Sat, Apr 20, 2024
[Archive]
Volume 18, Issue 9 (September 2020)
IJRM 2020, 18(9): 777-784 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Lozeie M, Bagheri M, abdi Rad I, Hossein-Zadeh N, Nasirzadeh M. Zinc attenuates ecstasy-induced apoptosis through downregulation of caspase-3 in cultured TM3 cells: An experimental study. IJRM 2020; 18 (9) :777-784
URL: http://ijrm.ir/article-1-1488-en.html
URL: http://ijrm.ir/article-1-1488-en.html
1- Islamic Azad University of Tabriz Complex, Tabriz, Iran
2- Cellular and Molecular Research Center, Cellular and Molecular Medicine Institute, Urmia University of Medical Sciences, Urmia, Iran. , mortazabagheri@yahoo.com
3- Cellular and Molecular Research Center, Cellular and Molecular Medicine Institute, Urmia University of Medical Sciences, Urmia , Iran.
2- Cellular and Molecular Research Center, Cellular and Molecular Medicine Institute, Urmia University of Medical Sciences, Urmia, Iran. , mortazabagheri@yahoo.com
3- Cellular and Molecular Research Center, Cellular and Molecular Medicine Institute, Urmia University of Medical Sciences, Urmia , Iran.
Full-Text [PDF 557 kb]
(646 Downloads)
| Abstract (HTML) (1475 Views)
Full-Text: (458 Views)
1. Introduction
3, 4-Methylenedioxymethamphetamine (MDMA; “Ecstasy”) is commonly known as the most famous amphetamine derivative. The toxic effects of ecstasy include: hyperthermia, rhabdomyolysis, coagulopathy, or bleeding abnormalities, and acute renal failure (1, 2). MDMA increases the secretion of three neurotransmitters of serotonin, dopamine, and norepinephrine from the axon terminals in the synapse. Serotonin influences mood, appetite, and sleep. It also triggers the secretion of the oxytocin and vasopressin hormones that control social behaviors (3). Ecstasy affects the endocrine system and the hypothalamic-pituitary-thyroid axis, leading to an increase in body temperature (4). Ecstasy with effect on the hypothalamic-pituitary-adrenal axis leads to an increase in the secretion of adrenocorticotropin hormone and cortisol. As a result, the level of free radicals is increased, resulting in ecstasy-induced neurotoxic effects (5, 6).
Chronic use of ecstasy has direct effects on the reproductive system. Ecstasy causes damage to testicular tissue and reduces spermatic production by increasing oxidative stress and apoptosis (7, 8). The mechanism(s) of ecstasy-induced infertility is not fully understood (9). Recent studies have shown that the production of excess amounts of free radicals can lead to serious damage to sperm (10-13). It has been demonstrated that oxidative stress influences the human fertility; and most specialists do not assess their patients regarding oxidative stress (14). In males, the interstitial, or Leydig, cells are placed in the connective tissue nearby the sperm-producing tubules of the testicles (gonads), and produce testosterone (15). The synthesis of androgens is controlled by hypothalamus-pituitary-testicular axis via pituitary secretion of LH. Leydig cells have LH receptors and maintain spermatogenesis through the hypothalamic-pituitary-dependent feedback mechanism (15). The effect of ecstasy on Leydig cells and testicular tissue has not been broadly investigated. At least from the outlook of apoptosis in Leydig cells, there is no report. It has been indicated that ecstasy is toxic to Leydig cells, and leads to the decreased level of normal cells and the increased level of DNA damage in ecstasy-treated Leydig cells (16).
The effect of ecstasy on male reproductive system is may be due to the stimulation of oxidative stress (17). Zinc has a protective influence on human reproductive system by reducing free radicals (18). Zinc deficiency can therefore adversely affect the function of zinc-dependent proteins in biological systems (19). Moreover, the growing bodies of evidences suggest that zinc deficiency increases the secretion and production of inflammatory cytokines as well as the phenomenon of oxidative stress (19-22). In men, antioxidants prevent the harmful effects of oxidative free radicals on spermatogenesis and sperm health and reduce the amount of testicular oxidative stress (23).
This study was designed to investigate the impact of MDMA, zinc and zinc + ecstasy on TM3 cells as well as the mRNA level of caspase-3 in the treated and control groups.
2. Materials and Methods
2.1. Cell culture protocol
In the experimental study, we used Leydig Mouse Cells (TM3) obtained from the Pasteur Institute of Iran. The cells were cultured in DMEM/F12 (ATOCEL, Austria) enriched with 10% FBS (ATOCEL, Austria) and 1% penicillin-streptomycin (ATOCEL, Austria). Following the initial cell-plating density, culture flasks were incubated in humidified 5% CO2 incubator at 37ºC. Cells between passages 3 and 6 were used for various analyses. For cell passage, TM3 cells were detached by using 0.25% Trypsin-EDTA solution (ATOCEL, Austria).
