Mon, Jan 5, 2026
[Archive]
Volume 6, Issue 3 (7-2008)
IJRM 2008, 6(3): 71-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Khaki A, Heidari M, Ghaffari Novin M, Khaki A A. Adverse effects of ciprofloxacin on testis apoptosis and sperm parameters in rats. IJRM 2008; 6 (3) :71-0
URL: http://ijrm.ir/article-1-110-en.html
URL: http://ijrm.ir/article-1-110-en.html
1- Department of Veterinary Pathology, Islamic Azad University of Tabriz, Tabriz, Iran
2- Avesina Research Center, Tehran, Iran
3- Cellular and Molecular Biology Research Center, Shaheed Beheshti University M.C., Tehran, Iran ,mghaffarin@yahoo.com
4- Department of Anatomical Science, Tabriz University of Medical Science, Tabriz, Iran
2- Avesina Research Center, Tehran, Iran
3- Cellular and Molecular Biology Research Center, Shaheed Beheshti University M.C., Tehran, Iran ,
4- Department of Anatomical Science, Tabriz University of Medical Science, Tabriz, Iran
Full-Text [PDF 165 kb]
(1650 Downloads)
| Abstract (HTML) (4272 Views)
Full-Text: (1106 Views)
Introduction
In the last few years, a marked decrease in the quality of semen has been reported (1). These changes in semen quality are more likely to be due to environmental factors. Chemicals and drugs which are particularly misused are among these environmental factors (2). Antibiotics are commonly prescribed for a multitude of everyday condition. Not surprisingly, a proportion of male patients attending fertility clinics may have been prescribed antibiotics by their general practitioner to treat these unrelated infections (3). In addition, some patients requiring assisted conception occasionally show evidence of infection of the male reproductive tract (4, 5). The antibiotic ciprofloxacin is routinely used by urologists, andrologist and fertility specialists to treat such bacterial infections occurring prior to in vitro fertilization treatment, or when high concentration of leukocytes are present in the semen of these patients, irrespective of microbial evidence of infection (6).Ciprofloxacin is a synthetic antibacterial agent belonging to the family of fluoroquinolones with a very broad spectrum against of microbial pathogens, especially Gram-negative infectious diseases, that has been approved in more than 100 countries world-wide (7). Ciprofloxacin is well absorbed orally and induced its antibacterial action mainly by inhibition of DNA gyras, which is equivalent to topoisomerase II in mammalian cell (8, 9). It is known that Ciprofloxacin can be transported to the seminal fluid and can directly affect sperm cells resulting in physiological, metabolic and /or genetic changes. Ciprofloxacin was detected in the prostatic tissue and seminal fluid in high concentration (10). In vivo genotoxicity studies suggest ciprofloxacin as safe for therapeutic use (6). However, other studies have demonstrated ciprofloxacin to significantly impair both testicular function and structure (11, 12). Administration of ciprofloxacin significantly reduced sperm count, motility and daily sperm production in rats (11). Demir et al also showed that in healthy rats, ciprofloxacin caused recognizable histological damage associated with a mild decrease in testicular volume and sperm concentration (12).
The aim of the present study was to define the effect of ciprofloxacin on sperm parameters and germ cells apoptosis in rat by using TUNEL assay for measurement of DNA fragmentation.
Materials and methods
Experimental animals
Twenty adult Wistar albino male rats 8 weeks old and weighing 250±10g were obtained from animal facility of Pasture Institute of Iran. All animals were treated in accordance to the Principles of Laboratory Animal Care (13). All rats were fed a standard diet and water. The daily intake of animal water was monitored at least one week prior to start of ciprofloxacin treatment in order to determine the amount of water needed per experimental animal.Thereafter, the animals were separated at random into two groups: experimental group received 12.5 mg/kg oral ciprofloxacin (Sigma 33433, USA) via gavage for 60 continuous days, the control group receiving only water and food. The dosages ciprofloxacin were similar to those used in human therapy. Body weight daily intake of food and water were determined several times per week throughout the study. All chemicals used in the present study were purchased from Sigma chemical.
