Wed, Jul 9, 2025
[Archive]
Volume 19, Issue 7 (July 2021)
IJRM 2021, 19(7): 653-662 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Namavar Jahromi B, Borzou N, Parsanezhad M E, Anvar Z, Ghaemmaghami P, Sabetian S. Associations of insulin resistance, sex hormone-binding globulin, triglyceride, and hormonal profiles in polycystic ovary syndrome: A cross-sectional study. IJRM 2021; 19 (7) :653-662
URL: http://ijrm.ir/article-1-1860-en.html
URL: http://ijrm.ir/article-1-1860-en.html
Bahia Namavar Jahromi1 

 , Niloofar Borzou2
, Niloofar Borzou2 

 , Mohammad Ebrahim Parsanezhad1
, Mohammad Ebrahim Parsanezhad1 

 , Zahra Anvar3
, Zahra Anvar3 

 , Parvin Ghaemmaghami4
, Parvin Ghaemmaghami4 

 , Soudabeh Sabetian *5
, Soudabeh Sabetian *5 




 , Niloofar Borzou2
, Niloofar Borzou2 

 , Mohammad Ebrahim Parsanezhad1
, Mohammad Ebrahim Parsanezhad1 

 , Zahra Anvar3
, Zahra Anvar3 

 , Parvin Ghaemmaghami4
, Parvin Ghaemmaghami4 

 , Soudabeh Sabetian *5
, Soudabeh Sabetian *5 


1- Infertility Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. Department of Obstetrics and Gynecology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
2- Student Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
3- Infertility Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
4- School of Nursing and Midwifery, Shiraz University of Medical Sciences, Shiraz, Iran.
5- Infertility Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. ,soudabehsabet@gmail.com
2- Student Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
3- Infertility Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
4- School of Nursing and Midwifery, Shiraz University of Medical Sciences, Shiraz, Iran.
5- Infertility Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. ,
Full-Text [PDF 287 kb]
(1000 Downloads)
| Abstract (HTML) (2113 Views)
Full-Text: (426 Views)
- Introduction
Polycystic ovary syndrome (PCOS) classified as World Health Organization (WHO) group II ovulation disorders is one of the most common health problems in women of reproductive age recognized by chronic anovulation (1). PCOS is associated with insulin resistance (IR), hyperinsulinemia, and obesity. IR is detected in 50-70% of PCOS women with normal body mass index (BMI), whereas obese PCOS women show a higher prevalence of IR (2). IR along with high insulin levels stimulates the ovaries to produce more androgens. IR in PCOS increases the risk of diabetes and pre-diabetic states (3). About 60% of PCOS women exhibit high levels of serum androgens including testosterone, androstenedione, and dehydroepiandrosterone sulfate (DHEAS) (4). Low levels of sex hormone-binding globulin (SHBG) were reported to be associated with obesity, IR, hyperandrogenism, glucose intolerance, and type-2 diabetes in women with PCOS. It was observed that the therapeutic balance of SHBG can improve PCOS-dependent morbidities (5). Therefore, we hypothesized that SHBG levels might be a helpful biomarker for the diagnosis and treatment of PCOS or IR. Modifications of lifestyle and diet according to the medical guidelines and preventive strategies in general practice is the first line of treatment for PCOS (6). A proper prediabetes screening test is important to be performed for all PCOS women (7). On the other hand, IR can be estimated by various methods ranging from complex to simple techniques with different accuracies (8). To perform a standard method like hyperinsulinemic-euglycemic clamp (HEC) or the frequently sampled intravenous glucose tolerance test (FSIVGTT), multiple blood samples are collected (9). Due to the complexity of HEC and FSIVGTT, simple and semi-invasive methods are preferred for the clinical settings (10). Fasting blood sugar (FBS) and fasting insulin (FI) levels are suggested to detect pre-diabetic states in PCOS patients (11). FBS-to-FI ratio (FBS/FI), homeostasis model assessment of insulin resistance (HOMA-IR), and the quantitative insulin sensitivity check index (QUICKI) are important indicators of insulin sensitivity for patients with hyperinsulinemia (12). Although several tests are introduced to detect IR, no one could obtain general public for both clinical and research purposes (13).
It was previously reported that IR and lipid profiles are positively correlated with total testosterone and free androgenic index (14). Also, triglyceride (TG) was suggested to be considered as a valuable substitute marker for IR in women with PCOS (15). In this research, we studied the relationship between IR, SHBG, TG, and hormonal profiles of PCOS women including the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of each IR method calculated based on HOMA-IR.
It was previously reported that IR and lipid profiles are positively correlated with total testosterone and free androgenic index (14). Also, triglyceride (TG) was suggested to be considered as a valuable substitute marker for IR in women with PCOS (15). In this research, we studied the relationship between IR, SHBG, TG, and hormonal profiles of PCOS women including the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of each IR method calculated based on HOMA-IR.
- Materials and Methods
- 1. Study design
This cross-sectional study included 74 PCOS women diagnosed according to the Modified Rotterdam Criteria 2003 (16). In order to diagnose PCOS, two of the following criteria are required: (i) menstrual abnormalities (amenorrhea, oligomenorrhea), (ii) clinical and/or biochemical hyperandrogenism, and (iii) the ultrasound look of polycystic ovaries. Additionally, it is important to exclude other disorders such as Cushing’s syndrome, congenital adrenal hyperplasia, and androgen‐secreting tumors which have similar clinical presentation to PCOS.
- 2. Inclusion and exclusion criteria
PCOS women referred to the clinics affiliated to Shiraz University of Medical Sciences from January to December 2018, aged 18-48 yr, and willing to participate were enrolled in this study. The exclusion criteria were pregnancy, lactation, hypertension, and diabetes. The patients who took insulin-sensitizing agents, hormonal treatments, or corticosteroids in the past two months prior to the study were not included.
- 3. Sample collection and outcome measurement
Blood samples were collected from PCOS women after an overnight fasting for 10–12 hr. Serum was extracted by centrifugation at 3000 g for 20 min and stored at -20oC. Luteinizing hormone (LH), follicle-stimulating hormone (FSH), thyroid-stimulating hormone (TSH), and prolactin and testosterone levels were measured by immune radiometric assay (RIA kit IRMA tube, Korea). SHBG and DHEAS levels were measured by radioimmunoassay (Testo-RIA kit, France) and TG by ELISA (BioVendor ELISA kit, Germany). FI and FBS values were checked by immunoradiometric assay (IRMA kit, Hungary).
In this project, the following five indirect methods were employed to assess IR in PCOS patients: FBS, FI, FBS/FI ratio, HOMA-IR, and QUICKI. We used the following formulas: HOMA = FI (µ U/mL) × fasting glucose (mg/dL)/405 and QUICKI = 1/ (log (FI µU/mL) + log (fasting glucose mg/dL)). FBS ≥ 100 (mg/dl), FI ≥ 10 (uIU/mL), FBS/FI < 4.5, HOMA-IR ≥ 2.5, and QUICKI ≤ 0.33 were considered as the cut-off values in favor of IR (17, 18). BMI < 25 was considered normal. IR values were compared between the patients with BMI ≥ 25 and those with < 25. Also, SHBG ≥ 36 (nmol/L) was considered normal and data were compared between women with SHBG ≥ 36 and SHBG < 36 (nmol/L) (19). The values of hormones and TG were also compared between the mentioned groups. Previous studies showed that the results of HOMA-IR assessment were correlated significantly with the results of the clamp studies (20). Also, HOMA-IR had the highest sensitivity according to recent research (18). Therefore, we substituted HOMA-IR for the standard technique and compared the sensitivity, specificity, PPV, and NPV of the other methods to HOMA-IR.
In this project, the following five indirect methods were employed to assess IR in PCOS patients: FBS, FI, FBS/FI ratio, HOMA-IR, and QUICKI. We used the following formulas: HOMA = FI (µ U/mL) × fasting glucose (mg/dL)/405 and QUICKI = 1/ (log (FI µU/mL) + log (fasting glucose mg/dL)). FBS ≥ 100 (mg/dl), FI ≥ 10 (uIU/mL), FBS/FI < 4.5, HOMA-IR ≥ 2.5, and QUICKI ≤ 0.33 were considered as the cut-off values in favor of IR (17, 18). BMI < 25 was considered normal. IR values were compared between the patients with BMI ≥ 25 and those with < 25. Also, SHBG ≥ 36 (nmol/L) was considered normal and data were compared between women with SHBG ≥ 36 and SHBG < 36 (nmol/L) (19). The values of hormones and TG were also compared between the mentioned groups. Previous studies showed that the results of HOMA-IR assessment were correlated significantly with the results of the clamp studies (20). Also, HOMA-IR had the highest sensitivity according to recent research (18). Therefore, we substituted HOMA-IR for the standard technique and compared the sensitivity, specificity, PPV, and NPV of the other methods to HOMA-IR.
- 4. Ethical considerations
All participants signed a written informed consent form before enrollment and after a complete explanation of the study design. The Medical Ethics Committee of Shiraz University of Medical Sciences approved the study protocol (Code: IR.SUMS.REC.1394.S612).
- 5. Statistical analysis
In this cross-sectional study, 74 PCOS women were enrolled by simple random sampling. Data are presented as mean ± SD and were analyzed using SPSS software version 19.0 (Statistical Package for the Social Sciences software, version 19.0, SPSS Inc., Chicago, IL, USA). T tests were used to compare the study parameters. P < 0.05 was considered significant. The predictive ability of covariates (whose p-value < 0.25 in t tests) on IR (HOMA-IR ≥ 2.5) was evaluated using logistic regression analysis.
3. Results
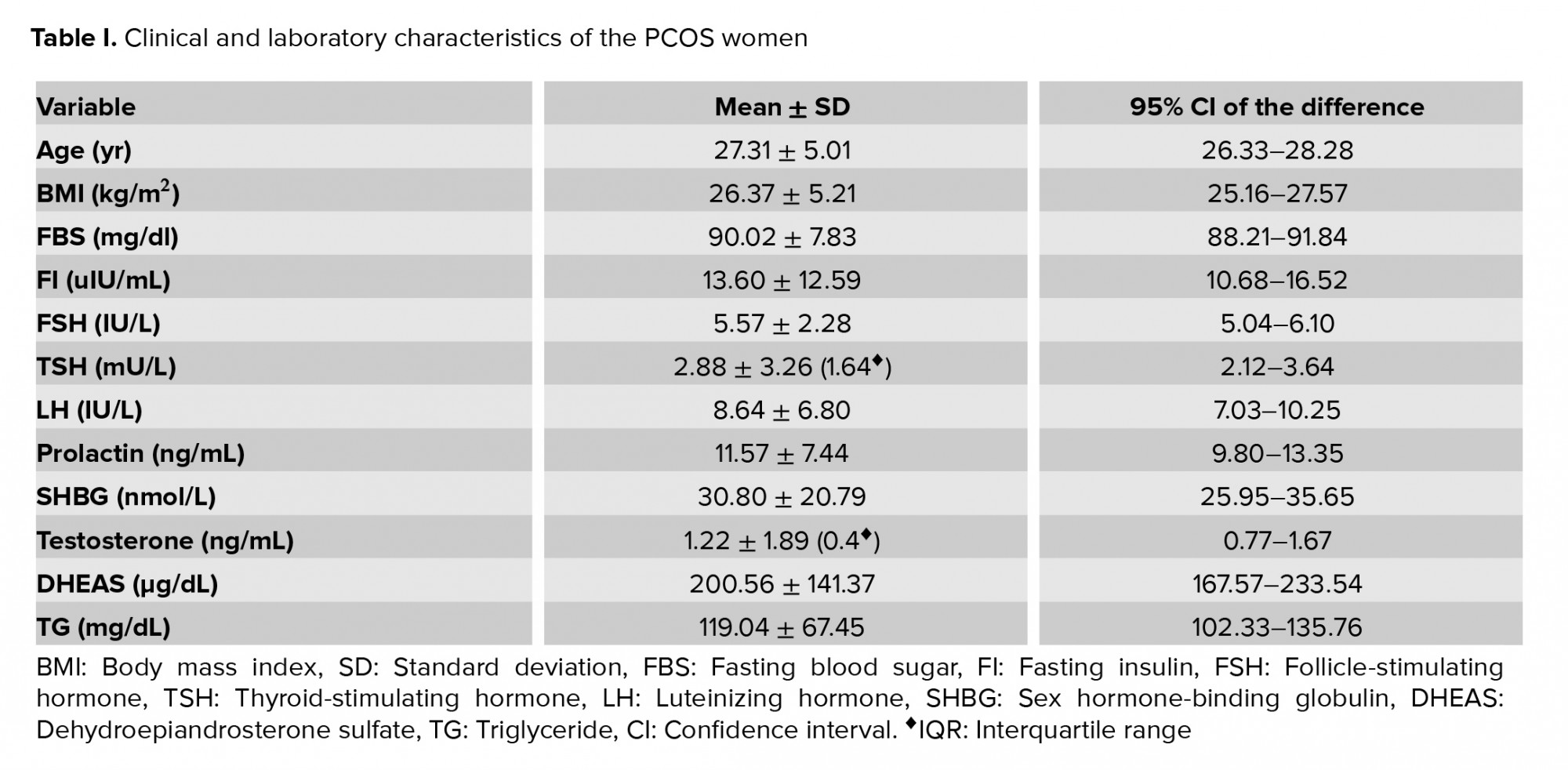
The demographic, clinical, and laboratory characteristics of the enrolled PCOS women are presented in Table I.
In this study, no significant correlation was observed between the BMI and IR in the PCOS patients. Table II presents a comparison of the hormonal profiles according to BMI and five indirect methods of IR.
TG was significantly higher in women with abnormal FBS (p = 0.002), QUICKI (p = 0.02), or HOMA-IR (p = 0.01). DHEAS was significantly higher among those with abnormal FI (p = 0.002). SHBG showed a significant negative association with FBS (p = 0.001).
HOMA-IR was applied as the standard test to determine IR. Table III shows the results of logistic regression analysis assessing the effectiveness of the covariates (whose p-value < 0.25 in t tests) including testosterone, SHBG, and TG in predicting the outcome of IR (if HOMA-IR ≥ 2.5 indicate IR). The results represented that the mentioned covariates were not significantly predictive of IR.
The measured variables and demographic data were classified according to the normal and abnormal SHBG levels, as presented in Table IV.
SHBG showed a significant negative association with TG (p = 0.02). The PCOS women with SHBG ≥ 36 (nmol/L) had statistically significant lower TG and higher FSH levels (p < 0.5). There were seven PCOS women with SHBG < 36 nmol/L, while all of the other measured variables were in the normal ranges.
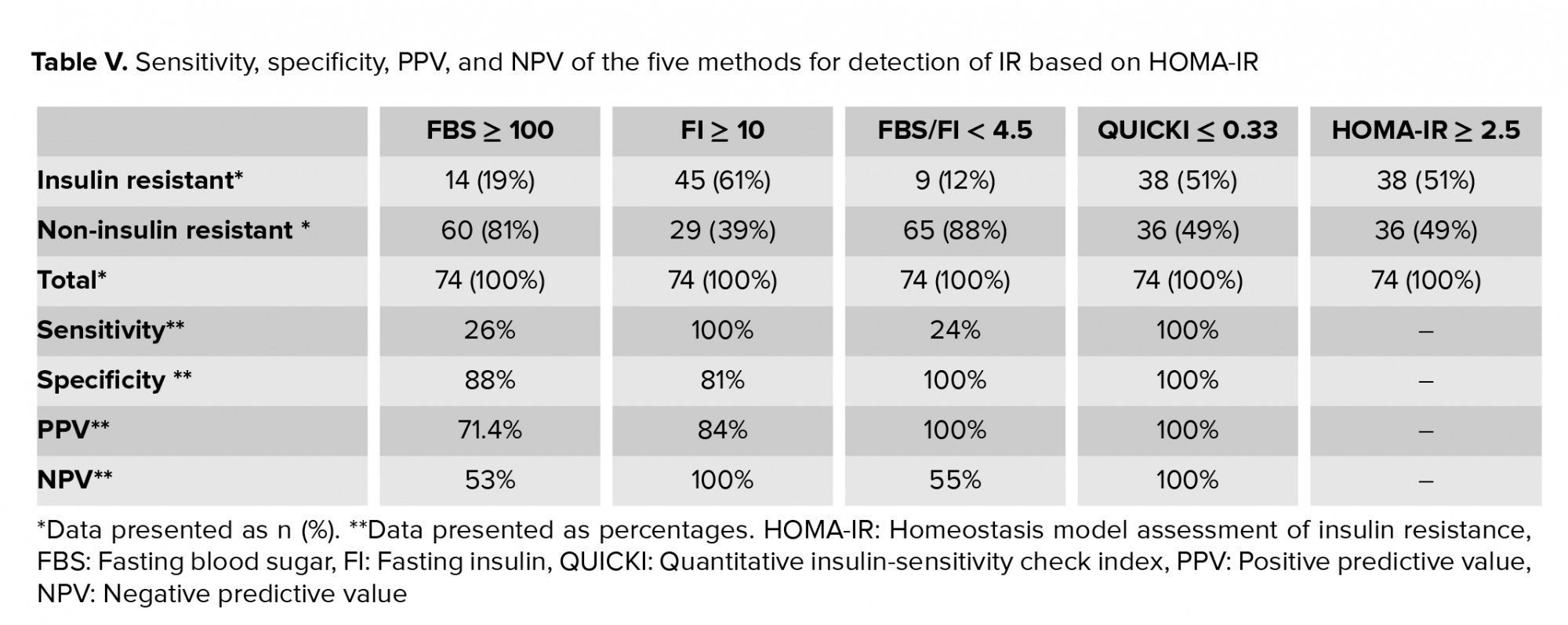
We compared the sensitivity, specificity, PPV, and NPV of FBS, FI, FBS/FI, and QUICKI to HOMA-IR (Table V).



4. Discussion
In this research, 74 PCOS women were studied for their serum SHBG, hormonal profiles, TG levels, and five indirect methods for the detection of IR. They were then classified according to their normal and abnormal BMI, IR, and SHBG levels, and the data were compared to detect any possible relationships. Also, sensitivity, specificity, PPV, and NPV of the five indirect methods of IR were calculated based on the HOMA-IR test.
Previous studies have shown BMI to be positively associated with IR and diabetes (21, 22). However, it has been showed that IR was not associated with waist circumference or BMI (23). Logically, we expected that the PCOS women with higher BMI would represent more IR. In this study, we observed higher mean levels of FBS, FI, and more other abnormal IR values among women with higher BMI but the differences were not statistically significant.
DHEAS is known as a general marker to diagnose extra amounts of adrenal precursor androgen (APA) in PCOS patients (24). Among the PCOS subjects of this study, we detected a significant association between DHEAS and FI levels (p = 0.002). We concluded that the higher levels of insulin could stimulate the production of APA leading to the increased levels of DHEAS.
We found that serum TG levels and IR have positive independent associations, so we think that the evaluation of TG might serve as a useful clinical biomarker to predict IR for PCOS patients to confirm the previous reports (15). The results of our study are in good agreement with the previous studies showing that TG has a significant correlation with IR detected by the indirect methods of QUICKI (p = 0.02), HOMA-IR (p = 0.01), and FBS (p = 0.002). On the other hand, lower SHBG levels were significantly associated with higher serum TG levels (p = 0.016) and FBS (p = 0.001) in our study. We know that SHBG binds the androgens with a high affinity to regulate the free sex hormones (25). Low levels of SHBG are associated with higher levels of free androgens that can manifest with hirsutism, acne, or irregular menstruations in PCOS patients (26). Although the specific role of SHBG in the glucose metabolism is not yet clear, several recent studies have implicated that the alterations in normal sex steroids physiology may have a role in the glucose homeostasis and low SHBG levels may precede the development of type-2 diabetes mellitus (27). In our study, the PCOS women with lower SHBG levels had higher mean FBS and TG levels, which is in good consistency with the hypothesis of the relationship between low SHBG with metabolic syndrome and abnormal glucose metabolism. SHBG ≥ 36 nmol/L was considered normal in this project (19). Out of the 74 PCOS patients enrolled in this study, 52 showed SHBG < 36 nmol/L. Interestingly, only seven PCOS women with normal IR, testosterone, and BMI showed abnormal SHBG < 36 nmol/L. The achieved results indicate that the measurement of SHBG might be a helpful biomarker for the diagnosis of PCOS and IR.
IR and hyperandrogenism play important roles in the metabolic features of PCOS and increase the risk of prediabetes state (28). PCOS women are prone to develop type-2 diabetes if ascertained lifestyle modifications are not adopted (29). Consistently, a balanced diet, weight loss, physical activity, and medications improve IR as well as prediabetes state (30). The American Diabetes Association (ADA) suggests an effective lifestyle modification to prevent type-2 diabetes for women who are diagnosed with prediabetes states (31). As IR affects a high percentage of PCOS women with no clear symptoms, a clinically easy-to-perform and practical test with high accuracy and sensitivity is required for earlier diagnosis of prediabetes conditions.
In this project, we compared the sensitivity, specificity, PPV, and NPV of the five indirect methods of IR and found that HOMA-IR, FI, and QUICKI had the highest NPV and sensitivity to reflect the potential prediabetes in PCOS women. In addition, HOMA-IR, QUICKI, and FBS/FI had the highest PPV and specificity. We believe that the performance of a test with a higher sensitivity helps for earlier diagnosis of prediabetes state and this knowledge accompanied by lifestyle changes protects the PCOS women from diabetes and other subsequent complications. We admit that absence of data from non-PCOS women, as a control group, is a limitation of this study design. In addition, it was not possible for us to perform HEC and FSIVGTT direct methods to detect accurate IR for comparison because of the complexity of the procedures.
5. Conclusion
SHBG had a significant negative association with FBS and TG. DHEAS displayed a significant positive association with FI. TG represented a strong positive relationship with HOMA-IR and FBS and a significant negative relationship with QUICKI and SHBG levels. Some PCOS patients expressed abnormal SHBG despite normal values of other studied variables. SHBG is speculated to be a potential biomarker to diagnose PCOS. FI and QUICKI had the highest NPV and sensitivity while FBS/FI and QUICKI had the highest PPV and specificity when considered HOMA-IR as a standard test.
Acknowledgements
This article has been partially extracted from the M.D. thesis by Dr. Niloofar Borzou and financially supported by the Vice Chancellor for Research of Shiraz University of Medical Sciences (grant number of 94-01-50-9099).
Conflicts of interest
The authors declare that they have no conflict of interest.
In this study, no significant correlation was observed between the BMI and IR in the PCOS patients. Table II presents a comparison of the hormonal profiles according to BMI and five indirect methods of IR.
TG was significantly higher in women with abnormal FBS (p = 0.002), QUICKI (p = 0.02), or HOMA-IR (p = 0.01). DHEAS was significantly higher among those with abnormal FI (p = 0.002). SHBG showed a significant negative association with FBS (p = 0.001).
HOMA-IR was applied as the standard test to determine IR. Table III shows the results of logistic regression analysis assessing the effectiveness of the covariates (whose p-value < 0.25 in t tests) including testosterone, SHBG, and TG in predicting the outcome of IR (if HOMA-IR ≥ 2.5 indicate IR). The results represented that the mentioned covariates were not significantly predictive of IR.
The measured variables and demographic data were classified according to the normal and abnormal SHBG levels, as presented in Table IV.
SHBG showed a significant negative association with TG (p = 0.02). The PCOS women with SHBG ≥ 36 (nmol/L) had statistically significant lower TG and higher FSH levels (p < 0.5). There were seven PCOS women with SHBG < 36 nmol/L, while all of the other measured variables were in the normal ranges.
We compared the sensitivity, specificity, PPV, and NPV of FBS, FI, FBS/FI, and QUICKI to HOMA-IR (Table V).





4. Discussion
In this research, 74 PCOS women were studied for their serum SHBG, hormonal profiles, TG levels, and five indirect methods for the detection of IR. They were then classified according to their normal and abnormal BMI, IR, and SHBG levels, and the data were compared to detect any possible relationships. Also, sensitivity, specificity, PPV, and NPV of the five indirect methods of IR were calculated based on the HOMA-IR test.
Previous studies have shown BMI to be positively associated with IR and diabetes (21, 22). However, it has been showed that IR was not associated with waist circumference or BMI (23). Logically, we expected that the PCOS women with higher BMI would represent more IR. In this study, we observed higher mean levels of FBS, FI, and more other abnormal IR values among women with higher BMI but the differences were not statistically significant.
DHEAS is known as a general marker to diagnose extra amounts of adrenal precursor androgen (APA) in PCOS patients (24). Among the PCOS subjects of this study, we detected a significant association between DHEAS and FI levels (p = 0.002). We concluded that the higher levels of insulin could stimulate the production of APA leading to the increased levels of DHEAS.
We found that serum TG levels and IR have positive independent associations, so we think that the evaluation of TG might serve as a useful clinical biomarker to predict IR for PCOS patients to confirm the previous reports (15). The results of our study are in good agreement with the previous studies showing that TG has a significant correlation with IR detected by the indirect methods of QUICKI (p = 0.02), HOMA-IR (p = 0.01), and FBS (p = 0.002). On the other hand, lower SHBG levels were significantly associated with higher serum TG levels (p = 0.016) and FBS (p = 0.001) in our study. We know that SHBG binds the androgens with a high affinity to regulate the free sex hormones (25). Low levels of SHBG are associated with higher levels of free androgens that can manifest with hirsutism, acne, or irregular menstruations in PCOS patients (26). Although the specific role of SHBG in the glucose metabolism is not yet clear, several recent studies have implicated that the alterations in normal sex steroids physiology may have a role in the glucose homeostasis and low SHBG levels may precede the development of type-2 diabetes mellitus (27). In our study, the PCOS women with lower SHBG levels had higher mean FBS and TG levels, which is in good consistency with the hypothesis of the relationship between low SHBG with metabolic syndrome and abnormal glucose metabolism. SHBG ≥ 36 nmol/L was considered normal in this project (19). Out of the 74 PCOS patients enrolled in this study, 52 showed SHBG < 36 nmol/L. Interestingly, only seven PCOS women with normal IR, testosterone, and BMI showed abnormal SHBG < 36 nmol/L. The achieved results indicate that the measurement of SHBG might be a helpful biomarker for the diagnosis of PCOS and IR.
IR and hyperandrogenism play important roles in the metabolic features of PCOS and increase the risk of prediabetes state (28). PCOS women are prone to develop type-2 diabetes if ascertained lifestyle modifications are not adopted (29). Consistently, a balanced diet, weight loss, physical activity, and medications improve IR as well as prediabetes state (30). The American Diabetes Association (ADA) suggests an effective lifestyle modification to prevent type-2 diabetes for women who are diagnosed with prediabetes states (31). As IR affects a high percentage of PCOS women with no clear symptoms, a clinically easy-to-perform and practical test with high accuracy and sensitivity is required for earlier diagnosis of prediabetes conditions.
In this project, we compared the sensitivity, specificity, PPV, and NPV of the five indirect methods of IR and found that HOMA-IR, FI, and QUICKI had the highest NPV and sensitivity to reflect the potential prediabetes in PCOS women. In addition, HOMA-IR, QUICKI, and FBS/FI had the highest PPV and specificity. We believe that the performance of a test with a higher sensitivity helps for earlier diagnosis of prediabetes state and this knowledge accompanied by lifestyle changes protects the PCOS women from diabetes and other subsequent complications. We admit that absence of data from non-PCOS women, as a control group, is a limitation of this study design. In addition, it was not possible for us to perform HEC and FSIVGTT direct methods to detect accurate IR for comparison because of the complexity of the procedures.
5. Conclusion
SHBG had a significant negative association with FBS and TG. DHEAS displayed a significant positive association with FI. TG represented a strong positive relationship with HOMA-IR and FBS and a significant negative relationship with QUICKI and SHBG levels. Some PCOS patients expressed abnormal SHBG despite normal values of other studied variables. SHBG is speculated to be a potential biomarker to diagnose PCOS. FI and QUICKI had the highest NPV and sensitivity while FBS/FI and QUICKI had the highest PPV and specificity when considered HOMA-IR as a standard test.
Acknowledgements
This article has been partially extracted from the M.D. thesis by Dr. Niloofar Borzou and financially supported by the Vice Chancellor for Research of Shiraz University of Medical Sciences (grant number of 94-01-50-9099).
Conflicts of interest
The authors declare that they have no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Biology
References
1. ESHRE Capri Workshop Group. Health and fertility in World Health Organization group 2 anovulatory women. Hum Reprod Update 2012; 18: 586-599. [DOI:10.1093/humupd/dms019] [PMID]
2. Al-Jefout M, Alnawaiseh N, Al-Qtaitat A. Insulin resistance and obesity among infertile women with different polycystic ovary syndrome phenotypes. Sci Rep 2017; 7: 1-9. [DOI:10.1038/s41598-017-05717-y] [PMID] [PMCID]
3. Baptiste CG, Battista MC, Trottier A, Baillargeon JP. Insulin and hyperandrogenism in women with polycystic ovary syndrome. J Steroid Biochem Mol Biol 2010; 122: 42-52. [DOI:10.1016/j.jsbmb.2009.12.010] [PMID] [PMCID]
4. Livadas S, Pappas C, Karachalios A, Marinakis E, Tolia N, Drakou M, et al. Prevalence and impact of hyperandrogenemia in 1,218 women with polycystic ovary syndrome. Endocrine 2014; 47: 631-638. [DOI:10.1007/s12020-014-0200-7] [PMID]
5. Deswal R, Yadav A, Dang AS. Sex hormone binding globulin-an important biomarker for predicting PCOS risk: A systematic review and meta-analysis. Syst Biol Reprod Med 2018; 64: 12-24. [DOI:10.1080/19396368.2017.1410591] [PMID]
6. Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: An endocrine society clinical practice guideline. J Clin Endocrinol Metab 2013; 98: 4565-4592. [DOI:10.1210/jc.2013-2350] [PMID] [PMCID]
7. Veltman-Verhulst SM, Goverde AJ, van Haeften TW, Fauser BC. Fasting glucose measurement as a potential first step screening for glucose metabolism abnormalities in women with anovulatory polycystic ovary syndrome. Hum Reprod 2013; 28: 2228-2234. [DOI:10.1093/humrep/det226] [PMID]
8. Trout KK, Homko C, Tkacs NC. Methods of measuring insulin sensitivity. Biol Res Nurs 2007; 8: 305-318. [DOI:10.1177/1099800406298775] [PMID]
9. Gutch M, Kumar S, Razi SM, Gupta KK, Gupta A. Assessment of insulin sensitivity/resistance. Indian J Endocrinol Metab 2015; 19: 160-164. [DOI:10.4103/2230-8210.146874] [PMID] [PMCID]
10. Henderson M, Rabasa-Lhoret R, Bastard JP, Chiasson JL, Baillargeon JP, Hanley J, et al. Measuring insulin sensitivity in youth: How do the different indices compare with the gold-standard method?. Diabetes Metab 2011; 37: 72-78. [DOI:10.1016/j.diabet.2010.06.008] [PMID]
11. Kite CS, Lahart IM, Afzal I, Broom D, Kyrou I, Randeva H, et al. Exercise interventions significantly reduce fasting insulin, but not fasting glucose, in women with polycystic ovary syndrome when compared with no intervention: A systematic review and meta-analysis. Diabetic Med 2018; 35: 60. 1-4.
12. Eftekhari MH, Ranjbar Zahedani M, Kohansal A. Comparison of metabolic syndrome components, inflammation and oxidative stress indices in normal weight obese and normal weight women: A case-control study. J Health Sci Surveillance Sys 2018; 6: 116-122.
13. Wu CZ, Lin JD, Hsia TL, Hsu CH, Hsieh CH, Chang JB, et al. Accurate method to estimate insulin resistance from multiple regression models using data of metabolic syndrome and oral glucose tolerance test. J Diabetes Investig 2014; 5: 290-296. [DOI:10.1111/jdi.12155] [PMID] [PMCID]
14. Cai J, Wu CH, Zhang Y, Wang YY, Xu WD, Lin TC, et al. High-free androgen index is associated with increased risk of non-alcoholic fatty liver disease in women with polycystic ovary syndrome, independent of obesity and insulin resistance. Int J Obes 2017; 41: 1341-1347. [DOI:10.1038/ijo.2017.116] [PMID]
15. Park SY, Cho YJ, Lee SR, Chung H, Jeong K. Triglyceride is a useful surrogate marker for insulin resistance in Korean women with polycystic ovary syndrome. Yonsei Med J 2015; 56: 785-792. [DOI:10.3349/ymj.2015.56.3.785] [PMID] [PMCID]
16. Group REASPCW. Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19: 41-47. [DOI:10.1093/humrep/deh098] [PMID]
17. Majid H, Masood Q, Khan AH. Homeostatic model assessment for insulin resistance (HOMA-IR): A better marker for evaluating insulin resistance than fasting insulin in women with polycystic ovarian syndrome. J Coll Physicians Surg Pak 2017; 27: 123-126.
18. Namvar Jahromi B, Dabaghmanesh MH, Parsanezhad ME, Fatehpoor F. Association of leptin and insulin resistance in PCOS: A case-controlled study. Int J Reprod Biomed 2017; 15: 423-428. [DOI:10.29252/ijrm.15.7.423]
19. Abu-Hijleh TM, Gammoh E, Al-Busaidi AS, Malalla ZH, Madan S, Mahmood N, et al. Common variants in the sex hormone-binding globulin (SHBG) gene influence SHBG levels in women with polycystic ovary syndrome. Ann Nutr Metab 2016; 68: 66-74. [DOI:10.1159/000441570] [PMID]
20. Barseem NF, Helwa MA. Homeostatic model assessment of insulin resistance as a predictor of metabolic syndrome: Consequences of obesity in children and adolescents. Egypt Pediatr Assoc Gazette 2015; 63: 19-24. [DOI:10.1016/j.epag.2014.12.001]
21. Okura T, Nakamura R, Fujioka Y, Kawamoto-Kitao S, Ito Y, Matsumoto K, et al. Body mass index≥ 23 is a risk factor for insulin resistance and diabetes in Japanese people: A brief report. PLoS One 2018; 13: e0201052. [DOI:10.1371/journal.pone.0201052] [PMID] [PMCID]
22. Chung JO, Cho DH, Chung DJ, Chung MY. Associations among body mass index, insulin resistance, and pancreatic β-cell function in Korean patients with new-onset type 2 diabetes. Korean J Intern Med 2012; 27: 66-71. [DOI:10.3904/kjim.2012.27.1.66] [PMID] [PMCID]
23. Gonzalez-Cantero J, Martin-Rodriguez JL, Gonzalez-Cantero A, Arrebola JP, Gonzalez-Calvin JL. Insulin resistance in lean and overweight non-diabetic Caucasian adults: Study of its relationship with liver triglyceride content, waist circumference and BMI. PLoS One 2018; 13: e0192663. [DOI:10.1371/journal.pone.0192663] [PMID] [PMCID]
24. Saito K, Matsuzaki T, Iwasa T, Miyado M, Saito H, Hasegawa T, et al. Steroidogenic pathways involved in androgen biosynthesis in eumenorrheic women and patients with polycystic ovary syndrome. J Steroid Biochem Mol Biol 2016; 158: 31-37. [DOI:10.1016/j.jsbmb.2016.02.010] [PMID]
25. Laurent MR, Hammond GL, Blokland M, Jardí F, Antonio L, Dubois V, et al. Sex hormone-binding globulin regulation of androgen bioactivity in vivo: Validation of the free hormone hypothesis. Sci Rep 2016; 6: 35539. 1-12. [DOI:10.1038/srep35539] [PMID] [PMCID]
26. Pavičić Baldani D, Škrgatić L, Bukvić Mokos Z, Trgovčić I. Hyperandrogenemia association with acne and hirsutism severity in Croatian women with polycystic ovary syndrome. Acta Dermatovenerol Croat 2013; 21: 105-112.
27. Le TN, Nestler JE, Strauss JF, Wickham EP. Sex hormone-binding globulin and type 2 diabetes mellitus. Trends Endocrinol Metab 2012; 23: 32-40. [DOI:10.1016/j.tem.2011.09.005] [PMID] [PMCID]
28. Stepto NK, Cassar S, Joham AE, Hutchison SK, Harrison CL, Goldstein RF, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod 2013; 28: 777-784. [DOI:10.1093/humrep/des463] [PMID]
29. Watson CS. Prediabetes: Screening, diagnosis and intervention. J Nurse Practitioner 2017; 13: 216-221. [DOI:10.1016/j.nurpra.2016.08.005]
30. Naderpoor N, Shorakae S, De Courten B, Misso ML, Moran LJ, Teede HJ. Metformin and lifestyle modification in polycystic ovary syndrome: Systematic review and meta-analysis. Hum Reprod Update 2015; 21: 560-574. [DOI:10.1093/humupd/dmv025] [PMID]
31. Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the study of diabetes (EASD). Diabetologia 2018; 61: 2461-2498. [DOI:10.1007/s00125-018-4729-5] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





