Wed, Apr 24, 2024
[Archive]
Volume 18, Issue 2 (February 2020)
IJRM 2020, 18(2): 129-134 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Martínez-Leal B, Ivette Álvarez-Banderas K, Sánchez-Dávila H, Dávila-Rodríguez I, Cortés-Gutiérrez E I. Human papillomavirus as a single infection in pregnant women from Northeastern Mexico: Cross-sectional study. IJRM 2020; 18 (2) :129-134
URL: http://ijrm.ir/article-1-1040-en.html
URL: http://ijrm.ir/article-1-1040-en.html
Bernardo Martínez-Leal1 

 , Karla Ivette Álvarez-Banderas2
, Karla Ivette Álvarez-Banderas2 

 , Homero Sánchez-Dávila2
, Homero Sánchez-Dávila2 

 , Imelda Dávila-Rodríguez3
, Imelda Dávila-Rodríguez3 

 , Elva Irene Cortés-Gutiérrez *
, Elva Irene Cortés-Gutiérrez * 

 4
4


 , Karla Ivette Álvarez-Banderas2
, Karla Ivette Álvarez-Banderas2 

 , Homero Sánchez-Dávila2
, Homero Sánchez-Dávila2 

 , Imelda Dávila-Rodríguez3
, Imelda Dávila-Rodríguez3 

 , Elva Irene Cortés-Gutiérrez *
, Elva Irene Cortés-Gutiérrez * 

 4
4
1- Universidad de Monterrey, Vicerrectoría Ciencias de la Salud, San Pedro Garza García, México.
2- Department of Clinical Dysplasia, Gynecology and Obstetrics No.23 Hospital, Mexican Institute of Social Security, Monterrey, Mexico.
3- Universidad Autónoma de Nuevo León, Faculty of Public Health and Nutrition, Monterrey, México.
4- Universidad Autónoma de Nuevo León, Faculty of Biological Sciences, San Nicolás de los Garza, México. , elvairenecortes@yahoo.com.mx
2- Department of Clinical Dysplasia, Gynecology and Obstetrics No.23 Hospital, Mexican Institute of Social Security, Monterrey, Mexico.
3- Universidad Autónoma de Nuevo León, Faculty of Public Health and Nutrition, Monterrey, México.
4- Universidad Autónoma de Nuevo León, Faculty of Biological Sciences, San Nicolás de los Garza, México. , elvairenecortes@yahoo.com.mx
Full-Text [PDF 264 kb]
(638 Downloads)
| Abstract (HTML) (2145 Views)
Thus, this case-control study was conducted to investigate the presence of HPV as single or multiple infections in cervical samples from pregnant and nonpregnant women using the INNO-LiPA test.
