Wed, Dec 3, 2025
[Archive]
Volume 6, Issue 5 (7-2008)
IJRM 2008, 6(5): 209-215 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mahmoudi R, Amiri I, Pasbakhsh P, Ragardi Kashani I, Abbasi M, Aboulhasani F, et al . The effects of vitrification on spindle apparatus of in vitro matured germinal vesicle in mice. IJRM 2008; 6 (5) :209-215
URL: http://ijrm.ir/article-1-126-en.html
URL: http://ijrm.ir/article-1-126-en.html
Reza Mahmoudi1 
 , Iraj Amiri2
, Iraj Amiri2 
 , Parichehr Pasbakhsh3
, Parichehr Pasbakhsh3 
 , Iraj Ragardi Kashani3
, Iraj Ragardi Kashani3 
 , Mehdi Abbasi3
, Mehdi Abbasi3 
 , Farid Aboulhasani3
, Farid Aboulhasani3 
 , Tooba Mehrannia4
, Tooba Mehrannia4 
 , Aligholi Sobhani *5
, Aligholi Sobhani *5 


 , Iraj Amiri2
, Iraj Amiri2 
 , Parichehr Pasbakhsh3
, Parichehr Pasbakhsh3 
 , Iraj Ragardi Kashani3
, Iraj Ragardi Kashani3 
 , Mehdi Abbasi3
, Mehdi Abbasi3 
 , Farid Aboulhasani3
, Farid Aboulhasani3 
 , Tooba Mehrannia4
, Tooba Mehrannia4 
 , Aligholi Sobhani *5
, Aligholi Sobhani *5 

1- Department of Anatomy, Faculty of Medicine, Yasouj University of Medical Sciences, Yasuj, Iran
2- Department of Anatomy, Faculty of Medicine, Hamedan University of Medical Sciences, Hamedan, Iran
3- Department of Anatomy, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran
4- Department of Anatomy, Faculty of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran
5- Department of Anatomy, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran ,Sobhania@tums.ac.ir
2- Department of Anatomy, Faculty of Medicine, Hamedan University of Medical Sciences, Hamedan, Iran
3- Department of Anatomy, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran
4- Department of Anatomy, Faculty of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran
5- Department of Anatomy, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran ,
Full-Text [PDF 109 kb]
(1094 Downloads)
| Abstract (HTML) (3555 Views)
Full-Text: (452 Views)
Introduction
The cryopreservation of oocytes and embryos is valuable for the treatment of infertility. Oocyte cryopreservation has wider clinical implications than embryo freezing. For patients undergoing in-vitro fertilization ( IVF ) , freezing the excess oocytes could avert repeated ovarian stimulation and oocyte retrieval from the patients themselves or be a source for oocyte donation (1-3).
From this point of view, cryopreservation of some oocytes is an alternative approach. In the cases of fertilization failure, the frozen oocytes cryopreservation was rarely successful in the past. This was principally because of low rates of survival, fertilization, and development (4). The majority of the research concerning oocyte cryopreservation has centered on mature oocytes. The mature oocyte is arrested at the metaphase II (MII) stage, which means that the chromosomes are attached to the labile microtubules of the second meiotic spindle (5).
Studies performed on animal models as well as in humans have shown that exposure to low temperatures and to cryoprotectant agents result in depolymerization of the microtubules, sometimes with attendant dispersal of chromosomes (6, 7). The microtubules of oocytes are vulnerable to cryoprotectants and thermal changes involved in cryopreservation. Although the microtubular system of mouse oocytes with respect to the distribution of pericentriolar material is different from that of human oocytes, it has been widely used as a model to study the spindle organization of human oocytes (8, 9). Disruption of the meiotic spindle may lead to impairment of fertilization of oocytes and the growth of embryos (10). One alternative approach to circumvent the problem of damaging the meiotic spindle would be freezing at the immature oocyte stage of development, when meiosis is arrested at the prophase I stage and the chromosomes are protected within the membrane of the germinal vesicle and when no microtubular structures have formed yet (6). Cryopreservation of immature oocytes at GV stage has been performed in mice, rats and humans and the results confirmed that immature human oocytes are able to survive cryopreservation and that surviving oocytes are able to mature to metaphase II, undergo fertilization in vitro and develop into blastocyst stage (11,12). However, there are few reports of pregnancy after cryopreservation of human oocytes at prophase I (13,14), therefore more detailed genetic screening and cytological analysis of the oocytes and embryos obtained after maturation and fertilization of vitrified oocytes need to be undertaken before this method is applied clinically. To determine the optimal conditions for vitrification of mouse immature oocyte, we examined the effects of step-wise and single step vitrification using conventional straws on morphological survival, in vitro maturation rates, meiotic spindles and chromosomes configuration.
Materials and methods
Chemical reagents
All chemicals were purchased from Sigma Chemical Co., St. Louis, MO, except for the ones specifically described.