2.2. MTT assay
TM3 cells were cultured at a density of 5 × 103 cells per well in a 96-well culture plate (SPL, Korea) and were kept at 37ºC under 5% CO2. The minimal effective concentration of ecstasy and zinc were evaluated regarding different concentrations of ecstasy and zinc. In this case, after a 24 hr stabilization period, cells were treated with ecstasy (0, 0.5, 1, 3, and 5 mM) for 24 hr and 48 hr. Also, to determine the effective concentration of zinc, TM3 cells were treated with 0, 4, 8, 16, and 32 µM zinc sulfate (sigma, USA) for 24 hr and 48 hr in a final volume of 100 µl. The protective effect of zinc on ecstasy induced apoptosis was tested by pretreatment with zinc. A pretreatment for 24 hour was performed with 8 µM zinc prior to ecstasy (5 mM) exposure. At the end of the incubation time, the supernatant media were discarded and replaced by 100 μl PBS containing 1 mg/ml MTT (3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide, Sigma).
Next, after 4 hr, 100 μl dimethyl sulfoxide (Merck, Germany) was added and kept for 15 min at room temperatture. Using a microplate reader (Bio-Tek, USA), each sample’s absorbance was measured at 545-630 nm. The experiments were repeated in triplicate and results expressed as % of non-treated control. Based on the results from cell survival assay, the effective concentration of ecstasy and zinc was determined. Then, TM3 cells were cultured in four groups and examined. The TM3 cells were cultured in free medium as control (group I), and medium containing ecstasy (5 mM) (group II), medium containing zinc (8 µM) (group III), and medium containing zinc (8 µM) prior to MDMA administration (5 mM) (group IV). In this regard, the TM3 cells were pre-treated with zinc (8 µM) for 24 hr before incubation with ecstasy (5 mM). The MMT assay was performed for evaluating the cell survival rate in the tested groups.
2.3. RNA extraction, cDNA synthesis, and real-time PCR (RT-PCR)
105 TM3 cells/wells were cultured in 6-well plates for 24 hr. The cells were examined in four groups including ecstasy (5 mM), zinc (8 µM), pre-treatment, and control. After 24 hr, while the well of pretreatment group was treated with zinc, the other wells were treated with free medium for 24 hr. The medium was changed with fresh medium. On the next day, the medium was aspirated from each well and exchanged with an ecstasy containing medium for wells of pre-treatment and ecstasy groups, with a zinc-containing medium for zinc group and with free medium for control group. After 24 hr of last treatment, the cells trypsinized and the cells pellet was used to extract RNA after centrifugation at 3000 rpm for 10 min. RNA extraction was carried out using the RNX Plus Solution Kit (SinaClon) (Catalog Number: RN7713C). Two sets of forward and reverse primers were used for the target gene (Caspase-3) (GCA GCT TTG TGT GTG TGA TTC and AGT TTC GGC TTT CCA GTC AG) and reference gene (beta-actin) (TAG GCG GAC TGT TAC TGA GC and GCT CCA ACC AAC TGC TGTC). The PCR program included 94oC for 30 sec; 60oC for 40 sec; and 72oC for 50 sec (35 cycles) (24). The RNA concentration of all specimens was confirmed and the synthesis of cDNA was done through the following compounds: total RNA was used to generate single-stranded cDNA with 2-step RT-PCR kit (Thermo Scientific RevertAid First Strand cDNA Synthesis Kit #K1622). The cDNA synthesis of the samples was carried out in thermocycler for 60 min, according to the program presented in the product certificate at 25oC for 5 min and 42oC for 60 min. Then, the synthesized cDNA was used in this step to perform real-time PCR. The RT-PCR was done using the Applied Biosystems StepOne RT-PCR System.
2.4. Ethical consideration
This study has been approved by the research ethics committee of the Urmia University of Medical Sciences (IR.UMSU.REC.1397.448).
2.5. Statistical analysis
The RT-PCR results were analyzed using the 2-ΔΔCt method. To analysis the data, the Statistical Package for the Social Sciences, version 20, SPSS Inc, Chicago, Illinois, USA (SPSS) software was used. The statistically significant data was determined using the one-way analysis of ANOVA, followed by Tukey's test. Additionally, to determine the significance of the results, the value of P was considered as 0.05.
