Sat, Jul 12, 2025
[Archive]
Volume 6, Issue 5 (7-2008)
IJRM 2008, 6(5): 181-186 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Darabi M R, Nasr-Esfahani M H, Baharvand H, Mardani M, Karimi-Jashni H. Fusion and development of 2-cell bovine embryos to tetraploid blastocyst with different voltages and durations. IJRM 2008; 6 (5) :181-186
URL: http://ijrm.ir/article-1-129-en.html
URL: http://ijrm.ir/article-1-129-en.html
Mohamad Reza Darabi1 
 , Mohamad Hosein Nasr-Esfahani *2
, Mohamad Hosein Nasr-Esfahani *2 
 , Hosein Baharvand3
, Hosein Baharvand3 
 , Mohmad Mardani4
, Mohmad Mardani4 
 , Hojatolah Karimi-Jashni5
, Hojatolah Karimi-Jashni5 


 , Mohamad Hosein Nasr-Esfahani *2
, Mohamad Hosein Nasr-Esfahani *2 
 , Hosein Baharvand3
, Hosein Baharvand3 
 , Mohmad Mardani4
, Mohmad Mardani4 
 , Hojatolah Karimi-Jashni5
, Hojatolah Karimi-Jashni5 

1- Department of Anatomy, Arak University of Medical Sciences, Arak, Iran
2- Department of Clinical and Experimental Embryology, Reproductive Medicine Research Center, Royan Institute, Isfahan campus, ACECR Tehran, Iran ,mh.nasr-esfahani@royaninstitute.org
3- Department of stem cell, Royan Institute, Tehran, Iran
4- Department of Anatomy, Isfahan University of Medical Sciences, Isfahan, Iran
5- Department of Anatomy, Jahrom University of Medical Sciences, Jahrom, Iran
2- Department of Clinical and Experimental Embryology, Reproductive Medicine Research Center, Royan Institute, Isfahan campus, ACECR Tehran, Iran ,
3- Department of stem cell, Royan Institute, Tehran, Iran
4- Department of Anatomy, Isfahan University of Medical Sciences, Isfahan, Iran
5- Department of Anatomy, Jahrom University of Medical Sciences, Jahrom, Iran
Full-Text [PDF 124 kb]
(1150 Downloads)
| Abstract (HTML) (2461 Views)
Full-Text: (425 Views)
Introduction
Although cloning mammals by transgenic nuclear transfer into oocyte cytoplasts has been performed successfully for nearly a decade, only a very small percentage of cloned embryos have developed to term, with a high incidence of developmental anomalies (1, 2). The impact of this technology has been spectacular, opening up new possibilities for producing recombinant proteins and therapeutic cloning, as animal cells can synthesize these proteins with appropriate post-translation modification (3, 4). Production of transgenic and knock-out mice and cattle is a choice for production of tetraploid embryos by aggregation of transgenic embryonic stem (ES) cells with tetraploid morulae (5, 6).
In tetraploid/diploid chimeras, a non-random distribution of cells occurs in the developing conceptus. In these types of chimeras, tetraploid cells readily contributes to extra-embryonic tissue compared to inner cell mass (7) and the offspring from these chimeras are formed solely by embryonic diploid cells. In aggregation of bovine embryonic ES-like cells with day 3 bovine diploid embryos, the ES-like cells have limited presence in various tissues, while in aggregation of bovine ES-like cells with bovine tetraploid embryos, the embryo proper is formed solely by ES-Like cells (8, 10).
Tetraploid embryos are produced by various procedures. One of the most accurate, repeatable and well defined, procedures is electrofusion. During this procedure, embryos are placed between two electrodes in fusion buffer, exposed to fusigenic stimulus for a very short period. This procedure was first reported in mice and in bovine using zona-enclosed blastomeres (11, 12).
Because required and optimum parameters reported for electrofusion by various studies are different, the aim of this study was to evaluate the effect of different voltages (0.5, 0.75, 1, 1.25 and 1.50 kV/cm) and durations (20, 40, 60, 80 and 100 μs) on fusion, cleavage and development of bovine embryos into blastocyst.
Materials and methods
This was an experimental study. All chemicals and reagents were purchased from Sigma chemicals, unless otherwise indicated. Ovaries were collected from local abattoirs from unstimulated heifers and cows, after 30 min of slaughter, transported to the laboratory in sterile 0.9% saline at 25 to 30 ˚C. Ovarian follicles (2 to 6 mm) were aspirated by 18-gauge needle primed with washing medium TCM-199 (Sigma M-2520).
In this study, over 1750 high quality (homogene and equal cell size) two-cell embryos were produced 33 to 35 hr post insemination. Nearly 570 two-cell embryo were used for each (control and experimental) group. The results in each group were repeated between 4 to 5 times.
