BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijrm.ir/article-1-84-en.html
2- Department of Obstetrics and Gynaecology, Queen Elizabeth II Research Institute for Mothers and Infants, University of Sydney, Australia , helena@med.usyd.edu.au
Endometriosis is an increasingly commonly recognised disease in Western Industrialised Society and is probably also common – but less widely recognised – in the developing world. In spite of a great deal of recent research it is still an enigmatic condition with a high degree of variability from patient to patient – with great variability in age of onset, severity and extent of pain symptoms, occurrence of infertility, anatomical locations , response to treatment and rate of recurrence following treatment. There is a major familial component to the disease and it is likely that a number of different genes contribute to the occurrence and variability of the condition.
In the majority of patients, endometriosis presents with pain symptoms associated with menstruation and pelvic tenderness during sexual intercourse or with a bowel motion. Pelvic pain may also occur erratically at other times, although the classical complaint is of severe, gnawing and dragging pain deep in the pelvis and back which begins in mid-cycle or in the luteal phase and builds up in severity to the onset of bleeding – so-called ‘secondary congestive dysmenorrhoea’.
The mechanisms of pain generation are not well understood, although it is recognised that endometriosis is an “inflammatory” condition with the presence of multiple leukocytes of different types in the endometriotic plaques and eutopic endometrium. Hence, there is the potential for the local production of pain-stimulating molecules within these tissues. Nevertheless, no information is available on pain stimulation pathways or mechanisms for sensory nerve activation in eutopic endometrium or endometriotic plaques.
We have recently made the surprising observation that fine, unmyelinated, sensory nerve fibres are present in the functional layer of eutopic endometrium of women with endometriosis (1, 2). These nerve fibres are also present in ectopic plaques of peritoneal endometriosis (3). By contrast, these nerve fibres are never found in the functional layer of endometrium from women without endometriosis. These observations have the potential to encourage new lines of research into the mechanisms of pain generation and into pain management in endometriosis. They also offer the potential to develop simpler and less invasive techniques for diagnosis of endometriosis.
Discovery of nerve fibres in eutopic endometrium
Small unmyelinated nerve fibres were stained and identified with the highly specific pan-neuronal marker Protein Gene Product 9.5 (PGP9.5) in the functional layer of endometrium in each one of 25 endometrial curettings and 10 full-thickness uterine blocks (from hysterectomy specimens) in women with endometriosis who complained of dysmenorrhoea and a range of related pain symptoms (mean density ± SD, 10 ± 7/mm2 in curettings, mean density ± SD, 11 ± 5/mm2 in hysterectomy tissue, respectively)(1). However, no nerve fibres were detected in the functional layer of endometrium from endometrial curettings or full-thickness uterine hysterectomy blocks in any of the women without endometriosis (n = 47 and 35, respectively) (1).
Much more detailed nerve fibres in the functional layer of endometrium in women with endometriosis showed staining with substance P (SP) (a marker for sensory A delta and sensory C nerve fibres), calcitonin gene-related peptide (CGRP)(a marker for sensory A delta and sensory C nerve fibres), vasoactive intestinal polypeptide (VIP) (a marker for sensory A delta, sensory C and cholinergic nerve fibres) and neuropeptide Y (NPY) (a marker for sensory A delta, sensory C and adrenergic nerve fibres), but not with tyrosine hydroxylase (TH) (a marker for adrenergic nerve fibres) or vesicular acetylcholine transporter (VAChT) (a marker for cholinergic nerve fibres) (2). Specific staining for neurofilament (NF) demonstrated myelinated A delta fibres only in the basal layer of endometrium in women with endometriosis, but not in those without endometriosis. Therefore, nerve fibres in the functional layer of endometrium in women with endometriosis were most likely to be all sensory C fibres. Since many inflammatory mediators can be released from endometrium (4, 5), sensory C fibres in the functional layer of endometrium may be sensitised and/or activated by those inflammatory mediators to induce pain symptoms in women with endometriosis.
