Fri, Apr 26, 2024
[Archive]
Volume 20, Issue 9 (September 2022)
IJRM 2022, 20(9): 745-752 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Jalaliani S, Davar R, Akbarzadeh F, Emami F, Eftekhar M. Addition of intramuscular to vaginal progesterone for luteal phase support in fresh embryo transfer cycles: A cross-sectional study. IJRM 2022; 20 (9) :745-752
URL: http://ijrm.ir/article-1-2473-en.html
URL: http://ijrm.ir/article-1-2473-en.html
1- Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. , samane.jalaliani@gmail.com
2- Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Department of Psychiatry, Psychiatry and Behavioral Sciences Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
2- Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Department of Psychiatry, Psychiatry and Behavioral Sciences Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
Full-Text [PDF 269 kb]
(488 Downloads)
| Abstract (HTML) (734 Views)
1. Introduction
The luteal phase starts after ovulation, supported by progesterone which increases implantation and the pregnancy rate in assisted reproductive technology (ART) cycles (1). Luteal phase deficiency is a common result of ART and is characterized by inadequate progesterone production, so luteal phase support (LPS) is needed for better implantation in the ART cycle (2). Progesterone supplementation is imperative to maintain implantation and early pregnancy until the luteo-placental shift, which occurs during the second trimester of pregnancy (3). Various studies have shown that supporting the luteal phase by administration of progesterone in fresh embryo transfer cycles increases the rate of clinical pregnancy and live birth rate (4-6). Progestogen can begin on the day of oocyte retrieval. or one day later, or the day of embryo transfer, and should continue until positive pregnancy test or 10-12 wk after gestation or until a negative serum human chorionic gonadotropin (HCG) (7).
The progesterone administration has variable types: oral, vaginal, oil-based intramuscular (IM), and subcutaneous progesterone (8). Vaginal and IM progesterone are preferred while oral progesterone alone is usually avoided because it is associated with inadequate bioavailability (9).
Some studies have shown that the use of vaginal progesterone causes a lower rate of miscarriage than IM progesterone (5, 10). The same percentage of pregnancies and miscarriages has been reported in participants receiving vaginal or IM progesterone (11). Therefore, there is an ongoing requirement to assess the LPS in fresh in vitro fertilization cycles (12).
However, there is a general agreement on the use of progesterone in fresh cycles; the choice of preparation, and its duration remains a matter of debate. So far, this study aimed to evaluate the effect of adding IM progesterone to vaginal progesterone on increasing pregnancy rate, and whether it reduces miscarriage in fresh embryo transfer cycles. The study also compared the results with those obtained from vaginal progesterone administration alone.
2. Materials and Methods
2.1. Study population
This analytical cross-sectional study reviewed the medical records of infertile women who had a fresh embryo transfer between March 2020 and February 2021 at the Yazd Reproductive Sciences Institute, Yazd, Iran. 448 fresh embryo transfer cycles were reviewed. Women with incomplete data were removed from the study.
The inclusion criteria were infertile women aged between 18-40 yr and candidates for the antagonist protocol and fresh embryo transfer. On the other hand, the women candidates for frozen embryo transfer; those with uterine malformation or adhesions, severe adenomyosis or endometriosis, severe male factor, severe maternal systemic disease, and candidates for preimplantation genetic testing were excluded from the study. A total of 355 participants met the inclusion criteria of the study.
2.2. Study protocol
Women were stimulated by gonadotropin from the 2nd day of cycle. The initial gonadotropin dose ranged from 150-300 IU per day. Follicular monitoring was done by vaginal sonography from the 6th day of stimulation. Gonadotropin dose was adjusted according to the ovarian response.
With follicular diameter ≥ 14 mm, gonadotropin releasing hormone (GnRH)-antagonist 0.25 mg was administered daily and continued until the day of triggering. When at least three follicles reached a mean diameter of 17 mm, 5,000-10,000 IU of HCG or dual triggering with hCG plus GnRH agonist was done. Oocyte retrieval was performed 34-36 hr after triggering. Embryo transfer was done on day 2 or 3 after oocyte retrieval.
