Thu, Jan 8, 2026
[Archive]
Volume 6, Issue 5 (7-2008)
IJRM 2008, 6(5): 187-192 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Haidari K, Salehnia M, Rezazadeh Valojerdi M, Pourbyranvand S. Ultrastructural study of isolated mouse preantral follicles co-cultured with cumulus cells with leukemia inhibitory factor. IJRM 2008; 6 (5) :187-192
URL: http://ijrm.ir/article-1-130-en.html
URL: http://ijrm.ir/article-1-130-en.html
1- Department of Anatomy, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
2- Department of Anatomy, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran ,mogdeh@dr.com
2- Department of Anatomy, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran ,
Keywords: Co-culture, In vitro maturation, Leukemia inhibitory factor, Mouse preantral follicle, Ultrastructure
Full-Text [PDF 314 kb]
(994 Downloads)
| Abstract (HTML) (3050 Views)
Full-Text: (501 Views)
Introduction
In recent years, much attention has been focused on the improvement of in vitro maturation (IVM) of follicles in different culture systems (1-5). Culture conditions including the formulation of the base medium, supplementations and physical environment such as the presence of cumulus cells influence the maturation and subsequent follicular development (6). Supplementation of media with growth factors and cytokine locally produced in ovarian microenvironment has been used for in vitro maturation of follicles and oocytes (7-10). Leukemia inhibitory factor (LIF) is a 43 kDa glycoprotein and may be an important modulator of ovarian function (11, 12). It also improves the rate of fertilization of mouse and sheep oocytes in vitro (13, 14). LIF exerted a positive influence on the quality of the blastocysts as revealed by significantly higher number of ICM cells and total number of cells. It has shown that the LIF had antiapoptotic roles in oocytes and it inhibited female germ cell death (15). LIF acts on the cells via its receptors which consist of two subunits, a low affinity binding subunit and a transmembrane molecule, gp 130 (16). The leukemia inhibitory factor receptors were detected on the oocyte and preimplantation embryos (17, 18). In spite of the effects of LIF on embryo development and implantation (13, 19), there are limited reports regarding to its effects on follicular maturation in vitro. In this regard, the study of Nilsson et al showed that LIF promotes the primordial to primary follicle transition after ovarian tissue culture (20). Our previous studies showed that the LIF could improve the growth of isolated follicles in simple and co-culture system. The LIF-treated groups had significantly (p< 0.001) lager size than their control (21, 22). Thus it seems that LIF could induce some subcellular changes which facilitate the follicular maturation. Furthermore, the ultrastructural analysis of cultured follicles could direct us to understand subcellular changes during in vitro culture and maturation. Thus the aim of this study was to describe the detail of ultrastructural changes of mouse preantral follicles cultured in simple and co-culture system in the presence of LIF in comparison with their control.
Materials and methods
Animals
Five female NMRI (National Medical Research Institute) mice (14-day-old) were cared according to the university guide for the care and use of laboratory animals and they were sacrificed by cervical dislocation. Their ovaries were removed and stored in α- minimal essential medium (α- MEM; Gibco, UK) supplemented with 5% fetal bovine serum (FBS; Gibco, UK).
Preparation of feeder layer
The cumulus monolayer was prepared by puncturing, squeezing and removing cumulus cells from fresh early antral follicles. The cells with 96% viability (using 0.4% trypan blue staining) and concentration of 2×105/ml (counting with neobar lam) were cultured in 20 µl droplets of α-MEM medium supplemented with 5% FBS in a humidified 5% CO2 at 37 °C under mineral oil until confluency was reached. The medium was refreshed on day 2 and then the feeder layer was used for co-culturing with isolated preantral follicles.
Preantral follicle isolation
The preantral follicles with a diameter of 140-170 µm were mechanically isolated from mouse ovaries (near 15 follicles per ovary) using 29-guage needles under stereomicroscope. Isolated follicles containing several layers of granulosa cells with a centrally located healthy oocyte and a thin layer of theca cells were distributed randomly for the following groups of studies: control without LIF, control with LIF, co-cultured group with LIF, and co-cultured group without LIF.