Collection of germinal vesicle (GV) stage oocytes
GV-oocytes were obtained from 4-weeks-old [F1 C57BL/6 x DBA/2: BDF1] (Japan SLC Inc., Shizuoka, Japan) strain female mice and sperm was collected from ICR male mice. The animals were kept under controlled condition (12h light: 12h dark) and fed water and pellets ad libitum. Female mice were stimulated by an i.p. injection of 7.5 IU pregnant mare serum gonadotropin (PMSG: Teikokuzouki, Tokyo, Japan). The animals were killed 48h later by cervical dislocation and the ovaries were removed in Hepes-buffered human tubal fluid medium (HTF: Irvine) supplemented with 5mg/ml of bovine serum albumin (BSA). The GV-stage oocytes of ovarian antral follicles were released by puncturing with a 28G injection needle under a stereomicroscope. The collected GV-stage oocytes were randomly allocated to three groups: 1) control without treatments, 2) step-wise vitrification, and 3) single-step vitrification.
Vitrification and warming
Vitrification of oocytes was done according to the method described by Kasai et al (15) with some modification. The solutions for vitrification and warming were prepared using PB1 plus 20% fetal bovine serum (FBS). The step-wise vitrification solutions consisted of solution A; which was composed of 10% (v/v) ethylene glycol, 4.5% (w/v) Ficoll-70, and 0.075M sucrose, solution B; which was composed of 20% (v/v) ethylene glycol, 9.0% (w/v) Ficoll-70, and 0.15 M sucrose, and solution C; which was composed of 30% (v/v) ethylene glycol, 18% (w/v) Ficoll-70, and 0.3 M sucrose. Single-step vitrification solutions consisted of 30% (w/v) ethylene glycol, 18% (w/v) Ficoll-70, and 0.3 M sucrose. The diluting solutions for warming were made of 0.5, 0.25 and 0.125 M sucrose in PB1. The collected cumulus-oocyte-complexes ( COC ) were randomly divided into either a stepwise vitrification group or a single-step vitrification group. In the stepwise group, the COC were exposed first to 200 μl droplets of solution A for 5 min followed by solution B for 2 min, and finally solution C for 1 min in 4-well-dish. In the single step group, the COC were exposed to 200 μl drop of solution C for 1 min. The procedures were performed at the temperature of 22-24ºC. Then 10-15 oocytes were loaded into a 0.25 ml plastic straw (IVM, I Aigle, and France). The straw was filled with 1 cm of vitrification medium, 0.5 cm of air, 2 cm of vitrification medium containing oocytes, 0.5 cm of air, and 3.5 cm of vitrification medium. The straw then was plunged in to liquid nitrogen.
After storage for 1-5 days, the straw was taken out of the liquid nitrogen, held in the air for 10 sec, and then plunged into water of 37ºC for 10 sec. The straw was removed from water and wiped dry. It was cut with scissors and the contents containing oocytes were expelled into 400 μl drop with a sequential series of 0.5, 0.25, and 0.125 M sucrose for 90 sec in each solution, and washed for 6 min in TCM 199 medium supplemented with 20% FBS.
Maturation of GV oocytes
The vitrified-warmed GV-stage oocytes or fresh GV-stage oocytes (as a control group) were cultured in 100 μl drop of in vitro maturation (IVM) medium composed of α-MEM supplemented with 0.23 mM sodium pyruvate, 1 mg/ml of fetuin, antibiotic and antimycotic solution (comprising 100 U of penicillin, 100 μg of streptomycin, and 0.25 μg of amphotericin B), 10 ng/ml of mouse epithelial growth factor of cultured-grade (EGF, Upstate Biotechnology Inc, Lake placid, NY), 75 mU/ml of follicle-stimulating hormone (Fertinome, Serono, Geneva, Switzerland), and 3 mg/ml of BSA under mineral oil of embryo-tested grade, and incubated at 37ºC in an atmosphere of 5% CO2 in humidified air. 16-18 h after culture, the oocytes with first polar body was defined as mature MII oocytes.
Spindle and chromosome staining
The staining procedure was done as described previously by Johnson and Pickering (16). Briefly, in-vitro matured oocytes were denuded from the expanded cumulus cells by incubation at 37°C with PB1 plus bovine testicular hyaluronidase (150 IU/ml; Sigma UK Ltd) and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.2) for 60 min at room temperature. After fixation, oocytes were washed in three changes of PBS plus bovine serum albumin (PBS–BSA; 1 mg/ml).
The fixed oocytes were treated by 0.01% Triton X100 (Pierce, IL, USA) for 30 min at room temperature for permeation. Then, they were incubated in a fluorescein isothiocyanate (FITC)-conjugated anti-tubulin mouse monoclonal antibody at a dilution of 1:100 (Sigma No. F2168, MI, USA) for 1 hour at room temperature. After washing three times in PBS–BSA, the oocytes were incubated in propidium iodide (PI, 50µg/ml in PBS-BSA) for 10 min at room temperature for chromosome staining. The oocytes were washed again in three changes of PBS–BSA and mounted in a 5 µl droplet of PBS–BSA on glass coverslip.
