Wed, Apr 24, 2024
[Archive]
Volume 18, Issue 6 (June 2020)
IJRM 2020, 18(6): 439-448 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Negara K S, Suwiyoga K, Sudewi R, Astawa N M, Kamasan Arijana G N, Tunas K et al . The role of caspase-dependent and caspase-independent pathways of apoptosis in the premature rupture of the membranes: A case-control study. IJRM 2020; 18 (6) :439-448
URL: http://ijrm.ir/article-1-1122-en.html
URL: http://ijrm.ir/article-1-1122-en.html
Ketut Surya Negara * 

 1, Ketut Suwiyoga2
1, Ketut Suwiyoga2 

 , Raka Sudewi3
, Raka Sudewi3 

 , Nyoman Mantik Astawa4
, Nyoman Mantik Astawa4 

 , Gusti Nyoman Kamasan Arijana5
, Gusti Nyoman Kamasan Arijana5 

 , Ketut Tunas6
, Ketut Tunas6 

 , Tjokorda Gede Astawa Pemayun2
, Tjokorda Gede Astawa Pemayun2 




 1, Ketut Suwiyoga2
1, Ketut Suwiyoga2 

 , Raka Sudewi3
, Raka Sudewi3 

 , Nyoman Mantik Astawa4
, Nyoman Mantik Astawa4 

 , Gusti Nyoman Kamasan Arijana5
, Gusti Nyoman Kamasan Arijana5 

 , Ketut Tunas6
, Ketut Tunas6 

 , Tjokorda Gede Astawa Pemayun2
, Tjokorda Gede Astawa Pemayun2 


1- Department of Obstetrics and Gynecology, Medical Faculty of Udayana University, Sanglah Hospital, Bali, Indonesia. , tutsuryanegara@gmail.com
2- Department of Obstetrics and Gynecology, Medical Faculty of Udayana University, Sanglah Hospital, Bali, Indonesia.
3- Department of Neurology, Medical Faculty of Udayana University, Sanglah Hospital Bali, Indonesia.
4- Laboratory of Veterinary Medicine, Udayana University Bali, Indonesia.
5- Biomedic Laboratory of Medical Faculty Udayana University, Bali, Indonesia.
6- Department of Public Health, Dhyana Pura University Bali, Indonesia.
2- Department of Obstetrics and Gynecology, Medical Faculty of Udayana University, Sanglah Hospital, Bali, Indonesia.
3- Department of Neurology, Medical Faculty of Udayana University, Sanglah Hospital Bali, Indonesia.
4- Laboratory of Veterinary Medicine, Udayana University Bali, Indonesia.
5- Biomedic Laboratory of Medical Faculty Udayana University, Bali, Indonesia.
6- Department of Public Health, Dhyana Pura University Bali, Indonesia.
Full-Text [PDF 620 kb]
(659 Downloads)
| Abstract (HTML) (2232 Views)