The culture medium
The selected preantral follicles were cultured individually in 20 µl droplets of α- MEM (Gibco; UK) supplemented with 5% FBS, 100 mIU/ml recombinant follicle stimulating hormone (rFSH or Gonal-f; Serono, Switzerland), 1% insulin, transferrin, and selenium (ITS; Gibco, UK), 20ng/ml murine recombinant epidermal growth factor (mrEGF; Sigma, Germany), 100 IU/ml penicillin and 50 µg/ml streptomycin under mineral oil in a humidified atmosphere of 5% CO2 in air at 37 °C for 4 days. In LIF treated groups, 50 ng/ml mouse recombinant LIF (Sigma, USA) was added to culture media based on the pilot study (21).The medium without LIF was considered as control and the media were refreshed every other day in both groups.
Electron microscopy
The follicles adhered to culture dish after 4 days culturing and the normal structure of follicles was lost. The follicles had diffuse appearance by follicular and thecal cells outgrowth thus the sampling were done on day 4 of culture. The cultured preantral follicles from 4 groups of study (simple and co-culture groups in the presence and absence of LIF) were collected randomly (n = 30 from each groups) and fixed in 2.5% glutaraldehyde in phosphate-buffered saline (PBS, pH 7.4) for 2 hours, and post fixed with 1% osmium tetroxide in the same buffer for 2 hours. After dehydration in an ascending series of ethanol, the specimens were placed in propylene oxide and embedded in Epon 812 (TAAB, UK). Semithin sections (0.5 µm) were stained with toluidine blue for light microscopy. Ultrathin sections (60-80 nm) were stained with uranyl acetate and lead citrate and examined by transmission electron microscopy (TEM) (Zeiss EM 900, Germany).
Results
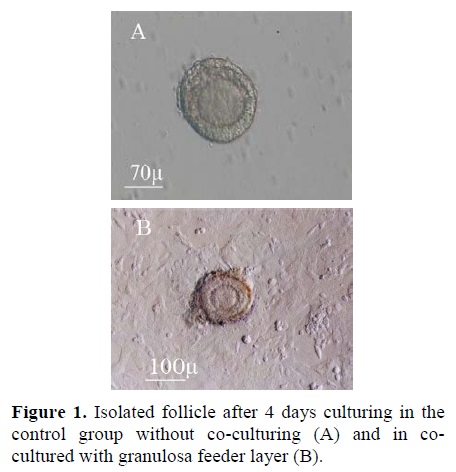
The morphology of the follicles after 4 days culturing is shown in figure 1. The growing granulosa and theca cells adhered to the culture plate and they had diffuse appearance. In vitro cultured preantral follicles consisted of an oocytes surrounded by several layers of polyhedral granulosa and flattened theca cells. The cultured follicles showed the granulosa cells were in close connection with oocyte (Figure 2A).
Ultrastructure of oocyte
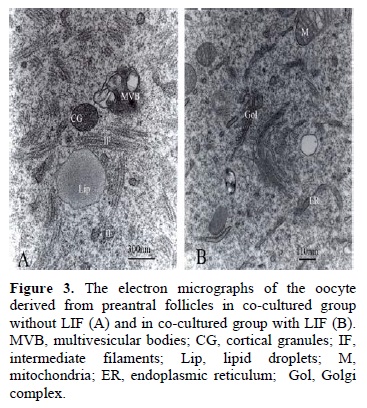
The electron micrograph of follicles in all studied groups demonstrated that the oocyte of cultured preantral follicles had a homogeneous cytoplasm and was surrounded by zona pellucida. The zona pellucida was as a thick layer around the oocytes. The projections of granulosa cells could be seen in the thickness of zona pellucida especially in its outer part. The long and torturous oocyte microvillus were extended into narrow perivitteline space (Figure 2A and B). The cortical granules were prominent in the co-cultured group (Figure 2A). In ooplasm, the most abundant organelles were the round or ovoid shaped mitochondria with light matrix and cristae and Golgi complex (Figure 2C). The aggregation of mitochondria around the germinal vesicle was prominent in the co-cultured follicles compared to the control group (Figure 2D). Also the intermediate filaments of cytokeratin with striated appearance in longitudinal sections were prominent in ooplasm of co-cultured groups without LIF (Figure 3A). In most samples, the endoplasmic reticulum cisternae were observed in association with mitochondria and Golgi complex, as figure 3B shows this phenomenon in co-cultured group with LIF. The multivesicular bodies were seen in ooplasm of all groups of study with different size and shape (Figure 2 A-C and 3A).The ultrastructure of co-cultured isolated follicles in the presence of LIF showed some similar characteristic to its control (the co-cultured group without LIF). However, some endoplasmic reticulum cisterns aligned from the nucleus envelope towards the cell membrane as a tubular network and others were close to the mitochondria and polyribosoms (Figure 2 B and C). The germinal vesicle of oocyte had an irregular outer border, and uncondensed chromatin (Figure 2D).
The ultrastructure of granulosa and theca cells
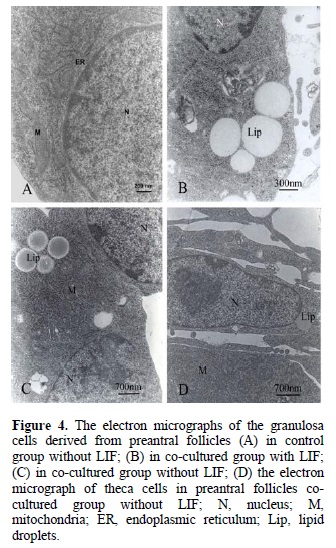
The granulosa cells had a normal morphology and an organelle distribution in all groups of study (Figure 4 A-C). Their nuclei were ovoid or round with euchromatin in the inner part and small peripheral aggregations of heterochromatin and no sign of cell death was observed. Most of them possessed abundant typical organelles of steroidogenesis, including: mitochondria with tubular cristae, a well developed smooth endoplasmic reticulum and several large lipid droplets. These characteristics were especially prominent in co-cultured group (Figure 4B and C). The theca cells were flattened cells with the same organelles distributions in experimental and control groups (Figure 4D).

Discussion
Our ultrastructural observations showed that the cultured follicles had normal morphology and there was no sign of degeneration in any groups of the study. In this regards Mazoochi et al previously showed that the incidence of cell death during in vitro maturation of follicles was not more than that in fresh control (23). However, isolated follicles cultured in co-cultured system with cumulus cells showed some maturation features in oocytes, including; more distribution of cortical granules, mitochondria (especially around the germinal vesicles) and the lipid droplets in vicinity with smooth endoplasmic reticulum (SER) cisternae compare to control group.
We suggest that using cumulus cells derived from antral follicles could increase the growth of isolated mouse follicles in vitro in a similar manner to in vivo. Therefore, using cumulus cells as a feeder cells could supplement the culture media for follicular growth. In agreement with this result, Quinn and Margalit showed that the culture of human embryos with their cumulus cells in insemination drops of medium produces a significantly greater proportion of fully expanded blastocysts compare to when the zygotes are transferred to fresh culture drops devoid of cumulus cells (24). Our observations showed that there were many contacts between granulosa cells and oocyte via their projections penetrated to the zona pellucida. As previously mentioned, the quantity and quality of junctional sites between oocytes and granulosa cells is very important for growth and maturation of oocyte (25, 26).
Another ultrastructural phenomenon, which we observed in the oocytes of cultured follicles, was the abundant mitochondria in the ooplasm and their aggregation around the envelope of germinal vesicle (GV) especially in both co-cultured groups. These observations were in agreement with our previous observation which showed that in co cultured and LIF treated groups, the size and growth of follicle significantly were increased compare to non treated groups (22). Increasingly, oocytes need the mitochondria during in vitro maturation and the number of mitochondria is very important for increasing the energy producing capacity of growing cells. Mitochondria have a vital role in the metabolism of energy–containing compounds in the ooplasm to provide ATP for fertilization and pre-implantation embryonic survival and development. During the transition of primary follicles to secondary follicles, the oocytes mitochondria become more numerous and are dispersed in the ooplasm. It has known that there is a major displacement of mitochondria during oocytes maturation that appears to correlate with the degree of developmental competence acquired by the oocytes.
The mitochondria in germinal vesicle oocytes (GV oocytes) are mainly distributed adjacent to the cortex but they are displaced during maturation to give the distinct patterns of mitochondrial distribution in metaphase II oocyte. As mentioned by Nishi et al (27), the aggregation of mitochondria around the nucleus was essential for maturation, fertilization and development.