In vitro maturation
High quality cumulus-oocyte-complexes (COCs), having homogenous evenly granulated cytoplasm surrounded by more than three layers compact granulosa cells, were rinsed 3 times in washing medium and twice in maturation medium (MM: washing medium supplemented with 1μg/ml 17β- Estradiol, 0.1 IU/ml HMG, 10% FCS and 1 mM L-glutamine) and subsequently were transferred in groups of 5 into 50μL drops of maturation medium under mineral oil. The COCs were then incubated (LabTec) for 22 to 24h in humidified atmosphere in 5% CO2 in air at 39˚C (12-14).
In vitro fertilization
After maturation, COCs were rinsed twice in fertilization medium (FM) and were inseminated, with final concentration of 1 to 2 million sperm for ml, in groups of 10 into 50 μL droplets of FM under mineral oil for 18 to 22h. Fertilization media also called Fert-TALP were prepared according to Parrish et al (15).
Live spermatozoa from fresh semen of fertile bulls were separated by centrifugation using Percoll gradients (90% and 45%; Seromed, Germany) at 2000 rpm for 25 min. The pellets were washed and diluted to the required concentration in FM. Capcitation of sperms occurred during the IVF culture (13-16).
In vitro Culture
Cleaning of spermatozoa and granulosa cells from inseminated COCs were carried out by vortexing (Ependorf) for 2 minutes in washing medium and subsequently transferred and cultured in SOF1 (17-21).
Then, 33 to 35h post insemination, 2-cell embryos were selected and categorized into two groups. One group without any exposure to electrical stimulation was taken as control group (UCG = unexposed control group) and was kept in SOF1 for further 37 to 39h. The 2-cell embryos in the other group were used for electrofusion.
Electrofusion
Two-cell embryos in groups of 5 to 10 were washed for 10-20 seconds in fusion buffer containing 0.3 M mannitol solution containing 0.1 mM MgS04, 0.05 mM CaCl2, pH=7.2-7.4, immediately transferred between electrodes in fusion chamber containing fusion buffer. Two stainless steel platform electrodes 1mm apart connected to electrofusion machine (CF-150B, Biological Laboratory Service, H-1165 Budapest, Zselyi A. U. 31. Hungary) form this chamber (9, 22). Two-cell embryos with inter-blastomeric axis placed parallel to electrodes were exposed to one pulse of direct current (DC) for certain voltage and duration. Embryos were observed at 60 min post electrofusion (7). Fused 2-cell embryos were called fused group (FG) and unfused 2-cells were called exposed control group (ECG). These embryos were transferred to SOF1 for further 37 to 39 h. At this stage embryos from UCG, FG, and ECG were transferred into SOF2 up to 10th day (SOF2 was the same as SOF1 without Na-pyrovate but containing 1%MEM essential amino acids, 10% FCS and 2 mM glucose) (17, 19, 20).
Embryo scoring
Cleavage was evaluated microscopically 72 h post-insemination in each group (FG, ECG, UCG). Morula and early blastocyst were assessed 168 h (7th day) post-insemination. Blastocyst and expanded blastocyst were assessed on the 8th to 10th day post insemination (23). Percentage of cleavage and blastocyst rate were evaluated with respect to the total number of embryos in each group. For cytogentic analysis by Giemsa staining two to eight-cell stage embryos from FG (4n chromosome) were processed according to modified Tarkowski protocol (24, 25). The number of chromosomes was determined under the light microscope at 100x magnification.
Statistical analysis
All statistical calculation including, coefficient of correlation and Chi-square were carried out using statistical package for social studies (SPSS-10) software, and Epi-Info statistical package, respectively.
Results
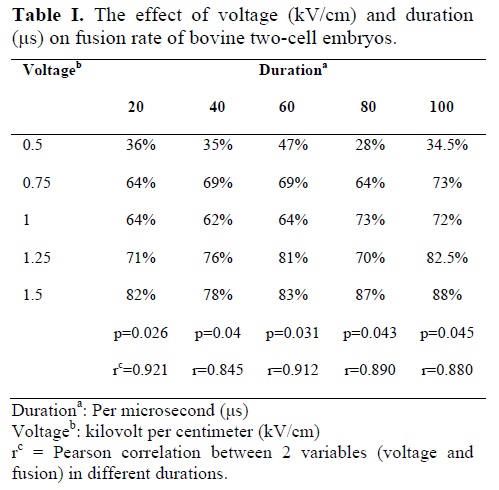
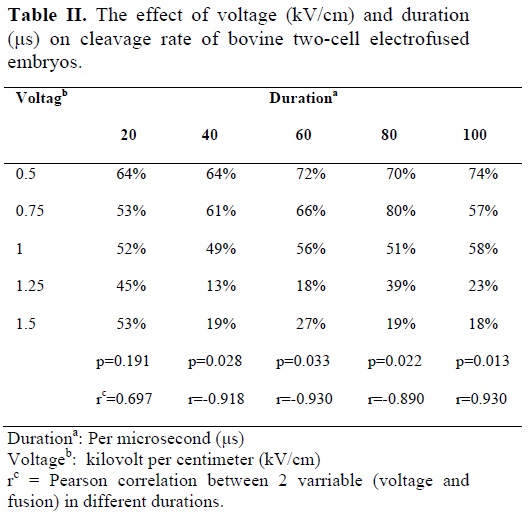
The results in table I show that, with increase in duration (20 to 100 μs) there is no significant increase in fusion rate in each different voltage (0.5 to 1.50 kV/cm). The results also showed that with increase in voltage, there is a steady increase in fusion rate, in each duration (Table I). Pearson analysis also confirms a high positive correlation between different voltages with fusion rate in different duration. The results in table II showed that with decrease in voltage from 1.5 to 0.5 kV/cm the percentage of cleavage steadily increases in different durations except in 20 μs duration. Pearson analysis also confirms a high negative significant correlation between different voltages with cleavage rate in different duration except 20 μs duration (Table II). Table II also shows that percentage of cleavage in the 1.5 and 1.25 kV/cm groups for 20 μs is significantly greater than that of groups of 40 to 100 μs, suggesting that during high voltage exposure, increase in duration reduces cleavage rate (p<0.05). The results also show that maximum cleavage rate was obtained in 0.75 kV/cm in 80 μs duration.