PROM affects approximately 10-12% of all pregnancies, varying from 6 to 19% of term pregnancies and 6 to 8% of preterm pregnancies. Almost 80% of the cases occur in term pregnancies (1, 2). In a study conducted at a tertiary teaching hospital in Indonesia, PROM was found in 14.62% of all deliveries; 84.43% occurred in term and 15.57% in preterm pregnancies (3). The etiology of PROM is multi-factorial and the mechanisms are still unclear. The weakening of the extracellular matrix within amnio-chorionic membrane due to collagen degradation is one of the processes that predispose to PROM (4, 5). During pregnancy, the weakening of amniotic membrane is hypothesized to be caused by distention of the fetal membrane and also by certain biochemical processes that result in remodeling and apoptosis. Microscopic observation of fetal membrane in PROM cases shows decreased collagen tissue, disturbance of the collagen structure, and also increased collagenolytic activity (6-8). The degradation of collagen is caused by matrix metalloproteinase (MMP). The MMPs can be activated by the apoptosis sequences and apoptosis itself can also be induced by MMPs activation (9-11).
The signals that induce apoptosis may come from both intracellular and extracellular compartments: the extrinsic/extracellular pathway (initiated through the stimulation of death receptors) and the intrinsic pathway (initiated through the release of signals from intracellular mitochondria/mitochondrial pathway). Both the intrinsic and extrinsic pathways are caspase-dependent and end up at the same point: the execution phase, marked by the activation of caspase-3 as the initiator of apoptosis (12-14). Some types of cell death may occur without caspase activation, especially in conditions where the cell is affected by infection or stress. In this situation, apoptosis is likely to occur through caspase-independent pathways that involve B-cell lymphoma 2 (Bcl-2) pro-apoptosis family members known as Bax. This caspase-independent pathway is triggered by infection-associated deoxyribonucleic acid (DNA) fragmentation due to mitochondrial damage. When a cell is exposed to DNA-damaging agents, intracellular p53 protein is activated and its increase then triggers apoptosis through the release of Bcl-2 proteins. This condition further increases the permeability of the mitochondrial membrane, releasing other apoptosis proteins such as cytochrome C, Smac, DIABLO, Apoptosis Inducing Factor (AIF), and endonuclease G (15-17).