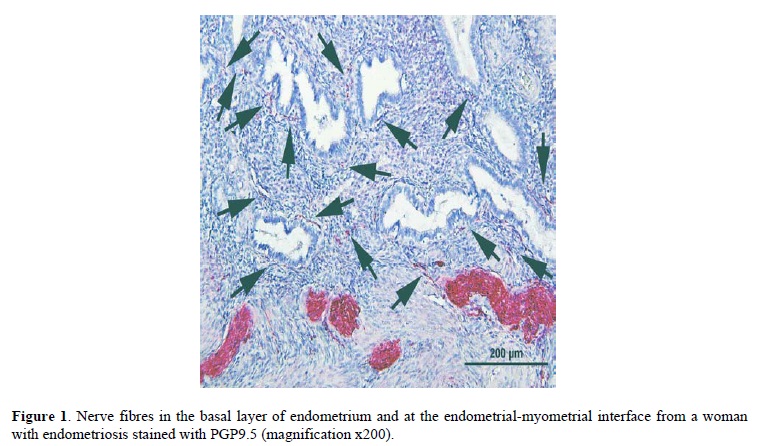
There was a higher density of nerve fibres stained with PGP9.5 in the basal layer of endometrium and in myometrium in women with endometriosis (mean density ± SD, 18 ± 8/mm2, 3.3 ± 1.2/mm2, respectively) than women without endometriosis (mean density ± SD, 0/mm2, and 0.9 ± 0.8/mm2, respectively) (1). Many small unmyelinated and large myelinated nerve fibres were present throughout the basal layer of endometrium in women with endometriosis (Figure 1), however small unmyelinated nerve fibres were rarely detected and myelinated fibres were never detected in the basal layer of endometrium in women without endometriosis (1). Obvious thick trunks were seen in the basal layer or at the endometrial-myometrial interface only in women with endometriosis and never in women without endometriosis (1).
Endometrium in women with endometriosis may produce substantial quantities of regulatory molecules with neurotrophic effects (eg nerve growth factor) to induce ingrowth of nerve fibres.
Different types of nerve fibres in eutopic endometrium in women with and without endometriosis
Nerve fibres in the functional layer of endometrium in women with endometriosis only stained with SP, CGRP, VIP and NPY, and were all considered to be sensory C fibres. There was a greater density of nerve fibres of VIP (mean density ± SD, 8.5 ± 2.3/mm2) and NPY (9.6 ± 2.8/mm2) (Figure 2) than SP (mean density ± SD, 1.0 ± 0.2/mm2) and CGRP (mean density ± SD, 1.7 ± 0.4/mm2)(2). Nerve fibres in the basal layer of endometrium in women with endometriosis were stained with SP, CGRP, TH, VIP, NPY and NF but not with VAChT. Therefore, nerve fibres in the basal layer of endometrium in women with endometriosis were most likely to be a mixture of sensory A delta, sensory C and adrenergic fibres (2). Nerve fibres in the basal layer of endometrium in women without endometriosis were stained with SP, CGRP, VIP and NPY but not NF, TH or VAChT. Therefore, nerve fibres in the basal layer of endometrium were most likely to be sensory C fibres with some myelinated, sensory A delta fibres (2). Nerve fibres in myometrium were stained with SP, CGRP, TH, VAChT, VIP and NPY in both women with and without endometriosis but the density was much less in those without endometriosis (2). Sensory C fibres in the functional layer of endometrium and adrenergic fibres in the basal layer of endometrium were present only in women with endometriosis and these nerve fibres may be associated with generating pain symptoms in women with endometriosis.

Effects of currently available drugs for treatment of endometriosis on endometrial nerve fibres
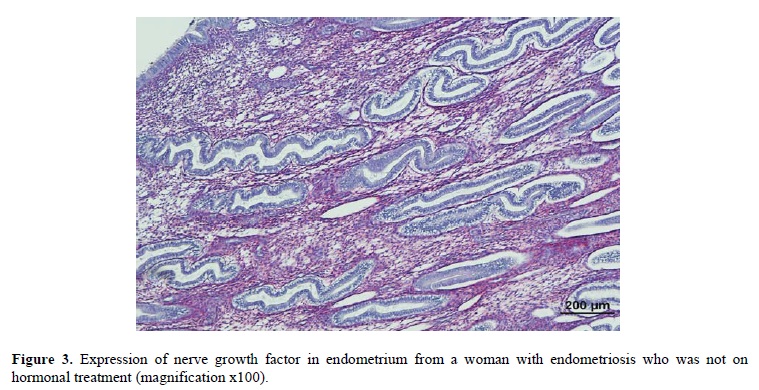
Currently available drugs to treat women with endometriosis-associated pain include danazol, gonadotropin-releasing hormone (GnRH) analogues, combined oral contraceptives (COCs) and progestogens (6) that can suppress endogenous oestrogen and progesterone production and local hormone action. Nerve fibre density in the functional (n = 27) and basal layers (n = 19) of endometrium and myometrium (n = 19) in hormonally treated women with endometriosis was significantly reduced compared with 10 untreated women with endometriosis (data not published). The density of nerve fibres in the functional and basal layers of endometrium (mean density ± SD, 0.2 ± 0.7/mm2, 0.9 ± 1.3/mm2, respectively) and myometrium (1.5 ± 0.8/mm2) in hormonally treated women with endometriosis was much lower than that of functional and basal endometrium (11 ± 5/mm2, 18 ± 8/mm2, respectively; p<0.001) and myometrium (3 ± 1/mm2)(Table I) in women with endometriosis who were not on hormone treatment. Nerve fibres in the functional and basal layers of endometrium in untreated women with endometriosis were stained with SP, CGRP, VIP and NPY, and SP, CGRP, VIP, NPY, NF and TH and respectively (Table I). However, after hormonal treatment, the small number of residual nerve fibres in the functional layer in women with endometriosis only stained with VIP and NPY, and in the basal layer with SP, CGRP, VIP