3. Results
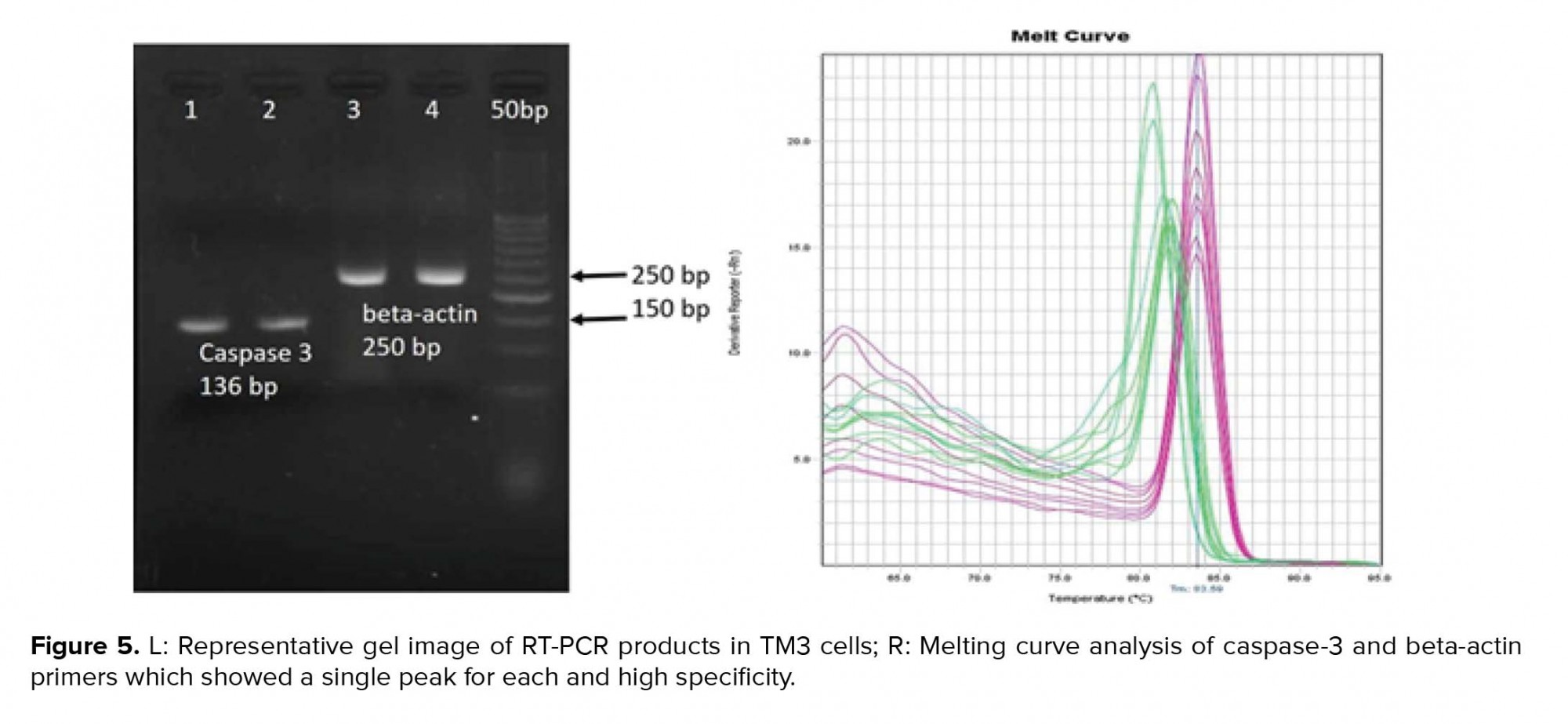
Cell viability was significantly reduced in the TM3 cells treated with different concentrations of ecstasy (0, 0.5, 1, 3, 5mM) for 24 hr and 48 hr. In both 24 hr and 48 hr exposure time, ecstasy decreased the cell viability in a dose/time-dependent manner with IC50 values of 5 mM for 24 hr and 1 mM for 48 hr exposure (Figures 1). In the case of zinc, our study indicated that cell viability became increased in comparison to the control in only lower concentration of zinc in 24 hr and 48 hr exposure time. However, cell viability was decreased regarding high concentrations of zinc (Figures 2). A concentration of 5 mM for ecstasy and 8 µM for zinc was found as effective concentrations (Figure 3). In group IV, cell viability became increased in comparison to groups I and II. Morever, in this study, amplification efficiencies were set at 90-105%. Our findings showed that the mean (± SE) of fold was 22.40 ± 7.5, 0.06 ± 0.02, and 0.009 ± 0.003 in group II, III, and IV, respectively. The mean of caspase-3 mRNA level (fold) was significantly increased by treatment with ecstasy in group II. The relative expression of caspase-3 gene was significantly decreased in the zinc + ecstasy group (group IV) compared with the ecstasy (5 mM) group (p = 0.001) (Figure 4). These results indicated that zinc has the inhibiting effect on apoptosis through caspase-3-mediated pathway in the TM3 cells. Figure 5 shows gel image and melting curve analysis of tested gene RT-PCR products.




4. Discussion
The present research studies the antioxidant activity of zinc. The foremost finding of the present investigation was the attenuation of caspase 3 gene expression by zinc following MDMA treatment. Pretreatment with zinc was protective against MDMA-induced apoptosis in TM3 cells. Our results are in agreement with the other studies and imply that zinc has protective and anti-apoptotic effects at low concentrations by inhibition of oxidative stress damage (16, 22). Several studies indicated that the consumption of ecstasy may result in damage of numerous organs such as the heart, liver, kidney, and central serotonergic (5-HT) systems, and death by unknown mechanisms (24-27). Montiel-Duarte and colleagues showed that exposure to ecstasy caused apoptosis of cultured rat liver cells (17). Also, Pourhassanali and colleagues showed that ethanol-induced toxicity in mouse Sertoli cells decreased via zinc pre-treatment (16).