Observation of spindles and chromosomes
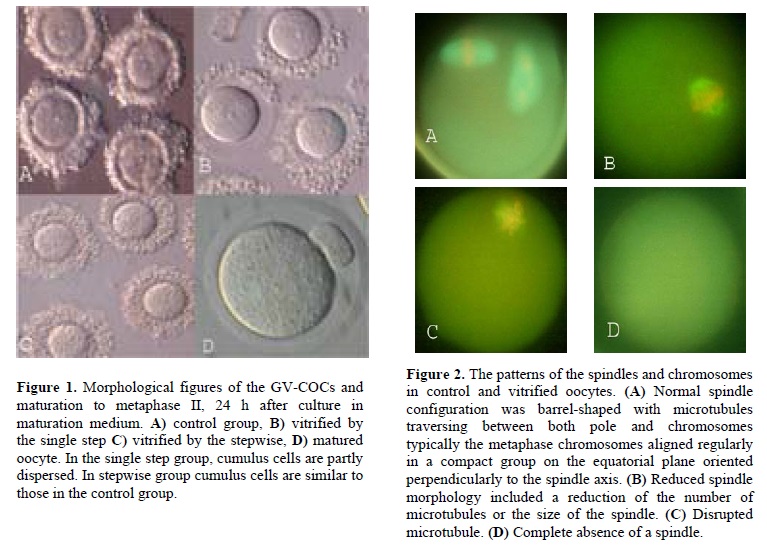
Fluorescence was observed using a microscope equipped with a fluorescent unit (OPTIPHOT 2, Nikon, Tokyo, Japan). A filter unit of EX465-495, DM505, BA515-555 nm was employed for the FITC green signal of the spindle. A filter unit of EX340-380, DM400, BA435-485 nm and EX540/25, DM565, BA605/55 nm was used to search for PI (red signal), respectively. In some experiments, a triple-band filter was used for observation of spindle and chromosome at same time. Oocytes were scored for normality of the spindle structure, distribution of the chromosomes and extrusion of the polar body. A normal oocyte was one that contained a barrel-shaped spindle and condensed chromosomes lying centrally on the equator of the spindle.
Abnormal spindle morphology included; a reduction of the number of microtubules or the size of the spindle, disruption of the spindle, or complete absence of a spindle. Dispersion of chromosomes was defined as abnormal (6). All oocytes of in-vitro matured experimental samples were scored for normality of these parameters. Images were acquired by a cooled CCD camera system (Penguin 600CL) and processed by Pixera software (Pixera, CA, USA).
Statistical analysis
Collected data were analyzed by one Way ANOVA test. The difference in values of survival, maturation rate, and spindle configuration were considered significant when p<0.05.
Results
A total of 553 GV oocytes whit cumulus cells ( COC ) were obtained from 24 ovaries that were used for stepwise and single step vitrified and non-vitrified (control) group. The number of oocytes collected averaged 23.04 per mouse ovary.
Morphology of GV oocytes
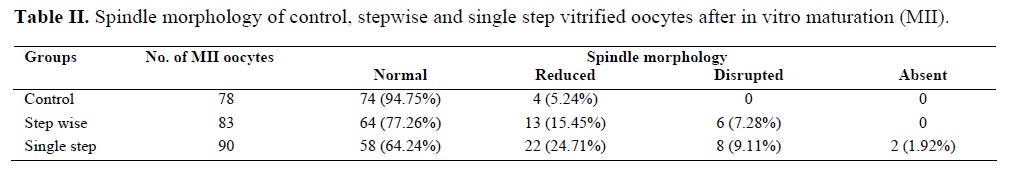
Morphological figure of nonvitrified (controls) and vitrified GV oocytes using either single step or stepwise method are shown in figure 1. In the single step group, cumulus cells are partly dispersed but in stepwise group cumulus cells are similar to those in the control group.
Survival and in vitro maturation of vitrified GV oocytes
The morphological survival and maturation rates of GV oocytes after different treatments including exposure to stepwise and single step vitrification in conventional straw, and non-vitrified oocytes are shown in table I.

The survival rate decreased after vitrification in both stepwise and single step groups after vitrification in conventional straw. Significantly more oocytes were damaged in single step group than in the stepwise vitrified group. The spindle morphology of the control oocytes and stepwise and single step vitrified in conventional straws are shown in table II. Normal spindle configuration was barrel-shaped with microtubules traversing between both pole and chromosomes typically the metaphase chromosomes aligned regularly in a compact group on the equatorial plane oriented perpendicularly to the spindle axis (Figure 2A). Abnormal spindle morphology included a reduction of the number of microtubules or the size of spindle (Figure 2B), disruption of the spindle (Figure 2C) or complete absence of a spindle (Figure 2D). Dispersion of chromosomes was defined as abnormal (Figure 2C) (6).
In comparison with the control group, vitrified either in stepwise or single step groups had significantly smaller (p<0.05) abnormal spindle configuration.
