Sun, Sep 22, 2024
[Archive]
Volume 18, Issue 10 (October 2020)
IJRM 2020, 18(10): 837-846 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ezati D, Vardiyan R, Talebi A, Anvari M, Pourentezari M. L-Carnitine reduces the negative effects of formalin on sperm parameters, chromatin condensation and apoptosis in mice: An experimental study. IJRM 2020; 18 (10) :837-846
URL: http://ijrm.ir/article-1-1649-en.html
URL: http://ijrm.ir/article-1-1649-en.html
1- Department of Biology and Anatomy, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
2- 2Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. ,prof_talebi@hotmail.com
3- Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
2- 2Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. ,
3- Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Full-Text [PDF 502 kb]
(814 Downloads)
| Abstract (HTML) (1901 Views)
Full-Text: (544 Views)
1. Introduction
While infertility is generally supposed to be a female problem, male-factor infertility is obvious in 50% of infertile pairs, with 30-40% of these cases being due to sperm abnormalities (1). Several factors are related with male infertility, containing an injury in the genital organ hurt, varicocele , seminal fluid infection, reproductive duct obstruction, endocrine and metabolic disease, and environmental factors such as alcohol intake and cigarette smoking (2). One of the experiment has revealed evidence of the damaging effects of environmental pollutants on male fertility (3). Formaldehyde (FA, CH2O) is a combustible, uncolored, and bitter chemical factor that is applied extremely in manufactories, hospitals, technological laboratories, and households (3, 4). It is known as indispensable chemical mixtures in the worldwide economic system. Extensive utilization of FA has augmented its environmental or vocational exposure (5). The generative system is adversely affected by FA, so that it declines sperm parameters like count, viability, motility, and morphology of sperm (6, 7). Facing with FA leads to testicular disturbance by reducing antioxidant enzymes performance and promoting lipid peroxidation, consequently stimulating the oxidative stress in testes (8, 9). FA also harms male germ cells and elevates transcription of Hsp70 and Bax gene in testis (10, 11). Increasing the DNA-protein crosslinks is the key pathway of FA toxicity that results in the activation of p53 and pro-apoptotic gene expression (12, 13). Regarding the results of abundant research, the use of antioxidants is able to inhibit the cellular damage caused by oxidation as well as improve of the sperm quality and genital operation (14, 15). Therefore, research into drugs or materials to reduce the side effects of formalin on the male reproductive system is necessary. L-Carnitine (LC) demonstrates a key role in the oxidation of long-chain fatty acid, and its active form, L-acetyl carnitine (ALC), supports mitochondria from metabolic toxins with its antioxidant factors. ALC also reinstates cell membranes and performs antiapoptotic actions (16). Moreover, LC absorbed by epididymal cells is freed into the epididymal lumen of the seminiferous epithelium. The condensation of LC in the epididymal lumen is nearly 2,000-fold greater than in the blood circulation, indicating that it plays a very important role in sperm parameters and metabolism (17).
Besides, LC also has some beneficial effects on spermatogenesis, sperm maturation, and sperm motility (18). It is a tiny ammonium compound involved in lipid metabolism in mammalian (19) It has also been revealed that LC and related compounds have antioxidant and anti-inflammatory impacts on several physiological situations (20). Meanwhile, it has been determined that LC can serve as a crucial antiapoptotic agent (21). Moreover, LC elevates the function of DNA-repairing enzyme as well as other associated repair pathways (22). The usage of LC and related compounds is a novel approach for improving fertility in humans. Recently, human and animal experiments have specified a potential role for using carnitine as an antioxidant and free radical sweeper that are able to raise seminal fluid quality. In addition, the pathway that carnitines regulate male fertility is not yet well-known and they may lead to several disorders such as nausea, vomiting, stomach upset, seizures, diarrhea, and heartburn (23, 24). Our previous experiment indicated that formalin has an adverse impact on sperm parameters and chromatin stability in mice.
This study was planned to assess the impacts of LC on the chromatin compaction and the percentage of apoptosis in male balb/c mice treated by formalin.
2. Materials and Methods
2.1. Animals and care
In this experimental study, twenty four adult balb/c mice (25-40 gr, 10-12 wk) were divided into three groups (n = 8/each): The control group (I) did not receive any injections or gavage; the formalin group )II( received 10 mg/kg formalin (25) intraperitoneally (I.P.); and the third group )III( was exposed to formalin and LC, injected I.P. daily with a dose of 10 mg/kg formalin, and the dose of 100 mg/kg LC (26) was kept in a solvent solution. The mice were kept in isolated cages for 31 days (almost a spermatogenesis period) and transmitted into a controlled location with a temperature ranging 25 ± 3ºC and mean average moisture of 50 ± 5%. They were fed “mice chow” and the water was available for them.
2.2. Epididymal sperm provision
Mice in all groups were anesthetized via ketamine and xylazine (10 mg/kg and 12 mg/kg, respectively) and their separated cauda epididymis was transmitted in 1 ml of pre-warmed Ham’s F10. Temperate rapturing was accomplished to swim-out spermatozoa into a culture medium and the containers were kept in the incubator (at 37°C, 5% CO2) for about 15 min (27).
2.3. Sperm examination
Sperm parameters were appraised for about 200 spermatozoa per animal. The sperm count and motility were measured using Maklr chamber. Motility was represented as the percentage of progressive, non progressive, and immotile sperm. Sperm viability and morphology were assessed via Eosin and Diff Quick staining tests, respectively (28).
2.4. Chromatin stability valuations
All the dyestuffs and reactants were bought from Sigma Aldrch Company (St Lous, MO, USA). The efficiency of dyestuffs was examined with and without acid denaturation of some sperm samples and they were accepted as positive and negative controls, respectively (28).
2.5. Aniline blue (AB) staining
Aniline blue selectively stains lysine-rich histones and is able to show those sperm chromatin condensation anomalies that are related to residual histones. (29). A semen stain was outspread on the glass slides to dry out in the open air. In brief, all the smears (derived from washed semen samples) were fixed in 4% buffered glutaraldehyde for 35 min at 20-25ºC. All smears were stained with 6% aqueous AB stain and blended with 5% acetic acid (pH = 3.4) for 8 min. The three categories of head staining concentrations were specified: unstained or gray/white stained (standard spermatozoa or AB-) and complete sperm head stained dark blue (atypical spermatozoa, AB+).