Although many studies on the proteins involved in apoptosis processes in PROM cases have been conducted, mostly focused only on the classic caspase-dependent pathways (18-20). As the result, the exact process by which apoptosis occurs in PROM remains unclear. To date, no study has investigated the role of caspase-independent pathways in the pathogenesis of PROM, especially the role of AIF as the main apoptosis protein involved in the pathway. Caspase-dependent activity can be measured by caspase-3 expression while the independent pathway by AIF expression. In this case-control study, we aim to evaluate the expression of caspase-3, AIF, and Bcl-2 as risk factors for PROM.
The participants had spontaneous vaginal delivery or through induced labor. After the delivery, tissue samples were taken from the edge of membrane rupture based on macroscopic examination. Immunohistochemistry examination was used to determine Caspase-3, AIF, and Bcl-2 expressions in the membrane. Cells that express the determined protein appear dark brown-stained with blue nucleus. In this study, the positive result was defined as being ≥ 10% of the cells that were stained. The examination was carried at the Integrated Biomedical Laboratory of the Medical Faculty, Udayana University, Bali, Indonesia.
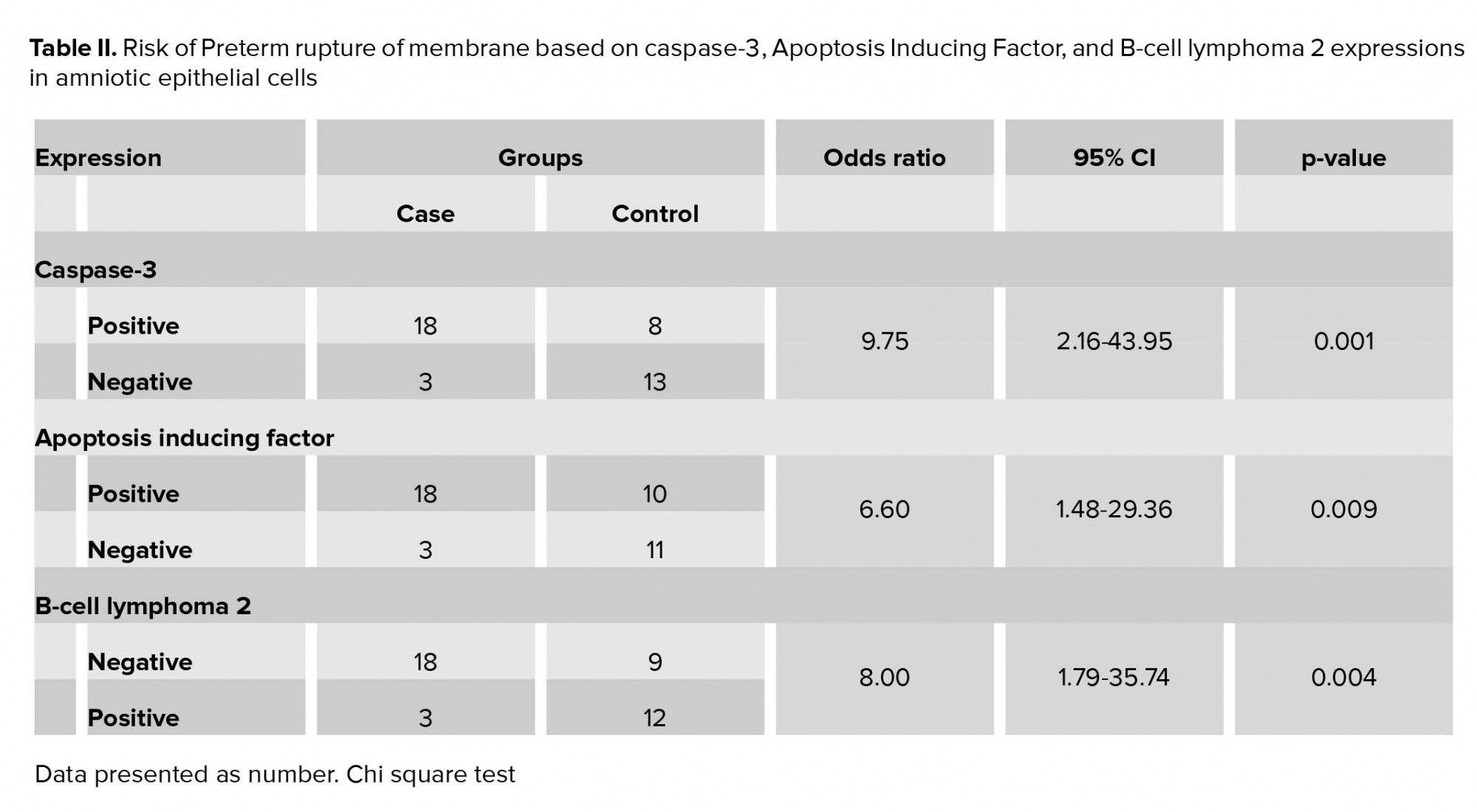
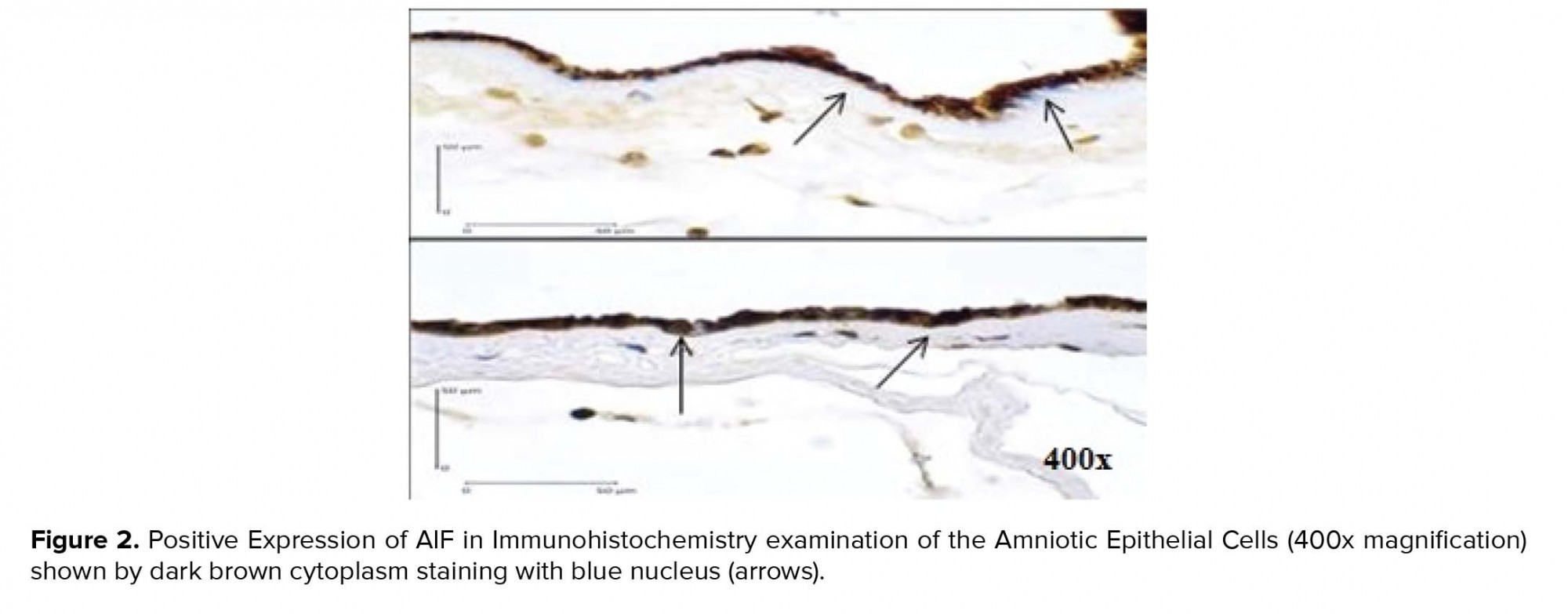
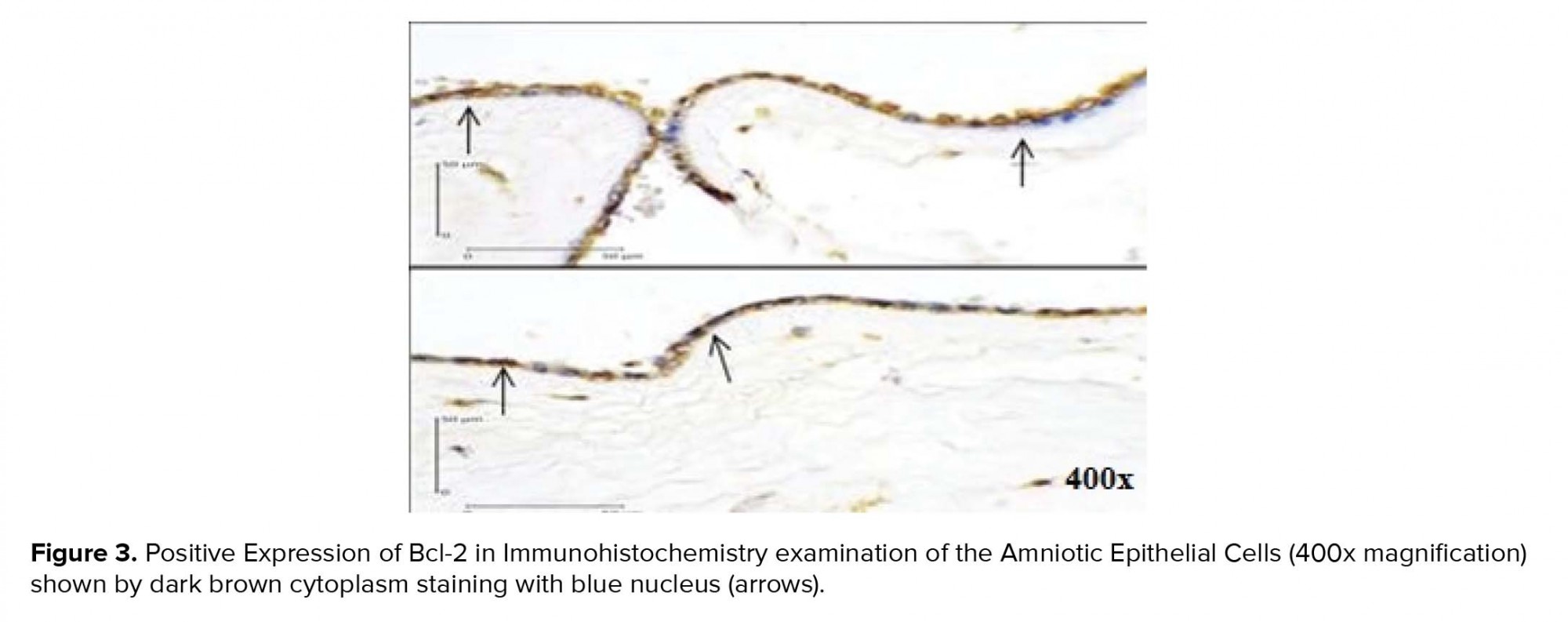
Using a Chi-square test to analyze the relationship between variables, we found a positive relationship between caspase-3 expression (Figure 1) and the increased risk of PROM by 9.75 times. Positive AIF expression (Figure 2) also significantly increased the risk of PROM by 6.60 times (Table II). The role of Bcl-2 anti-apoptosis protein expression (Figure 3) in PROM was also analyzed. We found that low expression of Bcl-2 was associated with an increased risk of PROM by 8.00 times (Table II).


4. Discussion
The role of caspase-3 in the incidence of PROM was investigated by Xu and Wang (13). The study evaluated the role of caspase-3 expression and MMP activity at amniochorionic membrane in cases of PROM. Amniotic membranes were obtained from several groups of women: women with spontaneous PROM (n = 8), women with spontaneous vaginal delivery at term (n = 8), and women with uncomplicated repeated cesarean section (n = 8). The caspase-3 peptide was evaluated with immunohistochemistry. Positive caspase-3 expression was almost 2-3 times higher in the PROM group (mean expression 62.86 ± 3.83%), as compared with spontaneous vaginal delivery (42.33 ± 2.99%) and the cesarean section group(20.97 ± 2.94%) which indicated an increased level of apoptosis in the amniotic cells (p < 0.005) (13).
The mechanism of apoptosis through the caspase-independent pathway does not require caspase as a messenger with a specific role in the cell-death mechanism. This pathway is induced mainly by mitochondrial pro-apoptosis protein molecules, AIF and endonuclease G. Translocation of these two proteins from mitochondria to the nucleus will cause nuclear DNA fragmentation (15-17). AIF is regarded as one of the main protein that mediates caspase-independent cell death. Studies found that apoptosis cannot be activated by caspase alone. Cell death can even be induced without the involvement of caspase, through the expression of Bax (or Bak). This process involves mitochondrial factors, such as AIF, which induces mitochondrial swelling, chromatin condensation, and cytochrome C release without caspase activation (18, 19, 21).
The increased apoptosis at the trophoblast chorionic layer of term pregnancies was investigated by Harirah and colleagues. They found an increased apoptotic index on the chorionic trophoblast of the distal part of the ruptured amniotic membrane which was three times higher compared to the artificial rupture in cesarean section. The choriodecidual layer of spontaneous delivery showed higher pro-apoptotic activity (high caspase-3 and low Bcl-2), compared to cesarean delivery (22). The Bcl-2 protein family is a key regulator of apoptosis which regulates the permeability of the outer mitochondrial membrane and the release of pro-apoptotic factors. They are also a key regulator of apoptosis because they connect the extrinsic and intrinsic pathways (15, 23, 24).
Both the intrinsic and extrinsic pathways may induce the activation of caspase in PROM; however, the intrinsic pathway is more dominant in term pregnancies. Studies also reported significant differences between Bcl-2, caspase-3, and caspase-9 in the supracervical area, which represent the intrinsic pathway activity. Fas and its ligand, (Fas-L), were also found in all amniotic membrane samples but there was no significant increase at the supracervical and the distal membrane areas. Thus, it is hypothesized that the extrinsic pathway is not involved in the remodeling of the amniotic membrane. Even though pro-apoptotic protein Bax expression was not significantly different, the anti-apoptotic protein Bcl-2 expression was found to be significantly decreased at the paracervical area (25-27).
Caspase-3 is the main family member of caspase that is actively involved in apoptosis. The activation of caspase-3 is the biochemical reaction that occurs before the initiation of apoptosis, and almost all apoptosis proceeds via this pathway. Thus, investigating the expression of caspase-3 may indicate the activity of cellular apoptosis. Immunohistochemistry examination of the amniotic membrane in PROM cases shows expression of caspase-3 on the amniotic epithelial cells and chorionic cytotrophoblast cells, while its expression is limited in mesenchymal and the reticular cells of the matrix. This indicates that apoptosis occurs at the amniotic and chorionic layers, and it plays important role in the regulation of the fetal membrane (13, 25, 27).
There are two major apoptotic pathways in the PROMs that can be initiated by infection, genotoxic agents, or other unknown factors. The first pathway utilizes tumor necrosis factor receptor-1 and the Fas receptors. These receptor proteins bind to their specific ligands, tumor necrosis factor and Fas L, respectively, and initiate signal transduction through two adapter proteins: tumor necrosis factor receptor type 1-associated death domain protein and Fas-associated protein with death domain. These domain proteins activate a group of proteases known as caspases. They also independently activate procaspase-8 into caspase-8, subsequently enabling caspase-3 (20, 28).
The other pathway may be initiated by p53, by increasing transactivator p53 proteins, which causes mitochondrial membrane damage and release cytochrome C. This event will then activate apoptosis protease-activating factor -1, which converts procaspase-9 into its active form, which in turn initiates the activation of the effector caspase-3, -7, and -6. The result of this process is proteolysis of structural proteins, homeostasis proteins, and some other proteins, ultimately programming cell death (20). Pro-apoptotic proteins that are also released by mitochondria during apoptosis are AIF, the endonuclease G, and CAD. AIF and endonuclease G independently cause DNA fragmentation and chromatin condensation (29-31).
Our study found caspase-3 expression in amniotic epithelial cells, which indicates the apoptotic process in the amnion; its expression was higher in PROM cases by 9.75 times. This suggests that caspase-3 expression is a risk factor for the PROM. Caspase-3 is an executor caspase that acts in the caspase-dependent, extrinsic and intrinsic pathways of apoptosis, which explains its dominant expression in apoptosis. Negara found that positive caspase-3 expression increased the risk of PROM by 7.3 times (OR 7.31; 95% CI 2.64-20.22; p = 0.001) (32). These findings indicate that caspase-dependent pathway of apoptosis plays a central role in the mechanism of PROM.
In the present study, low Bcl-2 expression was associated with an increased risk of PROM by eight times. This suggests that Bcl-2 is an anti-apoptotic protein that acts as a regulator of apoptosis, and its low level is a risk factor for PROM. Furthermore, the current study also found that high AIF expression was associated with an increased risk of PROM by 6.60 times. The previous study by Negara found that positive AIF expression is a risk factor of PROM by 5.10 times (OR5.10;95% CI 1.86-13.96; p = 0.001). AIF expression was more profound in the PROM group. It is a pro-apoptotic protein released by mitochondria in the caspase-independent pathway. Thus, high AIF expression indicates the involvement of caspase-independent pathway in PROM (33).
Our findings suggest that apoptosis via the caspase-dependent and caspase-independent pathways may be involved in PROM. It is evident that the high caspase-3 and AIF expressions and low Bcl-2 expression are significant risk factors that play important roles in the mechanism of PROM. This would imply that apoptosis mechanism in PROM may not be so simple. It may be comprised of various initiation processes and does not depend on only a specific molecular mechanism. This may explain the difficulty of predicting which pregnancy is more vulnerable to PROM. A complete understanding on the underlying process in PROM may assist clinician to apply preventive measures on high-risk pregnancies. Further studies are needed in the future to better define the complete process.
5. Conclusion
We found significant correlations between caspase-3, AIF, and Bcl-2 expressions with PROM. High expressions of caspase-3 and AIF were significant risk factors for the occurrence of PROM in term and preterm pregnancy. Low expression of Bcl-2 was also associated with the increased risk of PROM. Among the three variables, caspase-3 expression was the most dominant risk factor of PROM. We can conclude that apoptosis was a key component in the mechanism of PROM and that both caspase-dependent and caspase-independent apoptotic pathways were involved in PROM.
Clinical significance
Apoptosis process in PROM was thought to be mediated by a caspase-dependent pathway. This study shows that the caspase-independent pathway is also involved in PROM.
Acknowledgments
The authors would like to thank the President Director of the Sanglah Hospital, Bali, Indonesia, for the research authorization and support. This study received no financial sponsor. It is entirely conducted and funded by the authors
Conflict of Interest
The authors declare no conflict of interest regarding the publication of this paper.
Full-Text: (403 Views)
- Introduction
PROM affects approximately 10-12% of all pregnancies, varying from 6 to 19% of term pregnancies and 6 to 8% of preterm pregnancies. Almost 80% of the cases occur in term pregnancies (1, 2). In a study conducted at a tertiary teaching hospital in Indonesia, PROM was found in 14.62% of all deliveries; 84.43% occurred in term and 15.57% in preterm pregnancies (3). The etiology of PROM is multi-factorial and the mechanisms are still unclear. The weakening of the extracellular matrix within amnio-chorionic membrane due to collagen degradation is one of the processes that predispose to PROM (4, 5). During pregnancy, the weakening of amniotic membrane is hypothesized to be caused by distention of the fetal membrane and also by certain biochemical processes that result in remodeling and apoptosis. Microscopic observation of fetal membrane in PROM cases shows decreased collagen tissue, disturbance of the collagen structure, and also increased collagenolytic activity (6-8). The degradation of collagen is caused by matrix metalloproteinase (MMP). The MMPs can be activated by the apoptosis sequences and apoptosis itself can also be induced by MMPs activation (9-11).
The signals that induce apoptosis may come from both intracellular and extracellular compartments: the extrinsic/extracellular pathway (initiated through the stimulation of death receptors) and the intrinsic pathway (initiated through the release of signals from intracellular mitochondria/mitochondrial pathway). Both the intrinsic and extrinsic pathways are caspase-dependent and end up at the same point: the execution phase, marked by the activation of caspase-3 as the initiator of apoptosis (12-14). Some types of cell death may occur without caspase activation, especially in conditions where the cell is affected by infection or stress. In this situation, apoptosis is likely to occur through caspase-independent pathways that involve B-cell lymphoma 2 (Bcl-2) pro-apoptosis family members known as Bax. This caspase-independent pathway is triggered by infection-associated deoxyribonucleic acid (DNA) fragmentation due to mitochondrial damage. When a cell is exposed to DNA-damaging agents, intracellular p53 protein is activated and its increase then triggers apoptosis through the release of Bcl-2 proteins. This condition further increases the permeability of the mitochondrial membrane, releasing other apoptosis proteins such as cytochrome C, Smac, DIABLO, Apoptosis Inducing Factor (AIF), and endonuclease G (15-17).
Although many studies on the proteins involved in apoptosis processes in PROM cases have been conducted, mostly focused only on the classic caspase-dependent pathways (18-20). As the result, the exact process by which apoptosis occurs in PROM remains unclear. To date, no study has investigated the role of caspase-independent pathways in the pathogenesis of PROM, especially the role of AIF as the main apoptosis protein involved in the pathway. Caspase-dependent activity can be measured by caspase-3 expression while the independent pathway by AIF expression. In this case-control study, we aim to evaluate the expression of caspase-3, AIF, and Bcl-2 as risk factors for PROM.
- Materials and Methods
The participants had spontaneous vaginal delivery or through induced labor. After the delivery, tissue samples were taken from the edge of membrane rupture based on macroscopic examination. Immunohistochemistry examination was used to determine Caspase-3, AIF, and Bcl-2 expressions in the membrane. Cells that express the determined protein appear dark brown-stained with blue nucleus. In this study, the positive result was defined as being ≥ 10% of the cells that were stained. The examination was carried at the Integrated Biomedical Laboratory of the Medical Faculty, Udayana University, Bali, Indonesia.
- 1. Ethical consideration
- 2. Statistical analysis
- Results
Using a Chi-square test to analyze the relationship between variables, we found a positive relationship between caspase-3 expression (Figure 1) and the increased risk of PROM by 9.75 times. Positive AIF expression (Figure 2) also significantly increased the risk of PROM by 6.60 times (Table II). The role of Bcl-2 anti-apoptosis protein expression (Figure 3) in PROM was also analyzed. We found that low expression of Bcl-2 was associated with an increased risk of PROM by 8.00 times (Table II).