It has been demonstrated that ecstasy raises DNA break in sperm and modifies testes tissue (7). Ecstasy induces apoptosis in wide range of cell lines and organs such as testes, liver, and brain (28). In testes, the cell death occurs by apoptosis via hazardous materials (ecstasy, ethanol, deprivation of intra-testicular testosterone and serum levels of gonadotrophins, Sertoli cell toxicants, chemotherapeutic drug, etc.) (29). All these facts warn a serious necessity to train people around the toxicities of ecstasy especially in early reproductive age. The biological systems are sensitive to oxidative stress (30). For that reason, external consumption of antioxidant is known as one of the most general curative strategies against hazardous materials. In this regard, numerous antioxidants have been investigated in relation to the oxidative stress (31).
It has been demonstrated that MDMA results in intracellular Ca2+ overflow, depolarization of mitochondrial membrane, reactive oxygen species (ROS) production, and activation of Caspase-9 (32). At lower concentrations, ROS has been allied to the stimulation of cell survival reactions, but in the case of higher concentrations, it activates apoptosis via activation of caspases-3, -8, and -9 (33). Caspase-dependent apoptosis has been studied in several human diseases including cancer, neurological disorders, cardiovascular disorders, autoimmune diseases, and male infertility. Apoptosis has a central role in spermatogenesis (34).
In spermatogenesis, a lot of the developing germ cells pass away through apoptosis before maturity (35). "The physiological cell apoptosis occurs during life, but increased germ cell apoptosis results from external disturbances" (36). According to these findings in the present study the protective effect of zinc against ecstasy induced- apoptosis seems to be related to its potent antioxidant properties. Maintaining physiological concentrations of zinc and its tight control by MTs in each cell of the body is necessary to avoid oxidative stress, since not only zinc deficiency but also zinc overload are pro-oxidant conditions (due to the inhibition of mitochondrial respiration and antioxidant enzymes) (37, 38). Therefore, the protective effect of zinc might be associated to its antioxidant effects. Our findings for the first time not only demonstrated that ecstasy has cytotoxic effect on the TM3 cells and induced apoptosis via over-expression of caspase-3, but also zinc inhibited ecstasy-induced testicular injuries.
5. Conclusion
It can be concluded that dietary intake of zinc has a protective effect against MDMA consumption. These data suggest a possible underlying molecular mechanism for MDMA to induce the apoptosis signaling pathway by upregulation, and also, pretreatment with zinc attenuated apoptosis by down-regulation of caspase-3 gene expression in TM3 cells.
Acknowledgments
The authors are thankful to Dr. Shiva Roshan-Milani and Dr. Naser Khalaji whose valuable comments helped during the planning and development of this study. The author(s) received no specific funding for this work.
Conflicts of interest
None.
3, 4-Methylenedioxymethamphetamine (MDMA; “Ecstasy”) is commonly known as the most famous amphetamine derivative. The toxic effects of ecstasy include: hyperthermia, rhabdomyolysis, coagulopathy, or bleeding abnormalities, and acute renal failure (1, 2). MDMA increases the secretion of three neurotransmitters of serotonin, dopamine, and norepinephrine from the axon terminals in the synapse. Serotonin influences mood, appetite, and sleep. It also triggers the secretion of the oxytocin and vasopressin hormones that control social behaviors (3). Ecstasy affects the endocrine system and the hypothalamic-pituitary-thyroid axis, leading to an increase in body temperature (4). Ecstasy with effect on the hypothalamic-pituitary-adrenal axis leads to an increase in the secretion of adrenocorticotropin hormone and cortisol. As a result, the level of free radicals is increased, resulting in ecstasy-induced neurotoxic effects (5, 6).
Chronic use of ecstasy has direct effects on the reproductive system. Ecstasy causes damage to testicular tissue and reduces spermatic production by increasing oxidative stress and apoptosis (7, 8). The mechanism(s) of ecstasy-induced infertility is not fully understood (9). Recent studies have shown that the production of excess amounts of free radicals can lead to serious damage to sperm (10-13). It has been demonstrated that oxidative stress influences the human fertility; and most specialists do not assess their patients regarding oxidative stress (14). In males, the interstitial, or Leydig, cells are placed in the connective tissue nearby the sperm-producing tubules of the testicles (gonads), and produce testosterone (15). The synthesis of androgens is controlled by hypothalamus-pituitary-testicular axis via pituitary secretion of LH. Leydig cells have LH receptors and maintain spermatogenesis through the hypothalamic-pituitary-dependent feedback mechanism (15). The effect of ecstasy on Leydig cells and testicular tissue has not been broadly investigated. At least from the outlook of apoptosis in Leydig cells, there is no report. It has been indicated that ecstasy is toxic to Leydig cells, and leads to the decreased level of normal cells and the increased level of DNA damage in ecstasy-treated Leydig cells (16).
The effect of ecstasy on male reproductive system is may be due to the stimulation of oxidative stress (17). Zinc has a protective influence on human reproductive system by reducing free radicals (18). Zinc deficiency can therefore adversely affect the function of zinc-dependent proteins in biological systems (19). Moreover, the growing bodies of evidences suggest that zinc deficiency increases the secretion and production of inflammatory cytokines as well as the phenomenon of oxidative stress (19-22). In men, antioxidants prevent the harmful effects of oxidative free radicals on spermatogenesis and sperm health and reduce the amount of testicular oxidative stress (23).