2.6. Toluidine blue (TB) staining
To perform this staining, after the smears were dried out in the open air, they were fixed in freshly made 95% ethanol-acetone (1:1) at 5ºC for 35 min and then hydrolyzed in 0.2 NHCl at 5ºC for 8 min. The slides were cleaned in three changes of distilled water for 3 min and eventually stained with 0.06% TB in 60% McIlvaine buffer (pH = 3.6) for 15 min at 20-25ºC. The chromatin stability of spermatozoa was specified pursuant to metachromatic staining of sperm heads applied under a light microscopy at ×1000 eyepiece magnification (30, 31). Sperm heads with undamaged chromatin were light blue (TB-), those with mild abnormal chromatin were dark blue (TB+), and those with severely damaged chromatin and atypical packaging were deep violet and purple (TB+). About 200 sperm per slide were counted to compute the quantity of sperm with blue and violet head (32).
2.7. Chromomycin A3 staining
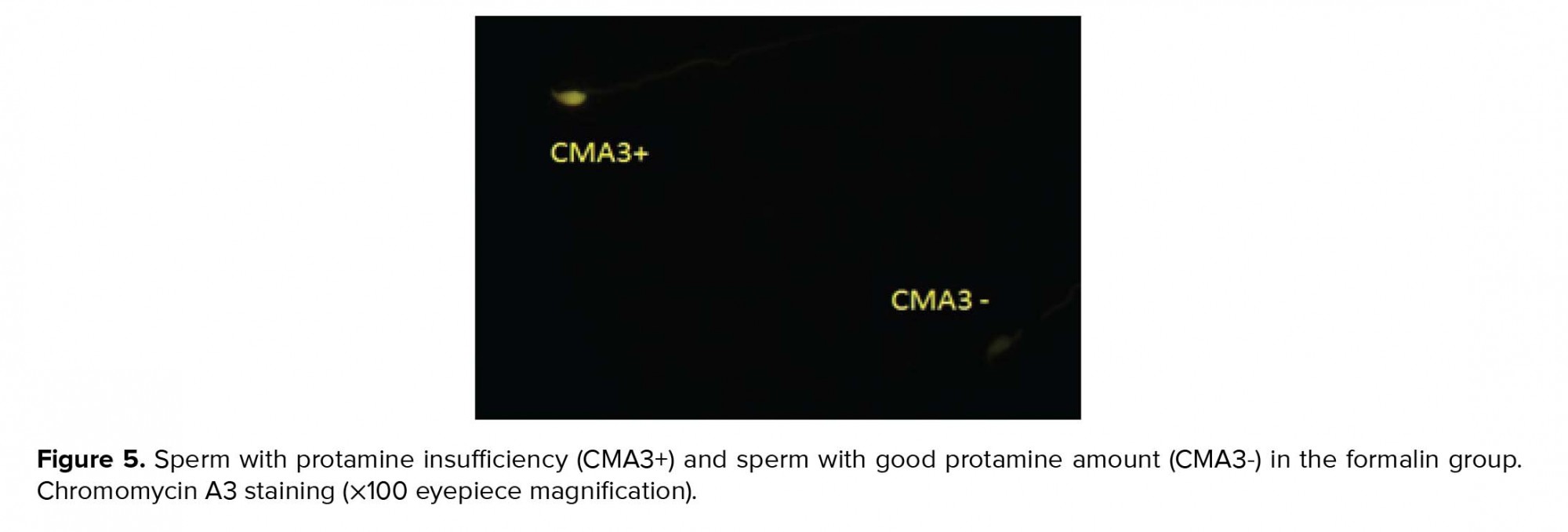
Spermatozoa recovered from washed sample and semen smears were fixed in Carnoy’s solution at 5ºC for 15 min. Each slide was treated with 160 ml of CMA3 (0.30 mg/ml) in McIlvan buffer for 25 min. The slides were cleaned in buffer and mounted with buffered glycerol. Fluorescence was executed via microscope. The spermatozoa were assessed in each sample. Two kinds of staining template were determined: bright yellow-stained (atypical chromatin packaging) and yellowish green-stained (standard chromatin packaging) (29).
2.8. Assessment of sperm apoptosis by TUNEL assay
The samples were fixed with methanol 100% (4 min) and incubated them in a blocking solution (3%H2O2 in methanol) for 20 min in a dark room. Then the slides were washed in phosphate-buffered saline (PBS) pH 7.4. The Permeability was accomplished by treating with 0.2% Triton X-100 and 0.2% sodium citrate for 5 min on ice. After cleaning with PBS, 30 μL of TUNEL reaction reagent (Roche, USA) was placed to each sample, they were incubated for 1 hour at 38ºC in a humid dark box. Next, after rinsing sufficiently with PBS and examination with fluorescence microscope under 100× magnification (33, 34), the nuclei of sperm cells with fragmented DNA (TUNEL+) indicated bright green color, while the nuclei of the normal cells (TUNEL-) presented pale green color.
2.9. Ethical consideration
All animal testing protocols were performed under the management of the Ethics Committee of the Shahid Sadoughi University of Medical Sciences (Code: IR.SSU.MEDICINE.REC.1396.239).
2.10. Statistical analysis
The results were analyzed using the SPSS software (Statistical Package for the Social Sciences, version 20.0, SPSS Inc., Chicago, Illinois, USA). Means were reported as mean ± standard deviation. One-way ANOVA was applied to evaluate the data, and LSD post-test was performed to determine the difference between the two groups. Two-sided p < 0.05 indicated a statistically significant difference between sperm evaluations.