All pregnant women who visited the Gynecology and Obstetrics Department of UMAE-23, Instituto Mexicano del Seguro Social (IMSS), Mexico, for the first time during pregnancy were invited to join the study. A gynecologist explained the aim of the study, the procedures, and the complications involved. Control samples were obtained from women attending early cancer detection programs at the same hospital. This was followed by an interview conducted by research assistants, in which all the participants responded to a questionnaire respecting reproductive history and sexual behavior.
None of the women included in the study presented cervical lesions, as assessed by clinical examination of the cervix, cytology, and colposcopy. Women with immunosuppressive diseases, as hepatitis C, hepatitis B, HIV, and diabetes mellitus, were eliminated from the study.
Cervical samples were obtained with a cytobrush in the course of gynecological exploration. The cytobrush was inserted into the endocervical canal, rotated 3 to 5 times, placed into the transport medium (Preserv-Cyt solution; Hologic, Bedford, MA), and immediately sent to the Laboratory of Molecular Cytogenetics of Centro de Investigación Biomédica del Noreste, IMSS, Mexico. Cervical scraping samples were frozen at -20ºC until DNA extraction. DNA was extracted using a Wizard Genomic DNA Purification Kit (Promega, Madison, WI). The DNA quantity and quality were verified using a NanoDrop® Jenway kit (Genova Life Science, OSA, UK).
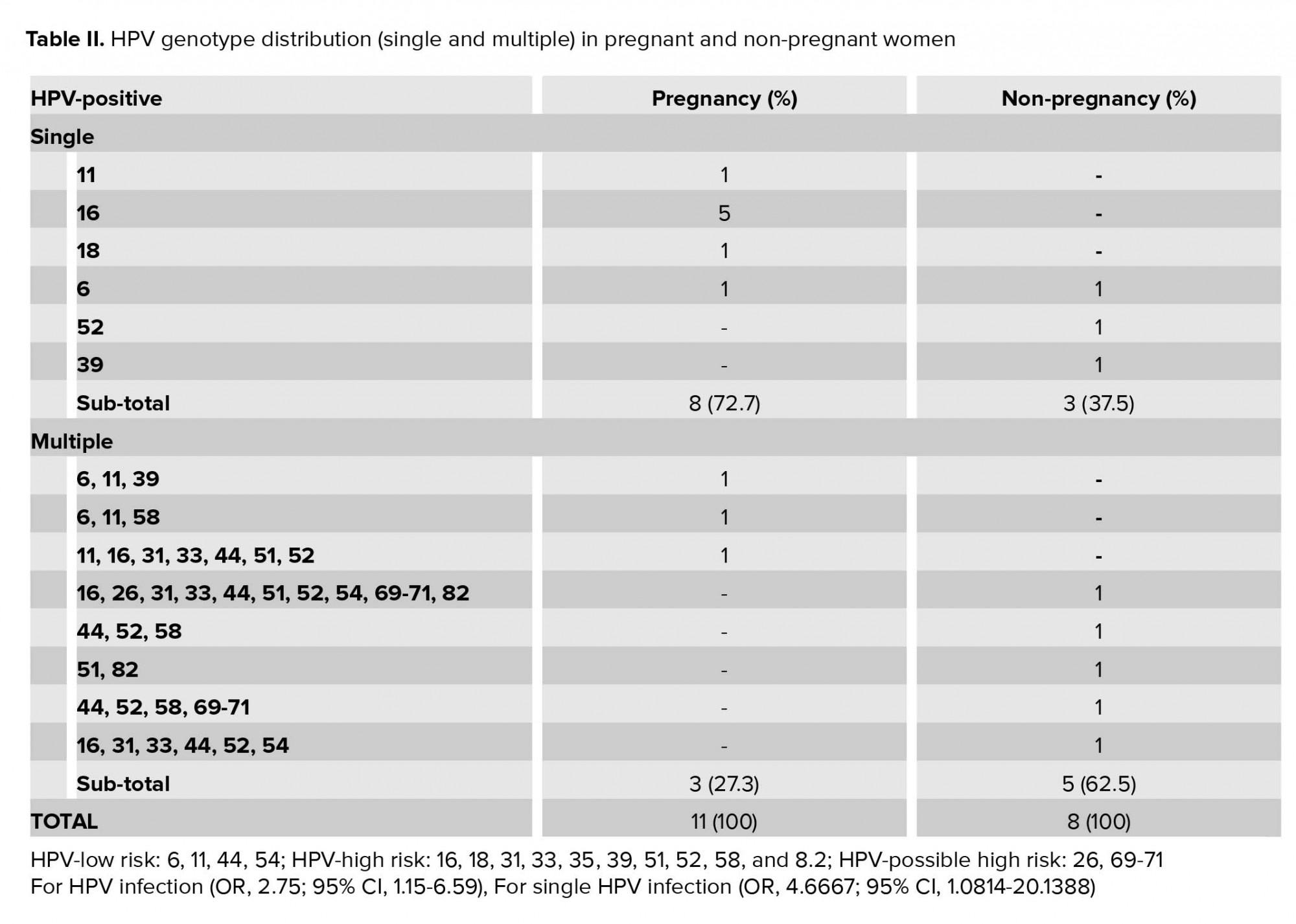
Among the 31 pregnant women studied here, 11 (35%) were HPV-positive. Of these, eight (73%) were infected with a single genotype and three (27%) were infected with multiple genotypes. HPV-16 was detected in six pregnant women (54%; five as a single infection and one as multiple infections). Among the 62 nonpregnant women, eight were HPV-positive (12%). Of these, three (36%) were infected with a single genotype and five (64%) were infected with multiple genotypes. The HPV-44 and HPV-52 genotypes were the most prevalent in all cases involving multiple infections (Table II).
A multivariate analysis showed that pregnancy was associated with an increased risk for multiple HPV infection (OR, 2.75; 95% CI, 1.15-6.59) and for single HPV infection (OR, 4.6667; 95% CI, 1.0814-20.1388).

4. Discussion
In this preliminary study, we determined the prevalence of HPV as single or multiple infections in pregnant women from Northeastern Mexico. Our findings revealed a significant increase in HPV prevalence among pregnant women (35%) compared with nonpregnant women (13%) (OR = 2.7).
Studies comparing the distribution of HPV types in pregnant and nonpregnant women are scarce in the Mexican population. Hernández-Girón et al. (10, 11) reported a higher prevalence of HR HPV infection in pregnant women compared with nonpregnant women, with detection rates of 37.2% (95% CI, 31-43) and 14.2% (95% CI, 12-16), respectively. Previous studies have reported similar findings (12-14).
Liu et al. (15) performed a meta-analysis of the risk of HPV infection in pregnant women from Asia, Europe, and North America and found a significantly increased risk among pregnant women (16%, 13%, and 30%, respectively), with a general OR of 1.42.
In contrast, Nobbenhuis (16) found no difference in HPV prevalence between pregnant and nonpregnant women. Such discrepancies may have arisen from the HPV detection method used and the number of detectable HPV genotypes. In our study, we used the INNO-LiPA test, which is able to identify 28 anogenital HPV genotypes and includes two internal controls and two positive controls. The INNO-LiPA test permits the simultaneous detection of multiple genotypes in a single sample.
Previous study reported a dependence of HPV prevalence on age (11), parity, smoking history, oral contraceptive use, and history of sexually transmitted infections. In our study, the groups under study were homogeneous in terms of these factors (17-19).
A possible explanation for the higher prevalence of HPV found in pregnant women respect to nonpregnant women is based that immunosuppression occurs during pregnancy because of the reduction of cellular immunity (7). It has been suggested that the high levels of estrogen and progesterone that occur during pregnancy decrease the cellular immunity, which is crucial for resolve the HPV infection. Our previous results showed that a high prevalence of HPV-52 and HPV-44 was involved in multiple infections in nonpregnant women (2). In contrast, a high prevalence of HPV-16 as a single infection was observed in pregnant women.
Interestingly, our results revealed that 78% of the pregnant women who were HPV-positive presented with a single infection compared with 28% of the nonpregnant women.
The transcriptional promoter of the E6/E7 oncogenic region of HPV-16 contains a steroid-hormone-receptor-binding element that activate HPV E6/E7 transcription, suggesting a hormonal stimulation effect on HPV replication. These observations indicate that pregnancy may have an effect on the patient’s capacity to eradicate multiple HPV infections (20). Yamasaki (21) reported that accelerated clearance of HPV may be caused by the normalization of the host’s immune system during the third trimester of pregnancy. Thus, the presence of HR HPV in the late period of pregnancy can be considered as being persistent and, therefore, represents a risk factor for neoplastic lesions.
In conclusion, a higher prevalence of HPV as a single infection in pregnant women was found, despite the number of women included in the study was relatively small. Further investigations are necessary to establish the biological role of HPV as a single infection and its participation in the development of precancerous lesions or cervical carcinoma.