This study was designed to investigate the impact of MDMA, zinc and zinc + ecstasy on TM3 cells as well as the mRNA level of caspase-3 in the treated and control groups.
2. Materials and Methods
2.1. Cell culture protocol
In the experimental study, we used Leydig Mouse Cells (TM3) obtained from the Pasteur Institute of Iran. The cells were cultured in DMEM/F12 (ATOCEL, Austria) enriched with 10% FBS (ATOCEL, Austria) and 1% penicillin-streptomycin (ATOCEL, Austria). Following the initial cell-plating density, culture flasks were incubated in humidified 5% CO2 incubator at 37ºC. Cells between passages 3 and 6 were used for various analyses. For cell passage, TM3 cells were detached by using 0.25% Trypsin-EDTA solution (ATOCEL, Austria).
2.2. MTT assay
TM3 cells were cultured at a density of 5 × 103 cells per well in a 96-well culture plate (SPL, Korea) and were kept at 37ºC under 5% CO2. The minimal effective concentration of ecstasy and zinc were evaluated regarding different concentrations of ecstasy and zinc. In this case, after a 24 hr stabilization period, cells were treated with ecstasy (0, 0.5, 1, 3, and 5 mM) for 24 hr and 48 hr. Also, to determine the effective concentration of zinc, TM3 cells were treated with 0, 4, 8, 16, and 32 µM zinc sulfate (sigma, USA) for 24 hr and 48 hr in a final volume of 100 µl. The protective effect of zinc on ecstasy induced apoptosis was tested by pretreatment with zinc. A pretreatment for 24 hour was performed with 8 µM zinc prior to ecstasy (5 mM) exposure. At the end of the incubation time, the supernatant media were discarded and replaced by 100 μl PBS containing 1 mg/ml MTT (3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide, Sigma).
Next, after 4 hr, 100 μl dimethyl sulfoxide (Merck, Germany) was added and kept for 15 min at room temperatture. Using a microplate reader (Bio-Tek, USA), each sample’s absorbance was measured at 545-630 nm. The experiments were repeated in triplicate and results expressed as % of non-treated control. Based on the results from cell survival assay, the effective concentration of ecstasy and zinc was determined. Then, TM3 cells were cultured in four groups and examined. The TM3 cells were cultured in free medium as control (group I), and medium containing ecstasy (5 mM) (group II), medium containing zinc (8 µM) (group III), and medium containing zinc (8 µM) prior to MDMA administration (5 mM) (group IV). In this regard, the TM3 cells were pre-treated with zinc (8 µM) for 24 hr before incubation with ecstasy (5 mM). The MMT assay was performed for evaluating the cell survival rate in the tested groups.
2.3. RNA extraction, cDNA synthesis, and real-time PCR (RT-PCR)
105 TM3 cells/wells were cultured in 6-well plates for 24 hr. The cells were examined in four groups including ecstasy (5 mM), zinc (8 µM), pre-treatment, and control. After 24 hr, while the well of pretreatment group was treated with zinc, the other wells were treated with free medium for 24 hr. The medium was changed with fresh medium. On the next day, the medium was aspirated from each well and exchanged with an ecstasy containing medium for wells of pre-treatment and ecstasy groups, with a zinc-containing medium for zinc group and with free medium for control group. After 24 hr of last treatment, the cells trypsinized and the cells pellet was used to extract RNA after centrifugation at 3000 rpm for 10 min. RNA extraction was carried out using the RNX Plus Solution Kit (SinaClon) (Catalog Number: RN7713C). Two sets of forward and reverse primers were used for the target gene (Caspase-3) (GCA GCT TTG TGT GTG TGA TTC and AGT TTC GGC TTT CCA GTC AG) and reference gene (beta-actin) (TAG GCG GAC TGT TAC TGA GC and GCT CCA ACC AAC TGC TGTC). The PCR program included 94oC for 30 sec; 60oC for 40 sec; and 72oC for 50 sec (35 cycles) (24). The RNA concentration of all specimens was confirmed and the synthesis of cDNA was done through the following compounds: total RNA was used to generate single-stranded cDNA with 2-step RT-PCR kit (Thermo Scientific RevertAid First Strand cDNA Synthesis Kit #K1622). The cDNA synthesis of the samples was carried out in thermocycler for 60 min, according to the program presented in the product certificate at 25oC for 5 min and 42oC for 60 min. Then, the synthesized cDNA was used in this step to perform real-time PCR. The RT-PCR was done using the Applied Biosystems StepOne RT-PCR System.
2.4. Ethical consideration
This study has been approved by the research ethics committee of the Urmia University of Medical Sciences (IR.UMSU.REC.1397.448).
2.5. Statistical analysis
The RT-PCR results were analyzed using the 2-ΔΔCt method. To analysis the data, the Statistical Package for the Social Sciences, version 20, SPSS Inc, Chicago, Illinois, USA (SPSS) software was used. The statistically significant data was determined using the one-way analysis of ANOVA, followed by Tukey's test. Additionally, to determine the significance of the results, the value of P was considered as 0.05.