Discussion
The present study analyses the effect of stepwise and single step vitrification using conventional straws on the morphological survival, maturation rate and spindle and chromosome configuration of mice oocytes frozen at the GV stage. Our results show that immature oocytes collected from stimulated ovaries are more resistant to the stepwise vitrified processes than single step vitrified oocytes. However, our results also demonstrate that cryopreserving immature oocytes using vitrification protocol has a deleterious effect on the spindle and chromosome configurations of the subsequently thawed oocytes. The results are like the results of previous studies that applied vitrification oocytes using grid or open pulled straws for human oocytes, and obtained survival rate of 63-92% (17-18). On the other hand, Toth et al (19) reported 58.5%survival rate for GV cryopreserved oocytes, which is lower than our results. In another study, in which immature bovine oocytes were vitrified using a mixture of 2.5M EG, ficoll and sucrose in (open pulled straws) OPS (20), a successful maturation rate of 60% was recorded. Also Cetin et al (21) vitrified immature bovine oocytes and 34.1% of oocytes reached the MII stage EG group. These results are also lower than our results. Similar to our study, Anon et al reported higher survival, maturation and developmental rate with using ultrarapid vitrivication accompanied with step-wise equilibration in mouse GV oocytes compare with single step vitrified group. In our study, GV oocytes exposed to the stepwise vitrification procedure had a drastic effect on the arrangement of microtubules and chromosomes, while the single step vitrification procedure seemed to more severely affect the spindle configuration of mouse oocytes. Approximately, 34% of the single step vitrified and 22% of the stepwise vitrified GV oocytes appeared to have abnormal or missing spindles compared to control oocytes, while in the manipulated controls, abnormal spindle patterns were detected in nearly 6% of the mouse GV oocytes. Our results are consistent with the report that vitrification of bovine blastocysts after a 16-step equilibration resulted in minimization of ultrastructural damage to the plasma membrane (22, 23). These results suggest that the process of gradual equilibrium conversion of CPAs appears to adjust permeability of the plasma membrane, which may contribute to maintain the connection between the oocyte and cumulus cells and/or may decrease rapid changes in osmotic pressure. In addition, the integrity of the normal characteristics of plasma membrane, especially in cryopreservation of GV oocytes, may be important for subsequent maturation and development abilities. Several authors have described that the main hurdle in developing successful protocols for the cryopreservation of mammalian oocytes is being able to preserve the integrity of the meiotic spindle when the oocytes are cooled (9).
Temperature fluctuations directly affect the cytoskeletal and chromosome organization of mature bovine (24) and human oocytes (7). The main consequence of cooling is pronounced depolymerization and the disappearance of microtubule organizing centers (25). Chilling leads to the disassembly of spindle fibers within minutes, followed by an equally rapid reassembly of the spindle after the return to normal temperatures. Magistrini and Szöllösi (26) reported that the meiotic spindles of mouse oocytes were sensitive to cooling, with complete disassembly occurring after 45–60 min at 0 °C. The effects of cooling on the spindle appeared to be reversible in the mouse oocyte, with normal spindle formation occurring after step-wise re-warming. Pickering et al. (6) found that the meiotic spindle of human oocytes completely disassembled, and this was accompanied by chromosomal dispersion in 60% of the oocytes after 30 min at room temperature. This effect appeared to be reversible in only 25–50% of the oocytes. The meiotic spindle becomes completely disassembled when in vitro-matured bovine oocytes are maintained for 10–20 min at 4 °C. When pig oocytes were kept for 5 min at 4 °C, microtubules in the spindles of most oocytes partially or completely disassembled (27). Wu and his coworker suggest that irreversible damage to the cytoskeleton of porcine GV-and MII-oocytes after vitrification could be an important factor affecting developmental competence (28). Disruption of the cytoskeleton may be intrinsic to the changes in shape and shrinkage related to cryopreservation procedures, which in turn may lead to irreversibly changes in the structure of the plasma membrane or cytoskeleton so that it can no longer adjust to changing conditions, including those associated with fertilization. Thus, even when the normal microfilament distribution of oocytes is restored after vitrification, irreversible alterations to other cell components may already have occurred such as the early release of cortical granule enzymes and zona hardening. These changes may either prevent fertilization completely or incompletely block polyspermy, both leading to decreased cleavage rates after insemination (29).
Conclusion
This study demonstrates that step-wise vitrification of mouse oocytes using conventional straw improves survival and maturation rate and preserves the spindle morphology and chromosomal pattern better than single step vitrification.
Acknowledgements
This project was financially supported by Research Deputy of Tehran University of Medical Sciences, so we would like to thank for their helps.
The cryopreservation of oocytes and embryos is valuable for the treatment of infertility. Oocyte cryopreservation has wider clinical implications than embryo freezing. For patients undergoing in-vitro fertilization ( IVF ) , freezing the excess oocytes could avert repeated ovarian stimulation and oocyte retrieval from the patients themselves or be a source for oocyte donation (1-3).
From this point of view, cryopreservation of some oocytes is an alternative approach. In the cases of fertilization failure, the frozen oocytes cryopreservation was rarely successful in the past. This was principally because of low rates of survival, fertilization, and development (4). The majority of the research concerning oocyte cryopreservation has centered on mature oocytes. The mature oocyte is arrested at the metaphase II (MII) stage, which means that the chromosomes are attached to the labile microtubules of the second meiotic spindle (5).
Studies performed on animal models as well as in humans have shown that exposure to low temperatures and to cryoprotectant agents result in depolymerization of the microtubules, sometimes with attendant dispersal of chromosomes (6, 7). The microtubules of oocytes are vulnerable to cryoprotectants and thermal changes involved in cryopreservation. Although the microtubular system of mouse oocytes with respect to the distribution of pericentriolar material is different from that of human oocytes, it has been widely used as a model to study the spindle organization of human oocytes (8, 9). Disruption of the meiotic spindle may lead to impairment of fertilization of oocytes and the growth of embryos (10). One alternative approach to circumvent the problem of damaging the meiotic spindle would be freezing at the immature oocyte stage of development, when meiosis is arrested at the prophase I stage and the chromosomes are protected within the membrane of the germinal vesicle and when no microtubular structures have formed yet (6). Cryopreservation of immature oocytes at GV stage has been performed in mice, rats and humans and the results confirmed that immature human oocytes are able to survive cryopreservation and that surviving oocytes are able to mature to metaphase II, undergo fertilization in vitro and develop into blastocyst stage (11,12). However, there are few reports of pregnancy after cryopreservation of human oocytes at prophase I (13,14), therefore more detailed genetic screening and cytological analysis of the oocytes and embryos obtained after maturation and fertilization of vitrified oocytes need to be undertaken before this method is applied clinically. To determine the optimal conditions for vitrification of mouse immature oocyte, we examined the effects of step-wise and single step vitrification using conventional straws on morphological survival, in vitro maturation rates, meiotic spindles and chromosomes configuration.
Materials and methods
Chemical reagents
All chemicals were purchased from Sigma Chemical Co., St. Louis, MO, except for the ones specifically described.
Collection of germinal vesicle (GV) stage oocytes
GV-oocytes were obtained from 4-weeks-old [F1 C57BL/6 x DBA/2: BDF1] (Japan SLC Inc., Shizuoka, Japan) strain female mice and sperm was collected from ICR male mice. The animals were kept under controlled condition (12h light: 12h dark) and fed water and pellets ad libitum. Female mice were stimulated by an i.p. injection of 7.5 IU pregnant mare serum gonadotropin (PMSG: Teikokuzouki, Tokyo, Japan). The animals were killed 48h later by cervical dislocation and the ovaries were removed in Hepes-buffered human tubal fluid medium (HTF: Irvine) supplemented with 5mg/ml of bovine serum albumin (BSA). The GV-stage oocytes of ovarian antral follicles were released by puncturing with a 28G injection needle under a stereomicroscope. The collected GV-stage oocytes were randomly allocated to three groups: 1) control without treatments, 2) step-wise vitrification, and 3) single-step vitrification.
Vitrification and warming
Vitrification of oocytes was done according to the method described by Kasai et al (15) with some modification. The solutions for vitrification and warming were prepared using PB1 plus 20% fetal bovine serum (FBS). The step-wise vitrification solutions consisted of solution A; which was composed of 10% (v/v) ethylene glycol, 4.5% (w/v) Ficoll-70, and 0.075M sucrose, solution B; which was composed of 20% (v/v) ethylene glycol, 9.0% (w/v) Ficoll-70, and 0.15 M sucrose, and solution C; which was composed of 30% (v/v) ethylene glycol, 18% (w/v) Ficoll-70, and 0.3 M sucrose. Single-step vitrification solutions consisted of 30% (w/v) ethylene glycol, 18% (w/v) Ficoll-70, and 0.3 M sucrose. The diluting solutions for warming were made of 0.5, 0.25 and 0.125 M sucrose in PB1. The collected cumulus-oocyte-complexes ( COC ) were randomly divided into either a stepwise vitrification group or a single-step vitrification group. In the stepwise group, the COC were exposed first to 200 μl droplets of solution A for 5 min followed by solution B for 2 min, and finally solution C for 1 min in 4-well-dish. In the single step group, the COC were exposed to 200 μl drop of solution C for 1 min. The procedures were performed at the temperature of 22-24ºC. Then 10-15 oocytes were loaded into a 0.25 ml plastic straw (IVM, I Aigle, and France). The straw was filled with 1 cm of vitrification medium, 0.5 cm of air, 2 cm of vitrification medium containing oocytes, 0.5 cm of air, and 3.5 cm of vitrification medium. The straw then was plunged in to liquid nitrogen.
After storage for 1-5 days, the straw was taken out of the liquid nitrogen, held in the air for 10 sec, and then plunged into water of 37ºC for 10 sec. The straw was removed from water and wiped dry. It was cut with scissors and the contents containing oocytes were expelled into 400 μl drop with a sequential series of 0.5, 0.25, and 0.125 M sucrose for 90 sec in each solution, and washed for 6 min in TCM 199 medium supplemented with 20% FBS.
Maturation of GV oocytes
The vitrified-warmed GV-stage oocytes or fresh GV-stage oocytes (as a control group) were cultured in 100 μl drop of in vitro maturation (IVM) medium composed of α-MEM supplemented with 0.23 mM sodium pyruvate, 1 mg/ml of fetuin, antibiotic and antimycotic solution (comprising 100 U of penicillin, 100 μg of streptomycin, and 0.25 μg of amphotericin B), 10 ng/ml of mouse epithelial growth factor of cultured-grade (EGF, Upstate Biotechnology Inc, Lake placid, NY), 75 mU/ml of follicle-stimulating hormone (Fertinome, Serono, Geneva, Switzerland), and 3 mg/ml of BSA under mineral oil of embryo-tested grade, and incubated at 37ºC in an atmosphere of 5% CO2 in humidified air. 16-18 h after culture, the oocytes with first polar body was defined as mature MII oocytes.
Spindle and chromosome staining
The staining procedure was done as described previously by Johnson and Pickering (16). Briefly, in-vitro matured oocytes were denuded from the expanded cumulus cells by incubation at 37°C with PB1 plus bovine testicular hyaluronidase (150 IU/ml; Sigma UK Ltd) and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.2) for 60 min at room temperature. After fixation, oocytes were washed in three changes of PBS plus bovine serum albumin (PBS–BSA; 1 mg/ml).
