Sat, Apr 20, 2024
[Archive]
Volume 5, Issue 2 (7-2007)
IJRM 2007, 5(2): 7-12 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rajangam S, Tilak P, N A, Devi R. Karyotyping and counseling in bad obstetric history and infertility. IJRM 2007; 5 (2) :7-12
URL: http://ijrm.ir/article-1-65-en.html
URL: http://ijrm.ir/article-1-65-en.html
1- Division of Human Genetics, Department Of Anatomy, St. John's Medical College, Bangalore, India , sjmcdhg@yahoo.co.in
2- Division of Human Genetics, Department Of Anatomy, St. John's Medical College, Bangalore, India
2- Division of Human Genetics, Department Of Anatomy, St. John's Medical College, Bangalore, India
Full-Text [PDF 53 kb]
(589 Downloads)
| Abstract (HTML) (3270 Views)
Full-Text: (399 Views)
Introduction
In literature, the terms recurrent miscarriage/ habitual abortion/ recurrent spontaneous abortion/ bad obstetric history/ recurrent or repetitive pregnancy loss, reproductive failure and infertility, have been used interchangeably. Even though discrepancies in the work-up parameters in this field exist, the genetic component has achieved near universal acceptance. The estimated chromosomal abnormality (CA) in live births is 9.2 per 1000; out of which the autosomal CA constitutes 75% and sex CA 25%. Among the autosomal CA, balanced rearrangements present in 5.2 per 1000 live births; whereas 0.6% have unbalanced autosomal CA (1).
In couples with bad obstetric history (BOH) percentages of CA vary from 1 to 25% for individuals or to 50% for couples. Most frequently occurring CA is balanced chromosomal rearrangement, i.e. translocation; other CAs seen usually are sex chromosomal mosaicism, inversions and ring chromosomes. Reciprocal translocations are found to be 60% and Robertsonian translocation 40% in couples experiencing recurrent pregnancy loss (2). Infertility is reported to affect up to 15% of couples. The infertile males definitely have the increased risk to be the carriers of CA (47, XXY; X mosaicism). The detection of CA is one of the fundamental diagnostic procedures for further management and treatment (3).
In this investigation, the term reproductive failure includes the couples and the female partners who have experienced BOH and the males diagnosed with infertility. In this article, CA was investigated in 1666 couples and 131 female partners who have had BOH and 73 infertile male partners, referred to Division of Human Genetics (DHG) for karyotyping and counseling.
Materials and methods
Results
As it is shown in table I, major CA was found in 83 cases of the 1870 total samples (4.4%). This include; 56 out of 1666 couples (3.4%), 15 out of 131 females (11.5%) and 12 out of 73 males (16.4%).
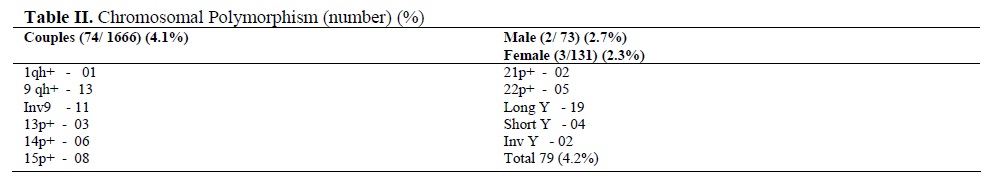
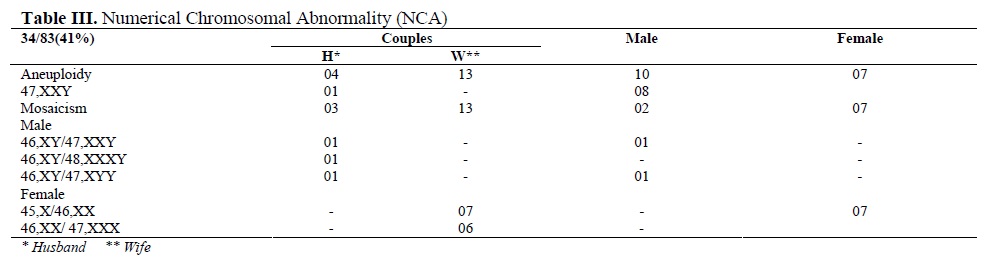
The noted chromosomal polymorphism/ heteromorphism/ variants are presented in table II. The chromosomal variants were present in 79 out of the 1870 (4.2%). Polymorphisms were more frequently associated to chromosomes 9 and Y. Numerical CA (34/83, 41%) were either Klinefelter or Turner syndrome which had the typical as well as the variant karyotypes or mosaicism for the sex chromosomes. Mosaicism involved mostly the X chromosome, except one instance of 47, XYY in a male partner along with normal cell line of 46, XY (Table III).


Discussion
For any given pregnancy, the reported risk of pregnancy loss is 15% and the likelihood of consecutive three losses, which is the classic definition of repetitive pregnancy loss (RPL), would be 0.34%. However, 1 to 2 % of couples experience three or more consecutive losses. Hence, medical help is sought in order to identify the causal factor as well as the strategy to alleviate the problem (4).
CA is included in the factors influencing the recurrence risk of RPL. Numerous studies have demonstrated that in around 5.5% of the couples, who have had RPL, one of the partners is the carrier for a balanced chromosomal rearrangement, in contrast to its incidence of less than 0.55% in the general population (5). It has been observed that the balanced chromosomal rearrangements have been detected to be present twice often in the female partners (6). In male the rearrangements are often associated with infertility (7).
Translocation (reciprocal and Robertsonian) is the most commonly observed CA (8). Overall, 75% are autosomal balanced translocation in the couples with pregnancy loss and this incidence is supposed to be 30 times higher than the report in the general population (5). The presence of the low - level sex chromosomal mosaic aneuploidy in the couples with the history of abortions has also been recorded. It may be due to age related phenomenon or an indicator of Turner or Klinefelter syndrome (9). It was thought that the frequency of CAs might be higher in couples with mixed problems in obstetric history than in couples with only abortions. However, it was not found to be significant, as various studies have quoted variable incidence from 2.9 to 23% (10-15). In a combined study done by collecting computerized database on 22,299 couples (44,398 individuals), 2.35% had CAs. Even from the pooled data, statistically significant differences have not been observed between the major CAs and the types of reproductive wastage and / or the presence or absence of normal live births (16). The incidence of CA in male infertility is reflected to the conditions such as hypogonadism, azoospermia or oligospermia. The CA frequency is found to be 4.6% and 13.7% in men with oligospermia and azoospermia respectively (17). The sex CA has the highest rate in azoospermic males, while autosomal CA predominates in the oligospermic males. Klinefelter syndrome with the classic karyotype of 47, XXY or mosaics with 46, XY / 47, XXY karyotypes are the frequently observed sex CA in infertile males. Robertsonian translocation is the frequently seen autosomal CA in infertile men (0.7%). A correlation has been observed between the XY bivalent and the chromosomes involved in Robertsonian trivalent in the pachytene stage of meiosis leading to the impairment of the germ cells (18). Pooling the data from the different series of infertile males, 0.5% reciprocal translocations were observed as compared to 0.1% in the newborn children. The correlation between reciprocal translocations with involvement of chromosomes 3, 4, 5, 6, 7, 9, 11, 13, 14, 15, 16, 17, 19, 20, 21, 22 and the impairment of sperm production is well known (19).
The incidence of CA in the present study has been found to be 3.4% in couples, 16.4% in the female partners with BOH and 11.5% in the males with infertility. The translocation and the sex chromosomes abnormalities (43/83, 51.8%) were the major observed CAs in the present study. In this study, the probands with X mosaicism have been considered as Turner or Klinefelter mosaic. The percentage of normal cell lines indicated that the probands might have started as normal zygote. In female patients with X mosaicism, there may be reduced amount of the genetic products transcribed from the critical region of the X chromosome, leading to BOH.
Mixed obstetric history in a couple may increase the probability of identifying the balanced translocation carrier status in one of the partners (20). The mechanism by which a balanced chromosomal translocation exerts a negative effect on the reproductive performance of the carrier is through production of unbalanced gametes during meiotic segregation. These carriers have an increased risk of abortions compared to the general population (15 to 50%). This risk is also increased, although to a lesser extent in the carriers of Robertsonian translocation.
In the present study, the incidence of the reciprocal and Robertsonian translocations in BOH were 65% (26/40) and 30% (12/40) respectively. The higher percentage of these two types of translocation in this study compared to the literature (50% and 2 to 25%) may be due to the sampling bias. As stated in the literature, the carrier status seemed to be prevalent in the females (57.5%).
Carriers of Balanced Complex Translocation (BCT) have a high risk of having spontaneous abortions or children with an unbalanced karyotype. BCT is ascertained in 68% of phenotypically normal couples because of their reproductive problems, around 23% in those born with multiple congenital anomalies and 8% through prenatal diagnosis (21). Infertility in a male BCT carrier is reflected as pre and post implantation losses rather than as disturbed spermatogenesis. Although the number of complex rearrangements seemed to be low, it is very important to look at the family history and to karyotype all the siblings before predicting the risk.
In literature, there have been reports of reciprocal translocation carriers with varying combination of the involved chromosomes, resulting in BOH and reproductive failure (22, 23). As far as the Robertsonian translocation is concerned, the frequencies of the chromosomes involved are as follows: 13; 14 (57.5 - 63 %), 14; 21 (8%), 13; 13 (19%) and other chromosomes (29%).
Even though frequently involved chromosomes in translocation in couples with BOH have been reported, there seems to be still a variation in the breakpoints as well as in the chromosomes, which enter into translocation. Statistical analysis has shown that although all the chromosome arms have been involved in translocations some seemed to be preferentially involved such as: 2q, 5q, 7p, 7q, 12q, 13q, 17q, 18q and 22q. The size of the chromosomal segments involved, the frequency of the break points and their positions have a vital role in reproduction. In translocations, break points are non-random, especially in couples with mixed obstetric history (24). The frequently involved chromosomes in the translocations in the present study were 4, 11, 15 and X. There were three X; autosomal translocations and a unique combination of translocation 1; 15 in the parent of a female carrier and translocation 13; 14 in a non- consanguineous couple.
The significance of the variants is the frequent subject of debate (23, 25). It has been suggested that chromosomal heteromorphisms does not induce miscarriage (26). The role played by the variants is still controversial, because of the large number of variants found in the normal population (26). In this study, the chromosomal polymorphisms were considered to be non- significant. The disparity observed in the present study with that of the literature is because of the differences in the sample size. Previous reported studies have included only couples with repeated spontaneous abortions/ recurrent miscarriages/ repeated pregnancy losses or couples with mixed obstetric history. Hence, the frequencies of the CAs vary from series to series, but definitely it has a greater frequency than in the general population.
This study has emphasized the importance of a proper work up of BOH and infertility, considering the different etiological factors including the karyotyping in order to find the cause for such a problem, thereby the affected couples may be effectively counseled.
In literature, the terms recurrent miscarriage/ habitual abortion/ recurrent spontaneous abortion/ bad obstetric history/ recurrent or repetitive pregnancy loss, reproductive failure and infertility, have been used interchangeably. Even though discrepancies in the work-up parameters in this field exist, the genetic component has achieved near universal acceptance. The estimated chromosomal abnormality (CA) in live births is 9.2 per 1000; out of which the autosomal CA constitutes 75% and sex CA 25%. Among the autosomal CA, balanced rearrangements present in 5.2 per 1000 live births; whereas 0.6% have unbalanced autosomal CA (1).
In couples with bad obstetric history (BOH) percentages of CA vary from 1 to 25% for individuals or to 50% for couples. Most frequently occurring CA is balanced chromosomal rearrangement, i.e. translocation; other CAs seen usually are sex chromosomal mosaicism, inversions and ring chromosomes. Reciprocal translocations are found to be 60% and Robertsonian translocation 40% in couples experiencing recurrent pregnancy loss (2). Infertility is reported to affect up to 15% of couples. The infertile males definitely have the increased risk to be the carriers of CA (47, XXY; X mosaicism). The detection of CA is one of the fundamental diagnostic procedures for further management and treatment (3).
In this investigation, the term reproductive failure includes the couples and the female partners who have experienced BOH and the males diagnosed with infertility. In this article, CA was investigated in 1666 couples and 131 female partners who have had BOH and 73 infertile male partners, referred to Division of Human Genetics (DHG) for karyotyping and counseling.
Materials and methods
This study was designed as a retrospective study. The data from 1666 couples and 131 female partners with BOH and 73 male partners with infertility referred to DHG has been considered for the study. Couples and the female partners were considered for the study only if they have had two or more than two abortions, neonatal deaths or offspring with multiple congenital anomalies. The reasons for the referral of the female and male partners alone were as follows; their spouses had the testing elsewhere, spouses genuinely could not come because their career was outside India, referral was only for the suspected partner and in-spite of the repeated requests the other partner did not turn up for the karyotyping.
The protocol for the preparation of the chromosomes for karyotyping was as follows: About 2ml of heparinized blood was collected from peripheral veins of the referred patients. Lymphocytes were grown in RPMI 1640 culture media containing antibiotics (penicillin/ streptomycin) and 15 % serum supplemention. Phytohemaglutinin (PHA) was added as the mitotic stimulant (0.5 ml of the innoculum) and the samples were incubated for 72 hours at 370C incubator. The cells were arrested at metaphase with 0.1% colchicine; chromosome elongation was accomplished by adding 1% ethydium bromide. Hypotonic treatment was done with Ohnuki solution (KCl / NaCOOH/ NaNo3) and cells were fixed with 3 changes of fixative (3:1, methanol: acetic acid). The prepared slides were stained with GTG (G-bands using Trypsin and Geimsa stain). Chromosomal analysis was done under 100x, magnification. Overall 15 metaphase spreads were screened and 5 metaphases were captured using a CCD camera. The captured picture was further enhanced by adjusting the sharpness, brightness and contrast and the printout was taken. According to ISCN standards, the karyotype was prepared.
Additional investigations for the complete work up of the referred patients include anatomical (scanning), endocrinological (hormonal assay), immunological (anti phospholipid profile–APL, anti cardio lipin- ACL) and environmental (infections, occupational hazards, cigarette smoking, alcohol, nutritional deficiency) factors.Results
As it is shown in table I, major CA was found in 83 cases of the 1870 total samples (4.4%). This include; 56 out of 1666 couples (3.4%), 15 out of 131 females (11.5%) and 12 out of 73 males (16.4%).
The noted chromosomal polymorphism/ heteromorphism/ variants are presented in table II. The chromosomal variants were present in 79 out of the 1870 (4.2%). Polymorphisms were more frequently associated to chromosomes 9 and Y. Numerical CA (34/83, 41%) were either Klinefelter or Turner syndrome which had the typical as well as the variant karyotypes or mosaicism for the sex chromosomes. Mosaicism involved mostly the X chromosome, except one instance of 47, XYY in a male partner along with normal cell line of 46, XY (Table III).
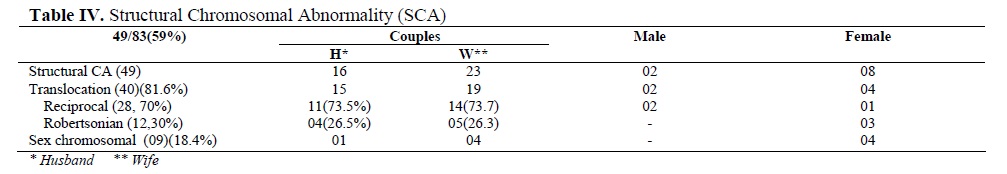
The structural CA (49/83, 59%) was translocation in 40 (81.6%) and the deletion/ duplication/ marker/ isochromosome associated with X in 9 patients (18.4%). They were:(Male: 46,XY/ 47,XY+ mar 01, Female: 45,X/ 47,XX+mar 01; 46,XX/ 47,XX+mar 01; 47,XX+frag 01; 46,X,Xq- 02; 46,X,Xp- 01; 46,X,Xp+01;45,X/46,X,iX(q)01) (Table IV). In total 28 cases had reciprocal (70%) and 9 had Robertsonian (30%) translocation.
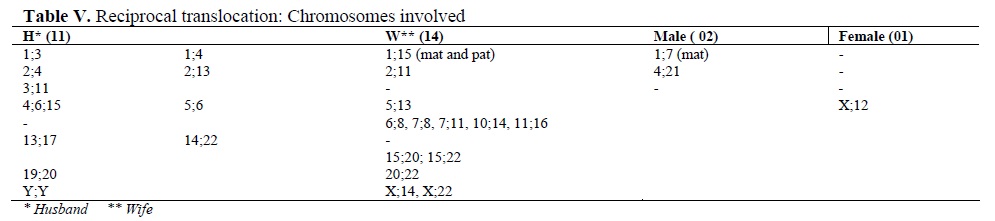
The frequently involved chromosomes in reciprocal translocation were 1, 11 and 15 followed by 2, 4, 13, 20, 22 and X. A unique case of translocation t(1; 15) was detected in a female partner wherein both parents of her also were the carriers. Among Robertsonian translocations t(13; 14) was prevalent. In one case, both partners had robt(13; 14). In total, 23 females were found to be translocation carriers (82.14%) (Tables V and VI).





Discussion
For any given pregnancy, the reported risk of pregnancy loss is 15% and the likelihood of consecutive three losses, which is the classic definition of repetitive pregnancy loss (RPL), would be 0.34%. However, 1 to 2 % of couples experience three or more consecutive losses. Hence, medical help is sought in order to identify the causal factor as well as the strategy to alleviate the problem (4).
CA is included in the factors influencing the recurrence risk of RPL. Numerous studies have demonstrated that in around 5.5% of the couples, who have had RPL, one of the partners is the carrier for a balanced chromosomal rearrangement, in contrast to its incidence of less than 0.55% in the general population (5). It has been observed that the balanced chromosomal rearrangements have been detected to be present twice often in the female partners (6). In male the rearrangements are often associated with infertility (7).
Translocation (reciprocal and Robertsonian) is the most commonly observed CA (8). Overall, 75% are autosomal balanced translocation in the couples with pregnancy loss and this incidence is supposed to be 30 times higher than the report in the general population (5). The presence of the low - level sex chromosomal mosaic aneuploidy in the couples with the history of abortions has also been recorded. It may be due to age related phenomenon or an indicator of Turner or Klinefelter syndrome (9). It was thought that the frequency of CAs might be higher in couples with mixed problems in obstetric history than in couples with only abortions. However, it was not found to be significant, as various studies have quoted variable incidence from 2.9 to 23% (10-15). In a combined study done by collecting computerized database on 22,299 couples (44,398 individuals), 2.35% had CAs. Even from the pooled data, statistically significant differences have not been observed between the major CAs and the types of reproductive wastage and / or the presence or absence of normal live births (16). The incidence of CA in male infertility is reflected to the conditions such as hypogonadism, azoospermia or oligospermia. The CA frequency is found to be 4.6% and 13.7% in men with oligospermia and azoospermia respectively (17). The sex CA has the highest rate in azoospermic males, while autosomal CA predominates in the oligospermic males. Klinefelter syndrome with the classic karyotype of 47, XXY or mosaics with 46, XY / 47, XXY karyotypes are the frequently observed sex CA in infertile males. Robertsonian translocation is the frequently seen autosomal CA in infertile men (0.7%). A correlation has been observed between the XY bivalent and the chromosomes involved in Robertsonian trivalent in the pachytene stage of meiosis leading to the impairment of the germ cells (18). Pooling the data from the different series of infertile males, 0.5% reciprocal translocations were observed as compared to 0.1% in the newborn children. The correlation between reciprocal translocations with involvement of chromosomes 3, 4, 5, 6, 7, 9, 11, 13, 14, 15, 16, 17, 19, 20, 21, 22 and the impairment of sperm production is well known (19).
The incidence of CA in the present study has been found to be 3.4% in couples, 16.4% in the female partners with BOH and 11.5% in the males with infertility. The translocation and the sex chromosomes abnormalities (43/83, 51.8%) were the major observed CAs in the present study. In this study, the probands with X mosaicism have been considered as Turner or Klinefelter mosaic. The percentage of normal cell lines indicated that the probands might have started as normal zygote. In female patients with X mosaicism, there may be reduced amount of the genetic products transcribed from the critical region of the X chromosome, leading to BOH.
Mixed obstetric history in a couple may increase the probability of identifying the balanced translocation carrier status in one of the partners (20). The mechanism by which a balanced chromosomal translocation exerts a negative effect on the reproductive performance of the carrier is through production of unbalanced gametes during meiotic segregation. These carriers have an increased risk of abortions compared to the general population (15 to 50%). This risk is also increased, although to a lesser extent in the carriers of Robertsonian translocation.
In the present study, the incidence of the reciprocal and Robertsonian translocations in BOH were 65% (26/40) and 30% (12/40) respectively. The higher percentage of these two types of translocation in this study compared to the literature (50% and 2 to 25%) may be due to the sampling bias. As stated in the literature, the carrier status seemed to be prevalent in the females (57.5%).
Carriers of Balanced Complex Translocation (BCT) have a high risk of having spontaneous abortions or children with an unbalanced karyotype. BCT is ascertained in 68% of phenotypically normal couples because of their reproductive problems, around 23% in those born with multiple congenital anomalies and 8% through prenatal diagnosis (21). Infertility in a male BCT carrier is reflected as pre and post implantation losses rather than as disturbed spermatogenesis. Although the number of complex rearrangements seemed to be low, it is very important to look at the family history and to karyotype all the siblings before predicting the risk.
In literature, there have been reports of reciprocal translocation carriers with varying combination of the involved chromosomes, resulting in BOH and reproductive failure (22, 23). As far as the Robertsonian translocation is concerned, the frequencies of the chromosomes involved are as follows: 13; 14 (57.5 - 63 %), 14; 21 (8%), 13; 13 (19%) and other chromosomes (29%).
Even though frequently involved chromosomes in translocation in couples with BOH have been reported, there seems to be still a variation in the breakpoints as well as in the chromosomes, which enter into translocation. Statistical analysis has shown that although all the chromosome arms have been involved in translocations some seemed to be preferentially involved such as: 2q, 5q, 7p, 7q, 12q, 13q, 17q, 18q and 22q. The size of the chromosomal segments involved, the frequency of the break points and their positions have a vital role in reproduction. In translocations, break points are non-random, especially in couples with mixed obstetric history (24). The frequently involved chromosomes in the translocations in the present study were 4, 11, 15 and X. There were three X; autosomal translocations and a unique combination of translocation 1; 15 in the parent of a female carrier and translocation 13; 14 in a non- consanguineous couple.
The significance of the variants is the frequent subject of debate (23, 25). It has been suggested that chromosomal heteromorphisms does not induce miscarriage (26). The role played by the variants is still controversial, because of the large number of variants found in the normal population (26). In this study, the chromosomal polymorphisms were considered to be non- significant. The disparity observed in the present study with that of the literature is because of the differences in the sample size. Previous reported studies have included only couples with repeated spontaneous abortions/ recurrent miscarriages/ repeated pregnancy losses or couples with mixed obstetric history. Hence, the frequencies of the CAs vary from series to series, but definitely it has a greater frequency than in the general population.
This study has emphasized the importance of a proper work up of BOH and infertility, considering the different etiological factors including the karyotyping in order to find the cause for such a problem, thereby the affected couples may be effectively counseled.
Type of Study: Original Article |
References
1. Harper PS. Practical Genetic Counseling. 6th Edition. London, UK, Hodder Arnold 2004: 306-310.
2. Simpson JL, Elias S. Genetics in Obstetrics and Gynecology. Philadelphia, USA: Saunders 2003: 101-132.
3. Mau-Holzmann UA. Somatic chromosomal abnormalities in infertile men and women. Cytogenet Genome Res 2005; 111: 317-336. [DOI:10.1159/000086906]
4. Brigham SA, Conlon C, Farquharson RG. A longitudinal study of pregnancy outcome following idiopathic recurrent miscarriage. Hum Reprod 1991; 14: 2868-2871. [DOI:10.1093/humrep/14.11.2868]
5. Fryns JP, Van Buggenhout G. Structural chromosome rearrangements in couples with recurrent fetal wastage. Eur J Obstet Gynecol Reprod Biol 1998; 81: 171-176. [DOI:10.1016/S0301-2115(98)00185-7]
6. Simpson JL, Bombard AT. Chromosomal abnormalities in spontaneous abortion: frequency, pathology and genetic counseling. In: Edmonds K, Bennett MJ (eds); Spontaneous abortion. London, UK: Blackwell 1987: 51.
7. Shreck R, Silverman N. Fetal loss. In: David L Rimoin, Michael Connor J, Reed E Pyeritz (Eds). 4th Edition. London UK: Churchill Livingstone 2002: 982-997.
8. Neri G, Serra A, Campana M, Tedeschi B. Reproductive risks for translocation carriers: Cytogenetic study and analysis of pregnancy outcome in 58 families. Am J Med Genet 1983; 16: 535-561. [DOI:10.1002/ajmg.1320160412]
9. Reinisch LC, Silvery KL, Dumms KW. Sex chromosome mosaicism in couples with repeated fetal loss. Am J Hum Genet 1981; 33: 117-A.
10. Duca D, Cioltei A, Ioan D, Maximilian C. The importance of cytogenetic investigation of the couples with multiple spontaneous abortions and malformed offsprings. Endocrinologie 1979; 17: 17-22.
11. Andrews T, Roberts DF. Chromosome analyses in couples with repeated pregnancy loss. J Biosoc Sci 1982; 14: 33-52. [DOI:10.1017/S0021932000013845]
12. Tharapel AT, Tharapel SA, Bannerman RM. Recurrent pregnancy losses and parental chromosome abnormalities: a review. Br J Obstet Gynaecol 1985; 92: 899-914. [DOI:10.1111/j.1471-0528.1985.tb03069.x]
13. Tho SP, Byrd JR, McDonough PG. Chromosome polymorphism in 110 couples with reproductive failure and subsequent pregnancy outcome. Fertil Steril 1982; 38: 688-694. [DOI:10.1016/S0015-0282(16)46695-1]
14. Radojcic-Badovinae A, Buretic- Tomljanovic A, Starcevic N, kapovic M, Vlastelic I, Randic L. Chromosome studies in patients with reproductive success. Am J Reprod Immunol 2000; 44: 279-283. [DOI:10.1111/j.8755-8920.2000.440505.x]
15. Duzan F, Atmaca M, Cetin GO, Bagci H. Cytogenetic studies in patients with reproductive failure. Acta Obstet Gynecol Scand 2003; 82: 53-56. [DOI:10.1034/j.1600-0412.2003.820109.x]
16. de Braekeleer M, Dao TN. Cytogenetic studies in couples experiencing repeated pregnancy losses. Hum Reprod 1990; 5: 519-528. [DOI:10.1093/oxfordjournals.humrep.a137135]
17. Van Assche E, Bonduelle M, Tournaye H, Joris H, Verheyen G, Devroey P, et al. Cytogenetics of infertile men. Hum Reprod 1996; 11: 1-26. [DOI:10.1093/humrep/11.suppl_4.1]
18. Johannisson R, Schwinger E, Wolff HH, vom Ende V, Lohrs U. The effect of 13; 14 Robertsonian translocation on germ-cell differentiation in fertile males. Cytogenet Cell Genet 1993; 63: 151-155. [DOI:10.1159/000133524]
19. Gabriel-Robez O, Rumpler Y. The meiotic pairing behavior in human spermatocytes carrier of chromosome anomalies and their repercussions on reproductive fitness. II Robertsonian and reciprocal translocations. A European collaborative study. Ann Genet 1996; 39: 17-25.
20. Tsenghi C, Metaxotou C, Kalpini-Mavrou A, Strataki-Benetou M, Matsaniotis N. Parental chromosome translocations and fetal loss. Obstet Gynecol 1981; 58: 456-458.
21. Schwartz S, Palmer CG. Chromosomal findings in 164 couples with repeated spontaneous abortions: with special considerations to prior reproductive history. Hum Genet1983; 63: 28-34. [DOI:10.1007/BF00285393]
22. Gorski JL, Kistenmacher ML, Punnett HH, Zackai EH, Emanuel BS. Reproductive risks for carriers of complex chromosome rearrangements: analysis of 25 families. Am J Med Genet 1988; 29: 247-261. [DOI:10.1002/ajmg.1320290202]
23. Bourrouillou G, Colombies P, Dastugue N. Chromosome studies in 2136 couples with spontaneous abortions. Hum Genet 1986; 74: 399-401. [DOI:10.1007/BF00280493]
24. Campana M, Serra A, Neri G. Role of chromosome aberrations in recurrent abortion: a study of 269 balanced translocations. Am J Med Genet 1986; 24: 341-356. [DOI:10.1002/ajmg.1320240214]
25. Simpson JL. Genetics for the obstetrician-gynecologist: questions and answers. Clin Obstet Gynecol 1976; 19: 981-1003. [DOI:10.1097/00003081-197612000-00023]
26. Portnoi MF, van den Akker J, Le Porrier N, Youssef S, Taillemite JL. A new case of ring chromosome 9. Sem Hop 1983; 59: 185-188.
27. del Porto G, D'Alessandro E, Grammatico P, Coghi IM, DeSanctis S, Giambenedetti M, et al. Chromosome heteromorphisms and early recurrent abortions. Hum Reprod 1993; 8: 755-788. [DOI:10.1093/oxfordjournals.humrep.a138135]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