Surgical procedure
On sixtieth day, the pentobarbital sodium (40 mg/kg) was administered intra peritoneal for anesthesia, and the peritoneal cavity was opened through a lower transverse abdominal incision. The reproductive organs (testes, epididymides and seminal vesicles) were carefully removed, washed in normal saline solution (0.9%), blotted and weighed. At the end of the experiment, the animals were anesthetized with diethyl ether and killed by decapitation according to recommendation of the Institutional Ethical Committee.
Epididymal spermatozoa motility, viability and count
Epididymal spermatozoa were collected by cutting the cauda region of the epididymis into small pieces in 5 ml of Ringer’s medium at 37°C. A sperm viability test was done by the method described by World Health Organization (14). Assessment of sperm count and motility were performed according to Freund and Carol (15). Briefly, both cauda epididymis from each rat were placed in 2 ml of normal saline pre-warmed to 37°C. Small cuts were made in the two cauda epididymis where the spermatozoa were obtained and suspended in the saline solution. Two hundred microlitres of the suspension was diluted with 800 ml of saline. A small amount of the diluted suspension was transferred to both chambers of a Neubauer haemocytometer using a Pasteur pipette by touching the edge of the cover slip and allowing each chamber to be filled by capillary action.
Light microscopy
The testis was fixed in 10% formalin and embedded in paraffin. Five-micron thick sections were prepared and stained with hematoxylin and eosin (HE). The specimens were examined under Olympus/3H light microscope. Two perpendicular diameters of 10 seminiferous tubules were measured each in 10 slides (10×10=100 seminiferous tubules in each groups), at 40× magnification, with the aid of an ocular reticule standardized with a stage micrometer. Values were recorded as mean diameter of seminiferous tubule (16). The diameters of venous were also measured at the same way.
TUNEL analysis of apoptosis
The in-situ DNA fragmentation was visualized by TUNEL method (17). Briefly, dewaxed tissue sections were predigested with 20 mg/ml proteinase K for 20 min and incubated in phosphate buffered saline solution (PBS) containing 3 % H2O2 for 10 min to block the endogenous peroxidase activity.
The sections were incubated with the TUNEL reaction mixture, fluorescein-dUTP (in situ Cell Death Detection, POD kit, Roche, Germany), for 60 min at 37°C. The slides were then rinsed three times with PBS and incubated with secondary antifluorescein-POD-conjugate for 30 min. After washing three times in PBS, diaminobenzidine-H2O2 (DAB, Roche, Germany) chromogenic reaction was added on sections and counterstained with hematoxylin. As a control for method specificity, the step using the TUNEL reaction mixture was omitted in negative control serial sections, and nucleotide mixture in reaction buffer was used instead. Apoptotic germ cells were quantified by counting the number of TUNEL stained nuclei per seminiferous tubular cross section. Cross sections of 100 tubules per specimen were assessed and the mean number of TUNEL positive germ cells per tubule cross- section was calculated.
Statistical analysis
Statistical comparisons were made using the Fisher -test for comparison of data in the control group with the experiment group. The results were expressed as mean ± S.E. (standard error). p-value less than 0.05 were considered significant.
Results
Weight of individual male reproductive organs
Weights of the testis, epididymis and seminal vesicle were significantly lower in treated animals with ciprofloxacin (12.5mg/kg/day), relative to the control group (p<0.05), which suggests that these antibiotics have the toxicity to male reproductive organs (Table I).

Sperm count, viability and motility
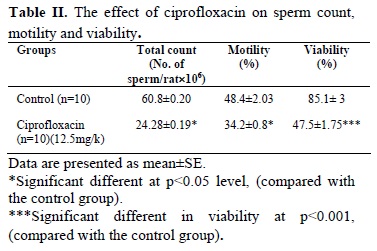
Administration of ciprofloxacin 12.5mg/kg/day, orally, for 60 consecutive days significantly reduced sperm count and motility in experimental group as compared with control group (p<0.05) (Table II). Moreover, sperm viability was significantly declined in the treatment group when compared to the control group (p<0.001)(Table II).