and NPY, (Table I). Nerve growth factor (NGF)(Figure 3) and nerve growth factor receptor 75 (NGFRp75) were over-expressed in endometrium in untreated women with endometriosis, however NGF and NGFRp75 were only very weakly expressed in endometrium in hormonally treated women with endometriosis (data not published). Since oestrogen and progestogen can regulate neurotrophin (7, 8), hormonal therapies for endometriosis may decrease neurotrophin production, resulting in reduced nerve fibre density in endometrium and myometrium in women with endometriosis. This may contribute significantly to the beneficial effect of hormonal therapies on symptoms.
Nerve fibres in peritoneal endometriotic plaques
Peritoneal endometriotic plaques are probably an important source of pain stimuli in many women with endometriosis (9-11) and several researchers have investigated the presence of nerve fibres in endometriotic plaques (3, 12-14). Nerve fibres in endometriotic plaques were stained with SP, CGRP and vesicular monoamine transporter (12), transforming growth factor β1 (TGFβ1)(13) and neurofilament (14). In our study, there was a greater density of nerve fibres stained with PGP9.5 in peritoneal endometriotic plaques in women with endometriosis (mean density ± SD, 16.3 ± 10.0/mm2) than normal peritoneum in women without endometriosis (mean density ± SD, 2.5 ± 1.3/mm2), and nerve fibres were a mixture of sensory A delta, sensory C, adrenergic and cholinergic fibres (3). Nerve fibres were present in greater concentration near endometriotic glands and large amounts of NGF were secreted near endometriotic glands (3). Over-expression of NGF may increase the number of nerve fibres, and many inflammatory mediators released from endometriotic plaques can directly or indirectly sensitise sensory nerve fibres to cause pain symptoms. Since absence of nerve fibres in the functional layer of endometrium after hormonal therapy was not always associated with complete alleviation of pain symptoms in women with endometriosis, there is a need for further investigation of the ways in which hormone therapies for endometriosis can affect nerve fibre density and function in endometriotic plaques.

Concept of endometriosis as an endometrial disease
A great deal of recent research has demonstrated multiple differences in function within the eutopic endometrium of women with endometriosis compared with women who do not have this disease (15). These differences include changes in expression of structural, metabolic and immune cellular proteins such as cytokeratins, integrins, heat shock proteins, actin, intercellular adhesion molecules, apoptosis proteins, transcription factors and a range of other immune and structural proteins (15-17). We have demonstrated differential expression of 119 out of 820 identified endometrial proteins in women with endometriosis compared with women without endometriosis (15).
More recently, it has been demonstrated that angiogenic activity is increased in the eutopic endometrium of endometriosis sufferers (18) and this appears to correlate with angiogenic activity in the typical peritoneal endometriotic ‘flare’ lesions (19). This may provide a contributory mechanism to the vascularisation and growth of new endometriotic plaques. Our own recent observations of unmyelinated sensory nerve fibres in eutopic endometrium (which are not present in “normal” endometrium) provide the first evidence of a reliable structural difference between endometriotic eutopic endometrium and “normal” endometrium. This provides the possibility of reliable diagnosis of endometriosis through an endometrial biopsy (20), and this is discussed in detail below.
The dramatically increased expression of nerve growth factor and nerve growth factor receptor in eutopic endometrium and in endometriotic plaques suggests that this abnormality may precede the growth of fine nerve fibres into these tissues. Hence, this could be an important primary abnormality within eutopic endometrium before the condition recognised as endometriosis actually develops.
Adolescent endometriosis and endometrial nerve fibres
Endometriosis has long been considered as a disease of women over the age of 20 years. However, recent data demonstrate a higher than expected incidence in teenagers (21). Endometriosis has been reported in young girls, even before their menarche (22).
It has been reported that these adolescent women with endometriosis have had uterine contractions with higher frequency, amplitude and basal pressure tone during their menstrual period than expected (23).
The expression of small unmyelinated nerve fibres in both basal and functional layers of the endometrium (1,2) of these young women may have played a role in producing these dynamic changes in the uterus. Furthermore, these changes in intensity of myometrial contractions may fully or partly contribute to the development of pelvic pain symptomatology. The multiplicity of molecular and cellular abnormalities demonstrated in the eutopic endometrium of women with endometriosis (16-18) suggests that primary abnormalities in this tissue may have a role in the genesis of endometriosis and perhaps even in the genesis of symptoms.
These young women with endometriosis frequently present with severe spasmodic central pelvic pain (of presumed uterine origin) which begins at or soon after menarche, and which later develops into a more widespread dragging type of deep pelvic pain and backache with the later development of typical peritoneal endometriosis. We believe that these nerve fibers which are present in the functional layer of endometrium of women with endometriosis, may have been present from before the development of typical endometriosis, having a role in atypical spasmodic pain symptomatology before the onset of the typical peritoneal disease. In these cases, the more extensive but dragging and congestive pelvic pain appearing with the onset of endometriosis can be attributable to more widespread disease involving the pelvic peritoneum and ovaries.
The presence of these nerve fibers in endometrium may be incorporated into a diagnostic tool using endometrial biopsy in the diagnosis of endometriosis in these young girls. This method will be less invasive, less costly and less demanding for these young women compared to more invasive laparoscopy and peritoneal biopsy, which currently remains the gold standard in the diagnosis of endometriosis.
From these data, we hypothesize that in these adolescent girls there may have been a primary inherited abnormality in the endometrium which may have been present well before the onset of typical endometriosis. We also believe that this primary abnormality in the endometrium may have implications for aetiology, pathophysiology, diagnosis and treatment of endometriosis in these young girls.
Endometrial biopsy for diagnosis of endometriosis
For many years, clinicians have been searching for the ideal means of making a definite diagnosis of endometriosis. Laparoscopy with histological confirmation is the current gold standard for endometriosis diagnosis. Yet, as a surgical procedure, laparoscopy still has some risks of injuring vessels or abdominal organs and because of the invasiveness of the procedure there is often a delay before it is contemplated. Moreover, visual inspection of the pelvis also has limitations, particularly for the diagnosis of early peritoneal and deep endometriotic lesions which may not be obvious.
A further problem is the inter-observer variability in assessing atypical lesions. Biopsy is often needed for confirmation. It is not surprising that considerable efforts are being made to improve diagnosis by other means with minimal risks and lower cost.
Based on the novel finding of the unique presence of small nerve fibres in the functional layer of endometrium of women with endometriosis; we conducted a pilot study to explore the possibility of using endometrial biopsy with a suction-enhanced Endosampler to detect endometrial small nerve fibres using immunohistochemistry with the specific pan-neuronal marker PGP9.5. We found that carefully collected endometrial biopsy has 100% specificity and 100% sensitivity in detecting endometriosis when compared with laparoscopy and peritoneal biopsy (20).
Endometrial biopsy is a less invasive procedure than laparoscopy and can usually be achieved without general anesthesia or strong analgesics. It may be useful for the early diagnosis of endometriosis in an outpatient setting. A special target group could be the adolescents with spasmodic dysmenorrhea or atypical pelvic pain and a strong family history of endometriosis, a group in whom delay in diagnosis is typically greater than older women. An endometrial biopsy may enable clinicians to make a more definitive early diagnosis and initiate early therapy. This shortening of diagnosis time will have positive impact on patient’s quality of life and help to protect their future fertility.
Conclusions
This novel observation of fine unmyelinated sensory nerve fibres, capable of mediating pain, in the functional layer of eutopic endometrium of women with endometriosis and in ectopic endometriotic plaques, has opened up a whole new field in the study of mechanisms of symptom development and expression in this condition. The observation also provides the potential for easier and earlier diagnosis. The linking of proteomic and genetic studies should also allow the identification of new mechanisms underlying the development, progression, variability and symptomatology of endometriosis.
Acknowledgements
The authors are grateful to Professor Peter Russell for specialised histopathology advice and for blinded assessment of histology.
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