The fixed oocytes were treated by 0.01% Triton X100 (Pierce, IL, USA) for 30 min at room temperature for permeation. Then, they were incubated in a fluorescein isothiocyanate (FITC)-conjugated anti-tubulin mouse monoclonal antibody at a dilution of 1:100 (Sigma No. F2168, MI, USA) for 1 hour at room temperature. After washing three times in PBS–BSA, the oocytes were incubated in propidium iodide (PI, 50µg/ml in PBS-BSA) for 10 min at room temperature for chromosome staining. The oocytes were washed again in three changes of PBS–BSA and mounted in a 5 µl droplet of PBS–BSA on glass coverslip.
Observation of spindles and chromosomes
Fluorescence was observed using a microscope equipped with a fluorescent unit (OPTIPHOT 2, Nikon, Tokyo, Japan). A filter unit of EX465-495, DM505, BA515-555 nm was employed for the FITC green signal of the spindle. A filter unit of EX340-380, DM400, BA435-485 nm and EX540/25, DM565, BA605/55 nm was used to search for PI (red signal), respectively. In some experiments, a triple-band filter was used for observation of spindle and chromosome at same time. Oocytes were scored for normality of the spindle structure, distribution of the chromosomes and extrusion of the polar body. A normal oocyte was one that contained a barrel-shaped spindle and condensed chromosomes lying centrally on the equator of the spindle.
Abnormal spindle morphology included; a reduction of the number of microtubules or the size of the spindle, disruption of the spindle, or complete absence of a spindle. Dispersion of chromosomes was defined as abnormal (6). All oocytes of in-vitro matured experimental samples were scored for normality of these parameters. Images were acquired by a cooled CCD camera system (Penguin 600CL) and processed by Pixera software (Pixera, CA, USA).
Statistical analysis
Collected data were analyzed by one Way ANOVA test. The difference in values of survival, maturation rate, and spindle configuration were considered significant when p<0.05.
Results
A total of 553 GV oocytes whit cumulus cells ( COC ) were obtained from 24 ovaries that were used for stepwise and single step vitrified and non-vitrified (control) group. The number of oocytes collected averaged 23.04 per mouse ovary.
Morphology of GV oocytes
Morphological figure of nonvitrified (controls) and vitrified GV oocytes using either single step or stepwise method are shown in figure 1. In the single step group, cumulus cells are partly dispersed but in stepwise group cumulus cells are similar to those in the control group.
Survival and in vitro maturation of vitrified GV oocytes
The morphological survival and maturation rates of GV oocytes after different treatments including exposure to stepwise and single step vitrification in conventional straw, and non-vitrified oocytes are shown in table I.

The survival rate decreased after vitrification in both stepwise and single step groups after vitrification in conventional straw. Significantly more oocytes were damaged in single step group than in the stepwise vitrified group. The spindle morphology of the control oocytes and stepwise and single step vitrified in conventional straws are shown in table II. Normal spindle configuration was barrel-shaped with microtubules traversing between both pole and chromosomes typically the metaphase chromosomes aligned regularly in a compact group on the equatorial plane oriented perpendicularly to the spindle axis (Figure 2A). Abnormal spindle morphology included a reduction of the number of microtubules or the size of spindle (Figure 2B), disruption of the spindle (Figure 2C) or complete absence of a spindle (Figure 2D). Dispersion of chromosomes was defined as abnormal (Figure 2C) (6).
In comparison with the control group, vitrified either in stepwise or single step groups had significantly smaller (p<0.05) abnormal spindle configuration.


Discussion
The present study analyses the effect of stepwise and single step vitrification using conventional straws on the morphological survival, maturation rate and spindle and chromosome configuration of mice oocytes frozen at the GV stage. Our results show that immature oocytes collected from stimulated ovaries are more resistant to the stepwise vitrified processes than single step vitrified oocytes. However, our results also demonstrate that cryopreserving immature oocytes using vitrification protocol has a deleterious effect on the spindle and chromosome configurations of the subsequently thawed oocytes. The results are like the results of previous studies that applied vitrification oocytes using grid or open pulled straws for human oocytes, and obtained survival rate of 63-92% (17-18). On the other hand, Toth et al (19) reported 58.5%survival rate for GV cryopreserved oocytes, which is lower than our results. In another study, in which immature bovine oocytes were vitrified using a mixture of 2.5M EG, ficoll and sucrose in (open pulled straws) OPS (20), a successful maturation rate of 60% was recorded. Also Cetin et al (21) vitrified immature bovine oocytes and 34.1% of oocytes reached the MII stage EG group. These results are also lower than our results. Similar to our study, Anon et al reported higher survival, maturation and developmental rate with using ultrarapid vitrivication accompanied with step-wise equilibration in mouse GV oocytes compare with single step vitrified group. In our study, GV oocytes exposed to the stepwise vitrification procedure had a drastic effect on the arrangement of microtubules and chromosomes, while the single step vitrification procedure seemed to more severely affect the spindle configuration of mouse oocytes. Approximately, 34% of the single step vitrified and 22% of the stepwise vitrified GV oocytes appeared to have abnormal or missing spindles compared to control oocytes, while in the manipulated controls, abnormal spindle patterns were detected in nearly 6% of the mouse GV oocytes. Our results are consistent with the report that vitrification of bovine blastocysts after a 16-step equilibration resulted in minimization of ultrastructural damage to the plasma membrane (22, 23). These results suggest that the process of gradual equilibrium conversion of CPAs appears to adjust permeability of the plasma membrane, which may contribute to maintain the connection between the oocyte and cumulus cells and/or may decrease rapid changes in osmotic pressure. In addition, the integrity of the normal characteristics of plasma membrane, especially in cryopreservation of GV oocytes, may be important for subsequent maturation and development abilities. Several authors have described that the main hurdle in developing successful protocols for the cryopreservation of mammalian oocytes is being able to preserve the integrity of the meiotic spindle when the oocytes are cooled (9).