3. Results
Cell viability was significantly reduced in the TM3 cells treated with different concentrations of ecstasy (0, 0.5, 1, 3, 5mM) for 24 hr and 48 hr. In both 24 hr and 48 hr exposure time, ecstasy decreased the cell viability in a dose/time-dependent manner with IC50 values of 5 mM for 24 hr and 1 mM for 48 hr exposure (Figures 1). In the case of zinc, our study indicated that cell viability became increased in comparison to the control in only lower concentration of zinc in 24 hr and 48 hr exposure time. However, cell viability was decreased regarding high concentrations of zinc (Figures 2). A concentration of 5 mM for ecstasy and 8 µM for zinc was found as effective concentrations (Figure 3). In group IV, cell viability became increased in comparison to groups I and II. Morever, in this study, amplification efficiencies were set at 90-105%. Our findings showed that the mean (± SE) of fold was 22.40 ± 7.5, 0.06 ± 0.02, and 0.009 ± 0.003 in group II, III, and IV, respectively. The mean of caspase-3 mRNA level (fold) was significantly increased by treatment with ecstasy in group II. The relative expression of caspase-3 gene was significantly decreased in the zinc + ecstasy group (group IV) compared with the ecstasy (5 mM) group (p = 0.001) (Figure 4). These results indicated that zinc has the inhibiting effect on apoptosis through caspase-3-mediated pathway in the TM3 cells. Figure 5 shows gel image and melting curve analysis of tested gene RT-PCR products.





4. Discussion
The present research studies the antioxidant activity of zinc. The foremost finding of the present investigation was the attenuation of caspase 3 gene expression by zinc following MDMA treatment. Pretreatment with zinc was protective against MDMA-induced apoptosis in TM3 cells. Our results are in agreement with the other studies and imply that zinc has protective and anti-apoptotic effects at low concentrations by inhibition of oxidative stress damage (16, 22). Several studies indicated that the consumption of ecstasy may result in damage of numerous organs such as the heart, liver, kidney, and central serotonergic (5-HT) systems, and death by unknown mechanisms (24-27). Montiel-Duarte and colleagues showed that exposure to ecstasy caused apoptosis of cultured rat liver cells (17). Also, Pourhassanali and colleagues showed that ethanol-induced toxicity in mouse Sertoli cells decreased via zinc pre-treatment (16).
It has been demonstrated that ecstasy raises DNA break in sperm and modifies testes tissue (7). Ecstasy induces apoptosis in wide range of cell lines and organs such as testes, liver, and brain (28). In testes, the cell death occurs by apoptosis via hazardous materials (ecstasy, ethanol, deprivation of intra-testicular testosterone and serum levels of gonadotrophins, Sertoli cell toxicants, chemotherapeutic drug, etc.) (29). All these facts warn a serious necessity to train people around the toxicities of ecstasy especially in early reproductive age. The biological systems are sensitive to oxidative stress (30). For that reason, external consumption of antioxidant is known as one of the most general curative strategies against hazardous materials. In this regard, numerous antioxidants have been investigated in relation to the oxidative stress (31).
It has been demonstrated that MDMA results in intracellular Ca2+ overflow, depolarization of mitochondrial membrane, reactive oxygen species (ROS) production, and activation of Caspase-9 (32). At lower concentrations, ROS has been allied to the stimulation of cell survival reactions, but in the case of higher concentrations, it activates apoptosis via activation of caspases-3, -8, and -9 (33). Caspase-dependent apoptosis has been studied in several human diseases including cancer, neurological disorders, cardiovascular disorders, autoimmune diseases, and male infertility. Apoptosis has a central role in spermatogenesis (34).
In spermatogenesis, a lot of the developing germ cells pass away through apoptosis before maturity (35). "The physiological cell apoptosis occurs during life, but increased germ cell apoptosis results from external disturbances" (36). According to these findings in the present study the protective effect of zinc against ecstasy induced- apoptosis seems to be related to its potent antioxidant properties. Maintaining physiological concentrations of zinc and its tight control by MTs in each cell of the body is necessary to avoid oxidative stress, since not only zinc deficiency but also zinc overload are pro-oxidant conditions (due to the inhibition of mitochondrial respiration and antioxidant enzymes) (37, 38). Therefore, the protective effect of zinc might be associated to its antioxidant effects. Our findings for the first time not only demonstrated that ecstasy has cytotoxic effect on the TM3 cells and induced apoptosis via over-expression of caspase-3, but also zinc inhibited ecstasy-induced testicular injuries.
5. Conclusion
It can be concluded that dietary intake of zinc has a protective effect against MDMA consumption. These data suggest a possible underlying molecular mechanism for MDMA to induce the apoptosis signaling pathway by upregulation, and also, pretreatment with zinc attenuated apoptosis by down-regulation of caspase-3 gene expression in TM3 cells.
Acknowledgments
The authors are thankful to Dr. Shiva Roshan-Milani and Dr. Naser Khalaji whose valuable comments helped during the planning and development of this study. The author(s) received no specific funding for this work.
Conflicts of interest
None.
Type of Study: Original Article |
Subject:
Reproductive Biology
References
1. Mohamed WMY, Ben Hamida S, Cassel JC, de Vasconcelos AP, Jones BC. MDMA: interactions with other psychoactive drugs. Pharmacol Biochem Behav 2011; 99: 759-774. [DOI:10.1016/j.pbb.2011.06.032] [PMID]
2. Rochester JA, Kirchner JT. Ecstasy (3,4-methylenedioxymethamphetamine): history, neurochemistry, and toxicology. J Am Board Fam Pract 1999; 12: 137-142. [DOI:10.3122/jabfm.12.2.137] [PMID]
3. Verrico CD, Miller GM, Madras BK. MDMA (Ecstasy) and human dopamine, norepinephrine, and serotonin transporters: implications for MDMA-induced neurotoxicity and treatment. Psychopharmacology (Berl) 2007; 189: 489-503. [DOI:10.1007/s00213-005-0174-5] [PMID]
4. Sprague JE, Banks ML, Cook VJ, Mills EM. Hypothalamic-pituitary-thyroid axis and sympathetic nervous system involvement in hyperthermia induced by 3,4-methylenedioxymethamphetamine (Ecstasy). J Pharmacol Exp Ther 2003; 305: 159-166. [DOI:10.1124/jpet.102.044982] [PMID]
5. Wetherell MA, Montgomery C. Basal functioning of the hypothalamic-pituitary-adrenal (HPA) axis and psychological distress in recreational ecstasy polydrug users. Psychopharmacology (Berl) 2014; 231: 1365-1375. [DOI:10.1007/s00213-013-3325-0] [PMID]
6. Barenys M, Gomez-Catalan J, Camps L, Teixido E, de Lapuente J, Gonzalez-Linares J, et al. MDMA (ecstasy) delays pubertal development and alters sperm quality after developmental exposure in the rat. Toxicol Lett 2010; 197: 135-142. [DOI:10.1016/j.toxlet.2010.05.009] [PMID]
7. Barenys M, Macia N, Camps L, de Lapuente J, Gomez-Catalan J, Gonzalez-Linares J, et al. Chronic exposure to MDMA (ecstasy) increases DNA damage in sperm and alters testes histopathology in male rats. Toxicol Lett 2009; 191: 40-46. [DOI:10.1016/j.toxlet.2009.08.002] [PMID]
8. MacLeod J. The role of oxygen in the metabolism and motility of human spermatozoa. Am J Physiol 1943; 138: 512-518. [DOI:10.1152/ajplegacy.1943.138.3.512]
9. Gharagozloo P, Aitken RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod 2011; 26: 1628-1640. [DOI:10.1093/humrep/der132] [PMID]
10. Kao SH, Chao HT, Chen HW, Hwang TIS, Liao TL, Wei YH. Increase of oxidative stress in human sperm with lower motility. Fertil Steril 2008; 89: 1183-1190. [DOI:10.1016/j.fertnstert.2007.05.029] [PMID]
11. Esteves SC, Agarwal A. Novel concepts in male infertility. Int Braz J Urol 2011; 37: 5-15. [DOI:10.1590/S1677-55382011000100002] [PMID]
12. Agarwal A, Allamaneni SSR. Free radicals and male reproduction. J Indian Med Assoc 2011; 109: 184-187.
13. Khosrowbeygi A. [The role of oxidative stress in male infertility: A review]. Arak Medical University Journal 2013; 15: 94-103. (in Persian)
14. Peak TC, Haney NM, Wang W, DeLay KJ, Hellstrom WJ. Stem cell therapy for the treatment of Leydig cell dysfunction in primary hypogonadism. World J Stem Cells 2016; 8: 306-315. [DOI:10.4252/wjsc.v8.i10.306] [PMID] [PMCID]
15. Ciapetti G, Cenni E, Pratelli L, Pizzoferrato A. In vitro evaluation of cell/ biomaterial interaction by MTT assay. Biomaterials 1993; 14: 359-364. [DOI:10.1016/0142-9612(93)90055-7]
16. Pourmasumi S, Sabeti P, Rahiminia T, Mangoli E, Tabibnejad N, Talebi AR. The etiologies of DNA abnormalities in male infertility: An assessmentand review. Int J Reprod Biomed 2017; 15: 331-344. [DOI:10.29252/ijrm.15.6.331] [PMID] [PMCID]
17. Montiel-Duarte C, Varela-Rey M, Osés-Prieto JA, López-Zabalza MJ, Beitia G, Cenarruzabeitia E, et al. 3,4-Methylenedioxymethamphetamine ("Ecstasy") induces apoptosis of cultured rat liver cells. Biochim Biophys Acta 2002; 1588: 26-32. [DOI:10.1016/S0925-4439(02)00112-6]
18. Tuerk MJ, Fazel N. Zinc deficiency. Curr Opin Gastroenterol 2009; 25: 136-143. [DOI:10.1097/MOG.0b013e328321b395] [PMID]
19. Yan M, Song Y, Wong CP, Hardin K, Ho E. Zinc deficiency alters DNA damage response genes in normal human prostate epithelial cells. J Nutr 2008; 138: 667-673. [DOI:10.1093/jn/138.4.667] [PMID] [PMCID]
20. Stefanidou M, Maravelias C, Dona A, Spiliopoulou C. Zinc: a multipurpose trace element. Arch Toxicol 2006; 80: 1-9. [DOI:10.1007/s00204-005-0009-5] [PMID]
21. Prasad AS. Impact of the discovery of human zinc deficiency on health. J Am Coll Nutr 2009; 28: 257-265. [DOI:10.1080/07315724.2009.10719780] [PMID]
22. Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME. Zinc and human health: an update. Arch Toxicol 2012; 86: 521-534. [DOI:10.1007/s00204-011-0775-1] [PMID]
23. Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health 2014; 32: 1-17. [DOI:10.5534/wjmh.2014.32.1.1] [PMID] [PMCID]
24. Pourhassanali N, Roshan-Milani S, Kheradmand F, Motazakker M, Bagheri M, Saboory E. Zinc attenuates ethanol-induced Sertoli cell toxicity and apoptosis through caspase-3 mediated pathways. Reprod Toxicol 2016; 61: 97-103. [DOI:10.1016/j.reprotox.2016.03.041] [PMID]
25. Carvalho M, Remião F, Milhazes N, Borges F, Fernandes E, Cen Monteiro Md, et al. Metabolism is required for the expression of ecstasy-induced cardiotoxicity in vitro. Chem Res Toxicol 2004; 17: 623-632. [DOI:10.1021/tx049960f] [PMID]
26. Bora F, Yılmaz F, Bora T. Ecstasy (MDMA) and its effects on kidneys and their treatment: A review. Iran J Basic Med Sci 2016; 19: 1151-1158.
27. Liechti ME. Effects of MDMA on body temperature in humans. Temperature 2014; 1: 192-200. [DOI:10.4161/23328940.2014.955433] [PMID] [PMCID]
28. Song BJ, Moon KH, Upreti VV, Eddington ND, Lee IJ. Mechanisms of MDMA (ecstasy)-induced oxidative stress, mitochondrial dysfunction, and organ damage. Curr Pharm Biotechnol 2010; 11: 434-443. [DOI:10.2174/138920110791591436] [PMID] [PMCID]
29. Dickerson SM, Walker DM, Reveron ME, Duvauchelle CL, Gore AC. The recreational drug ecstasy disrupts the hypothalamic-pituitarygonadal reproductive axis in adult male rats. Neuroendocrinology 2008; 88: 95-102. [DOI:10.1159/000119691] [PMID] [PMCID]
30. Café C, Torri C, Bertorelli L, Tartara F, Tancioni F, Gaetani P, et al. Oxidative events in neuronal and glial cell-enriched fractions of rat cerebral cortex. Free Radic Biol Med 1995; 19: 853-857. [DOI:10.1016/0891-5849(95)00086-D]
31. Soleimani Asl S, Pourheydar B, Dabaghian F, Nezhadi A, Roointan A, Mehdizadeh M. Ecstasy-induced caspase expression alters following ginger treatment. Basic Clin Neurosci 2013; 4: 329-333.
32. Montgomery T, Sitte H, McBean G. 4-Methylthioamphetamine (4-MTA) induces mitochondrial-dependent apoptosis in SH-SY5Y cells independently of dopamine and noradrenaline transporters. BMC Pharmacology 2010; 10 (Suppl.): A22. [DOI:10.1186/1471-2210-10-S1-A22] [PMCID]
33. Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta 2016; 1863: 2977-2992. [DOI:10.1016/j.bbamcr.2016.09.012] [PMID]
34. Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 1995; 270: 96-99. [DOI:10.1126/science.270.5233.96] [PMID]
35. Billig H, Furuta I, Rivier C, Tapanainen J, Parvinen M, Hsueh AJ. Apoptosis in testis germ cells: developmental changes in gonadotropin dependence and localization to selective tubule stages. Endocrinology 1995; 136: 5-12. [DOI:10.1210/endo.136.1.7828558] [PMID]
36. Pentikäinen V, Erkkilä K, Suomalainen L, Otala M, Pentikäinen MO, Parvinen M, et al. TNFα down-regulates the Fas ligand and inhibits germ cell apoptosis in the human testis. J Clin Endocrinol Metab 2001; 86: 4480-4488.
https://doi.org/10.1210/jc.86.9.4480 [DOI:10.1210/jcem.86.9.7861] [PMID]
37. Skulachev VP, Chistyakov VV, Jasaitis AA, Smirnova EG. Inhibition of the respiratory chain by zinc ions. Biochem Biophys Res Commun 1967; 26: 1-6. [DOI:10.1016/0006-291X(67)90242-2]
38. Maret W. The function of zinc metallothionein: a link between cellular zinc and redox state. J Nutr 2000; 130 (Suppl.): 1455S-1458S. [DOI:10.1093/jn/130.5.1455S] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








