Sat, Sep 21, 2024
[Archive]
Volume 20, Issue 7 (July 2022)
IJRM 2022, 20(7): 569-580 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Nguyen-Thanh T, Dang-Van P, Dang-Ngoc P, Kim W, Le-Minh T, Nguyen-Vu Q. Chronic scrotal heat stress causes testicular interstitial inflammation and fibrosis: An experimental study in mice. IJRM 2022; 20 (7) :569-580
URL: http://ijrm.ir/article-1-2189-en.html
URL: http://ijrm.ir/article-1-2189-en.html
Tung Nguyen-Thanh * 

 1, Phuoc Dang-Van2
1, Phuoc Dang-Van2 

 , Phuc Dang-Ngoc3
, Phuc Dang-Ngoc3 

 , Won Kim4
, Won Kim4 

 , Tam Le-Minh5
, Tam Le-Minh5 

 , Quoc-Huy Nguyen-Vu5
, Quoc-Huy Nguyen-Vu5 




 1, Phuoc Dang-Van2
1, Phuoc Dang-Van2 

 , Phuc Dang-Ngoc3
, Phuc Dang-Ngoc3 

 , Won Kim4
, Won Kim4 

 , Tam Le-Minh5
, Tam Le-Minh5 

 , Quoc-Huy Nguyen-Vu5
, Quoc-Huy Nguyen-Vu5 


1- Faculty of Basic Science, Hue University of Medicine and Pharmacy, Hue University, Hue, Vietnam. Institute of Biomedicine, Hue University of Medicine and Pharmacy, Hue University, Hue, Vietnam. , nttung@huemed-univ.edu.vn
2- Forensic Medicine Center in Thua Thien Hue Province, Hue, Vietnam.
3- Faculty of Medicine, Dong A University, Da Nang, Vietnam.
4- Department of Internal Medicine, Jeonbuk National University Medical School, Jeonju, Republic of Korea.
5- Department of Obstetrics and Gynecology, Hue University of Medicine and Pharmacy, Hue University, Hue, Vietnam.
2- Forensic Medicine Center in Thua Thien Hue Province, Hue, Vietnam.
3- Faculty of Medicine, Dong A University, Da Nang, Vietnam.
4- Department of Internal Medicine, Jeonbuk National University Medical School, Jeonju, Republic of Korea.
5- Department of Obstetrics and Gynecology, Hue University of Medicine and Pharmacy, Hue University, Hue, Vietnam.
Full-Text [PDF 3571 kb]
(669 Downloads)
| Abstract (HTML) (1040 Views)
1. Introduction
The scrotum temperatures of mammal animals are often lower than body temperature to accommodate normal spermatogenesis (1). Heat stress is a harmful factor for many biological systems in the body, such as the circulatory system, integumentary system, respiratory system, and male reproductive activity (2). An increase in scrotal temperatures in male mammals animal and men results to reduced sperm parameters, such as decreased sperm motility, and increases in the percentage of sperm with abnormal morphology (3).
There are many factors that affect spermatogenesis, heat stress causes cell and molecular changes, affecting gene expression that disrupts sperm production, resulting in reduced reproductive health (4). Spermatogenic cells were determined that sensitive to high temperatures (2). After testicular heat stress, many cells undergo extrinsic and intrinsic apoptosis signal pathway (5). Spermatogenic cells are affected by heat stress principally by primary spermatocytes and early spermatids (6). The heat-exposed mice showed degenerative changes with spermatic arrest in most of the seminiferous tubules (7). The heat stress also increased the sperm DNA fragmentation (8). The mechanism of sperm DNA damage may involve various types of oxidative reactions, DNA repair errors in the late stages of spermatogenesis, and functional abnormalities that reduce the protective ability of Sertoli cells (9).
Heat stress adversely affects human and animal health, including an increase in the inflammatory process in the body, largely in mammals (10). Several studies showed that heat stress increases inflammatory response and the release of inflammatory mediators such as cytokines in the kidney, muscle, and intestine (11, 12). Heat stress has been shown to systemically increase inflammation signals through the pathways of toll-like receptor 4, activator protein 1, and nuclear factor kappa-light-chain-enhancer of activated B (13, 14).
Several studies have shown that heat stress affects the induction of fibrosis in various tissues including muscle, liver, and lung (15-17). Heat stress increases the excessive accumulation of extracellular components, in which collagen plays an important role, and this process often occurs after trauma, inflammation leads to increased fibrosis, resulting in reduced function and causing chronic diseases in many tissues and organs (18). In addition, Dos Santos Hamilton and colleagues showed that there was a mild multifocal interstitial fibrosis in ram testicular exposed to heat stress (8).
Currently, studies on the effects of heat stress on testicular inflammation and fibrosis are limited. This study has investigated the histopathological changes related to inflammation and fibrosis in response to testicular chronic heat stress.
2. Materials and Methods
2.1. Animals
This experimental study was performed on a mice model. Animals were kept in an animal facility at a controlled temperature (25 ± 1ºC) and illuminated (12 hr light/dark cycle) with free access to food and water. Thirty 8-10 wk old male Swiss mice (Mus musculus) (20-23 gr) were divided into 3 groups (n = 10/each). The heat stress groups were submerged in a water bath at 37°C (H37°C group) and 40°C (H40°C group), while the control group was treated at 25°C.
2.2. Induction of chronic heat stress
The lower body of mice including the scrotum was soaked for 10 min in a temperature controlled water heated bath twice a day, 10 min apart, 5 consecutive weeks, and 6 days a week. Mice were checked for wounds or redness after being soaked and dried, then returned to the cages of each group. Mice were raised under the same environmental conditions, given free food and enough water, and monitored for general health.
2.3. Hematoxylin and eosin staining
The animal was sacrificed after 5 wk of heat stress. The testis' tissues were harvested for histological analysis. The testicular specimens were individually immersed into 4% buffered formaldehyde and dehydrated with graded concentrations of ethanol then embedded into paraffin. The paraffin blocks were cut thinly with a thickness of 5 µm and transferred into gelatinized slides. The sections were deparaffinized with xylene and then rehydrated through a descending series of ethanol and water. Slides were stained with hematoxylin and eosin (H&E) and then observed under a light microscope (19).
2.4. Johnson scoring system
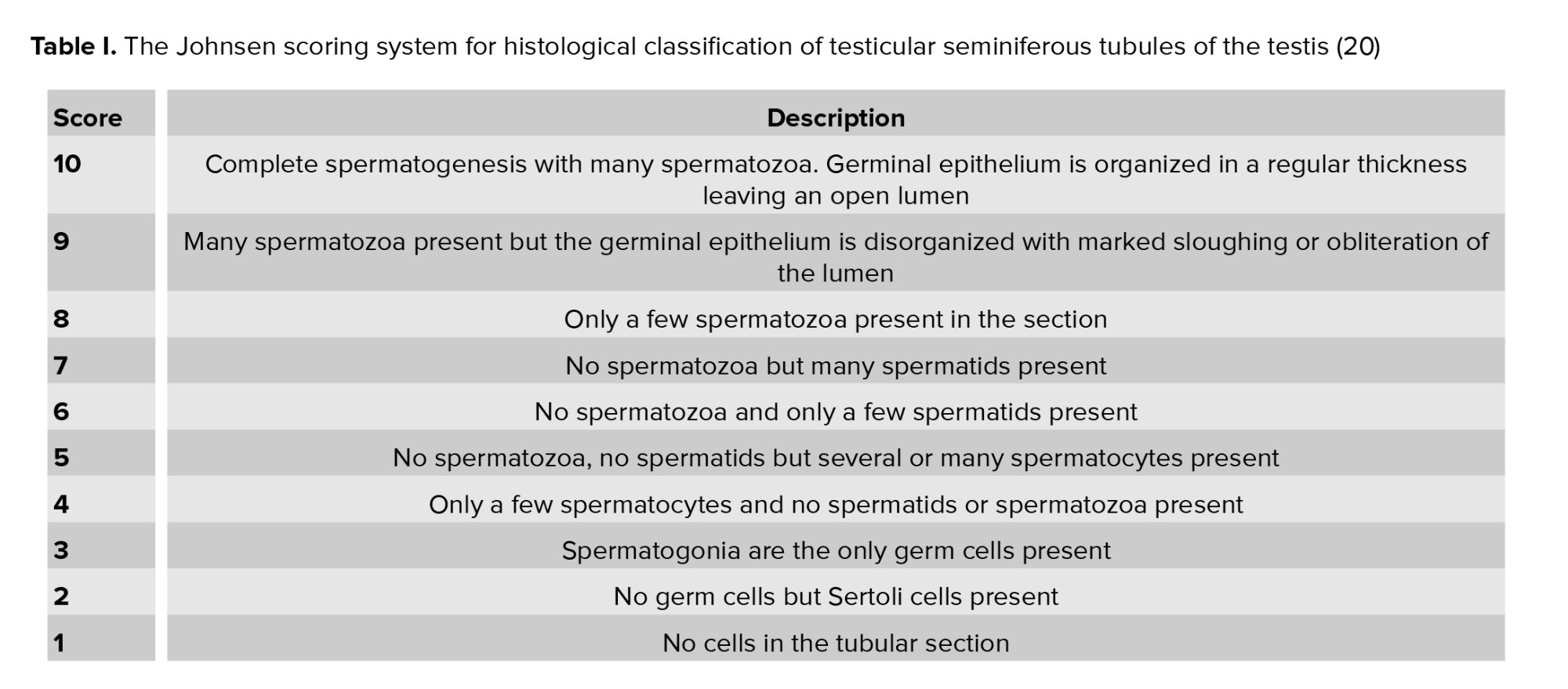
Testicular and sperm lesions were assessed with a testicular average score according to Johnsen (20). By using a 40× magnification, 30 testicular tubules for each animal were graded. Each tubular section was given a score from 1-10 according to the presence or absence of spermatogenous cells in the lumen as spermatozoa, spermatids, spermatocytes, spermatogonia, and Sertoli cells. The state of spermatogenesis was assessed as normal or good with a high Johnsenian score, in contrast to poor fertility or dysfunction of the semen function with a low Johnsenian score. A score of 10 means that all types of sperm cells arranged in an order in the lumen of the spermatogenesis indicate normal spermatogenesis, while point 1 indicates no epithelial maturation (Table I).
2.5. Picro sirius red staining
To evaluate the collagen accumulation, paraffin-embedded tissue sections were stained with picro sirius red and quantified by image analysis as described previously (21). Briefly, the sections were deparaffinized, hydrated, and then incubated with 0.1% picro sirius red solution (Sigma Chemical Co., St. Louis, MO, USA) for 1 hr. Tissue sections were cleared in xylene and mounted in a resinous medium. The ImageJ software (http://rsb.info.nih.gov/ij) was used to measure 10 randomly chosen non-overlapping fields of the picro sirius red-positive areas.
2.6. Immunohistochemistry staining
Immunohistochemical staining was performed as described previously (21). Briefly, the tissue sections were boiled in citrate buffer (Dako target retrieval solution, S1699, DAKO, Carpenteria, CA) to achieve antigen retrieval for 20 min. The slides were then incubated with peroxidase blocking solution (S2023, DAKO) for 15 min and protein block serum-free (X0909, DAKO) for 30 min. Tissue sections were incubated overnight at 4°C with primary antibodies: type I collagen (1:200; 1310-01; Southern Biotech, Birmingham, AL, USA), intercellular adhesion molecule-1 (ICAM-1) (1:200; sc1511; Santa Cruz Biotechnology, Santa Cruz, CA, USA), F4/80 (1:200; 14-4801-81; eBioscience, San Diego, CA, USA) and fibroblast-specific protein 1 (FSP-1)/S100A4 (1:200; ab41532; Abcam, Cambridge, UK). Second antibodies were incubated for 30 min at room temperature and then treated with horseradish peroxidase-conjugated streptavidin (P0397, DAKO) for 30 min. To visualize the immunocomplexes, the testicular sections were treated with 3-amino-9-ethyl carbazole substrate solution (K3464, DAKO). The F4/80-positive macrophages and FSP1-positive fibroblasts per high-power field were counted at a magnification of ×200. The Image J software was used to measure the fibrotic areas of type I collagen and ICAM-1 positive area in 10 randomly chosen non-overlapping fields at a magnification of ×200.
2.7. Ethical considerations
The animal experiment protocol was reviewed and approved by the Ethical Committee of Hue University of Medicine and Pharmacy, Hue, Vietnam (Certificate no. H2019/345). Mice were provided by the Pasteur Institute of Nha Trang, Vietnam.
2.8. Statistical analysis
Statistical analysis was performed using Predictive Analytics Software statistic 18 (SPSS Inc., Chicago, IL). The student’s t parametric test to assess statistical significance between the means of 2 independent groups with normal distribution. While data without normal distribution were analyzed using the nonparametric Mann-Whitney U test. All values are presented as mean ± standard deviation. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Heat stress impairs spermatogenesis in the testis
Normal mice without heat exposure showed histopathology of normal testicles with a full structure of spermatogenous cells arranged in order into the lumen (Figure 1a). The spermatogenetic cell located within invaginations of Sertoli cells.
In the germinal epithelium that include different developmental stages of germ cells, namely spermatogonia, primary and secondary spermatocytes, round and elongate spermatids, and spermatozoa (Figure 1b). Meanwhile, the heat-stress-exposed mice (37oC and 40oC) exhibited degenerated and disorganized features of spermatogenic epithelium and reduced spermatogenic cell numbers (Figure 1c, d, e, and f). In the H40oC groups, the complete arrest of spermatogenesis at the spermatocyte stage was the main feature. There were several multinucleated giant cells in seminiferous tubules (Figure 1f). Chronic heat exposure at 37oC and 40oC for 5 wk impaired histological architecture of testicular tissue, resulting in a significantly reduced Johnson score compared to the control group (Figure 2a), decreased ratio of high Johnsen score, and a gradual increase in the percentage of the low score (Figure 2b). Spermatozoa counted per seminiferous tubule was significantly reduced in the heat-stress-exposed group compared to those in the control group (Figure 2c).