Temperature fluctuations directly affect the cytoskeletal and chromosome organization of mature bovine (24) and human oocytes (7). The main consequence of cooling is pronounced depolymerization and the disappearance of microtubule organizing centers (25). Chilling leads to the disassembly of spindle fibers within minutes, followed by an equally rapid reassembly of the spindle after the return to normal temperatures. Magistrini and Szöllösi (26) reported that the meiotic spindles of mouse oocytes were sensitive to cooling, with complete disassembly occurring after 45–60 min at 0 °C. The effects of cooling on the spindle appeared to be reversible in the mouse oocyte, with normal spindle formation occurring after step-wise re-warming. Pickering et al. (6) found that the meiotic spindle of human oocytes completely disassembled, and this was accompanied by chromosomal dispersion in 60% of the oocytes after 30 min at room temperature. This effect appeared to be reversible in only 25–50% of the oocytes. The meiotic spindle becomes completely disassembled when in vitro-matured bovine oocytes are maintained for 10–20 min at 4 °C. When pig oocytes were kept for 5 min at 4 °C, microtubules in the spindles of most oocytes partially or completely disassembled (27). Wu and his coworker suggest that irreversible damage to the cytoskeleton of porcine GV-and MII-oocytes after vitrification could be an important factor affecting developmental competence (28). Disruption of the cytoskeleton may be intrinsic to the changes in shape and shrinkage related to cryopreservation procedures, which in turn may lead to irreversibly changes in the structure of the plasma membrane or cytoskeleton so that it can no longer adjust to changing conditions, including those associated with fertilization. Thus, even when the normal microfilament distribution of oocytes is restored after vitrification, irreversible alterations to other cell components may already have occurred such as the early release of cortical granule enzymes and zona hardening. These changes may either prevent fertilization completely or incompletely block polyspermy, both leading to decreased cleavage rates after insemination (29).
Conclusion
This study demonstrates that step-wise vitrification of mouse oocytes using conventional straw improves survival and maturation rate and preserves the spindle morphology and chromosomal pattern better than single step vitrification.
Acknowledgements
This project was financially supported by Research Deputy of Tehran University of Medical Sciences, so we would like to thank for their helps.
Type of Study: Original Article |
References
1. Wennerholm WB. Cryopreservation of embryos and oocytes: obstetric outcome and health in children. Hum Reprod 2000; 15: 18-25. [DOI:10.1093/humrep/15.suppl_5.18]
2. Aono N, Naganuma T, Abe Y, Hara K, Sasada H, Sato E, et al. Successful production of blastocysts following ultrarapid vitrification with step-wise equilibriation of germinal vesicle-stage mouse oocytes. J Reprod Dev 2003; 49: 501-506. [DOI:10.1262/jrd.49.501]
3. Chen SU, Lien YR, Chao KH, Ho HN, Yang YS, Lee TY. Effects of cryopreservation on meiotic spindles of oocytes and its dynamics after thawing: Clinical implications in oocyte freezing. Molecular and Cellular Endocrinology 2003; 202: 101-107. [DOI:10.1016/S0303-7207(03)00070-4]
4. Tucker MJ, Morton PC, Wright G, Sweitzer CL, Massey JB. Clinical application of human egg cryopreservation. Hum Reprod 1998; 13: 3156-3159. [DOI:10.1093/humrep/13.11.3156]
5. Boiso I, Marti M, Santalo J, Ponsa M, Barri PN, Veiga A. A confocal microscopy analysis of the spindle and chromosome configuration of human oocytes cryopreserved at the germinal vesicle and metaphase II stage. Hum Reprod 2002; 17: 1885-1891. [DOI:10.1093/humrep/17.7.1885]
6. Pickering SJ, Braude PR, Johnson MH, Cant A, Currie J. Transient cooling to room temperature can cause irreversible disruption of the meiotic spindle in the human oocyte. Fertil Steril 1990; 54: 102-108. [DOI:10.1016/S0015-0282(16)53644-9]
7. Almeida P, Bolton V. The effect of temperature fluctuations on the cytoskeletal organisation and chromosomal constitution of the human oocyte. Zygote 1995; 3: 357-365. [DOI:10.1017/S0967199400002793]
8. Van Blerkom J, Davis PW. Cytogenetic, cellular, and developmental consequences of cryopreservation of immature and mature mouse and human oocytes. Microsc Res Tech 1994; 27: 165-193. [DOI:10.1002/jemt.1070270209]
9. Joly C, Bchini O, Boulekbache H. Effects of 1,2-propanediol on the cytoskeletal organization of the mouse oocyte. Hum Reprod 1992; 7: 374-378. [DOI:10.1093/oxfordjournals.humrep.a137654]
10. Eroglu A, Toth TL, Toner M. Alterations of the cytoskeleton and polyploidy induced by cryopreservation of metaphase II mouse oocytes. Fertil Steril 1998; 69: 944-957. [DOI:10.1016/S0015-0282(98)00030-2]
11. Wu J, Zhang L, Wang X. In vitro maturation, fertilization and embryo development after ultrarapid freezing of immature human oocytes. Reproduction 2001; 121: 389-393. [DOI:10.1530/rep.0.1210389]
12. Oktay K, Demirtas E, Son WY, Lostritto K, Chian RC, Tan SL. In vitro maturation of germinal vesicle oocytes recovered after premature luteinizing hormone surge: description of a novel approach to fertility preservation. Fertil Steril. 2008; 89: e19-22. [DOI:10.1016/j.fertnstert.2007.02.028]
13. Kagawa N, Kuwayama M, Nakata K, Vajta G, Silber S, Manabe N, et al. Production of the first offspring from oocytes derived from fresh and cryopreserved pre-antral follicles of adult mice. Reprod Biomed Online 2007; 14: 693-699. [DOI:10.1016/S1472-6483(10)60670-0]
14. Chian RC, Gilbert L, Huang JY, Demirtas E, Holzer H, Benjamin A, et al. Live birth after vitrification of in vitro matured human oocytes. Fertil Steril 2008; 29: 1-5.
