian syndrome rat model. J Ayurveda Integr Med 2010; 1: 273-279. [DOI:10.4103/0975-9476.74090] [PMID] [PMCID]
30. Padalkar RK, Patil SM, Andure DV, Bhagat SS, Raut AM. Study of hormone and lipid profile in polycystic ovarian syndrome women between the age 18 to 30 years. J Pract Biochem Biophys 2017; 2: 11-15.
31. Hadi A, Arab A, Ghaedi E, Rafie N, Miraghajani M, Kafeshani M. Barberry (Berberis vulgaris L.) is a safe approach for management of lipid parameters: A systematic review and meta‐analysis of randomized controlled trials. Complement Ther Med 2019; 43: 117-124. [DOI:10.1016/j.ctim.2019.01.017] [PMID]
32. Derosa G, D'Angelo A, Bonaventura A, Bianchi L, Romano D, Maffioli P. Effects of berberine on lipid profile in subjects with low cardiovascular risk. Expert Opin Biol Ther 2013; 13: 475-482. [DOI:10.1517/14712598.2013.776037] [PMID]
33. Shamsa F, Ahmadiani A, Khosrokhavar R. Antihistaminic and anticholinergic activity of barberry fruit (Berberis vulgaris) in the guinea-pig ileum. J Ethnopharmacol 1999; 64: 161-166. [DOI:10.1016/S0378-8741(98)00122-6]
34. Simental-Mendía LE, Guerrero-Romero F. Effect of resveratrol supplementation on lipid profile in subjects with dyslipidemia: A randomized double-blind, placebo-controlled trial. Nutrition 2019; 58: 7-10. [DOI:10.1016/j.nut.2018.06.015] [PMID]
35. Cho IJ, Ahn JY, Kim S, Choi MS, Ha TY. Resveratrol attenuates the expression of HMG-CoA reductase mRNA in hamsters. Biochem Biophys Res Commun 2008; 367: 190-194. [DOI:10.1016/j.bbrc.2007.12.140] [PMID]
36. Yashiro T, Nanmoku M, Shimizu M, Inoue J, Sato R. Resveratrol increases the expression and activity of the low density lipoprotein receptor in hepatocytes by the proteolytic activation of the sterol regulatory element-binding proteins. Atherosclerosis 2012; 220: 369-374. [DOI:10.1016/j.atherosclerosis.2011.11.006] [PMID]
37. Bonnefont-Rousselot D. Resveratrol and cardiovascular diseases. Nutrients 2016; 8: 250-273. [DOI:10.3390/nu8050250] [PMID] [PMCID]
38. Victor VM, Rocha M, Bañuls C, Alvarez A, de Pablo C, Sanchez-Serrano M, et al. Induction of oxidative stress and human leukocyte/endothelial cell interactions in polycystic ovary syndrome patients with insulin resistance. J Clin Endocrinol Metab 2011; 96: 3115-3122. [DOI:10.1210/jc.2011-0651] [PMID]
39. Zhu X, Liu Q, Wang M, Liang M, Yang X, Xu X, et al. Activation of Sirt1 by resveratrol inhibits TNF-alpha induced inflammation in fibroblasts. PloS One 2011; 6: e27081. [DOI:10.1371/journal.pone.0027081] [PMID] [PMCID]
40. Zhu J, Yong W, Wu X, Yu Y, Lv J, Liu C, et al. Anti-inflammatory effect of resveratrol on TNF-α-induced MCP-1 expression in adipocytes. Biochem Biophys Res Commun 2008; 369: 471-477. [DOI:10.1016/j.bbrc.2008.02.034] [PMID]
41. Sebastiano M, Eens M, Messina S, AbdElgawad H, Pineau K, Beemster GTS, et al. Resveratrol supplementation reduces oxidative stress and modulates the immune response in free‐living animals during a viral infection. Functional Ecology 2018; 32: 2509-2519. [DOI:10.1111/1365-2435.13195]
42. Imenshahidi M, Hosseinzadeh H. Berberis vulgaris and berberine: An update review. Phytother Res 2016; 30: 1745-1764. [DOI:10.1002/ptr.5693] [PMID]
43. Li Z, Geng YN, Jiang JD, Kong WJ. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid Based Complement Alternat Med 2014; 2014: 289264-289276. [DOI:10.1155/2014/289264] [PMID] [PMCID]
44. Bergman RN, Finegood DT, Kahn SE. The evolution of β‐cell dysfunction and insulin resistance in type 2 diabetes. Eur J Clin Invest 2002; 32 (Suppl.): 35-45. [DOI:10.1046/j.1365-2362.32.s3.5.x] [PMID]
45. Firouzi S, Malekahmadi M, Ghayour-Mobarhan M, Ferns G, Rahimi HR. Barberry in the treatment of obesity and metabolic syndrome: possible mechanisms of action. Diabetes Metab Syndr Obes 2018; 11: 699-705. [DOI:10.2147/DMSO.S181572] [PMID] [PMCID]
46. Pérez-Rubio KG, González-Ortiz M, Martínez-Abundis E, Robles-Cervantes JA, Espinel-Bermúdez MC. Effect of berberine administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab Syndr Relat Disord 2013; 11: 366-369. [DOI:10.1089/met.2012.0183] [PMID]
47. Ashraf H, Heidari R, Nejati V, Ilkhanipoor M. [Preventive effect of berberis integerrima on the serum levels of glucose and lipids in streptozotocin (stz)-induced diabetes in rats]. J Fasa Univ Med Sci 2012; 2: 148-155. (in Persian)
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





