Thu, Apr 25, 2024
[Archive]
Volume 20, Issue 8 (August 2022)
IJRM 2022, 20(8): 643-650 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Seyedoshohadaei F, Abbasi S, Rezaie M, Allahvaisi A, Rezaie M J, Soufizade N et al . Myo-inositol effect on pregnancy outcomes in infertile women undergoing in vitro fertilization/intracytoplasmic sperm injection: A double-blind RCT. IJRM 2022; 20 (8) :643-650
URL: http://ijrm.ir/article-1-2029-en.html
URL: http://ijrm.ir/article-1-2029-en.html
Fariba Seyedoshohadaei1 

 , Shahin Abbasi *
, Shahin Abbasi * 

 2, Masomeh Rezaie1
2, Masomeh Rezaie1 

 , Azra Allahvaisi3
, Azra Allahvaisi3 

 , Mohammad Jafar Rezaie3
, Mohammad Jafar Rezaie3 

 , Nasrin Soufizade4
, Nasrin Soufizade4 

 , Khaled Rahmani5
, Khaled Rahmani5 




 , Shahin Abbasi *
, Shahin Abbasi * 

 2, Masomeh Rezaie1
2, Masomeh Rezaie1 

 , Azra Allahvaisi3
, Azra Allahvaisi3 

 , Mohammad Jafar Rezaie3
, Mohammad Jafar Rezaie3 

 , Nasrin Soufizade4
, Nasrin Soufizade4 

 , Khaled Rahmani5
, Khaled Rahmani5 


1- Department of Obstetrics and Gynecology, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran. Infertility Treatment Center of Besat Hospital, Kurdistan University of Medical Sciences, Sanandaj, Iran.
2- Department of Obstetrics and Gynecology, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran. , s.abbasi@muk.ac.ir
3- Infertility Treatment Center of Besat Hospital, Kurdistan University of Medical Sciences, Sanandaj, Iran. Department of Anatomy, School of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran.
4- Department of Obstetrics and Gynecology, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran.
5- Liver and Digestive Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences, Sanandaj, Iran.
2- Department of Obstetrics and Gynecology, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran. , s.abbasi@muk.ac.ir
3- Infertility Treatment Center of Besat Hospital, Kurdistan University of Medical Sciences, Sanandaj, Iran. Department of Anatomy, School of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran.
4- Department of Obstetrics and Gynecology, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran.
5- Liver and Digestive Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences, Sanandaj, Iran.
Full-Text [PDF 301 kb]
(590 Downloads)
| Abstract (HTML) (1234 Views)
1. Introduction
Nowadays assisted reproductive techniques (ARTs) are used to achieve fertility in infertile people. In vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) are effective ARTs (1). IVF and ICSI are the most appropriate treatments in infertility clinics. However, one of the most important problems of these methods is the low rate of pregnancy success (2).
Despite significant advances in ARTs, there are still problems in achieving desirable results through IVF and ICSI techniques. Using supplements in ARTs can be very helpful in increasing pregnancy rates (3).
The balance between the production and clearance of reactive oxygen species is critical to the health of the female reproductive system. Antioxidants are compounds that can decrease oxidative damage in infertile women (4).
Myo-inositol is a C6 sugar alcohol belonging to the B-group vitamins (5). Myo-inositol plays a key role in all aspects of cell physiology including lipid synthesis, cell morphogenesis, cell membrane structures, and cell membrane functions (6, 7).
Studies on inositol and its isoforms (particularly Myo-inositol) in IVF have demonstrated that this molecule reduces insulin resistance and improves ovarian function, oocyte quality, and embryo pregnancy rates (8). The inositol 1,4,5-triphosphate receptor channel is found in mammalian cells which are intracellular mediators for the release of calcium (9, 10). Evidence suggests that this receptor channel has a key role in regulating calcium signaling in the oocytes of mammals (11). The mechanisms of calcium release during oogenesis play an important role in the success of fertilization. Several reports point to the possible role of inositol phospholipids-calcium in oocyte development (12, 13).
The goal of this research was to assess the impact of Myo-inositol on pregnancy success in infertile women candidates for IVF and ICSI in the Infertility Treatment Center, Besat hospital, Kurdistan University of Medical Sciences, Sanandaj, Iran.
2. Materials and Methods
2.1. Study design, participants, and setting
This double-blind, randomized clinical trial was conducted in 70 infertile women referred to the Infertility Treatment Center, Besat hospital, Sanandaj, Iran, from May 2019 to September 2019 for IVF/ICSI cycles. The women were randomly divided into 2 intervention and control groups using permuted block randomization (n = 30/each).
Our inclusion criteria were age 20-40 yr, infertility for at least 1 year, candidates for IVF/ICSI treatments, not used contraception methods for at least 1 yr ago, agonist cycles, and having a regular menstrual cycle (24-35 days). All women with any underling diseses such as metabolic or endocrine disorders, uterine anomaly, frozen embryo transfer cycle and history of recurrent miscarriages were excluded.
The intervention group received 2000 mg of Myo-inositol (Inofolic sachet, Lo. Li Pharma. Tehran, Iran) plus 200 mcg of folic acid (Raha pharmaceutical Co., Isfahan, Iran) twice a day for 2 months from the third day of cycle until the end of the second month. The control group received 200 mcg of folic acid (Raha pharmaceutical Co., Isfahan, Iran) twice a day for 2 months from the 3rd day of cycle until the end of the 2nd month.
2.2. The stimulation protocol and the IVF/ICSI cycles
The ovarian hyperstimulation protocol was performed for all the participants in the studied groups according to the gonadotropin-releasing hormone agonist protocol.
Low dose oral contraceptive pills were administered from the 3rd day to the 23rd day of the 1st menstrual cycle. Sub-cutaneous injections of the gonadotropin-releasing hormone analog (Buserelin, Cinna Fact, Cinna Gen, Iran) were started on day 21 of the 1st menstrual cycle with a dose of 0.5 mg and continued until the 2nd day of the 2nd cycle. The dose was gradually reduced to 0.25 mg until oocyte retrieval. Ovulation induction was started from the 3rd day of the 2nd cycle with a recombinant follicle-stimulating hormone (Cinnal-F, Cinna Gen, Iran) injection (150-300 IU) based on the follicle-stimulating hormone and anti-Mullerian hormone levels and vaginal ultrasound monitoring. After stimulating the ovaries, 10000 IU of human chorionic gonadotropin (HCG; Iran Hormone Pharmacy, Iran) was injected when 2 follicles with the size of ≥ 18 mm were detected. After 34-36 hr, ultrasound-guided oocyte retrieval was performed under spinal anesthesia.
The fertilized oocytes were investigated after 24 hr. After 3 days, the quality of the embryos was assessed. Participants received 2-3 high-quality embryos. The luteal phase was supported with the daily administration of 400 mg of progesterone suppository rectally and vaginally or 50 mg progesterone ampoules (Iran Hormone Pharmacy, Iran) till the 12th wk of gestation.
2.3. Outcomes and data collection
The number of clinical pregnancy, miscarriage, preterm delivery, and live birth in both study groups were recorded and compared. Furthermore, the total number of oocytes, meiosis II (MII) oocytes, germinal vesicle oocytes, degenerated oocytes, and the quality of the embryos were evaluated in the 2 groups.
Clinical pregnancy was defined as a fetal cardiac activity in the 6th-7th wk of pregnancy. Pregnancy loss earlier than the 20th wk of gestation was defined as miscarriage. Preterm delivery was defined as the birth of a baby earlier than 37 wk gestation. Live birth was defined as a birth of a live infant ≥ 24 wk of gestation that showed signs of life.
The study was initially designed as a double -blind trial. Participants and physicians who evaluated the outcomes were unaware of the random assignment of individuals to control and intervention groups.
2.5. Ethical considerations
This project was conducted with the permission of the Ethics Committee of the Kurdistan University of Medical Sciences, Sanandaj, Iran (Code: IR.MUK.REC.1397.236).In addition, those who agreed to participate in this study signed awritten. Study participants were assured that their data would remain confidential to researchers.
2.6. Statistical analysis
The data from this research were analyzed through the Statistical Package for the Social Sciences v.21.0 software (SPSS Inc., Chicago, IL, United States), using the Mann-Whitney U test, the student’s t test, and the Chi-square test. Logistic regression was used to predict the results of ICSI. A p-value < 0.05 was considered as statistically significant.
3. Results
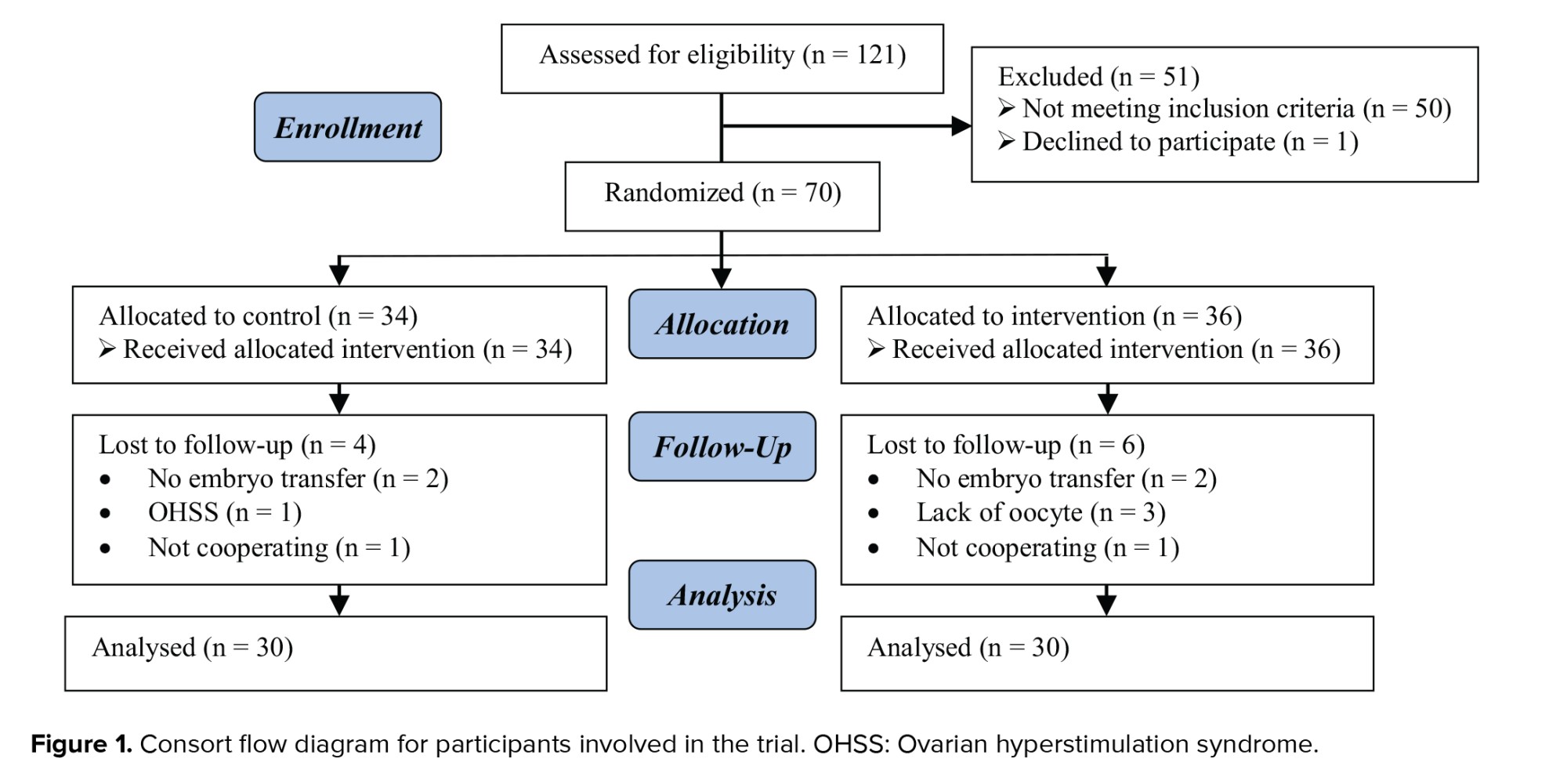
Initially, the 121 women were evaluated based on our inclusion criteria. Out of them, 50 women did not meet the inclusion criteria. 1 women was also excluded due to dissatisfaction to continue participating in the study. Finally, 70 women were assigned to 2 groups (36 and 34 women in intervention and control group, respectively). By the end of the study follow-up, 10 women (6 women in intervention and 4 women in control group) were excluded due to dissatisfaction to continue participating in the study or other reasons (not embryo transfer and OHSS). Finally, 60 women (30 women in each group) were remained in the study and entered into analysis (Figure 1).
As shown in table I, there were no significant differences between the 2 groups regarding the demographic characteristics including age, body mass index, familial history of infertility and miscarriage, irregular menstrual cycle, infertility type, and duration of infertility. In addition, the levels of follicle-stimulating hormone, luteinizing hormone, anti-Mullerian hormone, estrogen, and progesterone had no significant differences between the 2 groups (Table I).
Table II indicates that the mean numbers of oocytes, MII oocytes, and 2 pronuclear embryos were significantly higher in the intervention group than in the control group. In addition, the numbers of degenerated oocytes and grade A and B embryos were higher in the intervention group than in the controls. However, these differences were not statistically significant.
Table III shows the results of the pregnancy outcomes in the 2 study groups. The number of clinical pregnancies in the intervention group was significantly higher than in the control group (p = 0.04). The number of women who had live births in the intervention group was also higher than in the control group (p = 0.04). The pregnancy rate in the control group was lower than that of the intervention group. However, this difference was not significant. The miscarriage and preterm delivery rates did not differ between the 2 groups (Table III).



Infertility imposes a great deal of psychological and financial burden on the cases and their family. A failure of specialized and expensive treatments may result in negative psychological effects on couples. Despite the expansion of experience in ARTs and significant advancements in infertility treatment, the failure of fertility therapy is a major challenge (14). Oocyte quality is the main predictor of the success rate in IVF (15). Various studies have investigated ways for increasing the quality of oocytes and embryos (16, 17). The normal function of the ovaries is of great importance for the health of the reproductive tract. Antioxidant compounds are defensive barriers that maintain the active oxygen species balance. Disruption of the antioxidant balance can interfere with oocyte maturation, ovulation, fertilization, implantation, and embryo development (17). A study reported that compounds such as Myo-inositol could reduce oxidative stress by enhancing the cellular antioxidant defense (16). The effectiveness of inositol in modifying the process of ovulation has been extensively studied (18, 19).
The findings of a previous study showed that during IVF, pretreatment with Myo-inositol could help to improve the quality of the oocytes and embryos (20). Increased Myo-inositol levels in the follicular fluid can be effective in promoting follicle maturation and the development of high-quality oocytes (18). The results of the current study indicated that Myo-inositol enhanced the total number of oocytes and MII oocytes in women candidates for IVF/ICSI. Similar to our results, a pilot study of a prospective controlled observational trial demonstrated that Myo-inositol could increase the success rate of IVF in poor responders to gonadotropins with low-quality oocytes by increasing the recovery of MII oocytes and ovarian hypersensitivity to gonadotropins (21).
The findings of our study suggested that Myo-inositol affected the maturation of oocytes: it improved the fertilization rate (2 pronuclear embryos) and grade A embryos in the intervention group, and was associated with higher clinical pregnancy and live birth rates as compared with the control group. It should be noted that, except for secondary infertility causes, the 2 groups were homogeneous in terms of causes of infertility and all demographic characteristics (p > 0.05). The heterogeneity related to the control group causes may be due to the differences in using fresh (22 cases in the intervention group and 17 cases in the control group) vs. frozen (n = embryos in the groups (a higher proportion of women in the intervention group used fresh embryos that in the control group). Another reason maybe because of the effect of folic acid on the endometrium and in improving implantation.
The rates of preterm delivery and miscarriage in the intervention group were higher than in the control group. However, in general, most research has shown the beneficial effects of supplements on the outcomes of IVF treatment (21).
A meta-analysis that included 7 articles and 935 subjects, showed promising effects of Myo-inositol in promoting ARTs. Pretreatment with Myo-inositol increased the clinical pregnancy rate by 6.13% and also reduced the miscarriage rate by 27.08%. However, in contrast to these results, we found no significant effect of Myo-inositol in reducing miscarriage in the intervention group. Although the effects of Myo-inositol in infertile women undergoing ICSI or IVF and embryo transfer are promising, the optimal dose of inositol and the type of isomer have not been defined yet. Therefore, the effects of different genera and doses should be considered (22).
The results of this study showed that 4000 mg of Myo-inositol plus 400 mcg of folic acid can improve the rates of clinical pregnancy and live birth in IVF/ICSI cycles. A limited number of articles has shown similar results using 2000 mg. In addition, it has been reported that in women with poor oocyte quality, Myo-inositol and folic acid can improve pregnancy outcomes (23).
5. Conclusion
We can conclude that Myo-inositol, an antioxidant compound, can increase the pregnancy success rate in IVF/ICSI cycles, likely by improving the quality of the oocytes and embryos.
Acknowledgments
This research financially supported by the Kurdistan University of Medical Sciences, Sanandaj, Iran.
Conflict of Interest
The authors declare that there are no conflict of interest.
Full-Text: (149 Views)
1. Introduction
Nowadays assisted reproductive techniques (ARTs) are used to achieve fertility in infertile people. In vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) are effective ARTs (1). IVF and ICSI are the most appropriate treatments in infertility clinics. However, one of the most important problems of these methods is the low rate of pregnancy success (2).
Despite significant advances in ARTs, there are still problems in achieving desirable results through IVF and ICSI techniques. Using supplements in ARTs can be very helpful in increasing pregnancy rates (3).
The balance between the production and clearance of reactive oxygen species is critical to the health of the female reproductive system. Antioxidants are compounds that can decrease oxidative damage in infertile women (4).
Myo-inositol is a C6 sugar alcohol belonging to the B-group vitamins (5). Myo-inositol plays a key role in all aspects of cell physiology including lipid synthesis, cell morphogenesis, cell membrane structures, and cell membrane functions (6, 7).
Studies on inositol and its isoforms (particularly Myo-inositol) in IVF have demonstrated that this molecule reduces insulin resistance and improves ovarian function, oocyte quality, and embryo pregnancy rates (8). The inositol 1,4,5-triphosphate receptor channel is found in mammalian cells which are intracellular mediators for the release of calcium (9, 10). Evidence suggests that this receptor channel has a key role in regulating calcium signaling in the oocytes of mammals (11). The mechanisms of calcium release during oogenesis play an important role in the success of fertilization. Several reports point to the possible role of inositol phospholipids-calcium in oocyte development (12, 13).
The goal of this research was to assess the impact of Myo-inositol on pregnancy success in infertile women candidates for IVF and ICSI in the Infertility Treatment Center, Besat hospital, Kurdistan University of Medical Sciences, Sanandaj, Iran.
2. Materials and Methods
2.1. Study design, participants, and setting
This double-blind, randomized clinical trial was conducted in 70 infertile women referred to the Infertility Treatment Center, Besat hospital, Sanandaj, Iran, from May 2019 to September 2019 for IVF/ICSI cycles. The women were randomly divided into 2 intervention and control groups using permuted block randomization (n = 30/each).
Our inclusion criteria were age 20-40 yr, infertility for at least 1 year, candidates for IVF/ICSI treatments, not used contraception methods for at least 1 yr ago, agonist cycles, and having a regular menstrual cycle (24-35 days). All women with any underling diseses such as metabolic or endocrine disorders, uterine anomaly, frozen embryo transfer cycle and history of recurrent miscarriages were excluded.
The intervention group received 2000 mg of Myo-inositol (Inofolic sachet, Lo. Li Pharma. Tehran, Iran) plus 200 mcg of folic acid (Raha pharmaceutical Co., Isfahan, Iran) twice a day for 2 months from the third day of cycle until the end of the second month. The control group received 200 mcg of folic acid (Raha pharmaceutical Co., Isfahan, Iran) twice a day for 2 months from the 3rd day of cycle until the end of the 2nd month.
2.2. The stimulation protocol and the IVF/ICSI cycles
The ovarian hyperstimulation protocol was performed for all the participants in the studied groups according to the gonadotropin-releasing hormone agonist protocol.
Low dose oral contraceptive pills were administered from the 3rd day to the 23rd day of the 1st menstrual cycle. Sub-cutaneous injections of the gonadotropin-releasing hormone analog (Buserelin, Cinna Fact, Cinna Gen, Iran) were started on day 21 of the 1st menstrual cycle with a dose of 0.5 mg and continued until the 2nd day of the 2nd cycle. The dose was gradually reduced to 0.25 mg until oocyte retrieval. Ovulation induction was started from the 3rd day of the 2nd cycle with a recombinant follicle-stimulating hormone (Cinnal-F, Cinna Gen, Iran) injection (150-300 IU) based on the follicle-stimulating hormone and anti-Mullerian hormone levels and vaginal ultrasound monitoring. After stimulating the ovaries, 10000 IU of human chorionic gonadotropin (HCG; Iran Hormone Pharmacy, Iran) was injected when 2 follicles with the size of ≥ 18 mm were detected. After 34-36 hr, ultrasound-guided oocyte retrieval was performed under spinal anesthesia.
The fertilized oocytes were investigated after 24 hr. After 3 days, the quality of the embryos was assessed. Participants received 2-3 high-quality embryos. The luteal phase was supported with the daily administration of 400 mg of progesterone suppository rectally and vaginally or 50 mg progesterone ampoules (Iran Hormone Pharmacy, Iran) till the 12th wk of gestation.
2.3. Outcomes and data collection
The number of clinical pregnancy, miscarriage, preterm delivery, and live birth in both study groups were recorded and compared. Furthermore, the total number of oocytes, meiosis II (MII) oocytes, germinal vesicle oocytes, degenerated oocytes, and the quality of the embryos were evaluated in the 2 groups.
Clinical pregnancy was defined as a fetal cardiac activity in the 6th-7th wk of pregnancy. Pregnancy loss earlier than the 20th wk of gestation was defined as miscarriage. Preterm delivery was defined as the birth of a baby earlier than 37 wk gestation. Live birth was defined as a birth of a live infant ≥ 24 wk of gestation that showed signs of life.
The study was initially designed as a double -blind trial. Participants and physicians who evaluated the outcomes were unaware of the random assignment of individuals to control and intervention groups.
2.5. Ethical considerations
This project was conducted with the permission of the Ethics Committee of the Kurdistan University of Medical Sciences, Sanandaj, Iran (Code: IR.MUK.REC.1397.236).In addition, those who agreed to participate in this study signed awritten. Study participants were assured that their data would remain confidential to researchers.
2.6. Statistical analysis
The data from this research were analyzed through the Statistical Package for the Social Sciences v.21.0 software (SPSS Inc., Chicago, IL, United States), using the Mann-Whitney U test, the student’s t test, and the Chi-square test. Logistic regression was used to predict the results of ICSI. A p-value < 0.05 was considered as statistically significant.
3. Results
Initially, the 121 women were evaluated based on our inclusion criteria. Out of them, 50 women did not meet the inclusion criteria. 1 women was also excluded due to dissatisfaction to continue participating in the study. Finally, 70 women were assigned to 2 groups (36 and 34 women in intervention and control group, respectively). By the end of the study follow-up, 10 women (6 women in intervention and 4 women in control group) were excluded due to dissatisfaction to continue participating in the study or other reasons (not embryo transfer and OHSS). Finally, 60 women (30 women in each group) were remained in the study and entered into analysis (Figure 1).
As shown in table I, there were no significant differences between the 2 groups regarding the demographic characteristics including age, body mass index, familial history of infertility and miscarriage, irregular menstrual cycle, infertility type, and duration of infertility. In addition, the levels of follicle-stimulating hormone, luteinizing hormone, anti-Mullerian hormone, estrogen, and progesterone had no significant differences between the 2 groups (Table I).
Table II indicates that the mean numbers of oocytes, MII oocytes, and 2 pronuclear embryos were significantly higher in the intervention group than in the control group. In addition, the numbers of degenerated oocytes and grade A and B embryos were higher in the intervention group than in the controls. However, these differences were not statistically significant.
Table III shows the results of the pregnancy outcomes in the 2 study groups. The number of clinical pregnancies in the intervention group was significantly higher than in the control group (p = 0.04). The number of women who had live births in the intervention group was also higher than in the control group (p = 0.04). The pregnancy rate in the control group was lower than that of the intervention group. However, this difference was not significant. The miscarriage and preterm delivery rates did not differ between the 2 groups (Table III).




4. Discussion
In the present study, administration of myo-inositol in women candidates for IVF/ICSI was associated with a higher total number of oocytes and a higher number of M II oocytes. In addition, the clinical pregnancy and live birth rates were also higher in these women.Infertility imposes a great deal of psychological and financial burden on the cases and their family. A failure of specialized and expensive treatments may result in negative psychological effects on couples. Despite the expansion of experience in ARTs and significant advancements in infertility treatment, the failure of fertility therapy is a major challenge (14). Oocyte quality is the main predictor of the success rate in IVF (15). Various studies have investigated ways for increasing the quality of oocytes and embryos (16, 17). The normal function of the ovaries is of great importance for the health of the reproductive tract. Antioxidant compounds are defensive barriers that maintain the active oxygen species balance. Disruption of the antioxidant balance can interfere with oocyte maturation, ovulation, fertilization, implantation, and embryo development (17). A study reported that compounds such as Myo-inositol could reduce oxidative stress by enhancing the cellular antioxidant defense (16). The effectiveness of inositol in modifying the process of ovulation has been extensively studied (18, 19).
The findings of a previous study showed that during IVF, pretreatment with Myo-inositol could help to improve the quality of the oocytes and embryos (20). Increased Myo-inositol levels in the follicular fluid can be effective in promoting follicle maturation and the development of high-quality oocytes (18). The results of the current study indicated that Myo-inositol enhanced the total number of oocytes and MII oocytes in women candidates for IVF/ICSI. Similar to our results, a pilot study of a prospective controlled observational trial demonstrated that Myo-inositol could increase the success rate of IVF in poor responders to gonadotropins with low-quality oocytes by increasing the recovery of MII oocytes and ovarian hypersensitivity to gonadotropins (21).
The findings of our study suggested that Myo-inositol affected the maturation of oocytes: it improved the fertilization rate (2 pronuclear embryos) and grade A embryos in the intervention group, and was associated with higher clinical pregnancy and live birth rates as compared with the control group. It should be noted that, except for secondary infertility causes, the 2 groups were homogeneous in terms of causes of infertility and all demographic characteristics (p > 0.05). The heterogeneity related to the control group causes may be due to the differences in using fresh (22 cases in the intervention group and 17 cases in the control group) vs. frozen (n = embryos in the groups (a higher proportion of women in the intervention group used fresh embryos that in the control group). Another reason maybe because of the effect of folic acid on the endometrium and in improving implantation.
The rates of preterm delivery and miscarriage in the intervention group were higher than in the control group. However, in general, most research has shown the beneficial effects of supplements on the outcomes of IVF treatment (21).
A meta-analysis that included 7 articles and 935 subjects, showed promising effects of Myo-inositol in promoting ARTs. Pretreatment with Myo-inositol increased the clinical pregnancy rate by 6.13% and also reduced the miscarriage rate by 27.08%. However, in contrast to these results, we found no significant effect of Myo-inositol in reducing miscarriage in the intervention group. Although the effects of Myo-inositol in infertile women undergoing ICSI or IVF and embryo transfer are promising, the optimal dose of inositol and the type of isomer have not been defined yet. Therefore, the effects of different genera and doses should be considered (22).
The results of this study showed that 4000 mg of Myo-inositol plus 400 mcg of folic acid can improve the rates of clinical pregnancy and live birth in IVF/ICSI cycles. A limited number of articles has shown similar results using 2000 mg. In addition, it has been reported that in women with poor oocyte quality, Myo-inositol and folic acid can improve pregnancy outcomes (23).
5. Conclusion
We can conclude that Myo-inositol, an antioxidant compound, can increase the pregnancy success rate in IVF/ICSI cycles, likely by improving the quality of the oocytes and embryos.
Acknowledgments
This research financially supported by the Kurdistan University of Medical Sciences, Sanandaj, Iran.
Conflict of Interest
The authors declare that there are no conflict of interest.
Type of Study: Original Article |
Subject:
Fertility & Infertility
References
1. Ashrafi M, Jahanian Sadatmahalleh Sh, Akhoond MR, Ghaffari F, Zolfaghari Z. ICSI outcome in infertile couples with different causes of infertility: A cross-sectional study. Int J Fertil Steril 2013; 7: 88-95.
2. Andersen AN, Goossens V, Gianaroli L, Felberbaum R, de Mouzon J, Nygren KG. Assisted reproductive technology in Europe, 2003: Results generated from European registers by ESHRE. Hum Reprod 2007; 22: 1513-1525. [DOI:10.1093/humrep/dem053] [PMID]
3. Oehninger S, Gosden RG. Should ICSI be the treatment of choice for all cases of in-vitro conception? No, not in light of the scientific data. Hum Reprod 2002; 17: 2237-2242. [DOI:10.1093/humrep/17.9.2237] [PMID]
4. Showell MG, Mackenzie‐Proctor R, Jordan V, Hart RJ. Antioxidants for female subfertility. Cochrane Database Syst Rev 2017; 7: CD007807. [DOI:10.1002/14651858.CD007807.pub3]
5. Irvine RF. A short history of inositol lipids. J Lipid Res 2016; 57: 1987-1994. [DOI:10.1194/jlr.R071712] [PMID] [PMCID]
6. Chhetri DR. Myo-Inositol and its derivatives: Their emerging role in the treatment of human diseases. Front Pharmacol 2019; 10: 1172. [DOI:10.3389/fphar.2019.01172] [PMID] [PMCID]
7. Croze ML, Soulage CO. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013; 95: 1811-1827. [DOI:10.1016/j.biochi.2013.05.011] [PMID]
8. Simi G, Genazzani AR, Obino MER, Papini F, Pinelli S, Cela V, et al. Inositol and in vitro fertilization with embryo transfer. Int J Endocrinol 2017; 2017: 5469409. [DOI:10.1155/2017/5469409] [PMID] [PMCID]
9. Whitaker M. Calcium signalling in early embryos. Philos Trans R Soc Lond B Biol Sci 2008; 363: 1401-1418. [DOI:10.1098/rstb.2008.2259] [PMID] [PMCID]
10. Lee B, Yoon SY, Malcuit Ch, Parys JB, Fissore RA. Inositol 1,4,5-trisphosphate receptor 1 degradation in mouse eggs and impact on [Ca2+]i oscillations. J Cell Physiol 2010; 222: 238-247. [DOI:10.1002/jcp.21945] [PMID] [PMCID]
11. Berridge MJ. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev 2016; 96: 1261-1296. [DOI:10.1152/physrev.00006.2016] [PMID]
12. Machaca Kh. Ca2+ signaling differentiation during oocyte maturation. J Cell Physiol 2007; 213: 331-340. [DOI:10.1002/jcp.21194] [PMID]
13. Goud PT, Goud AP, Leybaert L, Oostveldt PV, Mikoshiba K, Diamond MP, et al. Inositol 1,4,5-trisphosphate receptor function in human oocytes: Calcium responses and oocyte activation-related phenomena induced by photolytic release of InsP3 are blocked by a specific antibody to the type I receptor. Mol Hum Reprod 2002; 8: 912-918. [DOI:10.1093/molehr/8.10.912] [PMID]
14. van Loendersloot LL, van Wely M, Limpens J, Bossuyt PMM, Repping S, van der Veen F. Predictive factors in in vitro fertilization (IVF): A systematic review and meta-analysis. Hum Reprod Update 2010; 16: 577-589. [DOI:10.1093/humupd/dmq015] [PMID]
15. Wang Sh, He G, Chen M, Zuo T, Xu W, Liu X. The role of antioxidant enzymes in the ovaries. Oxid Med Cell Longev 2017; 2017: 4371714. [DOI:10.1155/2017/4371714] [PMID] [PMCID]
16. Jiang WD, Wu P, Kuang ShY, Liu Y, Jiang J, Hu K, et al. Myo-inositol prevents copper-induced oxidative damage and changes in antioxidant capacity in various organs and the enterocytes of juvenile Jian carp (Cyprinus carpio var. Jian). Aquat toxicol 2011; 105: 543-551. [DOI:10.1016/j.aquatox.2011.08.012] [PMID]
17. Walters KA. Role of androgens in normal and pathological ovarian function. Reproduction 2015; 149: R193-R218. [DOI:10.1530/REP-14-0517] [PMID]
18. Papaleo E, Unfer V, Baillargeon JP, Chiu TT. Contribution of myo-inositol to reproduction. Eur J Obstet Gynecol Reprod Biol 2009; 147: 120-123. [DOI:10.1016/j.ejogrb.2009.09.008] [PMID]
19. Nestler JE, Jakubowicz DJ, Reamer P, Gunn RD, Allan G. Ovulatory and metabolic effects of d-chiro-inositol in the polycystic ovary syndrome. New Engl J Med 1999; 340: 1314-1320. [DOI:10.1056/NEJM199904293401703] [PMID]
20. Papaleo E, Unfer V, Baillargeon JP, Fusi F, Occhi F, De Santis L. Myo-inositol may improve oocyte quality in intracytoplasmic sperm injection cycles: A prospective, controlled, randomized trial. Fertil Steril 2009; 91: 1750-1754. [DOI:10.1016/j.fertnstert.2008.01.088] [PMID]
21. Caprio F, D'Eufemia MD, Trotta C, Campitiello MR, Ianniello R, Mele D, et al. Myo-inositol therapy for poor-responders during IVF: A prospective controlled observational trial. J Ovarian Res 2015; 8: 37. [DOI:10.1186/s13048-015-0167-x] [PMID] [PMCID]
22. Zheng X, Lin D, Zhang Y, Lin Y, Song J, Li S, et al. Inositol supplement improves clinical pregnancy rate in infertile women undergoing ovulation induction for ICSI or IVF-ET. Medicine (Baltimore) 2017; 96: e8842. [DOI:10.1097/MD.0000000000008842] [PMID] [PMCID]
23. Kofi Arhin S, Zhao Y, Lu XSh, Cherry M, Lu JQ. Effect of micronutrient supplementation on IVF outcomes: A systematic review of the literature. Reprode Biomed Online 2017; 35: 715-722. [DOI:10.1016/j.rbmo.2017.08.018] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