3.2. Heat stress induces testicular interstitial cell proliferation and inflammation in the testis
Ki-67 marker was used to determine the cell proliferating in normal and heat stress-exposed testis (Figure 3 a, b, c). There were many rounds and elongating spermatids positive with Ki-67 in the intra-seminiferous tubule in the control group. Meanwhile, there was no Ki-67-positive cell inside the seminiferous tubule in the H40°C group. In contrast, heat stress-induced increased interstitial cell proliferation. In the H40°C group, many interstitial cells positive with Ki-67 were observed inside testicular interstitial tissue. The heat stress exposed group (H37°C and H40°C group) significantly increased the number of Ki-67-positive cells in the testicular interstitial compared to those in the control group (p < 0.001) (Figure 4a).
ICAM-1 plays a key role in the induction and development of inflammation. The expression of ICAM-1 on testicular tissues was investigated at 5 wk after heat treatment. Immunohistochemistry staining of ICAM-1 is shown in figures 3d, e, and f. In the H40°C group, ICAM-1 was a strong expression in the cytoplasm and membrane of the testicular interstitial cells (Figure 3f). The total area fraction of ICAM-1 was quantified as shown in figure 4b. Heat stress-induced at 40°C significantly increased the area fraction of ICAM-1 compared to those in the control and 37°C groups (p < 0.001).
Macrophage marker F4/80 expression in testicular interstitial cells is shown in figures 3g, h, and i. Heat stress exposure at 40ºC strongly induced F4/80 expression in the cytoplasm and membrane of the testicular interstitial cells (Figure 3i). Heat stress exposure at 40ºC significantly increased the number of F4/80-positive macrophages in the testicular interstitial compared to those in the control and H37ºC groups (p < 0.001). Meanwhile, the number of F4/80-positive macrophages in the testicular interstitial in the H37ºC did not increase compared to those in the control group (Figure 4c).
3.3. Testicular interstitial fibrosis is activated following scrotal heat stress
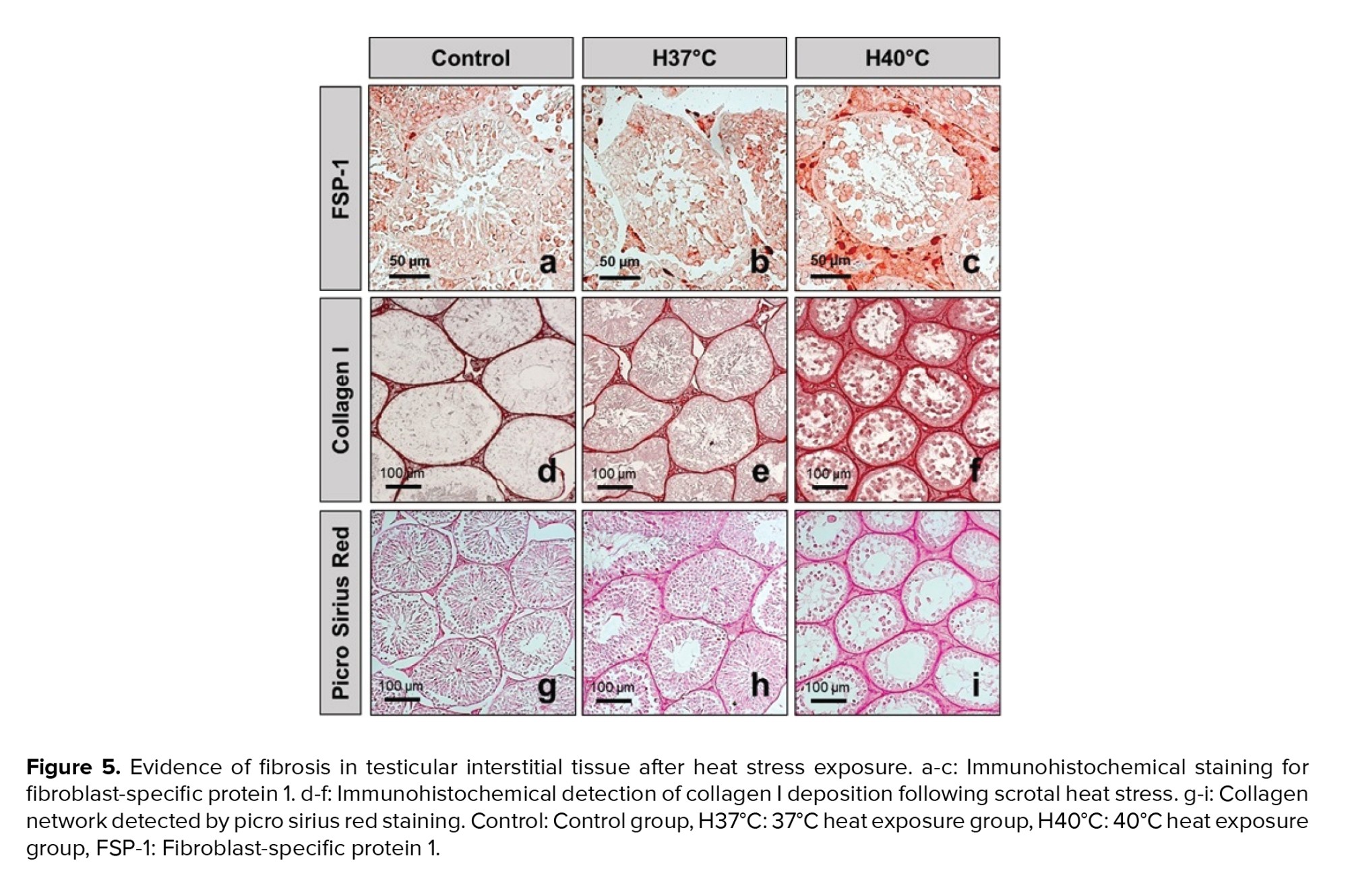
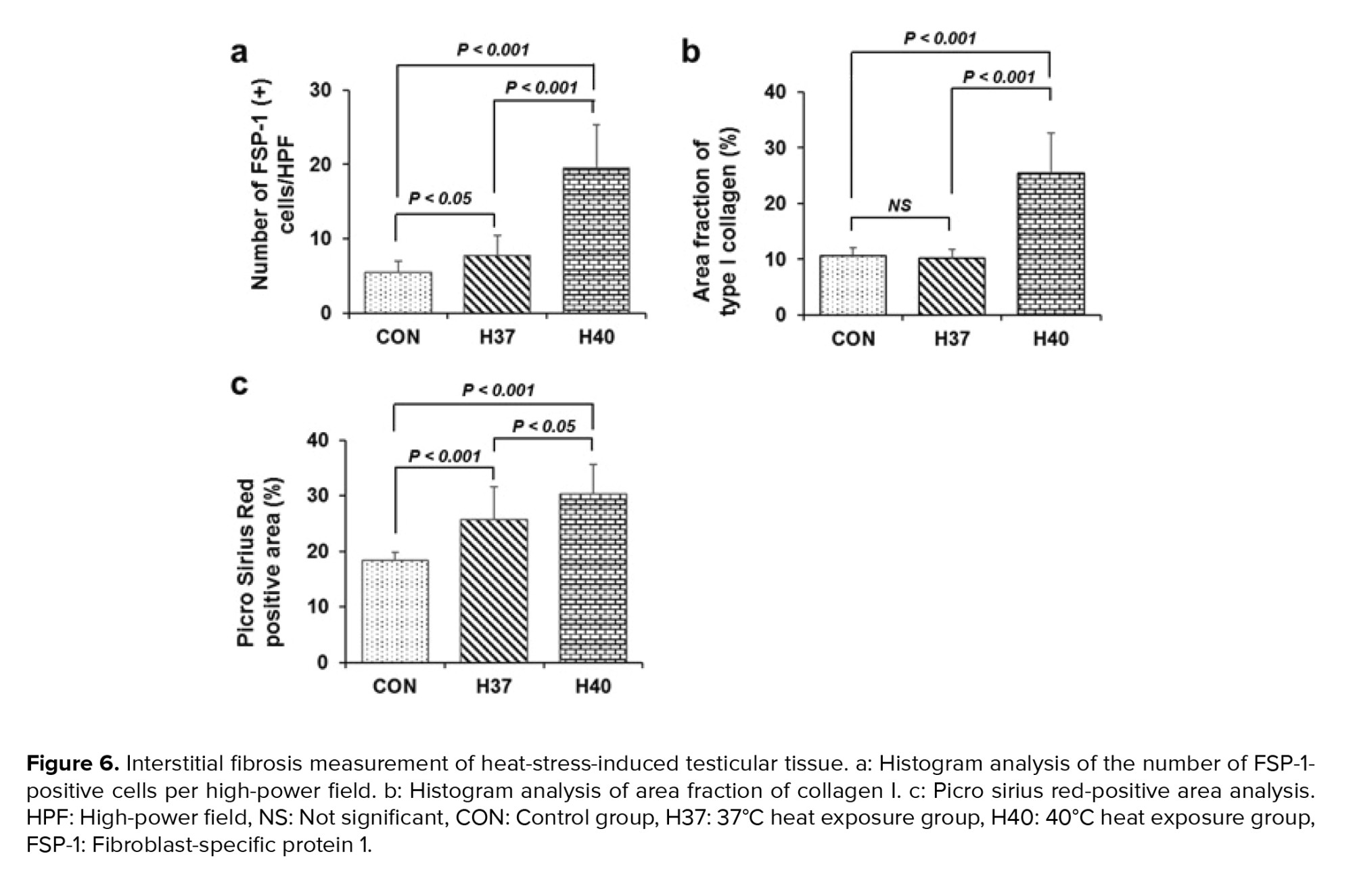
FSP-1 is considered a fibroblast-specific marker expressed in normal and fibrotic tissues. Interstitial FSP-1-positive fibroblasts were detected by immunohistochemistry as shown in figures 5a, b, and c. The number of FSP-1-positive fibroblasts in the testicular interstitial was significantly increased in the H40°C group compared to those in the control and H37°C groups (p < 0.001) (Figure 6a).
Immunohistochemical staining of collagen I deposition following scrotal heat stress is shown in figure 5d, e, and f. Extracellular matrix collagen I fibrils accumulation was strongly detected in interstitial tissue of testis exposed at 40°C (Figure 5d, e, f). The collagen I area fraction was significantly increased in the H40°C group compared to those in the control groups (p < 0.001). Meanwhile, heat stress exposure at 37°C did not increase the area fraction of collagen I compared to those in the control group (Figure 6b).
Histological visualization of collagen I and III fibers by picro sirius red staining is shown in figures 5g, h, i, and figure 6c. Collagen fibers accumulation in testicular interstitial was significantly induced in heat stress exposed groups (H37°C and H40°C) compared to those in the unexposed group (Figure 6c).




4. Discussion
This study showed that heat stress exposed degenerated and disorganized features of spermatogenic epithelium and reduced spermatogenic cell numbers in mice. In heat-stress-exposed groups, the complete arrest of spermatogenesis at the spermatocyte stage was the main feature. Previous studies showed that an increase in testicular temperatures in mammals impairs spermatogenesis, germ cells incur damage, decreased sperm motility, and increases the percentage of sperm with abnormal morphology (3, 22, 23). The effect of heat stress on testicular function and fertility ability in mice is also shown in Paul and colleagues' research (24). In addition, heat stress increases free radicals, oxidative stress, and increased sperm DNA fracture resulting in apoptosis of spermatogenic cells, and loss of sperm integrity.
Inflammation is the body's beneficial response to the elimination of damaging effects to the body. And inflammation should be actively tackled to avoid leading to chronic inflammation, tissue destruction, and progression to fibrosis (25). The damaged organization releases chemical factors that attract inflammatory white blood cells to the site of the lesion to increase the production of proinflammatory chemokines and cytokines, and these cells also secrete chemical intermediate which is harmful to the surrounding tissue; in addition, chronic inflammation causes fibrosis when severe, repetitive tissue damage or damage is disrupted, resulting in excessive buildup of fibrous connective tissue in tissue damage leading to fibrosis (26). In addition, the failure of inflammatory regulatory mechanisms to address inflammation results in chronic inflammation, persistent tissue damage, and fibrosis (25).
Heat stress increases inflammation in the testis by activating proinflammatory cytokines (interleukin 1 beta and tumor necrosis factor-alpha) and increasing their expression (27). This increases the migration of leukocytes into the reproductive system and increases the production of reactive oxygen species leading to oxidation imbalances, resulting in disorders of spermatogenesis, decreased sperm quality, and infertility (28, 29).
Immunohistochemical staining for ICAM-1 showed the heat stress-induced inflammatory interstitial cells in the testis. The H40ºC group significantly increased the number of F4/80-positive macrophages per high-power field in the testicular interstitial compared to those in the control and 37ºC groups. The results are consistent with earlier research, ICAM-1 has been implicated in a variety of inflammatory responses in testicular tissue, tumor cells, and synovial tissues (30, 31).
Ki67 cell proliferation marker staining in figure 2 shows that heat stress disrupted intra-seminiferous tubule cell proliferation, but induced increased interstitial cell proliferation. Previous studies have indicated that chronic heat stress stimulated Leydig cells in the interstitial proliferation (32, 33). It was also shown that the number of Leydig cells in the heat exposure group increased by 50% compared with the control group, this happens through the stimulation of cyclin proteins including proliferating cell nuclear antigen and cyclin D3. In addition, the Leydig cell's production of testosterone hormone is also interrupted because lipid metabolism is interrupted, leading to a decrease in spermatogenesis (34).
Heat stress increased interstitial fibrosis with increased expression of FSP-1, collagen I, and III in mice exposed to high temperatures. It was observed that heat stress degenerates germ cells in the testicles but insignificantly affects somatic cells, decreases germ epithelial thickness, and increases testicular tissue fibrosis (35). Inflammation involves 3 processes increasing blood supply to the damaged site, increasing capillary permeability, and increasing leukocytes into the tissue. Failure to remove the inflammatory agent will enhance the activation of macrophages, lymphocytes, and other cells that jointly produce cytokines and coordinate activities. If this process also fails to resolve the agent that will cause chronic inflammation in which inflammation and repair occur simultaneously, the result is that cytokines rise above normal levels with oxidative stress due to inflammation damaging spermatogenesis, and sperm DNA and apoptosis (29).
Immunohistochemical staining showed that the number of FSP-1-positive fibroblasts in the testicular interstitial was significantly increased in the 40ºC-exposed group compared to those in the control and 37ºC groups. The research results in morphological and histological features in ram testicular heat stress showed initial testicular degeneration and mild multifocal interstitial fibrosis (8). The upregulation of fibroblast-specific protein 1 has been determined that associated with the accumulation of myofibroblasts in inflamed and fibrotic kidneys (36).
5. Conclusion
In conclusion, heat stress adversely affects the testicular tissue and spermatogenesis. Chronic scrotal heat stress causes inflammation and progresses to testicular interstitial fibrosis.
Acknowledgments
This study was supported by the Hue university-level research projects in science and technology, Hue, Vietnam (DHH 2019-04-88). The authors also acknowledge the partial support of Hue University under the Core Research Program (Research group on Regenerative Medicine, NCM.DHH.2022.02).
Conflict of Interest
The authors declare that they have no conflict of interest.
Full-Text: (354 Views)
1. Introduction
The scrotum temperatures of mammal animals are often lower than body temperature to accommodate normal spermatogenesis (1). Heat stress is a harmful factor for many biological systems in the body, such as the circulatory system, integumentary system, respiratory system, and male reproductive activity (2). An increase in scrotal temperatures in male mammals animal and men results to reduced sperm parameters, such as decreased sperm motility, and increases in the percentage of sperm with abnormal morphology (3).
There are many factors that affect spermatogenesis, heat stress causes cell and molecular changes, affecting gene expression that disrupts sperm production, resulting in reduced reproductive health (4). Spermatogenic cells were determined that sensitive to high temperatures (2). After testicular heat stress, many cells undergo extrinsic and intrinsic apoptosis signal pathway (5). Spermatogenic cells are affected by heat stress principally by primary spermatocytes and early spermatids (6). The heat-exposed mice showed degenerative changes with spermatic arrest in most of the seminiferous tubules (7). The heat stress also increased the sperm DNA fragmentation (8). The mechanism of sperm DNA damage may involve various types of oxidative reactions, DNA repair errors in the late stages of spermatogenesis, and functional abnormalities that reduce the protective ability of Sertoli cells (9).
Heat stress adversely affects human and animal health, including an increase in the inflammatory process in the body, largely in mammals (10). Several studies showed that heat stress increases inflammatory response and the release of inflammatory mediators such as cytokines in the kidney, muscle, and intestine (11, 12). Heat stress has been shown to systemically increase inflammation signals through the pathways of toll-like receptor 4, activator protein 1, and nuclear factor kappa-light-chain-enhancer of activated B (13, 14).
Several studies have shown that heat stress affects the induction of fibrosis in various tissues including muscle, liver, and lung (15-17). Heat stress increases the excessive accumulation of extracellular components, in which collagen plays an important role, and this process often occurs after trauma, inflammation leads to increased fibrosis, resulting in reduced function and causing chronic diseases in many tissues and organs (18). In addition, Dos Santos Hamilton and colleagues showed that there was a mild multifocal interstitial fibrosis in ram testicular exposed to heat stress (8).
Currently, studies on the effects of heat stress on testicular inflammation and fibrosis are limited. This study has investigated the histopathological changes related to inflammation and fibrosis in response to testicular chronic heat stress.
2. Materials and Methods
2.1. Animals
This experimental study was performed on a mice model. Animals were kept in an animal facility at a controlled temperature (25 ± 1ºC) and illuminated (12 hr light/dark cycle) with free access to food and water. Thirty 8-10 wk old male Swiss mice (Mus musculus) (20-23 gr) were divided into 3 groups (n = 10/each). The heat stress groups were submerged in a water bath at 37°C (H37°C group) and 40°C (H40°C group), while the control group was treated at 25°C.
2.2. Induction of chronic heat stress
The lower body of mice including the scrotum was soaked for 10 min in a temperature controlled water heated bath twice a day, 10 min apart, 5 consecutive weeks, and 6 days a week. Mice were checked for wounds or redness after being soaked and dried, then returned to the cages of each group. Mice were raised under the same environmental conditions, given free food and enough water, and monitored for general health.
2.3. Hematoxylin and eosin staining
The animal was sacrificed after 5 wk of heat stress. The testis' tissues were harvested for histological analysis. The testicular specimens were individually immersed into 4% buffered formaldehyde and dehydrated with graded concentrations of ethanol then embedded into paraffin. The paraffin blocks were cut thinly with a thickness of 5 µm and transferred into gelatinized slides. The sections were deparaffinized with xylene and then rehydrated through a descending series of ethanol and water. Slides were stained with hematoxylin and eosin (H&E) and then observed under a light microscope (19).
2.4. Johnson scoring system
Testicular and sperm lesions were assessed with a testicular average score according to Johnsen (20). By using a 40× magnification, 30 testicular tubules for each animal were graded. Each tubular section was given a score from 1-10 according to the presence or absence of spermatogenous cells in the lumen as spermatozoa, spermatids, spermatocytes, spermatogonia, and Sertoli cells. The state of spermatogenesis was assessed as normal or good with a high Johnsenian score, in contrast to poor fertility or dysfunction of the semen function with a low Johnsenian score. A score of 10 means that all types of sperm cells arranged in an order in the lumen of the spermatogenesis indicate normal spermatogenesis, while point 1 indicates no epithelial maturation (Table I).
2.5. Picro sirius red staining
To evaluate the collagen accumulation, paraffin-embedded tissue sections were stained with picro sirius red and quantified by image analysis as described previously (21). Briefly, the sections were deparaffinized, hydrated, and then incubated with 0.1% picro sirius red solution (Sigma Chemical Co., St. Louis, MO, USA) for 1 hr. Tissue sections were cleared in xylene and mounted in a resinous medium. The ImageJ software (http://rsb.info.nih.gov/ij) was used to measure 10 randomly chosen non-overlapping fields of the picro sirius red-positive areas.
2.6. Immunohistochemistry staining
Immunohistochemical staining was performed as described previously (21). Briefly, the tissue sections were boiled in citrate buffer (Dako target retrieval solution, S1699, DAKO, Carpenteria, CA) to achieve antigen retrieval for 20 min. The slides were then incubated with peroxidase blocking solution (S2023, DAKO) for 15 min and protein block serum-free (X0909, DAKO) for 30 min. Tissue sections were incubated overnight at 4°C with primary antibodies: type I collagen (1:200; 1310-01; Southern Biotech, Birmingham, AL, USA), intercellular adhesion molecule-1 (ICAM-1) (1:200; sc1511; Santa Cruz Biotechnology, Santa Cruz, CA, USA), F4/80 (1:200; 14-4801-81; eBioscience, San Diego, CA, USA) and fibroblast-specific protein 1 (FSP-1)/S100A4 (1:200; ab41532; Abcam, Cambridge, UK). Second antibodies were incubated for 30 min at room temperature and then treated with horseradish peroxidase-conjugated streptavidin (P0397, DAKO) for 30 min. To visualize the immunocomplexes, the testicular sections were treated with 3-amino-9-ethyl carbazole substrate solution (K3464, DAKO). The F4/80-positive macrophages and FSP1-positive fibroblasts per high-power field were counted at a magnification of ×200. The Image J software was used to measure the fibrotic areas of type I collagen and ICAM-1 positive area in 10 randomly chosen non-overlapping fields at a magnification of ×200.

2.7. Ethical considerations
The animal experiment protocol was reviewed and approved by the Ethical Committee of Hue University of Medicine and Pharmacy, Hue, Vietnam (Certificate no. H2019/345). Mice were provided by the Pasteur Institute of Nha Trang, Vietnam.
2.8. Statistical analysis
Statistical analysis was performed using Predictive Analytics Software statistic 18 (SPSS Inc., Chicago, IL). The student’s t parametric test to assess statistical significance between the means of 2 independent groups with normal distribution. While data without normal distribution were analyzed using the nonparametric Mann-Whitney U test. All values are presented as mean ± standard deviation. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Heat stress impairs spermatogenesis in the testis
Normal mice without heat exposure showed histopathology of normal testicles with a full structure of spermatogenous cells arranged in order into the lumen (Figure 1a). The spermatogenetic cell located within invaginations of Sertoli cells.
In the germinal epithelium that include different developmental stages of germ cells, namely spermatogonia, primary and secondary spermatocytes, round and elongate spermatids, and spermatozoa (Figure 1b). Meanwhile, the heat-stress-exposed mice (37oC and 40oC) exhibited degenerated and disorganized features of spermatogenic epithelium and reduced spermatogenic cell numbers (Figure 1c, d, e, and f). In the H40oC groups, the complete arrest of spermatogenesis at the spermatocyte stage was the main feature. There were several multinucleated giant cells in seminiferous tubules (Figure 1f). Chronic heat exposure at 37oC and 40oC for 5 wk impaired histological architecture of testicular tissue, resulting in a significantly reduced Johnson score compared to the control group (Figure 2a), decreased ratio of high Johnsen score, and a gradual increase in the percentage of the low score (Figure 2b). Spermatozoa counted per seminiferous tubule was significantly reduced in the heat-stress-exposed group compared to those in the control group (Figure 2c).
3.2. Heat stress induces testicular interstitial cell proliferation and inflammation in the testis
Ki-67 marker was used to determine the cell proliferating in normal and heat stress-exposed testis (Figure 3 a, b, c). There were many rounds and elongating spermatids positive with Ki-67 in the intra-seminiferous tubule in the control group. Meanwhile, there was no Ki-67-positive cell inside the seminiferous tubule in the H40°C group. In contrast, heat stress-induced increased interstitial cell proliferation. In the H40°C group, many interstitial cells positive with Ki-67 were observed inside testicular interstitial tissue. The heat stress exposed group (H37°C and H40°C group) significantly increased the number of Ki-67-positive cells in the testicular interstitial compared to those in the control group (p < 0.001) (Figure 4a).
ICAM-1 plays a key role in the induction and development of inflammation. The expression of ICAM-1 on testicular tissues was investigated at 5 wk after heat treatment. Immunohistochemistry staining of ICAM-1 is shown in figures 3d, e, and f. In the H40°C group, ICAM-1 was a strong expression in the cytoplasm and membrane of the testicular interstitial cells (Figure 3f). The total area fraction of ICAM-1 was quantified as shown in figure 4b. Heat stress-induced at 40°C significantly increased the area fraction of ICAM-1 compared to those in the control and 37°C groups (p < 0.001).
Macrophage marker F4/80 expression in testicular interstitial cells is shown in figures 3g, h, and i. Heat stress exposure at 40ºC strongly induced F4/80 expression in the cytoplasm and membrane of the testicular interstitial cells (Figure 3i). Heat stress exposure at 40ºC significantly increased the number of F4/80-positive macrophages in the testicular interstitial compared to those in the control and H37ºC groups (p < 0.001). Meanwhile, the number of F4/80-positive macrophages in the testicular interstitial in the H37ºC did not increase compared to those in the control group (Figure 4c).
3.3. Testicular interstitial fibrosis is activated following scrotal heat stress
FSP-1 is considered a fibroblast-specific marker expressed in normal and fibrotic tissues. Interstitial FSP-1-positive fibroblasts were detected by immunohistochemistry as shown in figures 5a, b, and c. The number of FSP-1-positive fibroblasts in the testicular interstitial was significantly increased in the H40°C group compared to those in the control and H37°C groups (p < 0.001) (Figure 6a).
Immunohistochemical staining of collagen I deposition following scrotal heat stress is shown in figure 5d, e, and f. Extracellular matrix collagen I fibrils accumulation was strongly detected in interstitial tissue of testis exposed at 40°C (Figure 5d, e, f). The collagen I area fraction was significantly increased in the H40°C group compared to those in the control groups (p < 0.001). Meanwhile, heat stress exposure at 37°C did not increase the area fraction of collagen I compared to those in the control group (Figure 6b).
Histological visualization of collagen I and III fibers by picro sirius red staining is shown in figures 5g, h, i, and figure 6c. Collagen fibers accumulation in testicular interstitial was significantly induced in heat stress exposed groups (H37°C and H40°C) compared to those in the unexposed group (Figure 6c).






4. Discussion
This study showed that heat stress exposed degenerated and disorganized features of spermatogenic epithelium and reduced spermatogenic cell numbers in mice. In heat-stress-exposed groups, the complete arrest of spermatogenesis at the spermatocyte stage was the main feature. Previous studies showed that an increase in testicular temperatures in mammals impairs spermatogenesis, germ cells incur damage, decreased sperm motility, and increases the percentage of sperm with abnormal morphology (3, 22, 23). The effect of heat stress on testicular function and fertility ability in mice is also shown in Paul and colleagues' research (24). In addition, heat stress increases free radicals, oxidative stress, and increased sperm DNA fracture resulting in apoptosis of spermatogenic cells, and loss of sperm integrity.
Inflammation is the body's beneficial response to the elimination of damaging effects to the body. And inflammation should be actively tackled to avoid leading to chronic inflammation, tissue destruction, and progression to fibrosis (25). The damaged organization releases chemical factors that attract inflammatory white blood cells to the site of the lesion to increase the production of proinflammatory chemokines and cytokines, and these cells also secrete chemical intermediate which is harmful to the surrounding tissue; in addition, chronic inflammation causes fibrosis when severe, repetitive tissue damage or damage is disrupted, resulting in excessive buildup of fibrous connective tissue in tissue damage leading to fibrosis (26). In addition, the failure of inflammatory regulatory mechanisms to address inflammation results in chronic inflammation, persistent tissue damage, and fibrosis (25).
Heat stress increases inflammation in the testis by activating proinflammatory cytokines (interleukin 1 beta and tumor necrosis factor-alpha) and increasing their expression (27). This increases the migration of leukocytes into the reproductive system and increases the production of reactive oxygen species leading to oxidation imbalances, resulting in disorders of spermatogenesis, decreased sperm quality, and infertility (28, 29).
Immunohistochemical staining for ICAM-1 showed the heat stress-induced inflammatory interstitial cells in the testis. The H40ºC group significantly increased the number of F4/80-positive macrophages per high-power field in the testicular interstitial compared to those in the control and 37ºC groups. The results are consistent with earlier research, ICAM-1 has been implicated in a variety of inflammatory responses in testicular tissue, tumor cells, and synovial tissues (30, 31).
Ki67 cell proliferation marker staining in figure 2 shows that heat stress disrupted intra-seminiferous tubule cell proliferation, but induced increased interstitial cell proliferation. Previous studies have indicated that chronic heat stress stimulated Leydig cells in the interstitial proliferation (32, 33). It was also shown that the number of Leydig cells in the heat exposure group increased by 50% compared with the control group, this happens through the stimulation of cyclin proteins including proliferating cell nuclear antigen and cyclin D3. In addition, the Leydig cell's production of testosterone hormone is also interrupted because lipid metabolism is interrupted, leading to a decrease in spermatogenesis (34).
Heat stress increased interstitial fibrosis with increased expression of FSP-1, collagen I, and III in mice exposed to high temperatures. It was observed that heat stress degenerates germ cells in the testicles but insignificantly affects somatic cells, decreases germ epithelial thickness, and increases testicular tissue fibrosis (35). Inflammation involves 3 processes increasing blood supply to the damaged site, increasing capillary permeability, and increasing leukocytes into the tissue. Failure to remove the inflammatory agent will enhance the activation of macrophages, lymphocytes, and other cells that jointly produce cytokines and coordinate activities. If this process also fails to resolve the agent that will cause chronic inflammation in which inflammation and repair occur simultaneously, the result is that cytokines rise above normal levels with oxidative stress due to inflammation damaging spermatogenesis, and sperm DNA and apoptosis (29).
Immunohistochemical staining showed that the number of FSP-1-positive fibroblasts in the testicular interstitial was significantly increased in the 40ºC-exposed group compared to those in the control and 37ºC groups. The research results in morphological and histological features in ram testicular heat stress showed initial testicular degeneration and mild multifocal interstitial fibrosis (8). The upregulation of fibroblast-specific protein 1 has been determined that associated with the accumulation of myofibroblasts in inflamed and fibrotic kidneys (36).
5. Conclusion
In conclusion, heat stress adversely affects the testicular tissue and spermatogenesis. Chronic scrotal heat stress causes inflammation and progresses to testicular interstitial fibrosis.
Acknowledgments
This study was supported by the Hue university-level research projects in science and technology, Hue, Vietnam (DHH 2019-04-88). The authors also acknowledge the partial support of Hue University under the Core Research Program (Research group on Regenerative Medicine, NCM.DHH.2022.02).
Conflict of Interest
The authors declare that they have no conflict of interest.
Type of Study: Original Article |
Subject:
Cellular and Molecular Biology of Reproduction
References
1. Rockett JC, Mapp FL, Garges JB, Luft JC, Mori C, Dix DJ. Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice. Biol Reprod 2001; 65: 229-239. [DOI:10.1095/biolreprod65.1.229] [PMID]
2. Boni R. Heat stress, a serious threat to reproductive function in animals and humans. Mol Reprod Dev 2019; 86: 1307-1323. [DOI:10.1002/mrd.23123] [PMID]
3. Abdelhamid MHM, Walschaerts M, Ahmad G, Mieusset R, Bujan L, Hamdi S. Mild experimental increase in testis and epididymis temperature in men: Effects on sperm morphology according to spermatogenesis stages. Transl Androl Urol 2019; 8: 651-665. [DOI:10.21037/tau.2019.11.18] [PMID] [PMCID]
4. Cammack KM, Antoniou E, Hearne L, Lamberson WR. Testicular gene expression in male mice divergent for fertility after heat stress. Theriogenology 2009; 71: 651-661. [DOI:10.1016/j.theriogenology.2008.09.029] [PMID]
5. Durairajanayagam D, Agarwal A, Ong Ch. Causes, effects and molecular mechanisms of testicular heat stress. Reprod Biomed Online 2015; 30: 14-27. [DOI:10.1016/j.rbmo.2014.09.018] [PMID]
6. Setchell BP. The effect of heat on the testes of mammals. Anim Reprod 2006; 3: 81-91.
7. Mohajeri D, Kaffashi Elahi R. Effects of Nigella sativa on heat-induced testis damage in mouse. Bratisl Lek Listy 2015; 116: 264-269. [DOI:10.4149/BLL_2015_051] [PMID]
8. Dos Santos Hamilton TR, Perez Siqueira AF, de Castro LS, Mendes CM, Delgado JdC, de Assis PM, et al. Effect of heat stress on sperm DNA: Protamine assessment in Ram spermatozoa and testicle. Oxid Med Cell Longev 2018; 2018: 5413056. [DOI:10.1155/2018/5413056] [PMID] [PMCID]
9. Tesarik J, Mendoza-Tesarik R, Mendoza C. Sperm nuclear DNA damage: Update on the mechanism, diagnosis and treatment. Reprod Biomed Online 2006; 12: 715-721. [DOI:10.1016/S1472-6483(10)61083-8]
10. Cheng K, Song Zh, Li S, Yan E, Zhang H, Zhang L, et al. Effects of resveratrol on intestinal oxidative status and inflammation in heat-stressed rats. J Therm Biol 2019; 85: 102415. [DOI:10.1016/j.jtherbio.2019.102415] [PMID]
11. Sanchez-Lozada LG, Garcia-Arroyo FE, Gonzaga G, Silverio O, Blas-Marron MG, Munoz-Jimenez I, et al. Kidney injury from recurrent heat stress and rhabdomyolysis: Protective role of allopurinol and sodium bicarbonate. Am J Nephrol 2018; 48: 339-348. [DOI:10.1159/000494663] [PMID]
12. Abuajamieh M, Kvidera SK, Mayorga EJ, Kaiser A, Lei S, Seibert JT, et al. The effect of recovery from heat stress on circulating bioenergetics and inflammatory biomarkers. J Anim Sci 2018; 96: 4599-4610. [DOI:10.1093/jas/sky345] [PMID] [PMCID]
13. Ganesan S, Reynolds C, Hollinger K, Pearce SC, Gabler NK, Baumgard LH, et al. Twelve hours of heat stress induces inflammatory signaling in porcine skeletal muscle. Am J Physiol Regul Integr Comp Physiol 2016; 310: 1288-1296. [DOI:10.1152/ajpregu.00494.2015] [PMID] [PMCID]
14. Montilla SIR, Johnson TP, Pearce SC, Gardan-Salmon D, Gabler NK, Ross JW, et al. Heat stress causes oxidative stress but not inflammatory signaling in porcine skeletal muscle. Temperature (Austin) 2014; 1: 42-50. [DOI:10.4161/temp.28844] [PMID] [PMCID]
15. Hagiwara S, Iwasaka H, Matsumoto S, Noguchi T, Yoshioka H. Association between heat stress protein 70 induction and decreased pulmonary fibrosis in an animal model of acute lung injury. Lung 2007; 185: 287-293. [DOI:10.1007/s00408-007-9018-x] [PMID]
16. Shibaguchi T, Sugiura T, Fujitsu T, Nomura T, Yoshihara T, Naito H, et al. Effects of icing or heat stress on the induction of fibrosis and/or regeneration of injured rat soleus muscle. J Physiol Sci 2016; 66: 345-357. [DOI:10.1007/s12576-015-0433-0] [PMID]
17. Roncal-Jimenez CA, Sato Y, Milagres T, Andres Hernando A, Garcia G, Bjornstad P, et al. Experimental heat stress nephropathy and liver injury are improved by allopurinol. Am J Physiol Renal Physiol 2018; 315: F726-F733. [DOI:10.1152/ajprenal.00543.2017] [PMID] [PMCID]
18. Mann ChJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, et al. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle 2011; 1: 21. [DOI:10.1186/2044-5040-1-21] [PMID] [PMCID]
19. Thanh TN, Van PD, Cong ThD, Minh TL, Nguyen Vu QH. Assessment of testis histopathological changes and spermatogenesis in male mice exposed to chronic scrotal heat stress. J Anim Behav Biometeorol 2020; 8: 174-180. [DOI:10.31893/jabb.20023]
20. Johnsen SG. Testicular biopsy score count-a method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Hormones 1970; 1: 2-25. [DOI:10.1159/000178170] [PMID]
21. Nguyen-Thanh T, Kim D, Lee S, Kim W, Park SK, Kang KP. Inhibition of histone deacetylase 1 ameliorates renal tubulointerstitial fibrosis via modulation of inflammation and extracellular matrix gene transcription in mice. Int J Mol Med 2018; 41: 95-106. [DOI:10.3892/ijmm.2017.3218] [PMID] [PMCID]
22. Kanter M, Aktas C, Erboga M. Heat stress decreases testicular germ cell proliferation and increases apoptosis in short term: An immunohistochemical and ultrastructural study. Toxicol Ind Health 2013; 29: 99-113. [DOI:10.1177/0748233711425082] [PMID]
23. Shiraishi K, Matsuyama H, Takihara H. Pathophysiology of varicocele in male infertility in the era of assisted reproductive technology. Int J Urol 2012; 19: 538-550. [DOI:10.1111/j.1442-2042.2012.02982.x] [PMID]
24. Paul C, Murray AA, Spears N, Saunders PT. A single, mild, transient scrotal heat stress causes DNA damage, subfertility and impairs formation of blastocysts in mice. Reproduction 2008; 136: 73-84. [DOI:10.1530/REP-08-0036] [PMID]
25. Suthahar N, Meijers WC, Sillje HHW, de Boer RA. From inflammation to fibrosis-molecular and cellular mechanisms of myocardial tissue remodelling and perspectives on differential treatment opportunities. Current Heart Fail Rep 2017; 14: 235-250. [DOI:10.1007/s11897-017-0343-y] [PMID] [PMCID]
26. Wynn ThA, Ramalingam ThR. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat Med 2012; 18: 1028-1040. [DOI:10.1038/nm.2807] [PMID] [PMCID]
27. Delkhosh A, Shoorei H, Niazi V, Delashoub M, Gharamaleki MN, Ahani-Nahayati M, et al. Coenzyme Q10 ameliorates inflammation, oxidative stress, and testicular histopathology in rats exposed to heat stress. Hum Exp Toxicol 2021; 40: 3-15. [DOI:10.1177/0960327120940366] [PMID]
28. Beigi Harchegani A, Dahan H, Tahmasbpour E, Bakhtiari Kaboutaraki H, Shahriary A. Effects of zinc deficiency on impaired spermatogenesis and male infertility: The role of oxidative stress, inflammation and apoptosis. Hum Fertil 2020; 23: 5-16. [DOI:10.1080/14647273.2018.1494390] [PMID]
29. Azenabor A, Ekun AO, Akinloye O. Impact of inflammation on male reproductive tract. J Reprod Infertil 2015; 16: 123-129.
30. Hua S. Targeting sites of inflammation: Intercellular adhesion molecule-1 as a target for novel inflammatory therapies. Front Pharmacol 2013; 4: 127. [DOI:10.3389/fphar.2013.00127] [PMID] [PMCID]
31. Figenschau SL, Knutsen E, Urbarova I, Fenton C, Elston B, Perander M, et al. ICAM1 expression is induced by proinflammatory cytokines and associated with TLS formation in aggressive breast cancer subtypes. Sci Rep 2018; 8: 11720. [DOI:10.1038/s41598-018-29604-2] [PMID] [PMCID]
32. Aktas C, Kanter M. A morphological study on Leydig cells of scrotal hyperthermia applied rats in short-term. J Mol Histol 2009; 40: 31-39. [DOI:10.1007/s10735-009-9210-9] [PMID]
33. Oka S, Shiraishi K, Fujimoto M, Katiyar A, Takii R, Nakai A, et al. Role of heat shock factor 1 in conserving cholesterol transportation in Leydig cell steroidogenesis via steroidogenic acute regulatory protein. Endocrinology 2017; 158: 2648-2658. [DOI:10.1210/en.2017-00132] [PMID]
34. Li Z, Tian J, Cui G, Wang M, Yu D. Effects of local testicular heat treatment on Leydig cell hyperplasia and testosterone biosynthesis in rat testes. Reprod Fertil Dev 2015; 28: 1424-1432. [DOI:10.1071/RD14370] [PMID]
35. Rasooli A, Taha Jalali M, Nouri M, Mohammadian B, Barati F. Effects of chronic heat stress on testicular structures, serum testosterone and cortisol concentrations in developing lambs. Anim Reprod Sci 2010; 117: 55-59. [DOI:10.1016/j.anireprosci.2009.03.012] [PMID]
36. Le Hir M, Hegyi I, Cueni-Loffing D, Loffing J, Kaissling B. Characterization of renal interstitial fibroblast-specific protein 1/S100A4-positive cells in healthy and inflamed rodent kidneys. Histochem Cell Biol 2005; 123: 335-346. [DOI:10.1007/s00418-005-0788-z] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