4. Discussion
The role of caspase-3 in the incidence of PROM was investigated by Xu and Wang (13). The study evaluated the role of caspase-3 expression and MMP activity at amniochorionic membrane in cases of PROM. Amniotic membranes were obtained from several groups of women: women with spontaneous PROM (n = 8), women with spontaneous vaginal delivery at term (n = 8), and women with uncomplicated repeated cesarean section (n = 8). The caspase-3 peptide was evaluated with immunohistochemistry. Positive caspase-3 expression was almost 2-3 times higher in the PROM group (mean expression 62.86 ± 3.83%), as compared with spontaneous vaginal delivery (42.33 ± 2.99%) and the cesarean section group(20.97 ± 2.94%) which indicated an increased level of apoptosis in the amniotic cells (p < 0.005) (13).
The mechanism of apoptosis through the caspase-independent pathway does not require caspase as a messenger with a specific role in the cell-death mechanism. This pathway is induced mainly by mitochondrial pro-apoptosis protein molecules, AIF and endonuclease G. Translocation of these two proteins from mitochondria to the nucleus will cause nuclear DNA fragmentation (15-17). AIF is regarded as one of the main protein that mediates caspase-independent cell death. Studies found that apoptosis cannot be activated by caspase alone. Cell death can even be induced without the involvement of caspase, through the expression of Bax (or Bak). This process involves mitochondrial factors, such as AIF, which induces mitochondrial swelling, chromatin condensation, and cytochrome C release without caspase activation (18, 19, 21).
The increased apoptosis at the trophoblast chorionic layer of term pregnancies was investigated by Harirah and colleagues. They found an increased apoptotic index on the chorionic trophoblast of the distal part of the ruptured amniotic membrane which was three times higher compared to the artificial rupture in cesarean section. The choriodecidual layer of spontaneous delivery showed higher pro-apoptotic activity (high caspase-3 and low Bcl-2), compared to cesarean delivery (22). The Bcl-2 protein family is a key regulator of apoptosis which regulates the permeability of the outer mitochondrial membrane and the release of pro-apoptotic factors. They are also a key regulator of apoptosis because they connect the extrinsic and intrinsic pathways (15, 23, 24).
Both the intrinsic and extrinsic pathways may induce the activation of caspase in PROM; however, the intrinsic pathway is more dominant in term pregnancies. Studies also reported significant differences between Bcl-2, caspase-3, and caspase-9 in the supracervical area, which represent the intrinsic pathway activity. Fas and its ligand, (Fas-L), were also found in all amniotic membrane samples but there was no significant increase at the supracervical and the distal membrane areas. Thus, it is hypothesized that the extrinsic pathway is not involved in the remodeling of the amniotic membrane. Even though pro-apoptotic protein Bax expression was not significantly different, the anti-apoptotic protein Bcl-2 expression was found to be significantly decreased at the paracervical area (25-27).
Caspase-3 is the main family member of caspase that is actively involved in apoptosis. The activation of caspase-3 is the biochemical reaction that occurs before the initiation of apoptosis, and almost all apoptosis proceeds via this pathway. Thus, investigating the expression of caspase-3 may indicate the activity of cellular apoptosis. Immunohistochemistry examination of the amniotic membrane in PROM cases shows expression of caspase-3 on the amniotic epithelial cells and chorionic cytotrophoblast cells, while its expression is limited in mesenchymal and the reticular cells of the matrix. This indicates that apoptosis occurs at the amniotic and chorionic layers, and it plays important role in the regulation of the fetal membrane (13, 25, 27).
There are two major apoptotic pathways in the PROMs that can be initiated by infection, genotoxic agents, or other unknown factors. The first pathway utilizes tumor necrosis factor receptor-1 and the Fas receptors. These receptor proteins bind to their specific ligands, tumor necrosis factor and Fas L, respectively, and initiate signal transduction through two adapter proteins: tumor necrosis factor receptor type 1-associated death domain protein and Fas-associated protein with death domain. These domain proteins activate a group of proteases known as caspases. They also independently activate procaspase-8 into caspase-8, subsequently enabling caspase-3 (20, 28).
The other pathway may be initiated by p53, by increasing transactivator p53 proteins, which causes mitochondrial membrane damage and release cytochrome C. This event will then activate apoptosis protease-activating factor -1, which converts procaspase-9 into its active form, which in turn initiates the activation of the effector caspase-3, -7, and -6. The result of this process is proteolysis of structural proteins, homeostasis proteins, and some other proteins, ultimately programming cell death (20). Pro-apoptotic proteins that are also released by mitochondria during apoptosis are AIF, the endonuclease G, and CAD. AIF and endonuclease G independently cause DNA fragmentation and chromatin condensation (29-31).
Our study found caspase-3 expression in amniotic epithelial cells, which indicates the apoptotic process in the amnion; its expression was higher in PROM cases by 9.75 times. This suggests that caspase-3 expression is a risk factor for the PROM. Caspase-3 is an executor caspase that acts in the caspase-dependent, extrinsic and intrinsic pathways of apoptosis, which explains its dominant expression in apoptosis. Negara found that positive caspase-3 expression increased the risk of PROM by 7.3 times (OR 7.31; 95% CI 2.64-20.22; p = 0.001) (32). These findings indicate that caspase-dependent pathway of apoptosis plays a central role in the mechanism of PROM.
In the present study, low Bcl-2 expression was associated with an increased risk of PROM by eight times. This suggests that Bcl-2 is an anti-apoptotic protein that acts as a regulator of apoptosis, and its low level is a risk factor for PROM. Furthermore, the current study also found that high AIF expression was associated with an increased risk of PROM by 6.60 times. The previous study by Negara found that positive AIF expression is a risk factor of PROM by 5.10 times (OR5.10;95% CI 1.86-13.96; p = 0.001). AIF expression was more profound in the PROM group. It is a pro-apoptotic protein released by mitochondria in the caspase-independent pathway. Thus, high AIF expression indicates the involvement of caspase-independent pathway in PROM (33).
Our findings suggest that apoptosis via the caspase-dependent and caspase-independent pathways may be involved in PROM. It is evident that the high caspase-3 and AIF expressions and low Bcl-2 expression are significant risk factors that play important roles in the mechanism of PROM. This would imply that apoptosis mechanism in PROM may not be so simple. It may be comprised of various initiation processes and does not depend on only a specific molecular mechanism. This may explain the difficulty of predicting which pregnancy is more vulnerable to PROM. A complete understanding on the underlying process in PROM may assist clinician to apply preventive measures on high-risk pregnancies. Further studies are needed in the future to better define the complete process.
5. Conclusion
We found significant correlations between caspase-3, AIF, and Bcl-2 expressions with PROM. High expressions of caspase-3 and AIF were significant risk factors for the occurrence of PROM in term and preterm pregnancy. Low expression of Bcl-2 was also associated with the increased risk of PROM. Among the three variables, caspase-3 expression was the most dominant risk factor of PROM. We can conclude that apoptosis was a key component in the mechanism of PROM and that both caspase-dependent and caspase-independent apoptotic pathways were involved in PROM.
Clinical significance
Apoptosis process in PROM was thought to be mediated by a caspase-dependent pathway. This study shows that the caspase-independent pathway is also involved in PROM.
Acknowledgments
The authors would like to thank the President Director of the Sanglah Hospital, Bali, Indonesia, for the research authorization and support. This study received no financial sponsor. It is entirely conducted and funded by the authors
Conflict of Interest
The authors declare no conflict of interest regarding the publication of this paper.
Type of Study: Original Article |
Subject:
Pregnancy Health
References
1. Adeniji AO, Atanda OA. Interventions and neonatal outcomes in patients with premature rupture of fetal membranes at and beyond 34 weeks gestational age at a tertiary health facility in Nigeria. Br J Med Med Res 2013; 3: 1388-1397. [DOI:10.9734/BJMMR/2013/3428]
2. Endale T, Fentahun N, Gemada D, Hussen MA. Maternal and fetal outcomes in term premature rupture of membrane. World J Emerg Med 2016; 7: 147-152. [DOI:10.5847/wjem.j.1920-8642.2016.02.011] [PMID] [PMCID]
3. Budijaya M, Surya Negara IK. Labor profile with premature rupture of membranes (PROM) in Sanglah Hospital, Denpasar, Bali, Period January 1-31 December 2015. Int J Sci Res 2017; 6: 348-353.
4. Mercer BM. Preterm premature rupture of the membranes. Obstet Gynecol 2003; 101: 178-193.
https://doi.org/10.1097/00006250-200301000-00033 [DOI:10.1016/S0029-7844(02)02366-9] [PMID]
5. Rodrigo MRR, Kannamani A. Perinatal and maternal outcome in premature rupture of membranes. J Evol Med Dent Sci 2016; 5: 3245-3247. [DOI:10.14260/jemds/2016/753]
6. Thombre MK. A review of the etiology epidemiology prediction and interventions of preterm premature rupture of membranes. [MSc thesis]. US, Michigan State University; 2014.
7. Benirschke, K. Anatomy and Pathology of the Placental Membranes. In: Benirschke K, Burton GJ, Baergen RN. Pathology of Human placenta. 3rd Ed. Springer, USA; 2012: 249-307. [DOI:10.1007/978-3-642-23941-0_11]
8. Vishwakarma K, Patel SK, Yadav K, Pandey A. Impact of premature rupture of membranes on maternal & neonatal health in Central India. J Evidence Based Med Healthcare 2015; 2: 8505-8508. [DOI:10.18410/jebmh/2015/1165]
9. Tency I, Verstraelen H, Kroes I, Holtappels G, Verhasselt B, Vaneechoutte M, et al. Imbalances between matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs) in maternal serum during preterm labor. PLoS One 2012; 7: e49042. [DOI:10.1371/journal.pone.0049042] [PMID] [PMCID]
10. Garite TJ. Premature rupture of the membrane. In: Creasy RK, Resnik R. Maternal Fetal Medicine, Principle and Practice. 5th Ed. Netherland, Elsevier; 2004; 723-739.
11. Rangaswamy N, Kumar D, Moore RM, Mercer BM, Mansour JM, Redline R, et al. Weakening and rupture of human fetal membranes-biochemistry and biomechanics. Available at: https://www.intechopen.com/books/preterm-birth-mother-and-child/weakening-and-rupture-of-human-fetal-membranes-biochemistry-and-biomechanics.
12. Sukhikh GT, Kan NE, Tyutyunnik VL, Sannikova MV, Dubova EA, Pavlov KA, et al. The role of extracellular inducer of matrix metalloproteinases in premature rupture of membranes. J Matern Fetal Neonatal Med 2016; 29: 656-659. [DOI:10.3109/14767058.2015.1015416] [PMID]
13. Xu J, Wang HL. Role of caspase and MMPs in amniochorionic during PROM. J Reprod Contracept 2005; 16: 219-224.
14. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol 2007; 35: 495-516. [DOI:10.1080/01926230701320337] [PMID] [PMCID]
15. Hongmei Z. Extrinsic and intrinsic apoptosis signal pathway review. Available at: https://www.intechopen.com/books/apoptosis-and-medicine/extrinsic-and-intrinsic-apoptosis-signal-pathway-review.
16. Estaquier J, Vallette F, Vayssiere JL, Mignotte B. The mitochondrial pathways of apoptosis. Adv Mitochondrial Med 2011;942: 157-183. [DOI:10.1007/978-94-007-2869-1_7] [PMID]
17. Van Loo G, Schotte P, Van Gurp M, Demol H, Hoorelbeke B, Gevaert K, et al. Endonuclease G: a mitochondrial protein released in apoptosis and involved in caspase-independent DNA degradation. Cell Death Differ 2001; 8: 1136-1142. [DOI:10.1038/sj.cdd.4400944] [PMID]
18. Ashkenazi A, Salvesen G. Regulated cell death: signaling and mechanisms. Annu Rev Cell Dev Biol 2014; 30: 337-356. [DOI:10.1146/annurev-cellbio-100913-013226] [PMID]
19. Galluzzi L, Kepp O, Trojel-Hansen C, Kroemer G. Mitochondrial control of cellular life, stress, and death. Circ Res 2012; 111: 1198-1207. [DOI:10.1161/CIRCRESAHA.112.268946] [PMID]
20. Menon R, Fortunato SJ. The role of matrix degrading enzymes and apoptosis in rupture of membranes. J Soc Gynecol Investig 2004; 11: 427-437. [DOI:10.1016/j.jsgi.2004.04.001] [PMID]
21. Perfettini JL, Hospital V, Stahl L, Jungas T, Verbeke P, Ojcius DM. Cell death and inflammation during infection with the obligate intracellular pathogen, Chlamydia. Biochimie 2003; 85: 763-769. [DOI:10.1016/j.biochi.2003.08.006] [PMID]
22. Harirah HM, Borahay MA, Zaman W, Ahmed MS, Hankins GD. Increased apoptosis in chorionic trophoblasts of human fetal membranes with labor at term. Int J Clin Med 2012; 3: 136-142. [DOI:10.4236/ijcm.2012.32027] [PMID] [PMCID]
23. Parsons MJ, Green DR. Mitochondria in cell death. Essays Biochem 2010; 47: 99-114. [DOI:10.1042/bse0470099] [PMID]
24. Vaux DL. Apoptogenic factors released from mitochondria. Biochim Biophys Acta 2011; 1813: 546-550. [DOI:10.1016/j.bbamcr.2010.08.002] [PMID]
25. Saglam A, Ozgur C, Derwig I, Unlu BS, Gode F, Mungan T. The role of apoptosis in preterm premature rupture of the human fetal membranes. Arch Gynecol Obstet 2013: 288: 501-505. [DOI:10.1007/s00404-013-2774-3] [PMID]
26. Kataoka S, Furuta I, Yamada H, Kato EH, Ebina Y, Kishida T, et al. Increased apoptosis of human fetal membranes in rupture of the membranes and chorioamnionitis. Placenta 2002; 23: 224-231. [DOI:10.1053/plac.2001.0776] [PMID]
27. El Khwad M, Stetzer B, Moore RM, Kumar D, Mercer B, Arikat S, et al. Term human fetal membranes have a weak zone overlying the lower uterine pole and cervix before onset of labor. Biol Reprod 2005; 72: 720-726. [DOI:10.1095/biolreprod.104.033647] [PMID]
28. Reti NG, Lappas M, Riley C, Wlodek ME, Permezel M, Walker S, et al. Why do membranes rupture at term? Evidence of increased cellular apoptosis in the supracervical fetal membranes. Am J Obstet Gynecol 2007; 196: 484-e1-e10. [DOI:10.1016/j.ajog.2007.01.021] [PMID]
29. Candé C, Cohen I, Daugas E, Ravagnan L, Larochette N, Zamzami N, et al. Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie 2002; 84: 215-222. [DOI:10.1016/S0300-9084(02)01374-3]
30. Arnoult D, Gaume B, Karbowski M, Sharpe JC, Cecconi F, Youle RJ. Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax/Bak-mediated permeabilization. EMBO J 2003; 22: 4385-4399. [DOI:10.1093/emboj/cdg423] [PMID] [PMCID]
31. Akematsu T, Endoh H. Role of apoptosis-inducing factor (AIF) in programmed nuclear death during conjugation in Tetrahymena thermophila. BMC Cell Biol 2010; 11: 1-5. [DOI:10.1186/1471-2121-11-13] [PMID] [PMCID]
32. Negara KS, Suwiyoga K, Arijana K, Tunas K. Role of Caspase-3 as risk factors of premature rupture of membranes. Biomed Pharmacol J 2017; 10: 2091-2098. [DOI:10.13005/bpj/1332]
33. Negara KS, Suwiyoga K, Arijana K, Tunas K. Role of apoptosis inducing factor (AIF) as risk factors of premature rupture of membranes. Biomed Pharmacol J 2018; 11: 719-724. [DOI:10.13005/bpj/1425]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