3. Results
3.1. Assessment of sperm parameters
While the sperm count displayed a significant decrease in the formalin group in comparison with the other groups (p ≤ 0.001) it was significantly enhanced in the formalin + LC group in comparison with the other study groups (p ≤ 0.001) Table I. with regard to the sperm motility evaluation, progressive motility showed a significant decrease in the formalin group compared to other study groups, while it was significantly enhanced in the formalin + LC group in comparison with the formalin group (p = 0.001). Interestingly, no significant difference was observed between the groups with respect to nonprogressive motility (p ≤ 0.05). However, There was a significant enhancement in the immotile sperm in the formalin group compared to other study groups (p = 0.001) Table I. Further, a significant decrease in the sperm viability was observed in the formalin group in comparison with other study groups (p ≤ 0.001), while it was significantly enhanced in the formalin + LC group in comparison with the formalin group (p ≤ 0.001) Table I. Moreover, while a significant decrease was seen in the normal morphology of the sperm in the formalin group compared to the other groups, a significant rise in the normal morphology of sperm in the formalin + LC group compared to the formalin group was noted (p = 0.001, Figure 1, Table I).
3.2. Chromatin quality and sperm apoptosis assessment
As seen in Table II, the sperm chromatin integrity and apoptosis, the rates of spermatozoa with AB+, TB+, CMA3+, TUNEL+ showed a significant increase in the formalin group in comparison with the other groups, however it was significantly decreased in the formalin + LC group in comparison with the formalin group (Figures 2-5).






4. Discussion
Various experimental researches have demonstrated the negative impacts of formalin on the male fecundity index such as sperm parameters (35), however, till date, only a limited number of studies have been conducted to determine the effects of formalin on sperm parameters. It should be noted that the results of this study bring new information about the effects of formalin on chromatin compaction and DNA accuracy in mice. According to our results, a significant decline was observed in the sperm parameters in the formalin treated mice when compared with controls. Moladoust and colleagues reported that all semen parameters were detrimentally effected, with the FA displaying undermost count, motility, and viability. FA impel reactive oxygen species (36). In addition, Aitken and co-workers in their studies stated that generation which propels to lipid peroxidation in sperm membrane; wherefore, reduced sperm motility and enhancement chromatin damage accordingly happen (37). Also, ROS suppress intracellular enzymes and eventually decrease the ATP level results in reduced sperm motility (38). Fukushima and co-workers believed that cell cycle arrest and increased apoptosis level are the outcomes of ROS production which leads to decreased sperm count and viability (39).
Aziz and colleagues showed Sertoli stable cells are also exposed to damage by free radicals; when this occurs, the integrity of the germinal epithelium is impaired, resulting in a decrease in sperm count and anomalies in sperm morphology (40). Baird and co-authors showed that facing FA vapor (10 mg/m3 for 2 wk) may result in a reduction in the epididymis sperm source and motility in mice, this experiment also reported that the functions of the antioxidant enzymes were significantly reduced in the testis of mice facing with FA aspiration in comparison with the normal group. One of the rationales for the decrease in sperm motility may be the ability of FA to pass the blood dam, consequently bringing oxidative stress through augmenting reactive oxygen agents or reducing antioxidant activity in the luminal levels (41). In the work by Kose and co-workers focusing on the impact of FA on generative function in mice, the trial animals were faced with FA vapor (12 ppm/1h) for 40 days and the negative impacts of FA on sperm parameters (count, motility, and morphology) were detected (42). Our work indicated that formalin declined sperm motility. As stated by Alkan and colleagues immotile and atypical sperm are able to generate superoxidase, which has an oxidizing effect can decline the sperm quality like motility (43).
The results of this study also showed that high doses of formalin applied in this experiment lead to genotoxic impairment to mice sperm cells and also confirm other relevant outcomes about the mutagenic impacts of facing with formalin in lab animals (44). Duong and co-authors revealed the fundamental pathway that allows formalin to lead to generative and developmental disorders like chromosome instability, oxidative stress, enzymes dysfunction, apoptosis, and interfere with DNA methylation (45). Similar outcomes were obtained by Yoshikawa and co-workers , the sperm quantity in the FA-treated mice declined (50%) in comparison to the normal group in their study (46). In the work by Tang and colleague degradation and destruction to the genetic content of germ cells were detected in male mice facing with doses of 0.3, 3, and 30 mg/kg FA, injected IP for five days. The highest pathological alterations consisting of the decadence of testicular tissue, declined sperm quantity, and morphological alterations in the sperm head were detected in the those facing formalin (47). Expanding male fecundity problems, which is indicated by decrease in sperm condensation and the motility of spermatozoa, have been presented in Western countries (48).
Novel studies have demonstrated the important role of vitamins, nutrients, and minerals in sperm health (49). LC is available in high concentration in the epididymis and plays an important role in the development, maturation and metabolism of spermatozoa by amending sperm motility and exhibiting antioxidant and anti apoptotic confidants for the confirmation of the spermatozoa cell membrane (50). Similar to the results of this study, Banihani and colleagues reported that LC (0.6-1.2 mg /ml )raises human sperm motility and viability when the sperm is incubated at 38ºC (51). Similar progress in sperm motility in vitro has been revealed after pouring acetyl- L-carnitine (ALC) into human semen at 38ºC (52). This useful impact of LC on human sperm feature is owing to its antioxidant activity and also its role in the sperm metabolism pathway (18). LC, in addition to spermatozoa, reduces oxidative stress and destruction to DNA (53). Prevention of apoptosis via LC has been described in the culture medium of neuronal cells (54). LC increases the function of the DNA-repairing enzyme and other associated repair pathways (55). In this experiment, we proved that the quantity of apoptosis in sperm remarkably augmented in the formalin group in comparison with the normal group. Using LC combined with formalin shrinks the apoptosis in sperm, proposing that LC is able to preserve sperm from formalin damage. Subsequently, the outcomes of this study indicate that LC can be useful in increasing spermatogenesis and fertility in exposure to formalin by decreasing apoptosis. However, more research and experimental studies are needed to diagnose the right pathway of LC impacts in the sperm.
5. Conclusion
LC has improving effects on sperm parameters and chromatin density and can reduce the rate of apoptosis in spermatozoa. However, formalin, which is widely used in various industrial fields these days, can adversely effect the reproductive system. We can recommend the use of LC for those who are exposed to these harmful effects of formalin.
Acknowledgments
This experiment was funded by a grant from the Shahid Sadoughi University of Medical Sciences of Yazd, and it is published as part of the MS.c Thesis of Daniyal Ezati. The authors thank the Yazd Infertility Research Center and its colleagues for their valuable support.
Conflict of interest
The authors declare no conflict of interest.
While infertility is generally supposed to be a female problem, male-factor infertility is obvious in 50% of infertile pairs, with 30-40% of these cases being due to sperm abnormalities (1). Several factors are related with male infertility, containing an injury in the genital organ hurt, varicocele , seminal fluid infection, reproductive duct obstruction, endocrine and metabolic disease, and environmental factors such as alcohol intake and cigarette smoking (2). One of the experiment has revealed evidence of the damaging effects of environmental pollutants on male fertility (3). Formaldehyde (FA, CH2O) is a combustible, uncolored, and bitter chemical factor that is applied extremely in manufactories, hospitals, technological laboratories, and households (3, 4). It is known as indispensable chemical mixtures in the worldwide economic system. Extensive utilization of FA has augmented its environmental or vocational exposure (5). The generative system is adversely affected by FA, so that it declines sperm parameters like count, viability, motility, and morphology of sperm (6, 7). Facing with FA leads to testicular disturbance by reducing antioxidant enzymes performance and promoting lipid peroxidation, consequently stimulating the oxidative stress in testes (8, 9). FA also harms male germ cells and elevates transcription of Hsp70 and Bax gene in testis (10, 11). Increasing the DNA-protein crosslinks is the key pathway of FA toxicity that results in the activation of p53 and pro-apoptotic gene expression (12, 13). Regarding the results of abundant research, the use of antioxidants is able to inhibit the cellular damage caused by oxidation as well as improve of the sperm quality and genital operation (14, 15). Therefore, research into drugs or materials to reduce the side effects of formalin on the male reproductive system is necessary. L-Carnitine (LC) demonstrates a key role in the oxidation of long-chain fatty acid, and its active form, L-acetyl carnitine (ALC), supports mitochondria from metabolic toxins with its antioxidant factors. ALC also reinstates cell membranes and performs antiapoptotic actions (16). Moreover, LC absorbed by epididymal cells is freed into the epididymal lumen of the seminiferous epithelium. The condensation of LC in the epididymal lumen is nearly 2,000-fold greater than in the blood circulation, indicating that it plays a very important role in sperm parameters and metabolism (17).
Besides, LC also has some beneficial effects on spermatogenesis, sperm maturation, and sperm motility (18). It is a tiny ammonium compound involved in lipid metabolism in mammalian (19) It has also been revealed that LC and related compounds have antioxidant and anti-inflammatory impacts on several physiological situations (20). Meanwhile, it has been determined that LC can serve as a crucial antiapoptotic agent (21). Moreover, LC elevates the function of DNA-repairing enzyme as well as other associated repair pathways (22). The usage of LC and related compounds is a novel approach for improving fertility in humans. Recently, human and animal experiments have specified a potential role for using carnitine as an antioxidant and free radical sweeper that are able to raise seminal fluid quality. In addition, the pathway that carnitines regulate male fertility is not yet well-known and they may lead to several disorders such as nausea, vomiting, stomach upset, seizures, diarrhea, and heartburn (23, 24). Our previous experiment indicated that formalin has an adverse impact on sperm parameters and chromatin stability in mice.
This study was planned to assess the impacts of LC on the chromatin compaction and the percentage of apoptosis in male balb/c mice treated by formalin.
2. Materials and Methods
2.1. Animals and care
In this experimental study, twenty four adult balb/c mice (25-40 gr, 10-12 wk) were divided into three groups (n = 8/each): The control group (I) did not receive any injections or gavage; the formalin group )II( received 10 mg/kg formalin (25) intraperitoneally (I.P.); and the third group )III( was exposed to formalin and LC, injected I.P. daily with a dose of 10 mg/kg formalin, and the dose of 100 mg/kg LC (26) was kept in a solvent solution. The mice were kept in isolated cages for 31 days (almost a spermatogenesis period) and transmitted into a controlled location with a temperature ranging 25 ± 3ºC and mean average moisture of 50 ± 5%. They were fed “mice chow” and the water was available for them.
2.2. Epididymal sperm provision
Mice in all groups were anesthetized via ketamine and xylazine (10 mg/kg and 12 mg/kg, respectively) and their separated cauda epididymis was transmitted in 1 ml of pre-warmed Ham’s F10. Temperate rapturing was accomplished to swim-out spermatozoa into a culture medium and the containers were kept in the incubator (at 37°C, 5% CO2) for about 15 min (27).
2.3. Sperm examination
Sperm parameters were appraised for about 200 spermatozoa per animal. The sperm count and motility were measured using Maklr chamber. Motility was represented as the percentage of progressive, non progressive, and immotile sperm. Sperm viability and morphology were assessed via Eosin and Diff Quick staining tests, respectively (28).
2.4. Chromatin stability valuations
All the dyestuffs and reactants were bought from Sigma Aldrch Company (St Lous, MO, USA). The efficiency of dyestuffs was examined with and without acid denaturation of some sperm samples and they were accepted as positive and negative controls, respectively (28).
2.5. Aniline blue (AB) staining
Aniline blue selectively stains lysine-rich histones and is able to show those sperm chromatin condensation anomalies that are related to residual histones. (29). A semen stain was outspread on the glass slides to dry out in the open air. In brief, all the smears (derived from washed semen samples) were fixed in 4% buffered glutaraldehyde for 35 min at 20-25ºC. All smears were stained with 6% aqueous AB stain and blended with 5% acetic acid (pH = 3.4) for 8 min. The three categories of head staining concentrations were specified: unstained or gray/white stained (standard spermatozoa or AB-) and complete sperm head stained dark blue (atypical spermatozoa, AB+).
2.6. Toluidine blue (TB) staining
To perform this staining, after the smears were dried out in the open air, they were fixed in freshly made 95% ethanol-acetone (1:1) at 5ºC for 35 min and then hydrolyzed in 0.2 NHCl at 5ºC for 8 min. The slides were cleaned in three changes of distilled water for 3 min and eventually stained with 0.06% TB in 60% McIlvaine buffer (pH = 3.6) for 15 min at 20-25ºC. The chromatin stability of spermatozoa was specified pursuant to metachromatic staining of sperm heads applied under a light microscopy at ×1000 eyepiece magnification (30, 31). Sperm heads with undamaged chromatin were light blue (TB-), those with mild abnormal chromatin were dark blue (TB+), and those with severely damaged chromatin and atypical packaging were deep violet and purple (TB+). About 200 sperm per slide were counted to compute the quantity of sperm with blue and violet head (32).
2.7. Chromomycin A3 staining
Spermatozoa recovered from washed sample and semen smears were fixed in Carnoy’s solution at 5ºC for 15 min. Each slide was treated with 160 ml of CMA3 (0.30 mg/ml) in McIlvan buffer for 25 min. The slides were cleaned in buffer and mounted with buffered glycerol. Fluorescence was executed via microscope. The spermatozoa were assessed in each sample. Two kinds of staining template were determined: bright yellow-stained (atypical chromatin packaging) and yellowish green-stained (standard chromatin packaging) (29).
2.8. Assessment of sperm apoptosis by TUNEL assay
The samples were fixed with methanol 100% (4 min) and incubated them in a blocking solution (3%H2O2 in methanol) for 20 min in a dark room. Then the slides were washed in phosphate-buffered saline (PBS) pH 7.4. The Permeability was accomplished by treating with 0.2% Triton X-100 and 0.2% sodium citrate for 5 min on ice. After cleaning with PBS, 30 μL of TUNEL reaction reagent (Roche, USA) was placed to each sample, they were incubated for 1 hour at 38ºC in a humid dark box. Next, after rinsing sufficiently with PBS and examination with fluorescence microscope under 100× magnification (33, 34), the nuclei of sperm cells with fragmented DNA (TUNEL+) indicated bright green color, while the nuclei of the normal cells (TUNEL-) presented pale green color.
2.9. Ethical consideration
All animal testing protocols were performed under the management of the Ethics Committee of the Shahid Sadoughi University of Medical Sciences (Code: IR.SSU.MEDICINE.REC.1396.239).
2.10. Statistical analysis
The results were analyzed using the SPSS software (Statistical Package for the Social Sciences, version 20.0, SPSS Inc., Chicago, Illinois, USA). Means were reported as mean ± standard deviation. One-way ANOVA was applied to evaluate the data, and LSD post-test was performed to determine the difference between the two groups. Two-sided p < 0.05 indicated a statistically significant difference between sperm evaluations.
3. Results
3.1. Assessment of sperm parameters
While the sperm count displayed a significant decrease in the formalin group in comparison with the other groups (p ≤ 0.001) it was significantly enhanced in the formalin + LC group in comparison with the other study groups (p ≤ 0.001) Table I. with regard to the sperm motility evaluation, progressive motility showed a significant decrease in the formalin group compared to other study groups, while it was significantly enhanced in the formalin + LC group in comparison with the formalin group (p = 0.001). Interestingly, no significant difference was observed between the groups with respect to nonprogressive motility (p ≤ 0.05). However, There was a significant enhancement in the immotile sperm in the formalin group compared to other study groups (p = 0.001) Table I. Further, a significant decrease in the sperm viability was observed in the formalin group in comparison with other study groups (p ≤ 0.001), while it was significantly enhanced in the formalin + LC group in comparison with the formalin group (p ≤ 0.001) Table I. Moreover, while a significant decrease was seen in the normal morphology of the sperm in the formalin group compared to the other groups, a significant rise in the normal morphology of sperm in the formalin + LC group compared to the formalin group was noted (p = 0.001, Figure 1, Table I).
3.2. Chromatin quality and sperm apoptosis assessment
As seen in Table II, the sperm chromatin integrity and apoptosis, the rates of spermatozoa with AB+, TB+, CMA3+, TUNEL+ showed a significant increase in the formalin group in comparison with the other groups, however it was significantly decreased in the formalin + LC group in comparison with the formalin group (Figures 2-5).







4. Discussion
Various experimental researches have demonstrated the negative impacts of formalin on the male fecundity index such as sperm parameters (35), however, till date, only a limited number of studies have been conducted to determine the effects of formalin on sperm parameters. It should be noted that the results of this study bring new information about the effects of formalin on chromatin compaction and DNA accuracy in mice. According to our results, a significant decline was observed in the sperm parameters in the formalin treated mice when compared with controls. Moladoust and colleagues reported that all semen parameters were detrimentally effected, with the FA displaying undermost count, motility, and viability. FA impel reactive oxygen species (36). In addition, Aitken and co-workers in their studies stated that generation which propels to lipid peroxidation in sperm membrane; wherefore, reduced sperm motility and enhancement chromatin damage accordingly happen (37). Also, ROS suppress intracellular enzymes and eventually decrease the ATP level results in reduced sperm motility (38). Fukushima and co-workers believed that cell cycle arrest and increased apoptosis level are the outcomes of ROS production which leads to decreased sperm count and viability (39).
Aziz and colleagues showed Sertoli stable cells are also exposed to damage by free radicals; when this occurs, the integrity of the germinal epithelium is impaired, resulting in a decrease in sperm count and anomalies in sperm morphology (40). Baird and co-authors showed that facing FA vapor (10 mg/m3 for 2 wk) may result in a reduction in the epididymis sperm source and motility in mice, this experiment also reported that the functions of the antioxidant enzymes were significantly reduced in the testis of mice facing with FA aspiration in comparison with the normal group. One of the rationales for the decrease in sperm motility may be the ability of FA to pass the blood dam, consequently bringing oxidative stress through augmenting reactive oxygen agents or reducing antioxidant activity in the luminal levels (41). In the work by Kose and co-workers focusing on the impact of FA on generative function in mice, the trial animals were faced with FA vapor (12 ppm/1h) for 40 days and the negative impacts of FA on sperm parameters (count, motility, and morphology) were detected (42). Our work indicated that formalin declined sperm motility. As stated by Alkan and colleagues immotile and atypical sperm are able to generate superoxidase, which has an oxidizing effect can decline the sperm quality like motility (43).
The results of this study also showed that high doses of formalin applied in this experiment lead to genotoxic impairment to mice sperm cells and also confirm other relevant outcomes about the mutagenic impacts of facing with formalin in lab animals (44). Duong and co-authors revealed the fundamental pathway that allows formalin to lead to generative and developmental disorders like chromosome instability, oxidative stress, enzymes dysfunction, apoptosis, and interfere with DNA methylation (45). Similar outcomes were obtained by Yoshikawa and co-workers , the sperm quantity in the FA-treated mice declined (50%) in comparison to the normal group in their study (46). In the work by Tang and colleague degradation and destruction to the genetic content of germ cells were detected in male mice facing with doses of 0.3, 3, and 30 mg/kg FA, injected IP for five days. The highest pathological alterations consisting of the decadence of testicular tissue, declined sperm quantity, and morphological alterations in the sperm head were detected in the those facing formalin (47). Expanding male fecundity problems, which is indicated by decrease in sperm condensation and the motility of spermatozoa, have been presented in Western countries (48).
Novel studies have demonstrated the important role of vitamins, nutrients, and minerals in sperm health (49). LC is available in high concentration in the epididymis and plays an important role in the development, maturation and metabolism of spermatozoa by amending sperm motility and exhibiting antioxidant and anti apoptotic confidants for the confirmation of the spermatozoa cell membrane (50). Similar to the results of this study, Banihani and colleagues reported that LC (0.6-1.2 mg /ml )raises human sperm motility and viability when the sperm is incubated at 38ºC (51). Similar progress in sperm motility in vitro has been revealed after pouring acetyl- L-carnitine (ALC) into human semen at 38ºC (52). This useful impact of LC on human sperm feature is owing to its antioxidant activity and also its role in the sperm metabolism pathway (18). LC, in addition to spermatozoa, reduces oxidative stress and destruction to DNA (53). Prevention of apoptosis via LC has been described in the culture medium of neuronal cells (54). LC increases the function of the DNA-repairing enzyme and other associated repair pathways (55). In this experiment, we proved that the quantity of apoptosis in sperm remarkably augmented in the formalin group in comparison with the normal group. Using LC combined with formalin shrinks the apoptosis in sperm, proposing that LC is able to preserve sperm from formalin damage. Subsequently, the outcomes of this study indicate that LC can be useful in increasing spermatogenesis and fertility in exposure to formalin by decreasing apoptosis. However, more research and experimental studies are needed to diagnose the right pathway of LC impacts in the sperm.
5. Conclusion
LC has improving effects on sperm parameters and chromatin density and can reduce the rate of apoptosis in spermatozoa. However, formalin, which is widely used in various industrial fields these days, can adversely effect the reproductive system. We can recommend the use of LC for those who are exposed to these harmful effects of formalin.
Acknowledgments
This experiment was funded by a grant from the Shahid Sadoughi University of Medical Sciences of Yazd, and it is published as part of the MS.c Thesis of Daniyal Ezati. The authors thank the Yazd Infertility Research Center and its colleagues for their valuable support.
Conflict of interest
The authors declare no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Andrology
References
1. Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol 2015; 13: 37-45. [DOI:10.1186/s12958-015-0032-1] [PMID] [PMCID]
2. La Vignera S, Condorelli R, Vicari E, D'Agata R, Calogero AE. Diabetes mellitus and sperm parameters. J Androl 2012; 33: 145-153. [DOI:10.2164/jandrol.111.013193] [PMID]
3. Martino‐Andrade AJ, Chahoud I. Reproductive toxicity of phthalate esters. Mol Nutr Food Res 2010; 54: 148-157. [DOI:10.1002/mnfr.200800312] [PMID]
4. Golalipour MJ, Azarhoush R, Ghafari S, Gharravi AM, Fazeli SA, Davarian A. Formaldehyde exposure induces histopathological and morphometric changes in the rat testis. Folia Morphol 2007; 66: 167-171.
5. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Formaldehyde, 2-butoxyethanol and 1-tert-butoxypropan-2-ol. IARC Monogr Eval Carcinog Risks Hum 2006; 88: 1-487.
6. Vosoughi S, Khavanin A, Salehnia M, Mahabadi HA, Shahverdi A, Esmaeili V. Adverse effects of formaldehyde vapor on mouse sperm parameters and testicular tissue. Int J Fertil Steril 2013; 6: 250-267.
7. Tajaddini Mahani S, Behnam B, Abbassi M, Asgari H, Nazmara Z, Shirinbayan P, et al. Tsga10 expression correlates with sperm profiles in the adult formalin‐exposed mice. Andrologia 2016; 48: 1092-1099. [DOI:10.1111/and.12543] [PMID]
8. Zhou DX, Qiu SD, Zhang J, Tian H, Wang HX. The protective effect of vitamin E against oxidative damage caused by formaldehyde in the testes of adult rats. Asian J Androl 2006; 8: 584-588. [DOI:10.1111/j.1745-7262.2006.00198.x] [PMID]
9. Ozen OA, Kus MA, Kus I, Alkoc OA, Songur A. Protective effects of melatonin against formaldehyde-induced oxidative damage and apoptosis in rat testes: an immunohistochemical and biochemical study. Syst Biol Reprod Med 2008; 54: 169-176. [DOI:10.1080/19396360802422402] [PMID]
10. Afrigan L, Anarkooli IJ, Sohrabi D, Abdanipour A, Yazdinezhad A, Sayyar Z, et al. The effect of hydroethanolic extract of Matricaria chamomilla on the reproductive system of male rats exposed to formaldehyde. Andrologia 2019; 51: e13362. [DOI:10.1111/and.13362] [PMID]
11. Zhou D, Zhang J, Wang H. Assessment of the potential reproductive toxicity of long-term exposure of adult male rats to low-dose formaldehyde. Toxicol Ind Health 2011; 27: 591-598. [DOI:10.1177/0748233710393401] [PMID]
12. Wong VC, Cash HL, Morse JL, Lu S, Zhitkovich A. S-phase sensing of DNA-protein crosslinks triggers TopBP1-independent ATR activation and p53-mediated cell death by formaldehyde. Cell Cycle 2012; 11: 2526-2537. [DOI:10.4161/cc.20905] [PMID] [PMCID]
13. Tang BD, Wang X, Tang C, Zhang D, Wu SW, Li LN. Effect of formaldehyde on the reproductive toxicity in male Wistar rats. J Bengbu Med College 2018; 4: 187-195.
14. Jedlinska-Krakowska M, Bomba G, Jakubowski K, Rotkiewicz T, Jana B, Penkowski A. Impact of oxidative stress and supplementation with vitamins E and C on testes morphology in rats. J Reprod Dev 2006; 52: 203-209. [DOI:10.1262/jrd.17028] [PMID]
15. Yang HS, Han DK, Kim JR, Sim JC. Effects of α-tocopherol on cadmium-induced toxicity in rat testis and spermatogenesis. J Korean Med Sci 2006; 21: 445-451. [DOI:10.3346/jkms.2006.21.3.445] [PMID] [PMCID]
16. Abdelrazik H, Sharma R, Mahfouz R, Agarwal A. L-carnitine decreases DNA damage and improves the in vitro blastocyst development rate in mouse embryos. Fertil Steril 2009; 91: 589-596. [DOI:10.1016/j.fertnstert.2007.11.067] [PMID]
17. Mongioi L, Calogero AE, Vicari E, Condorelli RA, Russo GI, Privitera S, et al. The role of carnitine in male infertility. Andrology 2016; 4: 800-807. [DOI:10.1111/andr.12191] [PMID]
18. Agarwal A, Said TM. Carnitines and male infertility. Reprod Biomed Online 2004; 8: 376-384. [DOI:10.1016/S1472-6483(10)60920-0]
19. Vassiliadis S, Athanassakis I. A "conditionally essential" nutrient, L-carnitine, as a primary suspect in endometriosis. Fertil Steril 2011; 95: 2759-2760. [DOI:10.1016/j.fertnstert.2011.04.091] [PMID]
20. İzgüt-Uysal VN, Ağaç A, Derin N. Effect of L-carnitine on carrageenan-induced inflammation in aged rats. Gerontology 2003; 49: 287-292. [DOI:10.1159/000071709] [PMID]
21. Khushboo M, Murthy MK, Devi MS, Sanjeev S, Ibrahim KS, Kumar NS, et al. Testicular toxicity and sperm quality following copper exposure in Wistar albino rats: ameliorative potentials of L-carnitine. Environ Sci Pollut Res Int 2018; 25: 1837-1862. [DOI:10.1007/s11356-017-0624-8] [PMID]
22. Moretti S, Famularo G, Marcellini S, Boschini A, Santini G, Trinchieri V, et al. L-carnitine reduces lymphocyte apoptosis and oxidant stress in HIV-1-infected subjects treated with zidovudine and didanosine. Antioxid Redox Signal 2002; 4: 391-403. [DOI:10.1089/15230860260196191] [PMID]
23. Khademi A, Alleyassin A, Agha-Hosseini M, Safdarian L, Saeidi Saeidabadi H, Pooyan O. The effect of L-Carnitine on sperm parameters in patients candidated for intracytoplasmic sperm injection. Fertility Sterility 2005; 84: S212-S213. [DOI:10.1016/j.fertnstert.2005.07.539]
24. Onem G, Aral E, Enli Y, Oguz EO, Coskun E, Aybek H, et al. Neuroprotective effects of L-carnitine and vitamin E alone or in combination against ischemia-reperfusion injury in rats. J Surg Res 2006; 131: 124-130. [DOI:10.1016/j.jss.2005.12.017] [PMID]
25. Hegazy AA, Elsayed NE, Ahmad MM, Omar NM. Effect of formaldehyde on rat testis structure. Acad Anat Int 2017; 3: 15-23. [DOI:10.21276/aanat.2017.3.2.4]
26. Altun Z, Olgun Y, Ercetin P, Aktas S, Kirkim G, Serbetcioglu B, et al. Protective effect of acetyl‐l‐carnitine against cisplatin ototoxicity: role of apoptosis‐related genes and pro‐inflammatory cytokines. Cell Prolif 2014; 47: 72-80. [DOI:10.1111/cpr.12080] [PMID] [PMCID]
27. Mangoli E, Talebi AR, Anvari M, Pourentezari M. Effects of experimentally-induced diabetes on sperm parameters and chromatin quality in mice. Iran J Reprod Med 2013; 11: 53-60.
28. Talebi AR, Abbasi Sarcheshmeh A, Khalili MA, Tabibnejad N. Effects of ethanol consumption on chromatin condensation and DNA integrity of epididymal spermatozoa in rat. Alcohol 2011; 45: 403-409. [DOI:10.1016/j.alcohol.2010.10.005] [PMID]
29. Talebi AR, Khalili MA, Hossaini A. Assessment of nuclear DNA integrity of epididymal spermatozoa following experimental chronic spinal cord injury in the rat. Int J Androl 2007; 30: 163-169. [DOI:10.1111/j.1365-2605.2006.00736.x] [PMID]
30. Talebi AR, Moein MR, Tabibnejad N, Ghasemzadeh J. Effect of varicocele on chromatin condensation and DNA integrity of ejaculated spermatozoa using cytochemical tests. Andrologia 2008; 40: 245-251. [DOI:10.1111/j.1439-0272.2008.00852.x] [PMID]
31. Talebi AR, Vahidi S, Aflatoonian A, Ghasemi N, Ghasemzadeh J, Dehghani Firoozabadi R, et al. Cytochemical evaluation of sperm chromatin and DNA integrity in couples with unexplained recurrent spontaneous abortions. Andrologia 2012; 44 (Suppl.): 462-470. [DOI:10.1111/j.1439-0272.2011.01206.x] [PMID]
32. Pourentezari M, Talebi A, Abbasi A, Khalili MA, Mangoli E, Anvari M. Effects of acrylamide on sperm parameters, chromatin quality, and the level of blood testosterone in mice. Iran J Reprod Med 2014; 12: 335-342.
33. Gandini L, Lombardo F, Paoli D, Caponecchia L, Familiari G, Verlengia C, et al. Study of apoptotic DNA fragmentation in human spermatozoa. Hum Reprod 2000; 15: 830-839. [DOI:10.1093/humrep/15.4.830] [PMID]
34. Cankut S, Dinc T, Cincik M, Ozturk G, Selam B. Evaluation of sperm DNA fragmentation via halosperm technique and TUNEL assay before and after cryopreservation. Reprod Sci 2019; 26: 1575-1581. [DOI:10.1177/1933719119828096] [PMID]
35. Roshankhah S, Jalili C, Salahshoor MR. Effects of crocin on sperm parameters and seminiferous tubules in diabetic rats. Adv Biomed Res 2019; 8: 4-13. [DOI:10.4103/abr.abr_124_18] [PMID] [PMCID]
36. Moladoust H, Nasiri E, Gazor R, Mahdavi T, Ghorbani R, Rostampour M. Assessment of the protective impact of vitamin E on sex hormones and sperm parameters of formaldehyde-treated male rats: A preliminary investigation. Galen Medical Journal 2017; 6: 330-337.
37. Aitken RJ. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol Reprod Dev 2017; 84: 1039-1052. [DOI:10.1002/mrd.22871] [PMID]
38. Zhu Z, Kawai T, Umehara T, Hoque SAM, Zeng W, Shimada M. Negative effects of ROS generated during linear sperm motility on gene expression and ATP generation in boar sperm mitochondria. Free Radic Biol Med 2019; 141: 159-171. [DOI:10.1016/j.freeradbiomed.2019.06.018] [PMID]
39. Fukushima T, Hamada Y, Komiyama M, Matsuno Y, Mori C, Horii I. Early changes in sperm motility, acrosome reaction, and gene expression of reproductive organs in rats treated with sulfasalazine. Reprod Toxicol 2007; 23: 153-157. [DOI:10.1016/j.reprotox.2006.10.003] [PMID]
40. Aziz N, Saleh RA, Sharma RK, Lewis-Jones I, Esfandiari N, Thomas Jr AJ, et al. Novel association between sperm reactive oxygen species production, sperm morphological defects, and the sperm deformity index. Fertil Steril 2004; 81: 349-354. [DOI:10.1016/j.fertnstert.2003.06.026] [PMID]
41. Baird DJ, Pascoe TJ, Zhou X, Hajibabaei M. Building freshwater macroinvertebrate DNA-barcode libraries from reference collection material: formalin preservation vs specimen age. Journal of the North American Benthological Society 2011; 30: 125-130. [DOI:10.1899/10-013.1]
42. Köse E, Sarsılmaz M, Taş U, Kavaklı A, Türk G, Özlem Dabak D, et al. Rose oil inhalation protects against formaldehyde‐induced testicular damage in rats. Andrologia 2012; 44 (Suppl.): 342-348. [DOI:10.1111/j.1439-0272.2011.01187.x] [PMID]
43. Alkan İ, Yüksel M, Canat HL, Atalay HA, Can O, Özveri H, et al. Superoxide anion production by the spermatozoa of men with varicocele: relationship with Varicocele grade and semen parameters. World J Men Health 2018; 36: 255-262. [DOI:10.5534/wjmh.180028] [PMID] [PMCID]
44. Sapmaz HI, Yıldız A, Polat A, Vardı N, Köse E, Tanbek K, et al. Protective efficacy of Nigella sativa oil against the harmful effects of formaldehyde on rat testicular tissue. Asian Pacific Journal of Tropical Biomedicine 2018; 8: 548-553. [DOI:10.4103/2221-1691.245970]
45. Duong A, Steinmaus C, McHale CM, Vaughan CP, Zhang L. Reproductive and developmental toxicity of formaldehyde: A systematic review. Mutat Res 2011; 728: 118-138. [DOI:10.1016/j.mrrev.2011.07.003] [PMID] [PMCID]
46. Yoshikawa K, Oshima Y, Inagaki A, Sakuragawa A. Determination of formaldehyde in water samples by high-performance liquid chromatography with Methyl acetoacetate derivatization. Bull Environ Contam Toxicol 2018; 101: 672-677. [DOI:10.1007/s00128-018-2461-y] [PMID]
47. Tang M, Xie Y, Yi Y, Wang W. Effects of formaldehyde on germ cells of male mice. Wei Sheng Yan Jiu 2003; 32: 544-548.
48. Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, et al. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update 2017; 23: 646-659. [DOI:10.1093/humupd/dmx022] [PMID] [PMCID]
49. Balercia G, Regoli F, Armeni T, Koverech A, Mantero F, Boscaro M. Placebo-controlled double-blind randomized trial on the use of L-carnitine, L-acetylcarnitine, or combined L-carnitine and L-acetylcarnitine in men with idiopathic asthenozoospermia. Fertil Steril 2005; 84: 662-671. [DOI:10.1016/j.fertnstert.2005.03.064] [PMID]
50. Cheah Y, Yang W. Functions of essential nutrition for high quality spermatogenesis. Advances in Bioscience and Biotechnology 2011; 2: 182-197. [DOI:10.4236/abb.2011.24029]
51. Banihani S, Sharma R, Bayachou M, Sabanegh E, Agarwal A. Human sperm DNA oxidation, motility and viability in the presence of l‐carnitine during in vitro incubation and centrifugation. Andrologia 2012; 44 (Suppl.): 505-512. [DOI:10.1111/j.1439-0272.2011.01216.x] [PMID]
52. Hufana-Duran D, Duran PG, Monson R, Parrish J. Motility and membrane integrity of ejaculated bovine spermatozoa extended and cryopreserved in L-carnitine Tris-egg yolk extender. J ISSAAS 2017; 23: 56-67.
53. Berni A, Meschini R, Filippi S, Palitti F, De Amicis A, Chessa L. L-carnitine enhances resistance to oxidative stress by reducing DNA damage in Ataxia telangiectasia cells. Mutat Res 2008; 650: 165-174. [DOI:10.1016/j.mrgentox.2007.11.008] [PMID]
54. Zidan A, Hedya SE, Elfeky DM, Abdin AA. The possible anti-apoptotic and antioxidant effects of acetyl l-carnitine as an add-on therapy on a relapsing-remitting model of experimental autoimmune encephalomyelitis in rats. Biomed Pharmacother 2018; 103: 1302-1311. [DOI:10.1016/j.biopha.2018.04.173] [PMID]
55. de Moraes MS, Guerreiro G, Sitta A, de Moura Coelho D, Manfredini V, Wajner M, et al. Oxidative damage in mitochondrial fatty acids oxidation disorders patients and the in vitro effect of l-carnitine on DNA damage induced by the accumulated metabolites. Arch Biochem Biophys 2020; 679: 108206: 3-7. [DOI:10.1016/j.abb.2019.108206] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