Light microscopic study
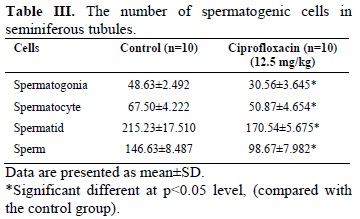
Histopathological study showed the cycle of spermatogenesis was regular in all males in the control group (Figure A). However, ciprofloxacin treatment resulted in a significant decrease in the number of spermatogenic cells (spermatogonia, spermatocyte, spermatid and sperm) in the seminiferous tubules (Figure B) (Table III). Intertubular spaces and veins congestion were increased in the treatment group as compared with those seen in the control group. Morphometeric study showed the diameter of seminiferous tubule (ST) was (250±0.77) and (285±0.81) µm in the experimental and control group, respectively.
This data is shown the diameter of (ST) was decreased in the experimental group when compared with the control group (Table IV). The diameter of veins was 0.921±0.01 µm and 1.73 ±.07 µm in the control and the experimental groups, respectively.


Result of apoptotic germ cells
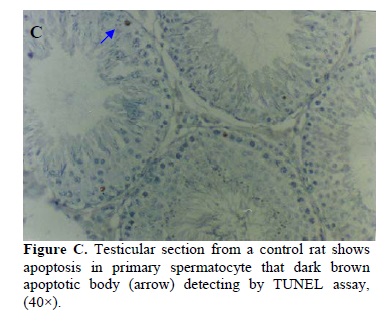
In this study, the number of TUNEL positive germ cells per tubule cross-section increased following ciprofloxacin treatment compared to the control group. The TUNEL positive spermatogonia and spermatocytes were the main germ cells undergoing apoptosis. Figures C and D demonstrate the typical histograms of TUNEL assay analyzed by light microscope in both groups. Using the in situ detection method by which the apoptotic cells can be identified by their darkly stained nuclei, we observed a low incidence of spontaneous apoptosis in normal rat testis from the control group. Although in low numbers, apoptotic germ cells were also observed in the control rats. The rate of total apoptotic cells (spermatogonia and spermatocytes) were 15.11±3.523 and 7.3±0.762 in the experimental and the control groups, respectively according to the definition of Clermont method (18).

Discussion
The therapeutic and prophylactic effects of ciprofloxacin on several infections have been well documented. However, the results of our experimental study reveal that prolonged administration of therapeutic dose of ciprofloxacin was promoted male reproductive toxicity in rats. Degenerative changes in the seminiferous tubules and reduction in sperm count and motility are the evidence for this toxicity.
The present study indicated that, administration of ciprofloxacin for 60 consecutive days, results in a marked reduction in sperm count, sperm motility and viability as compared to respective controls. This is in agreement with that of Abd-Allah et al, who reported that ciprofloxacin treatment for 15 days in rats caused in a marked reduction in sperm count and motility (11). In addition, Demir et al, has similarly shown that ciprofloxacin treatment for 10 days in rats resulted in a marked reduction in sperm count and motility (12). Previous studies in which male patients were given 250 mg ciprofloxacin twice a day did not show differences in sperm quality (19) and was without effect of spermatogenesis (20). In contrast, at pharmacologic concentration (100× physiologic), ciprofloxacin adversely affected human sperm motility with decrease rapid progression in vitro (21). The reasons for the discrepancy may be due to differences between in vitro and in vivo studies. Thus, up to 20% of each ciprofloxacin dose is metabolized in vivo, and ciprofloxacin metabolites may exert some biological activities that are not detectable by in vitro assays (22). On the other hand, the possible release of bacterial factors, from indigenous bacteria killed by the drug, should not be neglected.