The study population were divided into 2 groups based on the LPS regime: Group I received 400 mg of vaginal progesterone alone twice a day from the day of ovum pick up, and group II received 50 mg IM progesterone daily in addition to vaginal progesterone 400 mg twice a day from the day of ovum pick up. Chemical pregnancy was defined as serum beta hCG ≥ 50 IU/L, 14 days after embryo transfer. Clinical pregnancy was defined as presence of fetal heart activity in ultrasonography done 4 wk after embryo transfer. LPS was continued until 12 wk of gestation.
2.3. Data collection
Demographic characteristics, including age, duration and type of infertility, and body mass index, as well as laboratory information, including anti-mullerian hormone (AMH), endometrial thickness, embryo grading, and type of progesterone consumption were recorded for all women. Furthermore, the rates of positive or negative chemical and clinical pregnancy were recorded in this study.
2.4. Ethical considerations
The study protocol was reviewed and approved by the Ethics Committees of Yazd Reproductive Sciences Institute, Yazd, Iran (Code: IR.SSU.RSI.REC.1399.040). The data were coded and then recorded into the checklists to maintain data confidentiality.
2.5. Statistical analysis
Descriptive data were summarized as mean ± SD and/or percentage. The normality of the data was checked before the analysis by the one-sample Kolmogorov-Smirnov test. Moreover, Chi-square test was used to determine the relationship between progesterone intake and the pregnancy rate. The Independent-Sample t test was used to examine the effects of AMH, Age, and body mass index. The collected data were analyzed with Statistical Package for the Social Sciences, version 25.0, SPSS Inc, Chicago, Illinois, USA (SPSS). A p-value < 0.05 was considered statistically significant.
3. Results
This study was conducted on 355 women who received vaginal progesterone (n = 173; 48.7%) and vaginal progesterone along with IM progesterone (n = 182; 51.3%). The women's median age, body mass index, and median duration of infertility were the same between groups. Table I presents the general and demographic characteristics of the groups.
The infertility etiologies between groups was similar table II the mean age of the women was 34.11 ± 5.46 yr (age range: 18-40 yr). No statistically significant difference was observed between groups (Table I). The mean of anti-mullerian hormone (p = 0.315), infertility duration (p = 0.582), embryo grading (p = 0.376), and embryo number (p = 0.061) was the same between groups. The most frequent embryo grading were B (46.8%) and A (30.7%), respectively.
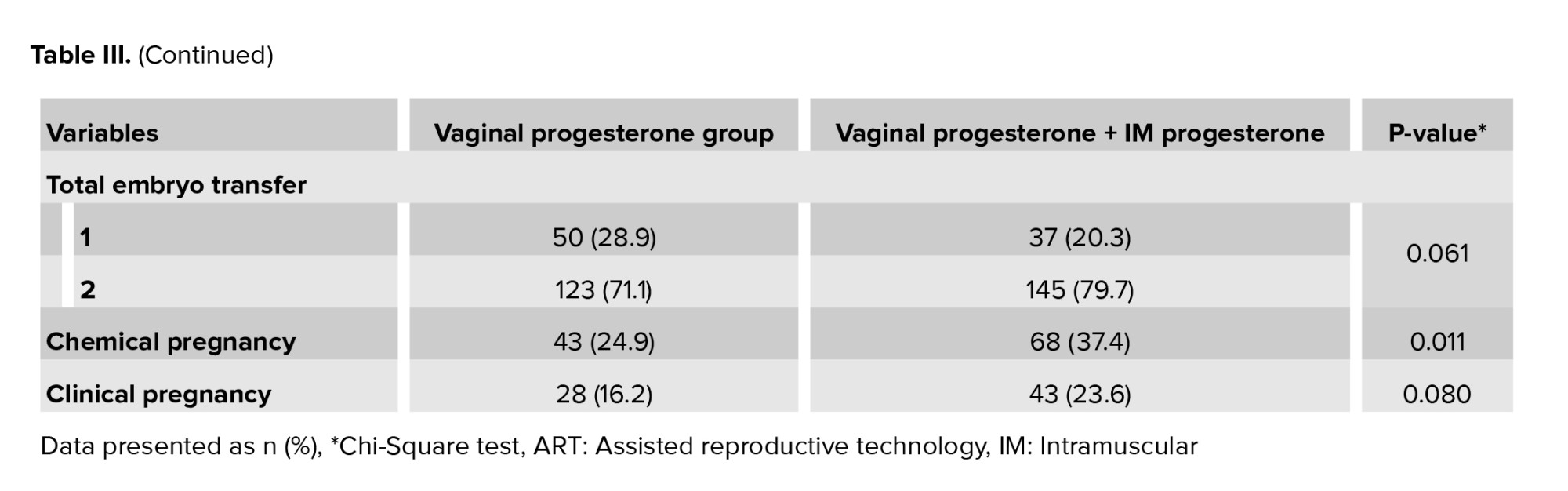
Chemical (37.4%) and clinical (23.6%) pregnancy rates were higher in the vaginal progesterone along with IM progesterone group, compared to the vaginal progesterone group (Table III). The statistical analysis showed that the difference was significant (p = 0.011) for chemical pregnancy; however, it was not significant in the clinical pregnancy (p = 0.080).



4. Discussion
The prescription of vaginal progesterone as an effective drug for luteal support has been well recognized in many studies (8, 13, 14). However, despite the common use of the progesterone for luteal support, the best route and dosing of progesterone is still unidentified (15). This study evaluated the effect of IM progesterone along with vaginal progesterone on in vitro fertilization cycle outcomes in fresh embryo transfer. This results showed that the distribution of AMH, infertility duration, fetal grade, and embryo number were the same between groups. Moreover, the rates of chemical and clinical pregnancy were higher in the vaginal progesterone and the IM progesterone group, compared to the group that received vaginal progesterone alone. However, chemical pregnancy showed a significant difference between groups. In a normal menstrual cycle after mid-cycle luteinizing hormone )LH) surge and monofollicular ovulation, peripheral progesterone concentration increased. "It is necessary for the secretory transformation of the endometrium, successful implantation and maintenance of early pregnancy. Insufficient progesterone secretion at the time of implantation may cause early pregnancy loss or lack of implantation" (16).
In the ovarian stimulation cycle, downregulation and pituitary desensitization with GnRH analogs results in the reduced endogenous release of gonadotropins in the early luteal phase. Furthermore, supraphysiological concentrations of estradiol and progesterone following ovarian stimulation and multiple corpus luteums have negative feedback on the hypothalamus and reduce the amount of LH released from the pituitary (17).
In fresh embryo transfer cycles, multiple corpora luteums are accessible in both ovaries. However, there is a relative mid-luteal phase hCG/LH deficiency after the aspiration of granulosa cells during oocyte retrieval (18). Exogenous progesterone is usually administered for LPS in the ovarian stimulation cycle and fresh embryo transfer (19). LPS via progesterone in fresh and frozen embryo transfer cycles increases pregnancy (5). LPS in ovarian stimulation cycles is required due to the iatrogenic effects of exogenous hormones on suppressing the secretion of endogenous gonadotropins (20).
A review article demonstrated that the oral, vaginal, subcutaneous, and IM use of progestrone is beneficial for clinical pregnancy rates and progesterone supplementation is considered mandatory for LPS in the ART cycle (21). IM progesterone results in higher concentration and more sustained serum levels than vaginal route; however, vaginal regimens achieve higher endometrial concentrations. It has been suggested that these higher local progesterone concentrations may not provide optimal support for better pregnancy outcome and IM progesterone has been suggested for better luteal support and greater uterine quiescence (13).
A survey on 408 ART centers in 82 countries found that about 77% of cycles used vaginal progesterone alone to support the luteal phase (8). However, the vaginal progesterone has some disadvantages, such as vaginal irritation or discharge in some women (22). Rare side effects like acute eosinophilic pneumonia have been reported after IM progesterone supplementation (23, 24).
One study reported that the addition of the IM progesterone to vaginal progesterone to support the luteal phase in fresh embryo transfer cycles increases pregnancy rate (10). Furthermore, the combined use of IM and vaginal progesterone in comparison to vaginal progesterone only leads to a reduced abortion rate and increased pregnancy rate (25). This study showed that the rate of chemical and clinical pregnancy was higher in the vaginal progesterone and the IM progesterone group, compared to the vaginal progesterone group. However, chemical pregnancy showed a significant difference between groups.
Similar to our study, a Cochrane review indicated that the combination therapy had no statistically significant differences between clinical pregnancy and miscarriage (26).
4.1. Limitations and suggestions
One limitation of this research is its retrospective nature. Moreover, according to our criteria, most of the gynecologic disorders that could affect endometrial receptivity were excluded. So, these results cannot cover the women with insufficient endometrial receptivity. Future studies are recommended to be conducted on the efficacy of vaginal progesterone and IM progesterone during the early implantation period.
5. Conclusion
This study attempted to show the effect of adding IM progesterone to vaginal progesterone for LPS on pregnancy rate in fresh embryo transfer cycles. The results showed that the rate of chemical and clinical pregnancy was higher in the vaginal progesterone and the IM progesterone group. Chemical pregnancy showed a significant difference between groups. In summary, the utilization of the IM progesterone and vaginal progesterone appears to have some benefits in terms of successful pregnancy. It is suggested to add IM progesterone to vaginal progesterone for LPS in routine protocols. Since available data are not strong enough, the efficacy of IM progesterone along with vaginal progesterone should be further investigated.
Acknowledgments
The study received no financial support. The authors are grateful to Farimah Shams for her assistance in statistical analysis and the laboratory staff of the Research and Clinical Center for Infertility, Yazd, Iran for their assistance.
Conflict of Interest
The authors declare that there is no conflict of interest.
Full-Text: (177 Views)
1. Introduction
The luteal phase starts after ovulation, supported by progesterone which increases implantation and the pregnancy rate in assisted reproductive technology (ART) cycles (1). Luteal phase deficiency is a common result of ART and is characterized by inadequate progesterone production, so luteal phase support (LPS) is needed for better implantation in the ART cycle (2). Progesterone supplementation is imperative to maintain implantation and early pregnancy until the luteo-placental shift, which occurs during the second trimester of pregnancy (3). Various studies have shown that supporting the luteal phase by administration of progesterone in fresh embryo transfer cycles increases the rate of clinical pregnancy and live birth rate (4-6). Progestogen can begin on the day of oocyte retrieval. or one day later, or the day of embryo transfer, and should continue until positive pregnancy test or 10-12 wk after gestation or until a negative serum human chorionic gonadotropin (HCG) (7).
The progesterone administration has variable types: oral, vaginal, oil-based intramuscular (IM), and subcutaneous progesterone (8). Vaginal and IM progesterone are preferred while oral progesterone alone is usually avoided because it is associated with inadequate bioavailability (9).
Some studies have shown that the use of vaginal progesterone causes a lower rate of miscarriage than IM progesterone (5, 10). The same percentage of pregnancies and miscarriages has been reported in participants receiving vaginal or IM progesterone (11). Therefore, there is an ongoing requirement to assess the LPS in fresh in vitro fertilization cycles (12).
However, there is a general agreement on the use of progesterone in fresh cycles; the choice of preparation, and its duration remains a matter of debate. So far, this study aimed to evaluate the effect of adding IM progesterone to vaginal progesterone on increasing pregnancy rate, and whether it reduces miscarriage in fresh embryo transfer cycles. The study also compared the results with those obtained from vaginal progesterone administration alone.
2. Materials and Methods
2.1. Study population
This analytical cross-sectional study reviewed the medical records of infertile women who had a fresh embryo transfer between March 2020 and February 2021 at the Yazd Reproductive Sciences Institute, Yazd, Iran. 448 fresh embryo transfer cycles were reviewed. Women with incomplete data were removed from the study.
The inclusion criteria were infertile women aged between 18-40 yr and candidates for the antagonist protocol and fresh embryo transfer. On the other hand, the women candidates for frozen embryo transfer; those with uterine malformation or adhesions, severe adenomyosis or endometriosis, severe male factor, severe maternal systemic disease, and candidates for preimplantation genetic testing were excluded from the study. A total of 355 participants met the inclusion criteria of the study.
2.2. Study protocol
Women were stimulated by gonadotropin from the 2nd day of cycle. The initial gonadotropin dose ranged from 150-300 IU per day. Follicular monitoring was done by vaginal sonography from the 6th day of stimulation. Gonadotropin dose was adjusted according to the ovarian response.
With follicular diameter ≥ 14 mm, gonadotropin releasing hormone (GnRH)-antagonist 0.25 mg was administered daily and continued until the day of triggering. When at least three follicles reached a mean diameter of 17 mm, 5,000-10,000 IU of HCG or dual triggering with hCG plus GnRH agonist was done. Oocyte retrieval was performed 34-36 hr after triggering. Embryo transfer was done on day 2 or 3 after oocyte retrieval.
The study population were divided into 2 groups based on the LPS regime: Group I received 400 mg of vaginal progesterone alone twice a day from the day of ovum pick up, and group II received 50 mg IM progesterone daily in addition to vaginal progesterone 400 mg twice a day from the day of ovum pick up. Chemical pregnancy was defined as serum beta hCG ≥ 50 IU/L, 14 days after embryo transfer. Clinical pregnancy was defined as presence of fetal heart activity in ultrasonography done 4 wk after embryo transfer. LPS was continued until 12 wk of gestation.
2.3. Data collection
Demographic characteristics, including age, duration and type of infertility, and body mass index, as well as laboratory information, including anti-mullerian hormone (AMH), endometrial thickness, embryo grading, and type of progesterone consumption were recorded for all women. Furthermore, the rates of positive or negative chemical and clinical pregnancy were recorded in this study.
2.4. Ethical considerations
The study protocol was reviewed and approved by the Ethics Committees of Yazd Reproductive Sciences Institute, Yazd, Iran (Code: IR.SSU.RSI.REC.1399.040). The data were coded and then recorded into the checklists to maintain data confidentiality.
2.5. Statistical analysis
Descriptive data were summarized as mean ± SD and/or percentage. The normality of the data was checked before the analysis by the one-sample Kolmogorov-Smirnov test. Moreover, Chi-square test was used to determine the relationship between progesterone intake and the pregnancy rate. The Independent-Sample t test was used to examine the effects of AMH, Age, and body mass index. The collected data were analyzed with Statistical Package for the Social Sciences, version 25.0, SPSS Inc, Chicago, Illinois, USA (SPSS). A p-value < 0.05 was considered statistically significant.
3. Results
This study was conducted on 355 women who received vaginal progesterone (n = 173; 48.7%) and vaginal progesterone along with IM progesterone (n = 182; 51.3%). The women's median age, body mass index, and median duration of infertility were the same between groups. Table I presents the general and demographic characteristics of the groups.
The infertility etiologies between groups was similar table II the mean age of the women was 34.11 ± 5.46 yr (age range: 18-40 yr). No statistically significant difference was observed between groups (Table I). The mean of anti-mullerian hormone (p = 0.315), infertility duration (p = 0.582), embryo grading (p = 0.376), and embryo number (p = 0.061) was the same between groups. The most frequent embryo grading were B (46.8%) and A (30.7%), respectively.
Chemical (37.4%) and clinical (23.6%) pregnancy rates were higher in the vaginal progesterone along with IM progesterone group, compared to the vaginal progesterone group (Table III). The statistical analysis showed that the difference was significant (p = 0.011) for chemical pregnancy; however, it was not significant in the clinical pregnancy (p = 0.080).




4. Discussion
The prescription of vaginal progesterone as an effective drug for luteal support has been well recognized in many studies (8, 13, 14). However, despite the common use of the progesterone for luteal support, the best route and dosing of progesterone is still unidentified (15). This study evaluated the effect of IM progesterone along with vaginal progesterone on in vitro fertilization cycle outcomes in fresh embryo transfer. This results showed that the distribution of AMH, infertility duration, fetal grade, and embryo number were the same between groups. Moreover, the rates of chemical and clinical pregnancy were higher in the vaginal progesterone and the IM progesterone group, compared to the group that received vaginal progesterone alone. However, chemical pregnancy showed a significant difference between groups. In a normal menstrual cycle after mid-cycle luteinizing hormone )LH) surge and monofollicular ovulation, peripheral progesterone concentration increased. "It is necessary for the secretory transformation of the endometrium, successful implantation and maintenance of early pregnancy. Insufficient progesterone secretion at the time of implantation may cause early pregnancy loss or lack of implantation" (16).
In the ovarian stimulation cycle, downregulation and pituitary desensitization with GnRH analogs results in the reduced endogenous release of gonadotropins in the early luteal phase. Furthermore, supraphysiological concentrations of estradiol and progesterone following ovarian stimulation and multiple corpus luteums have negative feedback on the hypothalamus and reduce the amount of LH released from the pituitary (17).
In fresh embryo transfer cycles, multiple corpora luteums are accessible in both ovaries. However, there is a relative mid-luteal phase hCG/LH deficiency after the aspiration of granulosa cells during oocyte retrieval (18). Exogenous progesterone is usually administered for LPS in the ovarian stimulation cycle and fresh embryo transfer (19). LPS via progesterone in fresh and frozen embryo transfer cycles increases pregnancy (5). LPS in ovarian stimulation cycles is required due to the iatrogenic effects of exogenous hormones on suppressing the secretion of endogenous gonadotropins (20).
A review article demonstrated that the oral, vaginal, subcutaneous, and IM use of progestrone is beneficial for clinical pregnancy rates and progesterone supplementation is considered mandatory for LPS in the ART cycle (21). IM progesterone results in higher concentration and more sustained serum levels than vaginal route; however, vaginal regimens achieve higher endometrial concentrations. It has been suggested that these higher local progesterone concentrations may not provide optimal support for better pregnancy outcome and IM progesterone has been suggested for better luteal support and greater uterine quiescence (13).
A survey on 408 ART centers in 82 countries found that about 77% of cycles used vaginal progesterone alone to support the luteal phase (8). However, the vaginal progesterone has some disadvantages, such as vaginal irritation or discharge in some women (22). Rare side effects like acute eosinophilic pneumonia have been reported after IM progesterone supplementation (23, 24).
One study reported that the addition of the IM progesterone to vaginal progesterone to support the luteal phase in fresh embryo transfer cycles increases pregnancy rate (10). Furthermore, the combined use of IM and vaginal progesterone in comparison to vaginal progesterone only leads to a reduced abortion rate and increased pregnancy rate (25). This study showed that the rate of chemical and clinical pregnancy was higher in the vaginal progesterone and the IM progesterone group, compared to the vaginal progesterone group. However, chemical pregnancy showed a significant difference between groups.
Similar to our study, a Cochrane review indicated that the combination therapy had no statistically significant differences between clinical pregnancy and miscarriage (26).
4.1. Limitations and suggestions
One limitation of this research is its retrospective nature. Moreover, according to our criteria, most of the gynecologic disorders that could affect endometrial receptivity were excluded. So, these results cannot cover the women with insufficient endometrial receptivity. Future studies are recommended to be conducted on the efficacy of vaginal progesterone and IM progesterone during the early implantation period.
5. Conclusion
This study attempted to show the effect of adding IM progesterone to vaginal progesterone for LPS on pregnancy rate in fresh embryo transfer cycles. The results showed that the rate of chemical and clinical pregnancy was higher in the vaginal progesterone and the IM progesterone group. Chemical pregnancy showed a significant difference between groups. In summary, the utilization of the IM progesterone and vaginal progesterone appears to have some benefits in terms of successful pregnancy. It is suggested to add IM progesterone to vaginal progesterone for LPS in routine protocols. Since available data are not strong enough, the efficacy of IM progesterone along with vaginal progesterone should be further investigated.
Acknowledgments
The study received no financial support. The authors are grateful to Farimah Shams for her assistance in statistical analysis and the laboratory staff of the Research and Clinical Center for Infertility, Yazd, Iran for their assistance.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Assisted Reproductive Technologies
References
1. Check JA. Luteal phase support for in vitro fertilization-embryo transfer-present and future methods to improve successful implantation. Clin Exp Obstet Gynecol 2021; 39: 422-428.
2. Lu B-J, Lin C-J, Lin B-Z, Huang L, Chien L-T, Chen C-H. ART outcomes following ovarian stimulation in the luteal phase: A systematic review and meta-analysis. J Assist Reprod Genet 2021; 38: 1927-1938. [DOI:10.1007/s10815-021-02237-7] [PMID] [PMCID]
3. Daya S. Luteal support: Progestogens for pregnancy protection. Maturitas 2009; 65: S29-S34. [DOI:10.1016/j.maturitas.2009.09.012] [PMID]
4. Yanushpolsky E, Hurwitz S, Greenberg L, Racowsky C, Hornstein M. Crinone vaginal gel is equally effective and better tolerated than intramuscular progesterone for luteal phase support in in vitro fertilization-embryo transfer cycles: A prospective randomized study. Fertil Steril 2010; 94: 2596-2599. [DOI:10.1016/j.fertnstert.2010.02.033] [PMID]
5. van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev 2015; 2015: CD009154. [DOI:10.1002/14651858.CD009154.pub3]
6. Miller CE, Zbella E, Webster BW, Doody KJ, Bush MR, Collins MG. Clinical comparison of ovarian stimulation and luteal support agents in patients undergoing GnRH antagonist IVF cycles. J Reprod Med 2013; 58: 153-160.
7. del Carmen Nogales M, Cruz M, de Frutos S, Martínez EM, Gaytán M, Ariza M, et al. Association between clinical and IVF laboratory parameters and miscarriage after single euploid embryo transfers. Reprod Biol Endocrinol 2021; 19: 186. [DOI:10.1186/s12958-021-00870-6] [PMID] [PMCID]
8. Vaisbuch E, de Ziegler D, Leong M, Weissman A, Shoham Z. Luteal-phase support in assisted reproduction treatment: Real-life practices reported worldwide by an updated website-based survey. Reprod Biomed Online 2014; 28: 330-335. [DOI:10.1016/j.rbmo.2013.10.022] [PMID]
9. Lee Ch-I, Chen H-H, Huang C-C, Lin P-Y, Lee T-H, Lee M-S. Early progesterone change associated with pregnancy outcome after fresh embryo transfer in assisted reproduction technology cycles with progesterone level of > 1.5 ng/ml on human chorionic gonadotropin trigger day. Front Endocrinol 2020; 11: 653. [DOI:10.3389/fendo.2020.00653] [PMID] [PMCID]
10. Mohammed A, Woad KJ, Mann GE, Craigon J, Raine-Fenning N, Robinson RS. Evaluation of progestogen supplementation for luteal phase support in fresh in vitro fertilization cycles. Fertil Steril 2019; 112: 491-502. [DOI:10.1016/j.fertnstert.2019.04.021] [PMID]
11. Acharya KS, Acharya CR, Bishop K, Harris B, Raburn D, Muasher SJ. Freezing of all embryos in in vitro fertilization is beneficial in high responders, but not intermediate and low responders: An analysis of 82,935 cycles from the society for assisted reproductive technology registry. Fertil Steril 2018; 110: 880-887. [DOI:10.1016/j.fertnstert.2018.05.024] [PMID]
12. Movahedi S, Aleyasin A, Agahosseini M, Safdarian L, Abroshan S, Khodaverdi S, et al. Endometrial preparation for women undergoing embryo transfer frozen-thawed embryo transfer with and without pretreatment with gonadotropin releasing hormone agonists. J Family Reprod Health 2018; 12: 191-196.
13. Pabuçcu EG, Pabuçcu R, Evliyaoglu Ozdegirmenci O, Bostancı Durmus A, Keskin M. Combined progesterone (IM+ V) versus vaginal progesterone for luteal support in cleavage-stage embryo transfer cycles of good prognosis patients. Gynecol Endocrinol 2016; 32: 366-369. [DOI:10.3109/09513590.2015.1127910] [PMID]
14. Venturella R, Vaiarelli A, Buffo L, D'alessandro P, Colamaria S, Pedri S, et al. Progesterone for preparation of the endometrium for frozen-thawed blastocyst transfer in vitro fertilization cycles: A prospective study on patients' opinions on a new subcutaneous formulation. Gynecol Endocrinol 2018; 34: 766-771. [DOI:10.1080/09513590.2018.1451508] [PMID]
15. Dashti S, Eftekhar M. Luteal-phase support in assisted reproductive technology: An ongoing challenge. Int J Reprod BioMed 2021; 19: 761-772. [DOI:10.18502/ijrm.v19i9.9708] [PMID] [PMCID]
16. Czyzyk A, Podfigurna A, Genazzani AR, Meczekalski B. The role of progesterone therapy in early pregnancy: From physiological role to therapeutic utility. Gynecol Endocrinol 2017; 33: 421-424. [DOI:10.1080/09513590.2017.1291615] [PMID]
17. Zhao J, Hao J, Li Y. Individualized luteal phase support after fresh embryo transfer: Unanswered questions, a review. Reprod Health 2022; 19: 19. [DOI:10.1186/s12978-021-01320-7] [PMID] [PMCID]
18. Tulic L, Tulic I, Bila J, Nikolic L, Dotlic J, Lazarevic-Suntov M, et al. Correlation of progesterone levels on the day of oocyte retrieval with basal hormonal status and the outcome of ART. Sci Rep 2020; 10: 22291. [DOI:10.1038/s41598-020-79347-2] [PMID] [PMCID]
19. Andersen CY, Andersen KV. Improving the luteal phase after ovarian stimulation: Reviewing new options. Reprod Biomed Online 2014; 28: 552-559. [DOI:10.1016/j.rbmo.2014.01.012] [PMID]
20. de Ziegler D, Pirtea P, Andersen CY, Ayoubi JM. Role of gonadotropin-releasing hormone agonists, human chorionic gonadotropin (hCG), progesterone, and estrogen in luteal phase support after hCG triggering, and when in pregnancy hormonal support can be stopped. Fertil Steril 2018; 109: 749-755. [DOI:10.1016/j.fertnstert.2018.03.006] [PMID]
21. Labarta E, Rodríguez C. Progesterone use in assisted reproductive technology. Best Pract Res Clin Obstet Gynaecol 2020; 69: 74-84. [DOI:10.1016/j.bpobgyn.2020.05.005] [PMID]
22. Griesinger G, Blockeel C, Sukhikh GT, Patki A, Dhorepatil B, Yang D-Z, et al. Oral dydrogesterone versus intravaginal micronized progesterone gel for luteal phase support in IVF: A randomized clinical trial. Hum Reprod 2018; 33: 2212-2221. [DOI:10.1093/humrep/dey306] [PMID] [PMCID]
23. Khan AM, Jariwala S, Lieman HJ, Klapper P. Acute eosinophilic pneumonia with intramuscular progesterone after in vitro fertilization. Fertil Steril 2008; 90: 1200. [DOI:10.1016/j.fertnstert.2007.10.073] [PMID]
24. Ahuja A, Ikladios O. Progesterone as a cause of eosinophilic pneumonia after in vitro fertilization. J Community Hosp Intern Med Perspect 2017; 7: 366-368. [DOI:10.1080/20009666.2017.1404418] [PMID] [PMCID]
25. Devine K, Richter KS, Widra EA, McKeeby JL. Vitrified blastocyst transfer cycles with the use of only vaginal progesterone replacement with endometrin have inferior ongoing pregnancy rates: Results from the planned interim analysis of a three-arm randomized controlled noninferiority trial. Fertil Steril 2018; 109: 266-275. [DOI:10.1016/j.fertnstert.2017.11.004] [PMID]
26. Glujovsky D, Pesce R, Fiszbajn G, Sueldo C, Hart RJ, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database Syst Rev 2010; 1: CD006359. [DOI:10.1002/14651858.CD006359.pub2] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