15. kassai M, Komi JH, Takakamo A, Tsudera H, Sakurai T. A simple method for mouse embryo cryopreservation in low toxicity vitrification solution, without appreciable loss of viability. J Reprod Fertil 1999; 89: 91-97. [DOI:10.1530/jrf.0.0890091]
16. Johnson MH, Pickering SJ. The effect of dimethylsulphoxide on the microtubular system of mouse oocytes. Development 1987; 100: 313-324.
17. Hong SW, Chung HM, Lim JM, Ko JJ, Yoon TK, Yee B. Improved human oocyte development after vitrification: a comparison of thawing methods. Fertil Steril 1999; 72: 142-146. [DOI:10.1016/S0015-0282(99)00199-5]
18. Yoon TK, Kim TJ, Park SE, Hong SW, Ko JJ, Chung HM. Live births after vitrification of oocytes in a stimulated in vitro fertilizationembryo transfer program. Fertil Steril 2003; 79: 1323-1326. [DOI:10.1016/S0015-0282(03)00258-9]
19. Toth TL, Baka SG, Veek LL, Jones HW, Muasher S, Lanzenddorf SE. Fertilization and in vitro development of cryopreserved human prophase I oocytes.Fertil Steril 1994; 61: 891-894. [DOI:10.1016/S0015-0282(16)56702-8]
20. Hurt AE, Landim F, Seidel GE, Squires EL. Vitrification of immature and mature equine and bovine oocytes in an ethylene glycol, ficoll and sucrose solution using open-pulled straws. Theriogenology 2000; 54: 119-128. [DOI:10.1016/S0093-691X(00)00330-7]
21. Cetin Y, Bastan A. Cryopreservation of immature bovine oocytes by vitrification in straws. Anim Reprod Sci 2006; 92: 29-36. [DOI:10.1016/j.anireprosci.2005.05.016]
22. Aono N, Abe Y, Hara K, Sasada H, Sato E,Yoshida H. Production of live offspring from mouse germinal vesicle-stage oocytes vitrified by a modified stepwise method, SWEID. Fertil Steril 2005; 84: 1078-1082. [DOI:10.1016/j.fertnstert.2005.03.077]
23. Kuwayama M, Fujikawa S, Nagai T. Ultrastructure of IVM-IVF bovine blastocysts vitrified after equilibration in glycerol 1, 2-propanediol using 2-step and 16-step procedures. Cryobiology 1994; 31: 415-422. [DOI:10.1006/cryo.1994.1051]
24. Saunders K, Parks JE. Effects of cryopreservation procedures on the cytology and fertilization rate of in vitro matured bovine oocytes. Biol Reprod 1999; 61: 178-187. [DOI:10.1095/biolreprod61.1.178]
25. Webb M, Howlet S, Maro B. Parthenogenesis and cytoskeletal organization in aging mouse eggs. J Embryol Exp Morphol 1986; 95: 131-145.
26. Magistrini M, Szöllösi D. Effects of cold and of isopropyl-N-phenylcarbamate on the second meiotic spindle of mouse oocytes. Eur J Cell Biol 1980; 22: 699-707.
27. Liu RH, Sun QY, Li YH, Jiao LH, Wang WH. Effects of cooling on meiotic spindle structure and chromosome alignment within in vitro matured porcine oocytes. Mol Reprod 2003; 65: 212-218. [DOI:10.1002/mrd.10282]
28. Wu C, Rui R, Dai J, Zhang C, Ju S, Xie B, et al. Effects of cryopreservation on the developmental competence, ultrastructure and cytoskeletal structure of porcine oocytes. Mol Reprod Dev 2006; 73: 1454-1462. [DOI:10.1002/mrd.20579]
29. Albarracin JL, Morato R, Rojas C, Mogas T. Effects of vitrification in open pulled straws on the cytology of in vitro matured prepubertal and adult bovine oocytes. Theriogenology 2005; 63: 890-901. [DOI:10.1016/j.theriogenology.2004.05.010]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |