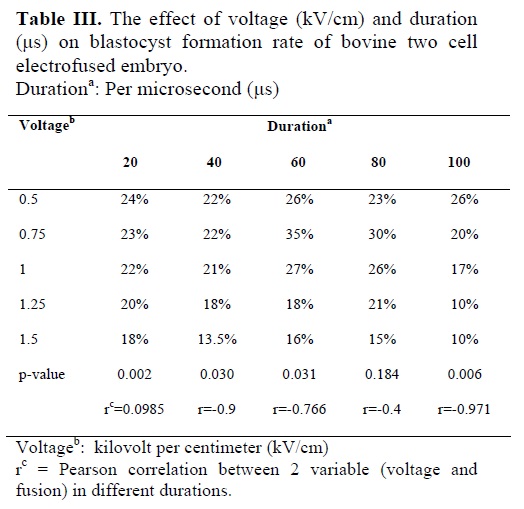
The results in table III show that with increase in voltage from 0.5 to 1.5 kV/cm the blastocyst formation rate decreases steadily during different duration (p<0.05), and with increase in duration of exposure during different voltage, blastocyst formation steadily decreases, however this reduction is not significant (p>0.05), suggesting that exposure to define voltage does not significantly reduces blastocyst formation rate in different duration. Pearson analysis also confirms a high negative significant correlation between different voltages with blastocyst formation rate in different duration except 60 and 80 μs duration (Table III).Maximum blastocyst formation rate was observed in 0.75 kV/cm in the duration of 60 μs.
The cleavage, morula and blastocyst formation rate in the ECG groups overall followed the same trend as the FG group. Therefore, the data were not shown. Mean cleavage and blastocyst formation rate in the UCG were 80.46 and 32.32% respectively. These values are not significantly higher than the values in the FG and ECG groups with the maximum blastocyst formation rate.
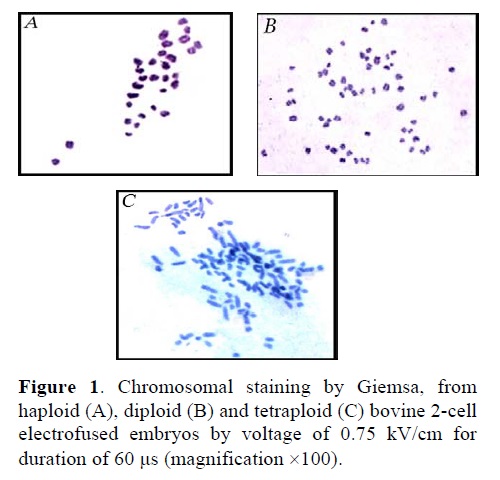
Figure 1 reveals the chromosomal analysis from putative tetraploid embryos produced by electrofusion (0.75 kV/cm for 60 μs).In total 37 (66%) of 56 electro-fused embryos at 2-8 cells could be analyzed and 3 (%8), 6 (%16) and 28 (%76) of 37 were haploid, diploid and tetraploid respectively.
Discusion
Electrofusion are routinely used for different technologic purposes. During electrofusion due to applied direct current electric field, the membranes are polarized and instabilized, results in attraction of other membrane (point membrane fusion) and formation of unstable flat membrane diaphragm, through reversible pore formation followed by reversible breakdown of the membrane or diaphragm. Under favorable conditions, the flat diaphragm deteriorates to allow cell mixing, indicating through cell-to-cell fusion (27). The obtained results suggest that the fusion rate is voltage dependent and with increases of voltage intensity from 0.5 to 1.5kV/cm, fusion rate increases to 88% (Table I), which is different to fusion rate in study of Lan Li et al (77% in goat) (3).
Possibly this difference is due to difference in species. However, increase in duration of electrical pulse to 100 μs did not affect the fusion rate in different voltage (Table I). The results of this study are in concordance with the result of Zhelev et al (28) in other cells, which suggest that there is a correlation between pulse intensity and pore formation. Similar results were obtained by Tatham et al (29) by fusing enucleated bovine oocytes (by different methods) with blastomeres (with different aged). These authors also showed that increase voltage intensity up to certain threshold level increase the fusion rate, after which fusion rate decreases.
However, they also showed that increasing in pulse duration has no fundamental effect on fusion rate up to the threshold level, which is in agreement with our results.
Following the fusion of 2-cell bovine embryos, the results of this study suggested that there is a negative correlation between voltage and cleavage rate in all duration, except in duration of 20 μs (Table II). This implying that exposure of bovine 2-cell embryos to high voltages has an inhibitory effect on cleavage rate. This is possibly due to large pore formation (and leakage of cytoplasm) over the two-blastomere membranes.
Therefore it could be suggested that for optimal cleavage rate, exposure of 2-cell bovine embryo to higher than 1kV/cm should be avoided. During this study a negative significant correlation was observed between blastocyst formation rate with increase in voltage in duration of 20, 40,and 100μs. Thus, exposure to high voltage during fusion, decrease blastocyst formation rate. Similar to cleavage rate, the duration of exposure within the range of this study (20 to 100 μs) had no significant effect on blastocyst formation rate. The fact that no significant correlation was observed between blastocyst formation rate and voltage in duration of 60 and 80 μs (Table III) suggest that for optimal developmental competence lower than 0.75kV/cm and higher than 1kV/cm should be avoided, and the best result can be obtained by exposing 2-cell bovine embryo to 0.75 kV/cm for 60 to 80 μs. Cytogenetic analysis of embryo showed that over 76% of fused embryos are true tetraploid. The value obtain in this study is close to value (%78) reported by Iwasaki et al (11) and significantly different with value (50%) reported by Prochazka et al (30) and by Curnow et al (12.5%) (9).
The lower developmental rate in high intensity is likely due to large pore formation and leakage of cytoplasmic material needed for development. The fusion rate obtained in this study when applying 1.5kV/cm for 100 μs was 88%, which is close to value (%76) reported by Curnow et al (9) when applied 1.4 kV/cm for 100 μs. For production of tetraploid blastocysts from bovine 2-cell embryos, exposure of 2-cell embryos to 0.75 kV/cm for 60μs is highly recommended. This value is close to value reported by Prochazka et al (30) (0.75 kV/cm for 50 μs), and different to Iwasaki et al (31) (2 pulses of 100 kV/cm for 10 to 25 μs) and and Xiangyung et al (32) (2 pulse of 100 kV/cm for 50 μs). Maybe this difference in electrofusion parameter is related to kind of fusion buffer, electrofusion machine, species of animal and etc. The mean blastocyst formation rate obtained when applying pulse of 0.75 kV/cm for 60 μs (35%) is nonsignificantly close to value in UCG (41%) and significantly different with value (18.8%) reported by Iwasaki et al (33). However, the overall developmental rate of embryos in fused group (FG) and exposed control group (ECG) is lower than UCG, suggesting that, alteration in distribution and behavior of microtubules and microfilaments might affect normal formation of mitotic spindle and the contractile ring, respectively (34). The lower developmental capacity of the fused embryo remained unclear, possibly this could be due to electrical stimulation, exposure to non-electrolyte medium or due to chromosomal construction of these embryos.
Although cloning mammals by transgenic nuclear transfer into oocyte cytoplasts has been performed successfully for nearly a decade, only a very small percentage of cloned embryos have developed to term, with a high incidence of developmental anomalies (1, 2). The impact of this technology has been spectacular, opening up new possibilities for producing recombinant proteins and therapeutic cloning, as animal cells can synthesize these proteins with appropriate post-translation modification (3, 4). Production of transgenic and knock-out mice and cattle is a choice for production of tetraploid embryos by aggregation of transgenic embryonic stem (ES) cells with tetraploid morulae (5, 6).
In tetraploid/diploid chimeras, a non-random distribution of cells occurs in the developing conceptus. In these types of chimeras, tetraploid cells readily contributes to extra-embryonic tissue compared to inner cell mass (7) and the offspring from these chimeras are formed solely by embryonic diploid cells. In aggregation of bovine embryonic ES-like cells with day 3 bovine diploid embryos, the ES-like cells have limited presence in various tissues, while in aggregation of bovine ES-like cells with bovine tetraploid embryos, the embryo proper is formed solely by ES-Like cells (8, 10).
Tetraploid embryos are produced by various procedures. One of the most accurate, repeatable and well defined, procedures is electrofusion. During this procedure, embryos are placed between two electrodes in fusion buffer, exposed to fusigenic stimulus for a very short period. This procedure was first reported in mice and in bovine using zona-enclosed blastomeres (11, 12).
Because required and optimum parameters reported for electrofusion by various studies are different, the aim of this study was to evaluate the effect of different voltages (0.5, 0.75, 1, 1.25 and 1.50 kV/cm) and durations (20, 40, 60, 80 and 100 μs) on fusion, cleavage and development of bovine embryos into blastocyst.
Materials and methods
This was an experimental study. All chemicals and reagents were purchased from Sigma chemicals, unless otherwise indicated. Ovaries were collected from local abattoirs from unstimulated heifers and cows, after 30 min of slaughter, transported to the laboratory in sterile 0.9% saline at 25 to 30 ˚C. Ovarian follicles (2 to 6 mm) were aspirated by 18-gauge needle primed with washing medium TCM-199 (Sigma M-2520).
In this study, over 1750 high quality (homogene and equal cell size) two-cell embryos were produced 33 to 35 hr post insemination. Nearly 570 two-cell embryo were used for each (control and experimental) group. The results in each group were repeated between 4 to 5 times.
In vitro maturation
High quality cumulus-oocyte-complexes (COCs), having homogenous evenly granulated cytoplasm surrounded by more than three layers compact granulosa cells, were rinsed 3 times in washing medium and twice in maturation medium (MM: washing medium supplemented with 1μg/ml 17β- Estradiol, 0.1 IU/ml HMG, 10% FCS and 1 mM L-glutamine) and subsequently were transferred in groups of 5 into 50μL drops of maturation medium under mineral oil. The COCs were then incubated (LabTec) for 22 to 24h in humidified atmosphere in 5% CO2 in air at 39˚C (12-14).
In vitro fertilization
After maturation, COCs were rinsed twice in fertilization medium (FM) and were inseminated, with final concentration of 1 to 2 million sperm for ml, in groups of 10 into 50 μL droplets of FM under mineral oil for 18 to 22h. Fertilization media also called Fert-TALP were prepared according to Parrish et al (15).
Live spermatozoa from fresh semen of fertile bulls were separated by centrifugation using Percoll gradients (90% and 45%; Seromed, Germany) at 2000 rpm for 25 min. The pellets were washed and diluted to the required concentration in FM. Capcitation of sperms occurred during the IVF culture (13-16).
In vitro Culture
Cleaning of spermatozoa and granulosa cells from inseminated COCs were carried out by vortexing (Ependorf) for 2 minutes in washing medium and subsequently transferred and cultured in SOF1 (17-21).
Then, 33 to 35h post insemination, 2-cell embryos were selected and categorized into two groups. One group without any exposure to electrical stimulation was taken as control group (UCG = unexposed control group) and was kept in SOF1 for further 37 to 39h. The 2-cell embryos in the other group were used for electrofusion.
Electrofusion
Two-cell embryos in groups of 5 to 10 were washed for 10-20 seconds in fusion buffer containing 0.3 M mannitol solution containing 0.1 mM MgS04, 0.05 mM CaCl2, pH=7.2-7.4, immediately transferred between electrodes in fusion chamber containing fusion buffer. Two stainless steel platform electrodes 1mm apart connected to electrofusion machine (CF-150B, Biological Laboratory Service, H-1165 Budapest, Zselyi A. U. 31. Hungary) form this chamber (9, 22). Two-cell embryos with inter-blastomeric axis placed parallel to electrodes were exposed to one pulse of direct current (DC) for certain voltage and duration. Embryos were observed at 60 min post electrofusion (7). Fused 2-cell embryos were called fused group (FG) and unfused 2-cells were called exposed control group (ECG). These embryos were transferred to SOF1 for further 37 to 39 h. At this stage embryos from UCG, FG, and ECG were transferred into SOF2 up to 10th day (SOF2 was the same as SOF1 without Na-pyrovate but containing 1%MEM essential amino acids, 10% FCS and 2 mM glucose) (17, 19, 20).
Embryo scoring
Cleavage was evaluated microscopically 72 h post-insemination in each group (FG, ECG, UCG). Morula and early blastocyst were assessed 168 h (7th day) post-insemination. Blastocyst and expanded blastocyst were assessed on the 8th to 10th day post insemination (23). Percentage of cleavage and blastocyst rate were evaluated with respect to the total number of embryos in each group. For cytogentic analysis by Giemsa staining two to eight-cell stage embryos from FG (4n chromosome) were processed according to modified Tarkowski protocol (24, 25). The number of chromosomes was determined under the light microscope at 100x magnification.
Statistical analysis
All statistical calculation including, coefficient of correlation and Chi-square were carried out using statistical package for social studies (SPSS-10) software, and Epi-Info statistical package, respectively.
Results
The results in table I show that, with increase in duration (20 to 100 μs) there is no significant increase in fusion rate in each different voltage (0.5 to 1.50 kV/cm). The results also showed that with increase in voltage, there is a steady increase in fusion rate, in each duration (Table I). Pearson analysis also confirms a high positive correlation between different voltages with fusion rate in different duration. The results in table II showed that with decrease in voltage from 1.5 to 0.5 kV/cm the percentage of cleavage steadily increases in different durations except in 20 μs duration. Pearson analysis also confirms a high negative significant correlation between different voltages with cleavage rate in different duration except 20 μs duration (Table II). Table II also shows that percentage of cleavage in the 1.5 and 1.25 kV/cm groups for 20 μs is significantly greater than that of groups of 40 to 100 μs, suggesting that during high voltage exposure, increase in duration reduces cleavage rate (p<0.05). The results also show that maximum cleavage rate was obtained in 0.75 kV/cm in 80 μs duration.
The results in table III show that with increase in voltage from 0.5 to 1.5 kV/cm the blastocyst formation rate decreases steadily during different duration (p<0.05), and with increase in duration of exposure during different voltage, blastocyst formation steadily decreases, however this reduction is not significant (p>0.05), suggesting that exposure to define voltage does not significantly reduces blastocyst formation rate in different duration. Pearson analysis also confirms a high negative significant correlation between different voltages with blastocyst formation rate in different duration except 60 and 80 μs duration (Table III).Maximum blastocyst formation rate was observed in 0.75 kV/cm in the duration of 60 μs.
The cleavage, morula and blastocyst formation rate in the ECG groups overall followed the same trend as the FG group. Therefore, the data were not shown. Mean cleavage and blastocyst formation rate in the UCG were 80.46 and 32.32% respectively. These values are not significantly higher than the values in the FG and ECG groups with the maximum blastocyst formation rate.
Figure 1 reveals the chromosomal analysis from putative tetraploid embryos produced by electrofusion (0.75 kV/cm for 60 μs).In total 37 (66%) of 56 electro-fused embryos at 2-8 cells could be analyzed and 3 (%8), 6 (%16) and 28 (%76) of 37 were haploid, diploid and tetraploid respectively.

Discusion
Electrofusion are routinely used for different technologic purposes. During electrofusion due to applied direct current electric field, the membranes are polarized and instabilized, results in attraction of other membrane (point membrane fusion) and formation of unstable flat membrane diaphragm, through reversible pore formation followed by reversible breakdown of the membrane or diaphragm. Under favorable conditions, the flat diaphragm deteriorates to allow cell mixing, indicating through cell-to-cell fusion (27). The obtained results suggest that the fusion rate is voltage dependent and with increases of voltage intensity from 0.5 to 1.5kV/cm, fusion rate increases to 88% (Table I), which is different to fusion rate in study of Lan Li et al (77% in goat) (3).
Possibly this difference is due to difference in species. However, increase in duration of electrical pulse to 100 μs did not affect the fusion rate in different voltage (Table I). The results of this study are in concordance with the result of Zhelev et al (28) in other cells, which suggest that there is a correlation between pulse intensity and pore formation. Similar results were obtained by Tatham et al (29) by fusing enucleated bovine oocytes (by different methods) with blastomeres (with different aged). These authors also showed that increase voltage intensity up to certain threshold level increase the fusion rate, after which fusion rate decreases.
However, they also showed that increasing in pulse duration has no fundamental effect on fusion rate up to the threshold level, which is in agreement with our results.

Following the fusion of 2-cell bovine embryos, the results of this study suggested that there is a negative correlation between voltage and cleavage rate in all duration, except in duration of 20 μs (Table II). This implying that exposure of bovine 2-cell embryos to high voltages has an inhibitory effect on cleavage rate. This is possibly due to large pore formation (and leakage of cytoplasm) over the two-blastomere membranes.

Therefore it could be suggested that for optimal cleavage rate, exposure of 2-cell bovine embryo to higher than 1kV/cm should be avoided. During this study a negative significant correlation was observed between blastocyst formation rate with increase in voltage in duration of 20, 40,and 100μs. Thus, exposure to high voltage during fusion, decrease blastocyst formation rate. Similar to cleavage rate, the duration of exposure within the range of this study (20 to 100 μs) had no significant effect on blastocyst formation rate. The fact that no significant correlation was observed between blastocyst formation rate and voltage in duration of 60 and 80 μs (Table III) suggest that for optimal developmental competence lower than 0.75kV/cm and higher than 1kV/cm should be avoided, and the best result can be obtained by exposing 2-cell bovine embryo to 0.75 kV/cm for 60 to 80 μs. Cytogenetic analysis of embryo showed that over 76% of fused embryos are true tetraploid. The value obtain in this study is close to value (%78) reported by Iwasaki et al (11) and significantly different with value (50%) reported by Prochazka et al (30) and by Curnow et al (12.5%) (9).

The lower developmental rate in high intensity is likely due to large pore formation and leakage of cytoplasmic material needed for development. The fusion rate obtained in this study when applying 1.5kV/cm for 100 μs was 88%, which is close to value (%76) reported by Curnow et al (9) when applied 1.4 kV/cm for 100 μs. For production of tetraploid blastocysts from bovine 2-cell embryos, exposure of 2-cell embryos to 0.75 kV/cm for 60μs is highly recommended. This value is close to value reported by Prochazka et al (30) (0.75 kV/cm for 50 μs), and different to Iwasaki et al (31) (2 pulses of 100 kV/cm for 10 to 25 μs) and and Xiangyung et al (32) (2 pulse of 100 kV/cm for 50 μs). Maybe this difference in electrofusion parameter is related to kind of fusion buffer, electrofusion machine, species of animal and etc. The mean blastocyst formation rate obtained when applying pulse of 0.75 kV/cm for 60 μs (35%) is nonsignificantly close to value in UCG (41%) and significantly different with value (18.8%) reported by Iwasaki et al (33). However, the overall developmental rate of embryos in fused group (FG) and exposed control group (ECG) is lower than UCG, suggesting that, alteration in distribution and behavior of microtubules and microfilaments might affect normal formation of mitotic spindle and the contractile ring, respectively (34). The lower developmental capacity of the fused embryo remained unclear, possibly this could be due to electrical stimulation, exposure to non-electrolyte medium or due to chromosomal construction of these embryos.
Type of Study: Original Article |
References
1. Nguyen VT, Sayaka W, Satoshi K, Hiroshi O, Takafusa H. Injection of somatic cell cytoplasm into oocytes before intracytoplasmic sperm injection impairs full-term development and increases placental weight in mice. Biol Reprod 2006; 74: 865-873. [DOI:10.1095/biolreprod.105.047803]
2. Shinozawa T, Sugawara A, Matsumoto A, Han Y-J, Tomioka I, Inai K, et al. Development of rat tetraploid and chimeric embryos aggregated with diploid cells. Zygot 2006; 14: 287-297 [DOI:10.1017/S096719940600387X]
3. Lan LI, Wei SHEN, Lingjiang MIN, Yujian SUN, Jixian DENG, Quingjie PAN. Nuclear transfer of goat somatic cells transgenic for human lactoferrin gene. Front Biol Chaina 2008; 3: 269-274. [DOI:10.1007/s11515-008-0052-8]
4. Jingjuan JI, Tonghang GUO, Xianhong TONG, Lihua LUO, Guixiang ZHOU, Yingyun FU, et al. Experimental cloning of embryos through human-rabbit interspecies nuclear transfer. Front Biol Chaina 2007; 2: 80-84. [DOI:10.1007/s11515-007-0014-6]
5. Polejaeva IA, Campbell KHS. New advances in somatic cell nuclear transfer: Applications in transgenesis. Theriogenology 2000; 53: 117-126. [DOI:10.1016/S0093-691X(99)00245-9]
6. Skrzyszowska M, Smorag Z, Slomski R, Katska-Ksiazkiewicz L, Kalak R. Generation of transgenic rabbits by the novel technique of chimeric somatic cell cloning. Biol Reprod 2006; 74: 1114-1120. [DOI:10.1095/biolreprod.104.039370]
7. James RM, Klerkx A, Keighren M, Flockhart JH, West JD. Restricted distribution of tetraploid cells in mouse tetraploid-diploid chimaeras. Developmental Biology 1995; 167: 213- 226. [DOI:10.1006/dbio.1995.1018]
8. James RM, West JD. A chimaeric animal model for confined placental mosaicism. Human Genetic 1994; 93: 603-604. [DOI:10.1007/BF00202833]
9. Curnow EC, Gunn LM, Trounson AQ. Electrofusion of two-cell bovine embryos for the production of tetraploid blastocysts in vitro. Molecular Reproduction Development 2000; 56: 372-377.
https://doi.org/10.1002/1098-2795(200007)56:3<372::AID-MRD7>3.0.CO;2-W [DOI:10.1002/1098-2795(200007)56:33.0.CO;2-W]
10. Berg H. Fusion of blastomeres and blastoctsts of mouse embryos. Bioelectrochemistry Bioenergetic 1982; 9: 223-228. [DOI:10.1016/0302-4598(82)80178-5]
11. Iwasaki S, Kono T, Fukatsu H, Nakahara T. Production of bovine tetraploid embryos by electrofusion and their developmental capacity in vitro. Gamete Research 1989; 24: 261-267. [DOI:10.1002/mrd.1120240303]
12. Tanghe S, Soom A, Mehrzad VJ, Maes D, Duchateau L, Kruif A. Cumulus contributions during bovine fertilization in vitro. Theriogenology 2003; 60: 135-149. [DOI:10.1016/S0093-691X(02)01360-2]
13. Khurana NK, Nieman H. Effects of oocyte quality, oxygen tension, embryo density, cumulus cells and energy substrates on cleavage and morula/blastocyst formation of bovine embryos. Theriogenology 2000; 54: 741-756. [DOI:10.1016/S0093-691X(00)00387-3]
14. Gandolfi F, Luciano MA, Modina S, Ponzini A, Pocar P, Armstrong DT, et al. The in vitro developmental competence of bovine oocytes can be related to the morphology of the ovary. Theriogenology 1997; 48:1153-1160. [DOI:10.1016/S0093-691X(97)00348-8]
15. Parrish JJ, Susko-Parrish JL, Winer MA, First NL. Capacitation of bovine sperm by heparin. Biol Reprod 1988; 38: 1171-1180. [DOI:10.1095/biolreprod38.5.1171]
16. Neglia G, Gasparrini B, Brienza VC, Palo RD, Campanile G, Presicce GA, et al. Bovine and buffalo in vitro embryo production using oocytes derived from abattoir ovaries or collected by transvaginal follicle aspiration. Theriogenology 2003; 59: 1123-1130. [DOI:10.1016/S0093-691X(02)01170-6]
17. Carolan C, Lonergan P, Langendonckt AV, Mermillod P. Factors affecting bovine embryo development in synthetic oviductal fluid following oocyte maturation and fertilization in vitro. Theriogenology 1995; 43: 1115-1128. [DOI:10.1016/0093-691X(95)00075-J]
18. Hevitt DA, England GCW. Synthetic oviductal fluid and oviductal cell co-cultue for canine oocyte maturation in vitro. Animal Reproduction Science 1999;55: 63-75 [DOI:10.1016/S0378-4320(98)00162-6]
19. Steeves TE, Gardner DK. Temporal and differential effects of amino acids on bovine embryo development in culture. Biol Reprod 1999; 61: 731-740. [DOI:10.1095/biolreprod61.3.731]
20. Yoshioka K, Othman AM, Taniguchi T, Yamanaka H, Sekikawa K. Differential pattern of blastulation in bovine morulae cultured in synthetic oviductal fluid medium containing FCS or BSA. Theriogenology 1997; 48: 997-1006. [DOI:10.1016/S0093-691X(97)00326-9]
21. Galli C, Duchi R, Crotti G, Turini P, Pondera N, Colleoni I, et al. Bovine embryo technologies. Theriogenology 2003; 59: 599-616. [DOI:10.1016/S0093-691X(02)01243-8]
22. Alexander K, Elena P, Loulia Z, Larissa V, Detlev G. Development of parthenogenetic rat embryos. Biol Reprod 2003; 68: 829-836. [DOI:10.1095/biolreprod.102.006494]
23. Gordon I. Culturing the early embryo. In: Laboratory Production of Cattle Embryo. 3rd Ed. Wallingford, CABI publication; 2003; 246-247.
24. Tarkowski AK. An air-drying method for chromosome preparations from mouse eggs. Cytogenetics 1966; 5: 394-400. [DOI:10.1159/000129914]
25. Yoshizawa M, Konno H, Zhu S, Kageyama S. Chromosomal diagnosis in each individual blastomere of 5-to 10-cell bovine embryos derived from in vitro fertilization. Theriogenology 1999; 51: 1239-1250. [DOI:10.1016/S0093-691X(99)00068-0]
26. Lechniak D. The incidence of polyploidy and mixoploidy in early bovine embryos derived from in vitro fertilization. Genetic Sel Evolution 1996; 28: 321-328. [DOI:10.1186/1297-9686-28-4-321]
27. Chernomerdik LV, Sowers AE. Physical and ultrastructural evidence that integrin of the spectrin network controls the macroscopic fusion produce morphology following the electrofusion of erythrocyte ghosts. Biophysical Journal 1991; 60: 1026-1037. [DOI:10.1016/S0006-3495(91)82140-3]
28. Zhelev DV, Dimitrov D, Doinov SP. Correlation between physical parameters in electro fusion and electroporation of protoplasts. Bioelectrochemistry and Bioenergeti 1988; 20: 155-167. [DOI:10.1016/S0302-4598(98)80013-5]
29. Tatham BG, Pushett DA, Giliam KJ, Dowsing AT, Mahavorasilpa TL, Trounson AO. Electrofusion of in vitro produced bovine embryonic cells for the production of isofusion contours for cells used in nuclear transfer. Journal of Reproduction and Fertility Supplementary 1995; 49: 549-553.
30. Prochazka R, Vodicka P, Zodova D, Rybar R, Motlik J. Development of in vivo derived diploid and tetraploid pig embryos in a modified medium NCSU 37. Theriogenology 2004; 62: 155-164 [DOI:10.1016/j.theriogenology.2003.08.017]
31. Iwasaki S, Campbell HS, Galli S, Akiama K. Production of live calves derived from embryonic stem like cells aggregated with tetraploid embryos. Biol Repropd 2000; 62: 470-475. [DOI:10.1095/biolreprod62.2.470]
32. Xiangyun Li, Wei Wei, Jun Yong, Qing Jia, Yuansong Yu, Keqian Di. The genetic heterozygozygosity and fitness of tetraploid embryos and embryonic stem cells are crucial parameters influencing survival of mice derived from embryonic stem cells by tetraploid embryo aggregation. Reproduction 2005; 130: 53-59 [DOI:10.1530/rep.1.00667]
33. Iwasaki Shizue, Yasuko Ito, Iwasaki Setsuo. In-vitro development of aggregates of bovine inner cell mass cells or bovine mammary cells and putative tetraploid embryos produced by electrofusion. Journal of Reproduction and Development 1999; 45: 65-71 [DOI:10.1262/jrd.45.65]
34. Suzuki H, Ogasavara I, Takahashi H, Imada Y, Toyokava K. Electrofusion of blastomeres of hamster 2-cell embryos and dynamic changes of the cytoskeletal distribution. Journal of Reproduction and Development 2001; 47: 227-235. [DOI:10.1262/jrd.47.227]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |


