Sun, Sep 22, 2024
[Archive]
Volume 17, Issue 11 (November 2019)
IJRM 2019, 17(11): 807-818 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Kamali F S, Shahrooz R, Najafi G, Razi M. Ameliorative effects of crocin on paraquat-induced oxidative stress in testis of adult mice: An experimental study. IJRM 2019; 17 (11) :807-818
URL: http://ijrm.ir/article-1-1721-en.html
URL: http://ijrm.ir/article-1-1721-en.html
1- Department of Histology and Embryology, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran.
2- Department of Histology and Embryology, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran. ,rasoul_Shahrooz@yahoo.com
3- Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran.
2- Department of Histology and Embryology, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran. ,
3- Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran.
Full-Text [PDF 9328 kb]
(1037 Downloads)
| Abstract (HTML) (2517 Views)
In line with male infertility disorders, it has been shown that PQ adversely affects the spermatogenesis (16), reduces the sperm quality, including count, motility, and morphology (4), and results in chromosomal aberrations in spermatozoa, spermatid, and preleptotene spermatogonial cells (17). Spermatogenesis is a dynamic system, which is characterized by cell proliferation and differentiation. Similar to hypermitotic tissues, the physiologic cellular proliferation of testicular tissue is maintained with controlled apoptosis. Indeed, the apoptosis is processed through intrinsic (mitochondria-dependent) and extrinsic (death receptor Fas and Fas-ligand-dependent) pathways (18).
Crocin (CCN) has attracted scientific attention and finally found a considerable place in pharmaceutical science due to its anti-inflammatory, antioxidant (19), anti-spasm (20), anti-depression (21), anti-cancer (22) and neuroprotective properties (23). A study showed that the CCN increases antioxidant enzymes and testosterone. However, it reduced testicular histological destructive changes in CP-treated mice (24).
Considering the oxidative stress as the main mechanism of PQ action and minding the adverse effect of free radicals on mitochondrial membrane integrity, the current study was performed to investigate the protective effect of CCN against PQ-related derangements.
The PQ, with the formulation of SL20%, was taken from EXIR Co. (Tehran, Iran) and the CCN was obtained from Sigma-Aldrich Co. (Cas NO: 17304, St. Louis, MO, USA). Commercial kits for superoxide dismutase (SOD) was obtained from RANDOX reagents Co. (Rondaxlab, Crumlin, BT 29, UK). All other chemicals were commercial products of analytical grade.
2.2. Animals and grouping
To perform the current original study, 28 mature Albino mice (20-25 gr) were randomly divided into 4 control and experimental groups (n= 7/each). The animals received food and water at libitum, in a standard light/dark and temperature condition.
1) The control group (Con) received 0.1 ml/day, IP of normal saline. 2) CCN-sole group received only CCN (200 mg/kg /day, IP) (25). 3) The sham-control group (PQ), received PQ (5 mg/kg/day, IP) (26). 4) The experimental group (PQ+CCN), received CCN (200 mg/kg/day, IP) along with PQ. All four groups were treated for 35 continuous days.
2.3. Tissue sampling and weight determination
Following the test termination, the animals were euthanized by overdose administration of ketamine and xylazine (Alfasan, Woerden, the Netherland) (three times more than the anesthesia dose of ketamine 120 mg/kg and xylazine 15 mg/kg). The total body weights of animals were recorded before and after the trial. Moreover, after the trial, the testicular weights as well as testicular weight relative to the total body weight were determined and compared between the groups. Next, the left testes were considered for further molecular and biochemical analyses at -70ºC. The right testicles were fixed in 10% formal saline for further histological and morphometric studies.
2.4. Histological analyses
Following fixation (72 hr), routine tissue passage and paraffin embedding were conducted. Then, the samples were cut (5-6 µm) using a rotary microtome (Lites, Germany). The histomorphometric analyses were performed after Hematoxylin-Eosin staining. For this purpose, the tubular repopulation (RI), differentiation (TDI) and spermiogenesis (SPI) indices were analyzed. The seminiferous tubules with more than 3 layers were marked as those tubules with positive TDI. Moreover, the those seminiferous tubules with developing spermatozoa were marked as tubules with positive SPI. Moreover, the seminiferous tubules with high percentages of active spermatogonia (with a dark nucleus) were considered as positive RI. Finally, the percentages of tubules with positive TDI, SPI, and RI were evaluated in 3 cross sections from each animal (total 21 cross sections from each group). Moreover, the Leydig and Sertoli cell distribution per mm2 of testicular tissue were counted in 3 cross sections from each animal (total 21 cross sections from each group).
The histological photomicrographs were taken using an onboard camera (SONY Zeiss, Cyber-Shot, Japan) and edited/combined with Adobe Photoshop CS10 (Adobe System Inc., Mountain View, CA, USA).
2.5. Assessment of testicular antioxidant status
In order to evaluate the biochemical activity of the SOD and malondialdehyde (MDA) content in testicular tissue, the tissue samples were weighed and 0.6 gr of each tissue were homogenized in 10 volumes of ice-cold 50 mM potassium phosphate buffer (pH 7.4) with 0.3 M KBr and a set of antiproteolytic agents (containing: 0.5 mM phenyl methylsulfonyl fluoride, 3 mM diethylenetriaminepentaacetic acid, 90 mg of aprotinin l-1, 10 mg of pepstatin l-1, 10 mg of chymostatin l-1, and 10 mg of leupeptin l-1). Thereafter, the SOD activity of homogenates was assayed by using commercial standard kits (ZB-SOD-96A, Zellbio, GmbH). Finally, the rates of absorbance for samples were measured at 420 nm and compared between the groups. The MDA contents of testicles were measured by using the thiobarbituric acid (TBA) reaction as described previously and the absorbance rates of samples were measured at 532 nm (27). Finally, the MDA was evaluated based on the Lowry technique (28) and the results for MDA was presented based on nmol/mg of tissue.
2.6. RNA isolation and semi-quantitative reverse transcriptase polymerase chain reaction (RT-PCR)
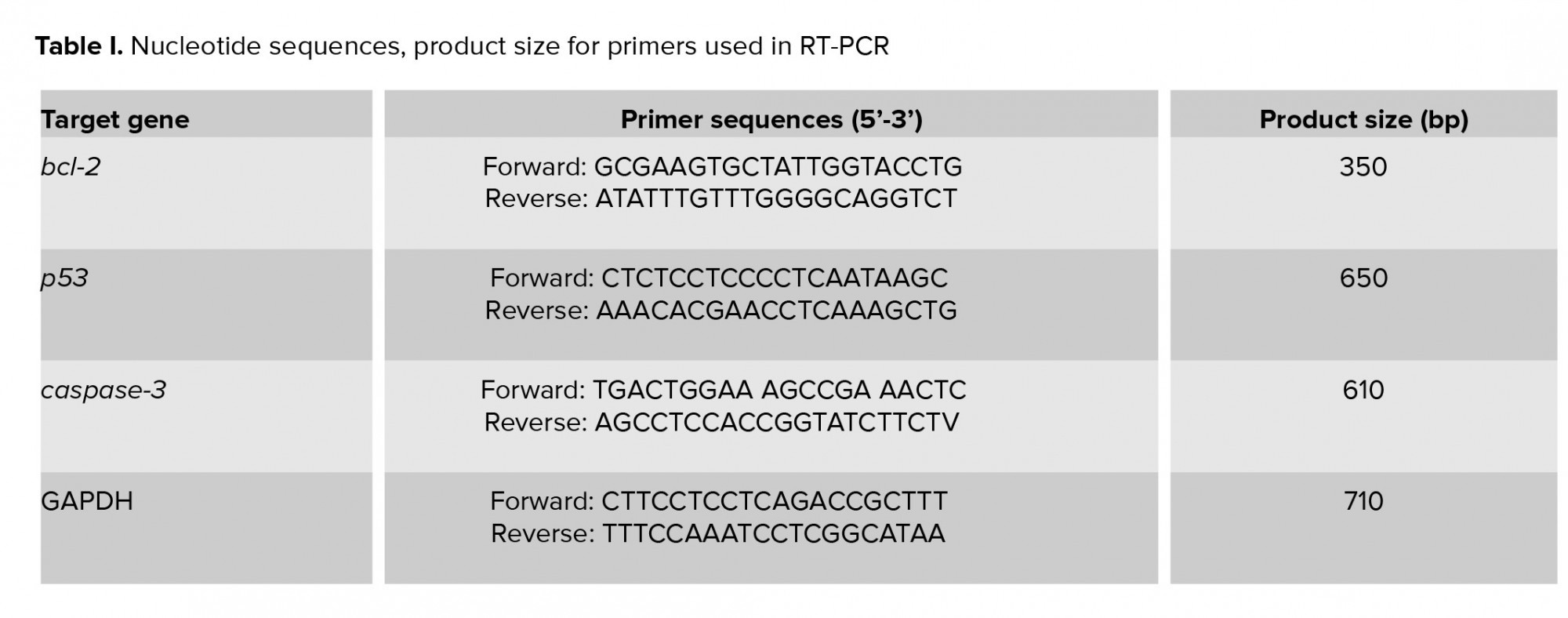
To perform RT-PCR test, 0.3 gr from each sample (n= 7 from each group) were used for RNA extraction by using TRIzol®-based total RNA isolation kit (GIBCO BRL, Gaithersburg, Maryland, USA). Next, the quality and concentration of extracted mRNA were evaluated by NanoDrop-1000 spectrophotometer (Thermo Scientific, Washington, USA) at 260 nm and A260/280 = 1.8-2.0 and stored in -70°C. “The cDNA was prepared by using 1 µg of RNA from each sample and used as a template for the amplification by the PCR with the specific forward and reverse primers presented in Table I. The PCR amplification conditions were 35-40 cycles of 95ºC for 20 sec; annealing temperature [50ºC for caspase-3 (45 s), 62ºC for Bcl-2 (1 min), 59ºC for Bax (1 min), 52ºC for p53 (1 min), and 57ºC for GAPDH (1 min)]; elongation: 72ºC for 1 min and 72ºC for 5 min. Specific primers (29) were designed and manufactured by Gen-Fanavarn Co. (Tehran, Iran). The amplified products (10 µl, including 7 µl from the sample and 3 µl from loading buffer) were electrophoresed in 1.5% agarose gels, stained with ethidium bromide, and viewed using ultraviolet (UV) trans-illuminator (ATP technology, Iran) and visualized by Gel-Pro analyses software (ATP, version 2.1 for window 7). In order to quantify the target gene amplification, the ratio between product genes and the GAPDH (internal control) was calculated to normalize (30).
2.7.The DNA ladder test
To assess DNA fragmentation the DNA ladder was performed using Cina Pure-DNA extraction kit (Sinaclon, Iran). In this process, 35 mg of testicles was homogenized with 100μl protease buffer in the microcentrifuge tubes. Then, incubation of the tubes at 55ºC for 2hr has been down. Next, 100 μl of samples were added into the new microtubes and precipitated with 300μl of precipitation solutions (isopropanol based) for 5min and centrifuged (12000 g) for 10 min. The tubes were decanted and placed on a tissue paper for 2-3 sec and 1 ml buffer solution (ethanol-based) was added to pellets and mixed through 5-sec. following centrifugation (2xafter the supernatant was poured off and the pellets were dried at 65ºC for 5 min. Finally, the unsolved residues were precipitated by centrifugation at 12,000 g for 30 sec and the DNA containing supernatant was removed. The DNA content was assessed using a NanoDrop-1000 spectrophotometer (Thermo Scientific, Washington, USA). Then, the DNA quantity was estimated and a volume of 2μg DNA (15-17μl of eluted DNA) was added to the loading buffer (50% glycerol, 2 mm ethylenediaminetetraacetic acid, and 0.40% bromophenol blue), and DNA solution was loaded on a 1% agarose gel (70-V constant voltage, 70 min). The PST1 was used as a marker to identify the DNA amount. Gels were stained with ethidium bromide and visualized by Gel Doc 2000 system (ATP, Tehran, Iran).
2.8. Ethical consideration
The procedure was carried out based on the guidelines of the Ethics Committee of Urmia University, Faculty of Veterinary Medicine (Approval letter IRB protocol No: AECVU-185-2018).
2.9. Statistical analysis
The variance normality and homogeneity of the data were evaluated by Kolmogorov-Smirnov and Levene’s tests, respectively. Then, all the data were analyzed by one-way ANOVA with the appropriate post-hoc (Turkey’s multiple comparisons).The appropriate analysis of covariance (ANCOVA) to analyze the relationship between cell number (as covariant) with mean alteration of genes expression. The SPSS software (Statistical Package for the Social Sciences, version 22.00, California, USA) was used for statistical and correlation analyses. A p< 0.05 was considered as a statistically significant and all data were presented as mean ± SD.
Observations revealed no statistically significant difference in total body weight between all groups before and after the experiment. More analyses showed decreased testicular weight and size in PQ-sole group vs the control and other experimental groups (p< 0.02). Accordingly, the testicular weight relative to the total body weight was decreased in the PQ-sole group vs the control group. The animals in the PQ + CCN-treated group compared to the PQ-sole group represented more testicular weight to the total body weight ratio (Figures 1A, 1B, 1C, 1D, and 1E).
3.2.Histological findings
Light microscopic analyses showed a remarkable (p< 0.01) increment in the percentage of seminiferous tubules with positive RI, TDI, and SPI in the PQ-sole group vs the control and other experimental groups. Meanwhile, the animals in the PQ + CCN-treated group exhibited ameliorated spermatogenesis (RI, TDI, and SPI) vs the PQ-sole group (p<0.03, p< 0.01, and p< 0.02) (Figures 2B, 2C, and 2D). More histological analyses represented remarkable edema in the connective tissue of testicular tissue of the PQ-sole vs the control and other experimental groups (Figures 2A and 2E). Finally, the animals in the PQ + CCN-treated group exhibited increased numbers of Leydig and Sertoli cells per mm2 of tissue compared to the PQ-sole group (p< 0.02, p< 0.01) (Figures 2F and 2G).
3.3.RT-PCR results
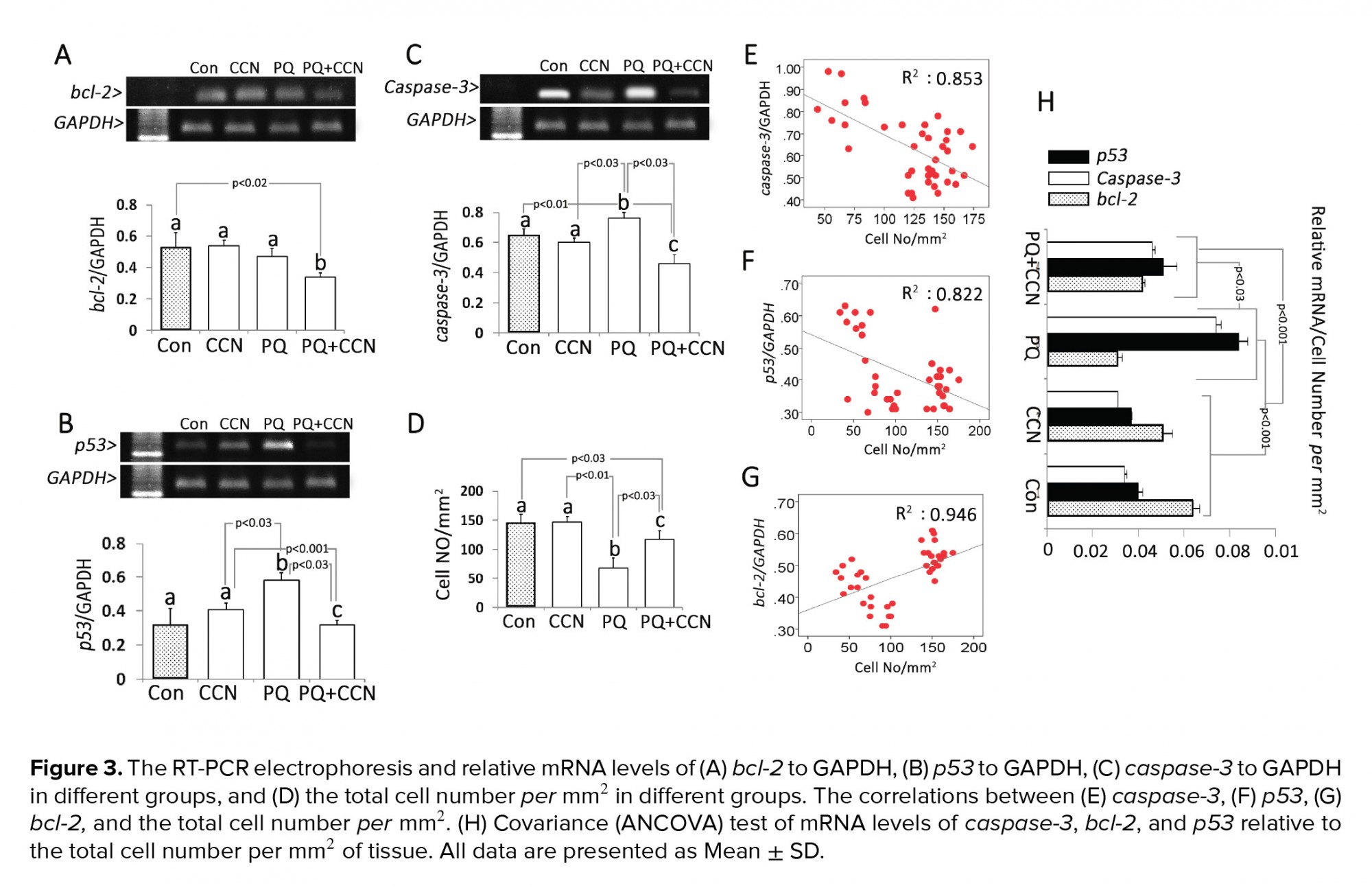
Semi quantitative RT-PCR showed that the mRNA levels of bcl-2 and caspase-3 decreased in the PQ + CCN-treated groups compared to the control and other experimental groups (p< 0.02 and p< 0.03). The mRNA level of p53 was increased in the PQ-sole group vs the control and other experimental groups (p< 0.03) (Figures 3A, 3B, and 3C). To understand the subject, the total testicular cell numbers per mm2 of tissue in different groups and the correlation between the cellular number and mRNA levels were estimated. Observations represented a positive correlation between the mRNA levels and mRNA levels of bcl-2, p53, and caspase-3 (Figures 3E, 3F, and 3G). Moreover, the covariance (ANCOVA) test was performed in which the cellular population of the tissue per mm2 act as the covariant. Observations showed a significant enhancement in the mRNA levels of caspase-3, and p53 relative to cellular population (per mm2 of tissue) was decreased in the PQ + CCN-treated group vs the PQ-sole group. However, the mRNA level of bcl-2 relative to cellular population (per mm2 of tissue) was increased in the PQ + CCN-treated group compared to the PQ-sole group (Figure 3H).
3.4.Biochemical findings
The animals in CCN and PQ-sole groups exhibited diminished (p< 0.01) levels of SOD in comparison with the control and PQ + CCN-treated groups. Moreover, the animals in the PQ-sole group represented a remarkable (p< 0.01) increment in the testicular MDA content vs the control and other experimental groups (Figures 4A and 4B). In order to better understand the biochemical changes, the covariance (ANCOVA) test was performed in which the cellular population of tissue per mm2 act as the covariant. Observations showed a significant (p< 0.02) enhancement in tissue SOD level relative to testicular cellularity in the CCN-sole and PQ + CCN-treated groups vs the PQ-sole group (Figure 4C). The same results were obtained for the MDA contents of the testicles (Figure 4D).
3.5.DNA ladder test
The PQ-induced DNA fragmentation was evaluated by DNA ladder test. The results showed that the PQ caused a violent DNA fragmentation. However, the animals in the CCN + PQ-treated group exhibited inhibited DNA fragmentation vs the PQ-sole group (Figure 5).




Turning back to the relation between oxidative stress and mitochondria-dependent apoptosis, one should consider that the ROS-induced lipid peroxidation adversely affects the mitochondrial metabolism, vital functions, including respiration and oxidative phosphorylation, inner membrane barrier properties, and maintenance of mitochondrial membrane potential (41). In line with this issue, the bcl-2, as stabilizing the protein in the mitochondrial membrane, is involved in maintaining the membrane integrity. Thus, any reduction in bcl-2 expression and/or the interaction of lipid peroxidation products with bcl-2 trigger the mitochondrial membrane disintegration (42). Accordingly, the bcl-2 and bcl-xL in the mitochondria prevent the apoptogenic factors, including cytochrome c and/or apoptosis-inducing factor (AIF) release from mitochondrial inter-membrane space into the cytoplasm because the released cytochrome c and AIF directly activate the caspases, as finishers of the apoptosis pathway (43). Our findings showed that the CCN significantly upregulated the bcl-2 expression and remarkably diminished the caspase-3 expression in the PQ + CCN-treated group. On the other hand, the animals in the PQ + CCN-treated group exhibited diminished DNA damage (hall mark of apoptosis) as well. Taking together, we can come close to this fact that upregulation of antioxidant potential simultaneous with enhanced expression of bcl-2 in the CCN-treated group inhibited the intrinsic apoptosis pathway by maintaining the mitochondrial membrane integrity.
Aside from all possible mechanisms and/or pathways discussed earlier, the p53-dependent checkpoint activity stops cell cycle in mitotically dividing germ cells with DNA double-strand breaks (DSBs). Indeed, the DSBs trigger the p53 expression, which in turn initiates the DNA repairing pathways. When the DNA damage is present before entry into S phase, p53 halts the cell cycle in G1 stage via p21 (cyclin-dependent kinase inhibitor cdkn1a)-dependent pathway (44). However, considering the decreased DNA fragmentation and diminished expression of p53 in the PQ + CCN-treated group, we can suggest that CCN could fairly diminish the DNA damage and as a consequent could potentially inhibit DNA damage. The limitations of our work suggests future studies in this field to investigate on the probability of the direct role of PQ on testis and evaluating the ultrastructure of the blood testes barrier in PQ poisoning. Moreover, the probable effects of PQ and CCN on hypothalamus-hypophyses and gonadal axis in molecular and gene expression level.
Acknowledgments
This manuscript was financially supported by the Department of Basic Sciences, Division of Comparative Histology and Embryology, Faculty of Veterinary Medicine, Urmia University. The authors wish to thank the Urmia University for financial supports.
Conflict of interest
The authors have no conflicts of interest to declare.
Full-Text: (433 Views)
- Introduction
In line with male infertility disorders, it has been shown that PQ adversely affects the spermatogenesis (16), reduces the sperm quality, including count, motility, and morphology (4), and results in chromosomal aberrations in spermatozoa, spermatid, and preleptotene spermatogonial cells (17). Spermatogenesis is a dynamic system, which is characterized by cell proliferation and differentiation. Similar to hypermitotic tissues, the physiologic cellular proliferation of testicular tissue is maintained with controlled apoptosis. Indeed, the apoptosis is processed through intrinsic (mitochondria-dependent) and extrinsic (death receptor Fas and Fas-ligand-dependent) pathways (18).
Crocin (CCN) has attracted scientific attention and finally found a considerable place in pharmaceutical science due to its anti-inflammatory, antioxidant (19), anti-spasm (20), anti-depression (21), anti-cancer (22) and neuroprotective properties (23). A study showed that the CCN increases antioxidant enzymes and testosterone. However, it reduced testicular histological destructive changes in CP-treated mice (24).
Considering the oxidative stress as the main mechanism of PQ action and minding the adverse effect of free radicals on mitochondrial membrane integrity, the current study was performed to investigate the protective effect of CCN against PQ-related derangements.
- Materials and methods
The PQ, with the formulation of SL20%, was taken from EXIR Co. (Tehran, Iran) and the CCN was obtained from Sigma-Aldrich Co. (Cas NO: 17304, St. Louis, MO, USA). Commercial kits for superoxide dismutase (SOD) was obtained from RANDOX reagents Co. (Rondaxlab, Crumlin, BT 29, UK). All other chemicals were commercial products of analytical grade.
2.2. Animals and grouping
To perform the current original study, 28 mature Albino mice (20-25 gr) were randomly divided into 4 control and experimental groups (n= 7/each). The animals received food and water at libitum, in a standard light/dark and temperature condition.
1) The control group (Con) received 0.1 ml/day, IP of normal saline. 2) CCN-sole group received only CCN (200 mg/kg /day, IP) (25). 3) The sham-control group (PQ), received PQ (5 mg/kg/day, IP) (26). 4) The experimental group (PQ+CCN), received CCN (200 mg/kg/day, IP) along with PQ. All four groups were treated for 35 continuous days.
2.3. Tissue sampling and weight determination
Following the test termination, the animals were euthanized by overdose administration of ketamine and xylazine (Alfasan, Woerden, the Netherland) (three times more than the anesthesia dose of ketamine 120 mg/kg and xylazine 15 mg/kg). The total body weights of animals were recorded before and after the trial. Moreover, after the trial, the testicular weights as well as testicular weight relative to the total body weight were determined and compared between the groups. Next, the left testes were considered for further molecular and biochemical analyses at -70ºC. The right testicles were fixed in 10% formal saline for further histological and morphometric studies.
2.4. Histological analyses
Following fixation (72 hr), routine tissue passage and paraffin embedding were conducted. Then, the samples were cut (5-6 µm) using a rotary microtome (Lites, Germany). The histomorphometric analyses were performed after Hematoxylin-Eosin staining. For this purpose, the tubular repopulation (RI), differentiation (TDI) and spermiogenesis (SPI) indices were analyzed. The seminiferous tubules with more than 3 layers were marked as those tubules with positive TDI. Moreover, the those seminiferous tubules with developing spermatozoa were marked as tubules with positive SPI. Moreover, the seminiferous tubules with high percentages of active spermatogonia (with a dark nucleus) were considered as positive RI. Finally, the percentages of tubules with positive TDI, SPI, and RI were evaluated in 3 cross sections from each animal (total 21 cross sections from each group). Moreover, the Leydig and Sertoli cell distribution per mm2 of testicular tissue were counted in 3 cross sections from each animal (total 21 cross sections from each group).
The histological photomicrographs were taken using an onboard camera (SONY Zeiss, Cyber-Shot, Japan) and edited/combined with Adobe Photoshop CS10 (Adobe System Inc., Mountain View, CA, USA).
2.5. Assessment of testicular antioxidant status
In order to evaluate the biochemical activity of the SOD and malondialdehyde (MDA) content in testicular tissue, the tissue samples were weighed and 0.6 gr of each tissue were homogenized in 10 volumes of ice-cold 50 mM potassium phosphate buffer (pH 7.4) with 0.3 M KBr and a set of antiproteolytic agents (containing: 0.5 mM phenyl methylsulfonyl fluoride, 3 mM diethylenetriaminepentaacetic acid, 90 mg of aprotinin l-1, 10 mg of pepstatin l-1, 10 mg of chymostatin l-1, and 10 mg of leupeptin l-1). Thereafter, the SOD activity of homogenates was assayed by using commercial standard kits (ZB-SOD-96A, Zellbio, GmbH). Finally, the rates of absorbance for samples were measured at 420 nm and compared between the groups. The MDA contents of testicles were measured by using the thiobarbituric acid (TBA) reaction as described previously and the absorbance rates of samples were measured at 532 nm (27). Finally, the MDA was evaluated based on the Lowry technique (28) and the results for MDA was presented based on nmol/mg of tissue.
2.6. RNA isolation and semi-quantitative reverse transcriptase polymerase chain reaction (RT-PCR)
To perform RT-PCR test, 0.3 gr from each sample (n= 7 from each group) were used for RNA extraction by using TRIzol®-based total RNA isolation kit (GIBCO BRL, Gaithersburg, Maryland, USA). Next, the quality and concentration of extracted mRNA were evaluated by NanoDrop-1000 spectrophotometer (Thermo Scientific, Washington, USA) at 260 nm and A260/280 = 1.8-2.0 and stored in -70°C. “The cDNA was prepared by using 1 µg of RNA from each sample and used as a template for the amplification by the PCR with the specific forward and reverse primers presented in Table I. The PCR amplification conditions were 35-40 cycles of 95ºC for 20 sec; annealing temperature [50ºC for caspase-3 (45 s), 62ºC for Bcl-2 (1 min), 59ºC for Bax (1 min), 52ºC for p53 (1 min), and 57ºC for GAPDH (1 min)]; elongation: 72ºC for 1 min and 72ºC for 5 min. Specific primers (29) were designed and manufactured by Gen-Fanavarn Co. (Tehran, Iran). The amplified products (10 µl, including 7 µl from the sample and 3 µl from loading buffer) were electrophoresed in 1.5% agarose gels, stained with ethidium bromide, and viewed using ultraviolet (UV) trans-illuminator (ATP technology, Iran) and visualized by Gel-Pro analyses software (ATP, version 2.1 for window 7). In order to quantify the target gene amplification, the ratio between product genes and the GAPDH (internal control) was calculated to normalize (30).
2.7.The DNA ladder test
To assess DNA fragmentation the DNA ladder was performed using Cina Pure-DNA extraction kit (Sinaclon, Iran). In this process, 35 mg of testicles was homogenized with 100μl protease buffer in the microcentrifuge tubes. Then, incubation of the tubes at 55ºC for 2hr has been down. Next, 100 μl of samples were added into the new microtubes and precipitated with 300μl of precipitation solutions (isopropanol based) for 5min and centrifuged (12000 g) for 10 min. The tubes were decanted and placed on a tissue paper for 2-3 sec and 1 ml buffer solution (ethanol-based) was added to pellets and mixed through 5-sec. following centrifugation (2xafter the supernatant was poured off and the pellets were dried at 65ºC for 5 min. Finally, the unsolved residues were precipitated by centrifugation at 12,000 g for 30 sec and the DNA containing supernatant was removed. The DNA content was assessed using a NanoDrop-1000 spectrophotometer (Thermo Scientific, Washington, USA). Then, the DNA quantity was estimated and a volume of 2μg DNA (15-17μl of eluted DNA) was added to the loading buffer (50% glycerol, 2 mm ethylenediaminetetraacetic acid, and 0.40% bromophenol blue), and DNA solution was loaded on a 1% agarose gel (70-V constant voltage, 70 min). The PST1 was used as a marker to identify the DNA amount. Gels were stained with ethidium bromide and visualized by Gel Doc 2000 system (ATP, Tehran, Iran).

2.8. Ethical consideration
The procedure was carried out based on the guidelines of the Ethics Committee of Urmia University, Faculty of Veterinary Medicine (Approval letter IRB protocol No: AECVU-185-2018).
2.9. Statistical analysis
The variance normality and homogeneity of the data were evaluated by Kolmogorov-Smirnov and Levene’s tests, respectively. Then, all the data were analyzed by one-way ANOVA with the appropriate post-hoc (Turkey’s multiple comparisons).The appropriate analysis of covariance (ANCOVA) to analyze the relationship between cell number (as covariant) with mean alteration of genes expression. The SPSS software (Statistical Package for the Social Sciences, version 22.00, California, USA) was used for statistical and correlation analyses. A p< 0.05 was considered as a statistically significant and all data were presented as mean ± SD.
- Results
Observations revealed no statistically significant difference in total body weight between all groups before and after the experiment. More analyses showed decreased testicular weight and size in PQ-sole group vs the control and other experimental groups (p< 0.02). Accordingly, the testicular weight relative to the total body weight was decreased in the PQ-sole group vs the control group. The animals in the PQ + CCN-treated group compared to the PQ-sole group represented more testicular weight to the total body weight ratio (Figures 1A, 1B, 1C, 1D, and 1E).
3.2.Histological findings
Light microscopic analyses showed a remarkable (p< 0.01) increment in the percentage of seminiferous tubules with positive RI, TDI, and SPI in the PQ-sole group vs the control and other experimental groups. Meanwhile, the animals in the PQ + CCN-treated group exhibited ameliorated spermatogenesis (RI, TDI, and SPI) vs the PQ-sole group (p<0.03, p< 0.01, and p< 0.02) (Figures 2B, 2C, and 2D). More histological analyses represented remarkable edema in the connective tissue of testicular tissue of the PQ-sole vs the control and other experimental groups (Figures 2A and 2E). Finally, the animals in the PQ + CCN-treated group exhibited increased numbers of Leydig and Sertoli cells per mm2 of tissue compared to the PQ-sole group (p< 0.02, p< 0.01) (Figures 2F and 2G).
3.3.RT-PCR results
Semi quantitative RT-PCR showed that the mRNA levels of bcl-2 and caspase-3 decreased in the PQ + CCN-treated groups compared to the control and other experimental groups (p< 0.02 and p< 0.03). The mRNA level of p53 was increased in the PQ-sole group vs the control and other experimental groups (p< 0.03) (Figures 3A, 3B, and 3C). To understand the subject, the total testicular cell numbers per mm2 of tissue in different groups and the correlation between the cellular number and mRNA levels were estimated. Observations represented a positive correlation between the mRNA levels and mRNA levels of bcl-2, p53, and caspase-3 (Figures 3E, 3F, and 3G). Moreover, the covariance (ANCOVA) test was performed in which the cellular population of the tissue per mm2 act as the covariant. Observations showed a significant enhancement in the mRNA levels of caspase-3, and p53 relative to cellular population (per mm2 of tissue) was decreased in the PQ + CCN-treated group vs the PQ-sole group. However, the mRNA level of bcl-2 relative to cellular population (per mm2 of tissue) was increased in the PQ + CCN-treated group compared to the PQ-sole group (Figure 3H).
3.4.Biochemical findings
The animals in CCN and PQ-sole groups exhibited diminished (p< 0.01) levels of SOD in comparison with the control and PQ + CCN-treated groups. Moreover, the animals in the PQ-sole group represented a remarkable (p< 0.01) increment in the testicular MDA content vs the control and other experimental groups (Figures 4A and 4B). In order to better understand the biochemical changes, the covariance (ANCOVA) test was performed in which the cellular population of tissue per mm2 act as the covariant. Observations showed a significant (p< 0.02) enhancement in tissue SOD level relative to testicular cellularity in the CCN-sole and PQ + CCN-treated groups vs the PQ-sole group (Figure 4C). The same results were obtained for the MDA contents of the testicles (Figure 4D).
3.5.DNA ladder test
The PQ-induced DNA fragmentation was evaluated by DNA ladder test. The results showed that the PQ caused a violent DNA fragmentation. However, the animals in the CCN + PQ-treated group exhibited inhibited DNA fragmentation vs the PQ-sole group (Figure 5).





- Discussion
Turning back to the relation between oxidative stress and mitochondria-dependent apoptosis, one should consider that the ROS-induced lipid peroxidation adversely affects the mitochondrial metabolism, vital functions, including respiration and oxidative phosphorylation, inner membrane barrier properties, and maintenance of mitochondrial membrane potential (41). In line with this issue, the bcl-2, as stabilizing the protein in the mitochondrial membrane, is involved in maintaining the membrane integrity. Thus, any reduction in bcl-2 expression and/or the interaction of lipid peroxidation products with bcl-2 trigger the mitochondrial membrane disintegration (42). Accordingly, the bcl-2 and bcl-xL in the mitochondria prevent the apoptogenic factors, including cytochrome c and/or apoptosis-inducing factor (AIF) release from mitochondrial inter-membrane space into the cytoplasm because the released cytochrome c and AIF directly activate the caspases, as finishers of the apoptosis pathway (43). Our findings showed that the CCN significantly upregulated the bcl-2 expression and remarkably diminished the caspase-3 expression in the PQ + CCN-treated group. On the other hand, the animals in the PQ + CCN-treated group exhibited diminished DNA damage (hall mark of apoptosis) as well. Taking together, we can come close to this fact that upregulation of antioxidant potential simultaneous with enhanced expression of bcl-2 in the CCN-treated group inhibited the intrinsic apoptosis pathway by maintaining the mitochondrial membrane integrity.
Aside from all possible mechanisms and/or pathways discussed earlier, the p53-dependent checkpoint activity stops cell cycle in mitotically dividing germ cells with DNA double-strand breaks (DSBs). Indeed, the DSBs trigger the p53 expression, which in turn initiates the DNA repairing pathways. When the DNA damage is present before entry into S phase, p53 halts the cell cycle in G1 stage via p21 (cyclin-dependent kinase inhibitor cdkn1a)-dependent pathway (44). However, considering the decreased DNA fragmentation and diminished expression of p53 in the PQ + CCN-treated group, we can suggest that CCN could fairly diminish the DNA damage and as a consequent could potentially inhibit DNA damage. The limitations of our work suggests future studies in this field to investigate on the probability of the direct role of PQ on testis and evaluating the ultrastructure of the blood testes barrier in PQ poisoning. Moreover, the probable effects of PQ and CCN on hypothalamus-hypophyses and gonadal axis in molecular and gene expression level.
- Conclusion
Acknowledgments
This manuscript was financially supported by the Department of Basic Sciences, Division of Comparative Histology and Embryology, Faculty of Veterinary Medicine, Urmia University. The authors wish to thank the Urmia University for financial supports.
Conflict of interest
The authors have no conflicts of interest to declare.
Type of Study: Original Article |
References
1. Lock EA, Wilks MF. Paraquat. In: Handbook of Pesticide Toxicology, 3rd Ed. Academic Press, San Diego 2010. [DOI:10.1016/B978-0-12-374367-1.00083-5]
2. Senarathna L, Eddleston M, Wilks MF, Woollen BH, Tomenson JA, Roberts DM, et al. Prediction of outcome after paraquat poisoning by measurement of the plasma paraquat concentration. QJM 2009; 102: 251-259. [DOI:10.1093/qjmed/hcp006] [PMID] [PMCID]
3. Gunnell D, Eddleston M, Phillips MR, Konradsen F. The global distribution of fatal pesticide self-poisoning: systematic review. BMC Public Health 2007; 7: 357. [DOI:10.1186/1471-2458-7-357] [PMID] [PMCID]
4. Ashoka DKHM, De Silva WAJP. Mechanism of paraquat action shows interference in spermatogenesis and epididymal maturation of sperm in mice. J Biol 2013; 01: 66-76
5. Agarwal A, Sharma RK, Desai NR, Prabakaran S, Tavares A, Sabanegh E. Role of oxidative stress in pathogenesis of varicocele and infertility. Urology 2009; 73: 461-469. [DOI:10.1016/j.urology.2008.07.053] [PMID]
6. Schiefer HB, Irvine DG, Buzik SC. Understanding toxicology: Chemicals, their benefits and risks. CRC Press: New York; 1997.
7. Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, et al. Rotenone, paraquat, and Parkinson's disease. Environmen Health Perspect 2011; 119: 866-872. [DOI:10.1289/ehp.1002839] [PMID] [PMCID]
8. Ossowska K, Śmiałowska M, Kuter K, Wierońska J, Zięba B, Wardas J, et al. Degeneration of dopaminergic mesocortical neurons and activation of compensatory processes induced by a long-term paraquat administration in rats: implications for Parkinson's disease. Neuroscience 2006; 141: 2155-2165. [DOI:10.1016/j.neuroscience.2006.05.039] [PMID]
9. Bus JS, Gibson JE. Paraquat: model for oxidant-initiated toxicity. Environm Health Perspect 1984; 55: 37-46. [DOI:10.1289/ehp.845537] [PMID] [PMCID]
10. Karaguzel E, Kadihasanoglu M, Kutlu O. Mechanisms of testicular torsion and potential protective agents. Nat Rev Urol 2014; 11: 391-399. [DOI:10.1038/nrurol.2014.135] [PMID]
11. Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev 2008; 1: 15-24. [DOI:10.4161/oxim.1.1.6843] [PMID] [PMCID]
12. Kutikov A, Casale P, White MA, Meyer WA, Chang A, Gosalbez R, et al. Testicular compartment syndrome: a new approach to conceptualizing and managing testicular torsion. Urology 2008; 72: 786-789. [DOI:10.1016/j.urology.2008.03.031] [PMID]
13. Moghimian M, Soltani M, Abtahi H, Shokoohi M. Effect of vitamin c on tissue damage and oxidative stress following tunica vaginalis flap coverage after testicular torsion. J Pediatr Surg 2017; 52: 1651-1655. [DOI:10.1016/j.jpedsurg.2017.07.001] [PMID]
14. Moghimian M, Abtahi-Evari SH, Shokoohi M, Amiri M, Soltani M. Effect of Syzygium aromaticum (clove) extract on seminiferous tubules and oxidative stress after testicular torsion in adult rats. Physiol Pharmacol 2017; 21: 343-350.
15. Shokri F, Shokoohi M, Niazkar HR, Roudi Rasht Abadi A, Kalarestaghi H, Ahin M. Investigation the Spermatogenesis and Testis Structure in diabetic rats after treatment with galega officinalis extract. Crescent Journal of Medical and Biological Sciences 2019; 6: 31-36.
16. Fathi N, Hossinipanah M, Hajihossini M, Ranjbar A. Effects of paraquat on testicular histomorphometry of male rats. Biol Forum 2015; 7: 573-575.
17. D'Souza UJ, Narayana K, Zain A, Raju S, Nizam HM, Noriah O. Dermal exposure to the herbicide-paraquat results in genotoxic and cytotoxic damage to germ cells in the male rat. Folia Morphol 2006; 65: 6-10.
18. Elumalai P, Gunadharini DN, Senthilkumar K, Banudevi S, Arunkumar R, Benson C, et al. Induction of apoptosis in human breast cancer cells by nimbolide through extrinsic and intrinsic pathway. Toxicol Lett 2012; 215: 131-142. [DOI:10.1016/j.toxlet.2012.10.008] [PMID]
19. Zhang Z, Wang CZ, Wen XD, Shoyama Y, Yuan CS. Role of saffron and its constituents on cancer chemoprevention. Pharm Biol 2013; 51: 920-924. [DOI:10.3109/13880209.2013.771190] [PMID] [PMCID]
20. Dianat M, Esmaeilizadeh M, Badavi M, Samarbaf-zadeh AR, Naghizadeh B. Protective effects of crocin on ischemia-reperfusion induced oxidative stress in comparison with vitamin E in isolated rat hearts. Jundishapur J Nat Pharm Prod 2014; 9: e17187. [DOI:10.17795/jjnpp-17187]
21. Talaei A, Hassanpour Moghadam M, Sajadi Tabassi SA, Mohajeri SA. Crocin, the main active saffron constituent, as an adjunctive treatment in major depressive disorder: a randomized, double-blind, placebo-controlled, pilot clinical trial. J Affect Disord 2015; 174: 51-56. [DOI:10.1016/j.jad.2014.11.035] [PMID]
22. Amin A, Bajbouj K, Koch A, Gandesiri M, Schneider-Stock R. Defective autophagosome formation in p53-null colorectal cancer reinforces crocin-induced apoptosis. Int J Mol Sci 2015; 16: 1544-1561. [DOI:10.3390/ijms16011544] [PMID] [PMCID]
23. Farshid AA, Tamaddonfard E. Histopathological and behavioral evaluations of the effects of crocin, safranal and insulin on diabetic peripheral neuropathy in rats. Avic J Phytomed 2015; 5: 469-478.
24. Bakhtiary Z, Shahrooz R, Ahmadi A, Zarei L. Evaluation of antioxidant effects of crocin on sperm quality in cyclophosphamide treated adult mice. Vet Res Forum 2014; 5: 213-218.
25. Bakhtiary Z, Shahrooz R, Ahmadi A, Malekinejad H, Mostafavi M. Study of protective effects of crocin on testicular histomorphometry and serological parameters in cyclophosphamide on treated adult mice. The Journal of Urmia University of Medical Sciences 2014; 25: 663-673.
26. Kuter K, Nowak P, Golembiowska K, Ossowska K. Increased reactive oxygen species production in the brain after repeated low-dose pesticide paraquat exposure in rats. A comparison with peripheral tissues. Neurochem Res 2010; 35: 1121-1130. [DOI:10.1007/s11064-010-0163-x] [PMID]
27. Nagababu E, Rifkind JM, Boindala S, Nakka L. Assessment of antioxidant activity of eugenol in vitro and in vivo. Methods Mol Biol 2010; 610: 165-180. [DOI:10.1007/978-1-60327-029-8_10] [PMID] [PMCID]
28. Seevaratnam R, Patel BP, Hamadeh MJ. Comparison of total protein concentration in skeletal muscle as measured by the Bradford and Lowry assays. J Biochem 2009; 145: 791-797. [DOI:10.1093/jb/mvp037] [PMID]
29. Faião-Flores F, Coelho PR, Toledo Arruda-Neto JD, Maria-Engler SS, Tiago M, Capelozzi VL, et al. Apoptosis through Bcl-2/Bax and cleaved caspase up-regulation in melanoma treated by boron neutron capture therapy. PLoS One 2013; 8: e59639. [DOI:10.1371/journal.pone.0059639] [PMID] [PMCID]
30. Shamsi-Gamchi N, Razi M, Behfar M. Testicular torsion and reperfusion: evidences for biochemical and molecular alterations. Cell Stress Chaperones 2018; 23: 429-439. [DOI:10.1007/s12192-017-0855-0] [PMID] [PMCID]
31. Ko EY, Sabanegh ESJr, Agarwal A. Male infertility testing: reactive oxygen species and antioxidant capacity. Fertil Steril 2014; 102: 1518-1527. [DOI:10.1016/j.fertnstert.2014.10.020] [PMID]
32. Tremellen K. Oxidative stress and male infertility-a clinical perspective. Hum Reprod Update 2008; 14: 243-258. [DOI:10.1093/humupd/dmn004] [PMID]
33. Agarwal A, Sharma RK, Sharma R, Assidi M, Abuzenadah AM, Alshahrani S, et al. Characterizing semen parameters and their association with reactive oxygen species in infertile men. Reprod Biol Endocrinol 2014; 12: 33. [DOI:10.1186/1477-7827-12-33] [PMID] [PMCID]
34. Shokoohi M, Shoorei H, Soltani M, Abtahi‐Eivari SH, Salimnejad R, Moghimian M. Protective effects of the hydroalcoholic extract of Fumaria parviflora on testicular injury induced by torsion/detorsion in adult rats. Andrologia 2018; 50: e13047. [DOI:10.1111/and.13047] [PMID]
35. Shokoohi M, Olad Saheb Madarek E, Khaki A, Shoorei H, Khaki AA, Soltani M, et al. Investigating the effects of onion juice on male fertility factors and pregnancy rate after testicular torsion/detorsion by intrauterine insemination method. Int J Women's Health Reprod Sci 2018; 6: 499-505. [DOI:10.15296/ijwhr.2018.82]
36. Khosravanian N, Razi M, Farokhi F, Khosravanian H. Testosterone and vitamin E administration up-regulated varicocele-reduced Hsp70-2 protein expression and ameliorated biochemical alterations. J Assist Reprod Genet 2014; 31: 341-354. [DOI:10.1007/s10815-013-0165-0] [PMID] [PMCID]
37. Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol 2014; 24: R453-R462. [DOI:10.1016/j.cub.2014.03.034] [PMID] [PMCID]
38. Tiwari S, Tiwari S, Singh M, Singh A, Prasad SM. Generation mechanisms of reactive oxygen species in the plant cell: Boon or Bane - Revisiting the Role of ROS. Reactive Oxygen Species in Plants. London: John Wiley & Sons Ltd.; 2017. Chapter 49; p.1-22. [DOI:10.1002/9781119324928.ch1]
39. Weydert CJ, Cullen JJ. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc 2010; 5: 51-66. [DOI:10.1038/nprot.2009.197] [PMID] [PMCID]
40. Dar RA, Brahman PK, Khurana N, Wagay JA, Lone ZA, Ganaie MA, et al. Evaluation of antioxidant activity of crocin, podophyllotoxin and kaempferol by chemical, biochemical and electrochemical assays. Arabian Journal of Chemistry 2017; 10: S1119-S1128. [DOI:10.1016/j.arabjc.2013.02.004]
41. Subramaniam SR, Chesselet MF. Mitochondrial dysfunction and oxidative stress in Parkinson's disease. Prog Neurobiol 2013; 106: 17-32. [DOI:10.1016/j.pneurobio.2013.04.004] [PMID] [PMCID]
42. Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem 2011; 351: 41-58. [DOI:10.1007/s11010-010-0709-x] [PMID]
43. Khavarimehr M, Nejati V, Razi M, Najafi G. Ameliorative effect of omega-3 on spermatogenesis, testicular antioxidant status and preimplantation embryo development in streptozotocin-induced diabetes in rats. Int Urol Nephrol 2017; 49: 1545-1560. [DOI:10.1007/s11255-017-1636-5] [PMID]
44. Liu D, Ou L, Clemenson GD Jr, Chao C, Lutske ME, Zambetti GP, et al. Puma is required for p53-induced depletion of adult stem cells. Nat Cell Biol 2010; 12: 993-998. [DOI:10.1038/ncb2100] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