It has been reported that the decrease in sperm count and motility are valid indices of male infertility in laboratory animals (23, 24). However, sperm motility is often used as a marker of chemical-induced testicular toxicity (25). They have also stated that the disruption of seminiferous epithelium is indicative of male reproductive hazard. Therefore, our experimental results suggest a gonadotoxic potential of ciprofloxacin. One of the reason for this effect can be explained on the basis that, ciprofloxacin interferes with the energy production process required for sperm vitality and motility (26). Apoptosis of select germ cells occurs normally in the testis and is essential for the normal maintenance of spermatogenesis (27-29). However, the relatively small increase in the percentage of germ cell apoptosis can result in defective spermatogenesis leading to infertility (30). Increases in the incidence of germ cell apoptosis often are observed as a result of various forms of physical or chemical injury to the testis (31). The present study is demonstrated that apoptosis in male rat germ cells is induced by ciprofloxacin. These results indicate that ciprofloxacin like other chemical agents may directly interfere in the process of spermatogenesis. This increase in germ cell apoptosis is possibly partly due to an increased peroxide radical generation in the testis following ciprofloxacin treatment (32). This increase then induces DNA single-strand breaks and chromosomal aberrations as demonstrated by in vitro genotoxicity studies (33, 34). In addition, previous study showed that caspase-3, which has an important role in apoptosis, could be activated by ciprofloxacin (35, 36). Therefore, ciprofloxacin could also induce apoptotic pathways through activation of Caspases. In conclusion, this study may suggest that ciprofloxacin has potential testicular toxicity as evidenced by decreased sperm count and motility, increase apoptotic cells and pathological testis changes.
In the last few years, a marked decrease in the quality of semen has been reported (1). These changes in semen quality are more likely to be due to environmental factors. Chemicals and drugs which are particularly misused are among these environmental factors (2). Antibiotics are commonly prescribed for a multitude of everyday condition. Not surprisingly, a proportion of male patients attending fertility clinics may have been prescribed antibiotics by their general practitioner to treat these unrelated infections (3). In addition, some patients requiring assisted conception occasionally show evidence of infection of the male reproductive tract (4, 5). The antibiotic ciprofloxacin is routinely used by urologists, andrologist and fertility specialists to treat such bacterial infections occurring prior to in vitro fertilization treatment, or when high concentration of leukocytes are present in the semen of these patients, irrespective of microbial evidence of infection (6).Ciprofloxacin is a synthetic antibacterial agent belonging to the family of fluoroquinolones with a very broad spectrum against of microbial pathogens, especially Gram-negative infectious diseases, that has been approved in more than 100 countries world-wide (7). Ciprofloxacin is well absorbed orally and induced its antibacterial action mainly by inhibition of DNA gyras, which is equivalent to topoisomerase II in mammalian cell (8, 9). It is known that Ciprofloxacin can be transported to the seminal fluid and can directly affect sperm cells resulting in physiological, metabolic and /or genetic changes. Ciprofloxacin was detected in the prostatic tissue and seminal fluid in high concentration (10). In vivo genotoxicity studies suggest ciprofloxacin as safe for therapeutic use (6). However, other studies have demonstrated ciprofloxacin to significantly impair both testicular function and structure (11, 12). Administration of ciprofloxacin significantly reduced sperm count, motility and daily sperm production in rats (11). Demir et al also showed that in healthy rats, ciprofloxacin caused recognizable histological damage associated with a mild decrease in testicular volume and sperm concentration (12).
The aim of the present study was to define the effect of ciprofloxacin on sperm parameters and germ cells apoptosis in rat by using TUNEL assay for measurement of DNA fragmentation.
Materials and methods
Experimental animals
Twenty adult Wistar albino male rats 8 weeks old and weighing 250±10g were obtained from animal facility of Pasture Institute of Iran. All animals were treated in accordance to the Principles of Laboratory Animal Care (13). All rats were fed a standard diet and water. The daily intake of animal water was monitored at least one week prior to start of ciprofloxacin treatment in order to determine the amount of water needed per experimental animal.Thereafter, the animals were separated at random into two groups: experimental group received 12.5 mg/kg oral ciprofloxacin (Sigma 33433, USA) via gavage for 60 continuous days, the control group receiving only water and food. The dosages ciprofloxacin were similar to those used in human therapy. Body weight daily intake of food and water were determined several times per week throughout the study. All chemicals used in the present study were purchased from Sigma chemical.
Surgical procedure
On sixtieth day, the pentobarbital sodium (40 mg/kg) was administered intra peritoneal for anesthesia, and the peritoneal cavity was opened through a lower transverse abdominal incision. The reproductive organs (testes, epididymides and seminal vesicles) were carefully removed, washed in normal saline solution (0.9%), blotted and weighed. At the end of the experiment, the animals were anesthetized with diethyl ether and killed by decapitation according to recommendation of the Institutional Ethical Committee.