Acknowledgments
This study was financially supported by the Fondo Sectorial de Investigación en Salud y Seguridad Social SS/IMSS/ISSSTE-CONACYT (293539), Mexico.
The authors would like to thank Sanjuana L. Guardado Limón for her excellent technical assistance. They are also grateful to Fernando Hernandez Garza MD (ϯ) from the Dysplasia Clinic, UMAE-23, IMSS, for his valuable support in the clinical diagnosis of the patients.
Conflict of Interest
The authors declare that there is no conflict of interest regarding this study.
Full-Text: (347 Views)
- Introduction
Thus, this case-control study was conducted to investigate the presence of HPV as single or multiple infections in cervical samples from pregnant and nonpregnant women using the INNO-LiPA test.
- Materials and Methods
- 1. Subjects and sample collection
All pregnant women who visited the Gynecology and Obstetrics Department of UMAE-23, Instituto Mexicano del Seguro Social (IMSS), Mexico, for the first time during pregnancy were invited to join the study. A gynecologist explained the aim of the study, the procedures, and the complications involved. Control samples were obtained from women attending early cancer detection programs at the same hospital. This was followed by an interview conducted by research assistants, in which all the participants responded to a questionnaire respecting reproductive history and sexual behavior.
None of the women included in the study presented cervical lesions, as assessed by clinical examination of the cervix, cytology, and colposcopy. Women with immunosuppressive diseases, as hepatitis C, hepatitis B, HIV, and diabetes mellitus, were eliminated from the study.
Cervical samples were obtained with a cytobrush in the course of gynecological exploration. The cytobrush was inserted into the endocervical canal, rotated 3 to 5 times, placed into the transport medium (Preserv-Cyt solution; Hologic, Bedford, MA), and immediately sent to the Laboratory of Molecular Cytogenetics of Centro de Investigación Biomédica del Noreste, IMSS, Mexico. Cervical scraping samples were frozen at -20ºC until DNA extraction. DNA was extracted using a Wizard Genomic DNA Purification Kit (Promega, Madison, WI). The DNA quantity and quality were verified using a NanoDrop® Jenway kit (Genova Life Science, OSA, UK).
- 2. Detection/genotyping
- 3. Ethical consideration
- Statistical analysis
- Results
Among the 31 pregnant women studied here, 11 (35%) were HPV-positive. Of these, eight (73%) were infected with a single genotype and three (27%) were infected with multiple genotypes. HPV-16 was detected in six pregnant women (54%; five as a single infection and one as multiple infections). Among the 62 nonpregnant women, eight were HPV-positive (12%). Of these, three (36%) were infected with a single genotype and five (64%) were infected with multiple genotypes. The HPV-44 and HPV-52 genotypes were the most prevalent in all cases involving multiple infections (Table II).
A multivariate analysis showed that pregnancy was associated with an increased risk for multiple HPV infection (OR, 2.75; 95% CI, 1.15-6.59) and for single HPV infection (OR, 4.6667; 95% CI, 1.0814-20.1388).


4. Discussion
In this preliminary study, we determined the prevalence of HPV as single or multiple infections in pregnant women from Northeastern Mexico. Our findings revealed a significant increase in HPV prevalence among pregnant women (35%) compared with nonpregnant women (13%) (OR = 2.7).
Studies comparing the distribution of HPV types in pregnant and nonpregnant women are scarce in the Mexican population. Hernández-Girón et al. (10, 11) reported a higher prevalence of HR HPV infection in pregnant women compared with nonpregnant women, with detection rates of 37.2% (95% CI, 31-43) and 14.2% (95% CI, 12-16), respectively. Previous studies have reported similar findings (12-14).
Liu et al. (15) performed a meta-analysis of the risk of HPV infection in pregnant women from Asia, Europe, and North America and found a significantly increased risk among pregnant women (16%, 13%, and 30%, respectively), with a general OR of 1.42.
In contrast, Nobbenhuis (16) found no difference in HPV prevalence between pregnant and nonpregnant women. Such discrepancies may have arisen from the HPV detection method used and the number of detectable HPV genotypes. In our study, we used the INNO-LiPA test, which is able to identify 28 anogenital HPV genotypes and includes two internal controls and two positive controls. The INNO-LiPA test permits the simultaneous detection of multiple genotypes in a single sample.
Previous study reported a dependence of HPV prevalence on age (11), parity, smoking history, oral contraceptive use, and history of sexually transmitted infections. In our study, the groups under study were homogeneous in terms of these factors (17-19).
A possible explanation for the higher prevalence of HPV found in pregnant women respect to nonpregnant women is based that immunosuppression occurs during pregnancy because of the reduction of cellular immunity (7). It has been suggested that the high levels of estrogen and progesterone that occur during pregnancy decrease the cellular immunity, which is crucial for resolve the HPV infection. Our previous results showed that a high prevalence of HPV-52 and HPV-44 was involved in multiple infections in nonpregnant women (2). In contrast, a high prevalence of HPV-16 as a single infection was observed in pregnant women.
Interestingly, our results revealed that 78% of the pregnant women who were HPV-positive presented with a single infection compared with 28% of the nonpregnant women.
The transcriptional promoter of the E6/E7 oncogenic region of HPV-16 contains a steroid-hormone-receptor-binding element that activate HPV E6/E7 transcription, suggesting a hormonal stimulation effect on HPV replication. These observations indicate that pregnancy may have an effect on the patient’s capacity to eradicate multiple HPV infections (20). Yamasaki (21) reported that accelerated clearance of HPV may be caused by the normalization of the host’s immune system during the third trimester of pregnancy. Thus, the presence of HR HPV in the late period of pregnancy can be considered as being persistent and, therefore, represents a risk factor for neoplastic lesions.
In conclusion, a higher prevalence of HPV as a single infection in pregnant women was found, despite the number of women included in the study was relatively small. Further investigations are necessary to establish the biological role of HPV as a single infection and its participation in the development of precancerous lesions or cervical carcinoma.
Acknowledgments
This study was financially supported by the Fondo Sectorial de Investigación en Salud y Seguridad Social SS/IMSS/ISSSTE-CONACYT (293539), Mexico.
The authors would like to thank Sanjuana L. Guardado Limón for her excellent technical assistance. They are also grateful to Fernando Hernandez Garza MD (ϯ) from the Dysplasia Clinic, UMAE-23, IMSS, for his valuable support in the clinical diagnosis of the patients.
Conflict of Interest
The authors declare that there is no conflict of interest regarding this study.
Type of Study: Original Article |
Subject:
Pregnancy Health
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69-90. [DOI:10.3322/caac.20107] [PMID]
2. Aguilar- Lemarroy A, Vallejo-Ruiz, Cortés Gutiérrez EI, Salgado-Bernabé ME, Ramos-González NP, Ortega-Cervantes, L, et al. Human papillomavirus infections in Mexican women with normal cytology, precancerous lesions, and cervical cancer: type-specific prevalence and HPV coinfections. J Med Virol 2015; 87: 871-884. [DOI:10.1002/jmv.24099] [PMID]
3. Schlecht NF, Kulaga S, Robitaille J, Ferraira S, Santos M, Miyamura RA, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA 2001; 286: 3106-3114. [DOI:10.1001/jama.286.24.3106] [PMID]
4. Clifford GM, Smith JS, Aguado T, Franceshi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer 2003; 89: 101-105. [DOI:10.1038/sj.bjc.6601024] [PMID] [PMCID]
5. Snijders PJ, Steenbergen RD, Heideman DA, Meijer CJ. HPV-mediated cervical carciogenesis: concepts and clinical implications. J Pathol 2005; 208: 152-164. [DOI:10.1002/path.1866] [PMID]
6. Torres-Mejia G, Ortega-Olvera C, Angeles-Llerenas A, Villalobos-Hernández AL, Salmerón-Castro J, Lazcano-Ponce E, et al. Utilization patterns of prevention and early diagnosis for cancer in women. Salud Publica Mex 2013; 55 (Suppl.): S241-S248. [DOI:10.21149/spm.v55s2.5121]
7. Sethi S, Mϋller M, Schneider A, Blettner M, Smith E, Turek L, et al. Serologic response to the E4, E6 and E7 proteins of human papillomavirus type 16 in pregnant women. Am J Obstet Gynecol 1998; 178: 360-364. [DOI:10.1016/S0002-9378(98)80026-4]
8. Banura C, Franceschi S, van Doorn LJ, Arsian A, Kleter B, Wabwire-Mangen F, et al. Prevalence, incidence and clearance of human papillomavirus infection among young primiparous pregnant women in Kampala Uganda. Int J Cancer 2008; 123: 2180-2187. [DOI:10.1002/ijc.23762] [PMID]
9. Schiffman M, Clifford G, Buonaguro FM. Classification of weakly carcinogenic human papillomavirus types: addressing the limits of epidemiology at the borderline. Infect Agent Cancer 2009; 4: 1-8. [DOI:10.1186/1750-9378-4-8] [PMID] [PMCID]
10. Hernández-Girón C, Smith JS, Lorincz A, Arreola Cháidez E, Lazcano E, Hernández-Avila M, et al. The prevalence of high-risk HPV infection in pregnant women from Morelos, México. Salud Publica Mex 2005; 47: 423-429. [DOI:10.1590/S0036-36342005000600006] [PMID]
11. Hernández-Girón C, Smith JS, Lorincz A, Lazcano E, Hernández-Avila M, Salmerón J. High-risk human papillomavirus detection and related risk factors among pregnant and nonpregnant women in Mexico. Sex Transm Dis 2005; 32: 613-618. [DOI:10.1097/01.olq.0000179888.47309.db] [PMID]
12. Gajewska M, Marianowski L, Wielgos M, Malejczyk M, Majewski S. The occurrence of genital types of human papillomavirus in normal pregnancy and in pregnant women with pregestational insulin dependent diabetes mellitus. Neuro Endocrinol Lett 2005; 26: 766-770.
13. Fife KH, Katz BP, Roush J, Handy VD, Brown DR, Hansell R. Cancer-associated human papillomavirus types are selectively increased in the cervix of women in the first trimester of pregnancy. Am J Obstet Gynecol 1996; 174: 1487-1493. [DOI:10.1016/S0002-9378(96)70593-8]
14. Morrison EA, Gammon MD, Goldberg GL, Vermund SH, Burk RD. Pregnancy and cervical infection with human papillomaviruses. Int J Gynaecol Obstet 1996; 54: 125-130. [DOI:10.1016/0020-7292(96)02694-X]
15. Liu P, Xu L, Sun Y, Wang Z. The prevalence and risk of human papillomavirus infection in pregnant women. Epidemiol Infect 2014; 142: 1567-1578. [DOI:10.1017/S0950268814000636] [PMID]
16. Nobbenhuis MA, Helmerhorst TJ, van den Brule AJ, Rozendaal L, Bezemer PD, Voorhorst FJ, et al. High-risk human papillomavirus clearance in pregnant women: trends for lower clearance during pregnancy with a catch-up postpartum. Br J Cancer 2002; 87: 75-80. [DOI:10.1038/sj.bjc.6600367] [PMID] [PMCID]
17. Tarney CM, Beltran TA, Klaric J, Han JJ. Tobacco use and prevalence of human papillomavirus in self-collected cervicovaginal swabs between 2009 and 2014. Obstet Gynecol 2018; 132: 45-51. [DOI:10.1097/AOG.0000000000002681] [PMID]
18. Silva KC, Rosa ML, Moyse N, Afonso LA, Oliveira LH, Cavalcanti SM. Risk factors associated with human papillomavirus infection in two populations from Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz 2009; 104: 885-891. [DOI:10.1590/S0074-02762009000600011] [PMID]
19. Lee H, Lee DH, Song YM, Lee K, Sung J, Ko G. Risk factors associated with human papillomavirus infection status in a Korean cohort. Epidemiol Infect 2014; 142: 1579-1589. [DOI:10.1017/S0950268813002549] [PMID]
20. Chan PK, Chang AR, Tam WH, Cheung JL, Cheng AF. Prevalence and genotype distribution of cervical human papillomavirus infection: comparison between pregnant women and non-pregnant controls. J Med Virol 2002; 67: 583-588. [DOI:10.1002/jmv.10142] [PMID]
21. Yamasaki K, Miura K, Shimada T, Miura S, Abe S, Murakami M, et al. Epidemiology of human papillomavirus genotypes in pregnant Japanese women. J Hum Genet 2011; 56: 313-315. [DOI:10.1038/jhg.2011.11] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