Epididymal spermatozoa motility, viability and count
Epididymal spermatozoa were collected by cutting the cauda region of the epididymis into small pieces in 5 ml of Ringer’s medium at 37°C. A sperm viability test was done by the method described by World Health Organization (14). Assessment of sperm count and motility were performed according to Freund and Carol (15). Briefly, both cauda epididymis from each rat were placed in 2 ml of normal saline pre-warmed to 37°C. Small cuts were made in the two cauda epididymis where the spermatozoa were obtained and suspended in the saline solution. Two hundred microlitres of the suspension was diluted with 800 ml of saline. A small amount of the diluted suspension was transferred to both chambers of a Neubauer haemocytometer using a Pasteur pipette by touching the edge of the cover slip and allowing each chamber to be filled by capillary action.
Histopathology
Light microscopy
The testis was fixed in 10% formalin and embedded in paraffin. Five-micron thick sections were prepared and stained with hematoxylin and eosin (HE). The specimens were examined under Olympus/3H light microscope. Two perpendicular diameters of 10 seminiferous tubules were measured each in 10 slides (10×10=100 seminiferous tubules in each groups), at 40× magnification, with the aid of an ocular reticule standardized with a stage micrometer. Values were recorded as mean diameter of seminiferous tubule (16). The diameters of venous were also measured at the same way.
TUNEL analysis of apoptosis
The in-situ DNA fragmentation was visualized by TUNEL method (17). Briefly, dewaxed tissue sections were predigested with 20 mg/ml proteinase K for 20 min and incubated in phosphate buffered saline solution (PBS) containing 3 % H2O2 for 10 min to block the endogenous peroxidase activity.
The sections were incubated with the TUNEL reaction mixture, fluorescein-dUTP (in situ Cell Death Detection, POD kit, Roche, Germany), for 60 min at 37°C. The slides were then rinsed three times with PBS and incubated with secondary antifluorescein-POD-conjugate for 30 min. After washing three times in PBS, diaminobenzidine-H2O2 (DAB, Roche, Germany) chromogenic reaction was added on sections and counterstained with hematoxylin. As a control for method specificity, the step using the TUNEL reaction mixture was omitted in negative control serial sections, and nucleotide mixture in reaction buffer was used instead. Apoptotic germ cells were quantified by counting the number of TUNEL stained nuclei per seminiferous tubular cross section. Cross sections of 100 tubules per specimen were assessed and the mean number of TUNEL positive germ cells per tubule cross- section was calculated.
Statistical analysis
Statistical comparisons were made using the Fisher -test for comparison of data in the control group with the experiment group. The results were expressed as mean ± S.E. (standard error). p-value less than 0.05 were considered significant.
Results
Weight of individual male reproductive organs
Weights of the testis, epididymis and seminal vesicle were significantly lower in treated animals with ciprofloxacin (12.5mg/kg/day), relative to the control group (p<0.05), which suggests that these antibiotics have the toxicity to male reproductive organs (Table I).

Sperm count, viability and motility
Administration of ciprofloxacin 12.5mg/kg/day, orally, for 60 consecutive days significantly reduced sperm count and motility in experimental group as compared with control group (p<0.05) (Table II). Moreover, sperm viability was significantly declined in the treatment group when compared to the control group (p<0.001)(Table II).



Light microscopic study
Histopathological study showed the cycle of spermatogenesis was regular in all males in the control group (Figure A). However, ciprofloxacin treatment resulted in a significant decrease in the number of spermatogenic cells (spermatogonia, spermatocyte, spermatid and sperm) in the seminiferous tubules (Figure B) (Table III). Intertubular spaces and veins congestion were increased in the treatment group as compared with those seen in the control group. Morphometeric study showed the diameter of seminiferous tubule (ST) was (250±0.77) and (285±0.81) µm in the experimental and control group, respectively.
This data is shown the diameter of (ST) was decreased in the experimental group when compared with the control group (Table IV). The diameter of veins was 0.921±0.01 µm and 1.73 ±.07 µm in the control and the experimental groups, respectively.


Result of apoptotic germ cells
In this study, the number of TUNEL positive germ cells per tubule cross-section increased following ciprofloxacin treatment compared to the control group. The TUNEL positive spermatogonia and spermatocytes were the main germ cells undergoing apoptosis. Figures C and D demonstrate the typical histograms of TUNEL assay analyzed by light microscope in both groups. Using the in situ detection method by which the apoptotic cells can be identified by their darkly stained nuclei, we observed a low incidence of spontaneous apoptosis in normal rat testis from the control group. Although in low numbers, apoptotic germ cells were also observed in the control rats. The rate of total apoptotic cells (spermatogonia and spermatocytes) were 15.11±3.523 and 7.3±0.762 in the experimental and the control groups, respectively according to the definition of Clermont method (18).


Discussion
The therapeutic and prophylactic effects of ciprofloxacin on several infections have been well documented. However, the results of our experimental study reveal that prolonged administration of therapeutic dose of ciprofloxacin was promoted male reproductive toxicity in rats. Degenerative changes in the seminiferous tubules and reduction in sperm count and motility are the evidence for this toxicity.
The present study indicated that, administration of ciprofloxacin for 60 consecutive days, results in a marked reduction in sperm count, sperm motility and viability as compared to respective controls. This is in agreement with that of Abd-Allah et al, who reported that ciprofloxacin treatment for 15 days in rats caused in a marked reduction in sperm count and motility (11). In addition, Demir et al, has similarly shown that ciprofloxacin treatment for 10 days in rats resulted in a marked reduction in sperm count and motility (12). Previous studies in which male patients were given 250 mg ciprofloxacin twice a day did not show differences in sperm quality (19) and was without effect of spermatogenesis (20). In contrast, at pharmacologic concentration (100× physiologic), ciprofloxacin adversely affected human sperm motility with decrease rapid progression in vitro (21). The reasons for the discrepancy may be due to differences between in vitro and in vivo studies. Thus, up to 20% of each ciprofloxacin dose is metabolized in vivo, and ciprofloxacin metabolites may exert some biological activities that are not detectable by in vitro assays (22). On the other hand, the possible release of bacterial factors, from indigenous bacteria killed by the drug, should not be neglected.
It has been reported that the decrease in sperm count and motility are valid indices of male infertility in laboratory animals (23, 24). However, sperm motility is often used as a marker of chemical-induced testicular toxicity (25). They have also stated that the disruption of seminiferous epithelium is indicative of male reproductive hazard. Therefore, our experimental results suggest a gonadotoxic potential of ciprofloxacin. One of the reason for this effect can be explained on the basis that, ciprofloxacin interferes with the energy production process required for sperm vitality and motility (26). Apoptosis of select germ cells occurs normally in the testis and is essential for the normal maintenance of spermatogenesis (27-29). However, the relatively small increase in the percentage of germ cell apoptosis can result in defective spermatogenesis leading to infertility (30). Increases in the incidence of germ cell apoptosis often are observed as a result of various forms of physical or chemical injury to the testis (31). The present study is demonstrated that apoptosis in male rat germ cells is induced by ciprofloxacin. These results indicate that ciprofloxacin like other chemical agents may directly interfere in the process of spermatogenesis. This increase in germ cell apoptosis is possibly partly due to an increased peroxide radical generation in the testis following ciprofloxacin treatment (32). This increase then induces DNA single-strand breaks and chromosomal aberrations as demonstrated by in vitro genotoxicity studies (33, 34). In addition, previous study showed that caspase-3, which has an important role in apoptosis, could be activated by ciprofloxacin (35, 36). Therefore, ciprofloxacin could also induce apoptotic pathways through activation of Caspases. In conclusion, this study may suggest that ciprofloxacin has potential testicular toxicity as evidenced by decreased sperm count and motility, increase apoptotic cells and pathological testis changes.
Type of Study: Original Article |
References
1. Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ 1992; 305: 609-613. [DOI:10.1136/bmj.305.6854.609]
2. Amann RP, Berndtson WE. Assessment of procedures for screening agents for effects on male reproduction: effects of dibromochloropropane (DBCP) on the rat. Fundam Appl Toxicol 1986; 7: 244-255. [DOI:10.1016/0272-0590(86)90154-5]
3. Sowerby E, Parsons J. Prevention of iatrogenic pelvic infection during in vitro fertilization-current practice in the UK. Hum Fertil 2004; 7,135-140. [DOI:10.1080/14647270410001720473]
4. Egbase PE, Udo EE, Al-Sharhan M, Grudzinskas JG. Prophylactic antibiotics and endocervical microbial inoculation of the endometrium at embryo transfer. Lancet 1999; 354: 651-652. [DOI:10.1016/S0140-6736(99)02415-0]
5. Peikrishvili R, Evrard B, Pouly JL, Janny LJ. Prophylactic antibiotic therapy (amoxicillin + clavulanic acid) before embryo transfer for IVF is useless. Results of a randomized study. Gynecol Obstet Biol Reprod 2004; 33: 713-719. [DOI:10.1016/S0368-2315(04)96632-X]
6. Herbold BA, Brendler-Schwaab SY, Ahr HJ. Ciprofloxacin: in vivo genotoxicity studies. Mutat Res 2001; 498:193-205. [DOI:10.1016/S1383-5718(01)00275-3]
7. Wolfson JS, Hooper DC. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother 1985; 28: 581-586. [DOI:10.1128/AAC.28.4.581]
8. Akasaka T, Kurosaka S, Uchida Y, Tanaka M, Sato K, Hayakawa I. Antibacterial activities and inhibitory effects of sitafloxacin (DU-6859a) and its optical isomers against type II topoisomerases. Antimicrob Agents Chemother 1998; 42:1284-1287. [DOI:10.1128/AAC.42.5.1284]
9. Liu Q, Wang JC. Similarity in the catalysis of DNA breakage and rejoining by type IA and IIA DNA topoisomerases. Proc Natl Acad Sci USA 1999; 96: 881-886. [DOI:10.1073/pnas.96.3.881]
10. Grabe M, Forsgren A, Bjork T. Concentrations of ciprofloxacin in serum and prostatic tissue in patients undergoing transurethral resection. Eur J Clin Microbiol 1986; 5: 211-212. [DOI:10.1007/BF02013991]
11. Abd-Allah AR, Aly HA, Moustafa AM, Abdel-Aziz AA, Hamada FM. Adverse testicular effects of some quinolone members in rats. Pharmacol Res 2000; 41: 211-219. [DOI:10.1006/phrs.1999.0580]
12. Demir A, Turker P, Onol FF, Sirvanci S, Findik A, Tarcan T. Effect of experimentally induced Escherichia coli epididymo-orchitis and ciprofloxacin treatment on rat spermatogenesis. Int J Urol 2007; 14: 268-272. [DOI:10.1111/j.1442-2042.2007.01682.x]
13. National Institutes of Health. The principles of laboratory animal care. National Institutes of Health publication 1985; 86: 23.
14. WHO. Laboratory manual for the examination of the human semen and sperm-cervical mucus interaction. New York: Cambridge University Press; 1999.
15. Freund M, Carol B. Factors affecting haemocytometer counts of sperm concentration in human semem. J Repord Fertil 1965; 8: 149-155. [DOI:10.1530/jrf.0.0080149]
16. Russel LD, Ettlin RA, Hikim APS, Clegg ED. Histological and histopathological evaluation of the testes. Clearwater, FL: Cache River Press; 1990.
17. Xiaozhong Yu, Kubota H, Wang R, Saegusa J, Ogawa Y, Ichihara G, et al. Involvement of Bcl-2 Family Genes and Fas Signaling Systemin Primary and Secondary Male Germ Cell Apoptosis Induced by 2-Bromopropane in Rat. J Toxicology and Applied Pharmacolog 2001; 174: 35-48. [DOI:10.1006/taap.2001.9187]
18. Clermont Y. Kinetics of spermatogenesis in mammals; seminiferous epithelium cycles and spermatogonial renewal.J Physiol Rev 1972; 52: 198-236. [DOI:10.1152/physrev.1972.52.1.198]
19. Merino G, Carranza-Lira S. Infection and male infertility: effect of diferent antibiotic regimens on semen quality. Arch Androl 1995; 35: 209- 212. [DOI:10.3109/01485019508987872]
20. Crotty KL, May R, Kulvicki A, Kumar D, Neal DE Jr. The effect of antimicrobial therapy on testicular aspirate flow cytometry. J Urol 1995; 153835-153838. [DOI:10.1016/S0022-5347(01)67731-0]
21. King K, Chan PJ, Patton WC, King A. Antibiotics: effect on cryopreserved-thawed human sperm motility in vitro. Fertil Steril 1997; 67:1146-1151. [DOI:10.1016/S0015-0282(97)81453-7]
22. Yao JDC, Moellering RC. Antibacterial agents. In: Manual of clinical microbiology. Balows A, Hausler WJ, Herrmann Jr KL, Isenberg HD, Shadomy HJ, eds. American Society for Microbiology , Washington D.C . 1991; 1065-1098.
23. Working PK, Chellman GJ. The testis, spermatogenesis and the excurrent duct system. In: Reproductive toxicology and infertility. Scialli AR, Zinaman MJ, eds. ISBN, McGraw Hill; 1993; 55-76.
24. Lemasters GK, Selevan SG. Toxic exposures and reproduction: a view of epidemiology and surveillance. In: Reproductive toxicology and infertility. Scialli AR, Zinaman MJ, eds. McGraw Hill; 1993; 307-321.
25. Bitman J, Cecil HC. Estrogenic activity of DDT analoges and polychlorinated biphenyls. J Agr Food Chem 1970; 18: 1108-1112. [DOI:10.1021/jf60172a019]
26. Folgero Bertheussen TK, Lindal S, Torbergsen T, Oian P. Mitochondrial disease and reduced sperm motility. Hum Reprod 1993; 8: 1863-1868. [DOI:10.1093/oxfordjournals.humrep.a137950]
27. Roosen-Runge EC. The process of spermatogenesis in mammals. Biol Rev Camb Philos Soc 1962; 37: 343-377. [DOI:10.1111/j.1469-185X.1962.tb01616.x]
28. Roosen-Runge EC. Germinal-cell loss in normal metazoan spermatogenesis. J Reprod Fertil 1973; 35: 339-348. [DOI:10.1530/jrf.0.0350339]
29. Print CG, Loveland KL. Germ cell suicide: new insights into apoptosis during spermatogenesis. Bioessays 2000; 22: 423-430.
https://doi.org/10.1002/(SICI)1521-1878(200005)22:5<423::AID-BIES4>3.0.CO;2-0 [DOI:10.1002/(SICI)1521-1878(200005)22:53.0.CO;2-0]
30. Moline JM, Golden AL, Bar-Chama N, Smith E, Rauch ME, Chapin RE, et al. Exposure to hazardous substances and male reproductive health: a research framework. Environ Health Perspect 2000; 108: 803-813. [DOI:10.1289/ehp.00108803]
31. Richburg JH. The relevance of spontaneous- and chemically-induced alterations in testicular germ cell apoptosis to toxicology. Toxicol Lett 2000; 112-113:79-86. [DOI:10.1016/S0378-4274(99)00253-2]
32. Weyers AI, Ugnia LI, Garcia Ovando H, Gorla NB. Ciprofloxacin increases hepatic and renal lipid hydroperoxides levels in mice. Biocell 2002; 26: 225-228.
33. Sanchez G, Hidalgo ME, Vivanco JM, Escobar J. Induced and photoinduced DNA damage by quinolones: ciprofloxacin, ofloxacin and nalidixic acid determined by comet assay. Photochem Photobiol 2005; 81: 819-822. [DOI:10.1562/2004-11-30-RA-386R.1]
34. Itoh T, Mitsumori K, Kawaguchi S, Sasaki YF. Genotoxic potential of quinolone antimicrobials in the in vitro comet assay and micronucleus test. Mutat Res 2006; 603: 135-144. [DOI:10.1016/j.mrgentox.2005.11.003]
35. Olivia A, Liping Z, Samir A, David PWJ, Tuan HK, Fazlul H S. Role of mitochondria in ciprofloxacin induced apoptosis in bladder cancer cell. Journal of Urology 2002; 167: 1288-1294. [DOI:10.1016/S0022-5347(05)65283-4]
36. Zhang JH, Zhang Y, Herman B. Caspases apoptosis and aging. J Ageing Res Rev 2003; 2: s357-366. [DOI:10.1016/S1568-1637(03)00026-6]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