In addition, the ultrastructure analysis of isolated follicles which were co-cultured in the presence of LIF showed some endoplasmic reticulum (ER) cisterns aligned from the nucleus envelope towards the cell membrane as a tubular network and the others were close to the mitochondria. Similar observations have been reported in the oocytes derived from fresh preantral follicles in several types of mammalian species (28-32). The role of this contact remains unclear. Motta et al (32) suggested that the mitochondrial-SER aggregations and mitochondrial–vesicle complex could be involved in the production of substrate needed for subsequent fertilization and early embryo development. The well-developed smooth endoplasmic reticulum of follicles seems to be responsible for lipid biosynthesis and consequent lipid droplets formation in later developmental stages of oocytes. Furthermore, ER can be the site of calcium storage needed for future events of oocytes such as fertilization and cortical reaction.
The cytoplasmic inclusions of mature oocytes of different mammalian species are characterized by the presence of multivesicular bodies, lipid droplets and myelin figures. They may play a role during the maturation of oocytes and in early embryo development (33-35). In electron micrograph of oocyte of co-cultured follicles, there were prominent multivesicular bodies and myelin-like lamellar bodies; however, there were a few fat droplets in the ooplasm of all groups. It seems that the lipid content of oocytes in preantral follicles was lower than that of mature oocytes which was reported earlier (35). However, the fate of lipid droplets during the oogenesis and embryogenesis in mammals is not clear so far.
Myelin figures, as described in follicles of other species (33) are thought to represent digestive vesicles responsible for degradation of aged and non-functional cellular structures. It seems that the lamellar bodies or myelin like structure are observed in the oocytes since oocyte is a long lived cell. The granulosa and theca cells are the main sources of steroid hormones. The electron micrograph of granulosa cells in our study showed a large number of round or cylindrical mitochondria with lamellar or tubular cristae as well as well-developed smooth endoplasmic reticulum tubules especially in co-cultured samples. Also, it was shown that the granulosa cells used for co-culture system gained ultrastructure characteristics typical of metabolically active and steroidogenic cells (32, 36). In conclusion, the oocyte and granulosa cells in co-cultured system showed more remarkable maturation features than that of control and this system could be effective to improve the maturation of isolated follicles, although it needs further studies.
Acknowledgements
This work was supported by a grant from Tarbiat Modares University.
In recent years, much attention has been focused on the improvement of in vitro maturation (IVM) of follicles in different culture systems (1-5). Culture conditions including the formulation of the base medium, supplementations and physical environment such as the presence of cumulus cells influence the maturation and subsequent follicular development (6). Supplementation of media with growth factors and cytokine locally produced in ovarian microenvironment has been used for in vitro maturation of follicles and oocytes (7-10). Leukemia inhibitory factor (LIF) is a 43 kDa glycoprotein and may be an important modulator of ovarian function (11, 12). It also improves the rate of fertilization of mouse and sheep oocytes in vitro (13, 14). LIF exerted a positive influence on the quality of the blastocysts as revealed by significantly higher number of ICM cells and total number of cells. It has shown that the LIF had antiapoptotic roles in oocytes and it inhibited female germ cell death (15). LIF acts on the cells via its receptors which consist of two subunits, a low affinity binding subunit and a transmembrane molecule, gp 130 (16). The leukemia inhibitory factor receptors were detected on the oocyte and preimplantation embryos (17, 18). In spite of the effects of LIF on embryo development and implantation (13, 19), there are limited reports regarding to its effects on follicular maturation in vitro. In this regard, the study of Nilsson et al showed that LIF promotes the primordial to primary follicle transition after ovarian tissue culture (20). Our previous studies showed that the LIF could improve the growth of isolated follicles in simple and co-culture system. The LIF-treated groups had significantly (p< 0.001) lager size than their control (21, 22). Thus it seems that LIF could induce some subcellular changes which facilitate the follicular maturation. Furthermore, the ultrastructural analysis of cultured follicles could direct us to understand subcellular changes during in vitro culture and maturation. Thus the aim of this study was to describe the detail of ultrastructural changes of mouse preantral follicles cultured in simple and co-culture system in the presence of LIF in comparison with their control.
Materials and methods
Animals
Five female NMRI (National Medical Research Institute) mice (14-day-old) were cared according to the university guide for the care and use of laboratory animals and they were sacrificed by cervical dislocation. Their ovaries were removed and stored in α- minimal essential medium (α- MEM; Gibco, UK) supplemented with 5% fetal bovine serum (FBS; Gibco, UK).
Preparation of feeder layer
The cumulus monolayer was prepared by puncturing, squeezing and removing cumulus cells from fresh early antral follicles. The cells with 96% viability (using 0.4% trypan blue staining) and concentration of 2×105/ml (counting with neobar lam) were cultured in 20 µl droplets of α-MEM medium supplemented with 5% FBS in a humidified 5% CO2 at 37 °C under mineral oil until confluency was reached. The medium was refreshed on day 2 and then the feeder layer was used for co-culturing with isolated preantral follicles.
Preantral follicle isolation
The preantral follicles with a diameter of 140-170 µm were mechanically isolated from mouse ovaries (near 15 follicles per ovary) using 29-guage needles under stereomicroscope. Isolated follicles containing several layers of granulosa cells with a centrally located healthy oocyte and a thin layer of theca cells were distributed randomly for the following groups of studies: control without LIF, control with LIF, co-cultured group with LIF, and co-cultured group without LIF.
The culture medium
The selected preantral follicles were cultured individually in 20 µl droplets of α- MEM (Gibco; UK) supplemented with 5% FBS, 100 mIU/ml recombinant follicle stimulating hormone (rFSH or Gonal-f; Serono, Switzerland), 1% insulin, transferrin, and selenium (ITS; Gibco, UK), 20ng/ml murine recombinant epidermal growth factor (mrEGF; Sigma, Germany), 100 IU/ml penicillin and 50 µg/ml streptomycin under mineral oil in a humidified atmosphere of 5% CO2 in air at 37 °C for 4 days. In LIF treated groups, 50 ng/ml mouse recombinant LIF (Sigma, USA) was added to culture media based on the pilot study (21).The medium without LIF was considered as control and the media were refreshed every other day in both groups.
Electron microscopy
The follicles adhered to culture dish after 4 days culturing and the normal structure of follicles was lost. The follicles had diffuse appearance by follicular and thecal cells outgrowth thus the sampling were done on day 4 of culture. The cultured preantral follicles from 4 groups of study (simple and co-culture groups in the presence and absence of LIF) were collected randomly (n = 30 from each groups) and fixed in 2.5% glutaraldehyde in phosphate-buffered saline (PBS, pH 7.4) for 2 hours, and post fixed with 1% osmium tetroxide in the same buffer for 2 hours. After dehydration in an ascending series of ethanol, the specimens were placed in propylene oxide and embedded in Epon 812 (TAAB, UK). Semithin sections (0.5 µm) were stained with toluidine blue for light microscopy. Ultrathin sections (60-80 nm) were stained with uranyl acetate and lead citrate and examined by transmission electron microscopy (TEM) (Zeiss EM 900, Germany).
Results
The morphology of the follicles after 4 days culturing is shown in figure 1. The growing granulosa and theca cells adhered to the culture plate and they had diffuse appearance. In vitro cultured preantral follicles consisted of an oocytes surrounded by several layers of polyhedral granulosa and flattened theca cells. The cultured follicles showed the granulosa cells were in close connection with oocyte (Figure 2A).
Ultrastructure of oocyte
The electron micrograph of follicles in all studied groups demonstrated that the oocyte of cultured preantral follicles had a homogeneous cytoplasm and was surrounded by zona pellucida. The zona pellucida was as a thick layer around the oocytes. The projections of granulosa cells could be seen in the thickness of zona pellucida especially in its outer part. The long and torturous oocyte microvillus were extended into narrow perivitteline space (Figure 2A and B). The cortical granules were prominent in the co-cultured group (Figure 2A). In ooplasm, the most abundant organelles were the round or ovoid shaped mitochondria with light matrix and cristae and Golgi complex (Figure 2C). The aggregation of mitochondria around the germinal vesicle was prominent in the co-cultured follicles compared to the control group (Figure 2D). Also the intermediate filaments of cytokeratin with striated appearance in longitudinal sections were prominent in ooplasm of co-cultured groups without LIF (Figure 3A). In most samples, the endoplasmic reticulum cisternae were observed in association with mitochondria and Golgi complex, as figure 3B shows this phenomenon in co-cultured group with LIF. The multivesicular bodies were seen in ooplasm of all groups of study with different size and shape (Figure 2 A-C and 3A).The ultrastructure of co-cultured isolated follicles in the presence of LIF showed some similar characteristic to its control (the co-cultured group without LIF). However, some endoplasmic reticulum cisterns aligned from the nucleus envelope towards the cell membrane as a tubular network and others were close to the mitochondria and polyribosoms (Figure 2 B and C). The germinal vesicle of oocyte had an irregular outer border, and uncondensed chromatin (Figure 2D).
The ultrastructure of granulosa and theca cells
The granulosa cells had a normal morphology and an organelle distribution in all groups of study (Figure 4 A-C). Their nuclei were ovoid or round with euchromatin in the inner part and small peripheral aggregations of heterochromatin and no sign of cell death was observed. Most of them possessed abundant typical organelles of steroidogenesis, including: mitochondria with tubular cristae, a well developed smooth endoplasmic reticulum and several large lipid droplets. These characteristics were especially prominent in co-cultured group (Figure 4B and C). The theca cells were flattened cells with the same organelles distributions in experimental and control groups (Figure 4D).




Discussion
Our ultrastructural observations showed that the cultured follicles had normal morphology and there was no sign of degeneration in any groups of the study. In this regards Mazoochi et al previously showed that the incidence of cell death during in vitro maturation of follicles was not more than that in fresh control (23). However, isolated follicles cultured in co-cultured system with cumulus cells showed some maturation features in oocytes, including; more distribution of cortical granules, mitochondria (especially around the germinal vesicles) and the lipid droplets in vicinity with smooth endoplasmic reticulum (SER) cisternae compare to control group.
We suggest that using cumulus cells derived from antral follicles could increase the growth of isolated mouse follicles in vitro in a similar manner to in vivo. Therefore, using cumulus cells as a feeder cells could supplement the culture media for follicular growth. In agreement with this result, Quinn and Margalit showed that the culture of human embryos with their cumulus cells in insemination drops of medium produces a significantly greater proportion of fully expanded blastocysts compare to when the zygotes are transferred to fresh culture drops devoid of cumulus cells (24). Our observations showed that there were many contacts between granulosa cells and oocyte via their projections penetrated to the zona pellucida. As previously mentioned, the quantity and quality of junctional sites between oocytes and granulosa cells is very important for growth and maturation of oocyte (25, 26).
Another ultrastructural phenomenon, which we observed in the oocytes of cultured follicles, was the abundant mitochondria in the ooplasm and their aggregation around the envelope of germinal vesicle (GV) especially in both co-cultured groups. These observations were in agreement with our previous observation which showed that in co cultured and LIF treated groups, the size and growth of follicle significantly were increased compare to non treated groups (22). Increasingly, oocytes need the mitochondria during in vitro maturation and the number of mitochondria is very important for increasing the energy producing capacity of growing cells. Mitochondria have a vital role in the metabolism of energy–containing compounds in the ooplasm to provide ATP for fertilization and pre-implantation embryonic survival and development. During the transition of primary follicles to secondary follicles, the oocytes mitochondria become more numerous and are dispersed in the ooplasm. It has known that there is a major displacement of mitochondria during oocytes maturation that appears to correlate with the degree of developmental competence acquired by the oocytes.
The mitochondria in germinal vesicle oocytes (GV oocytes) are mainly distributed adjacent to the cortex but they are displaced during maturation to give the distinct patterns of mitochondrial distribution in metaphase II oocyte. As mentioned by Nishi et al (27), the aggregation of mitochondria around the nucleus was essential for maturation, fertilization and development.
In addition, the ultrastructure analysis of isolated follicles which were co-cultured in the presence of LIF showed some endoplasmic reticulum (ER) cisterns aligned from the nucleus envelope towards the cell membrane as a tubular network and the others were close to the mitochondria. Similar observations have been reported in the oocytes derived from fresh preantral follicles in several types of mammalian species (28-32). The role of this contact remains unclear. Motta et al (32) suggested that the mitochondrial-SER aggregations and mitochondrial–vesicle complex could be involved in the production of substrate needed for subsequent fertilization and early embryo development. The well-developed smooth endoplasmic reticulum of follicles seems to be responsible for lipid biosynthesis and consequent lipid droplets formation in later developmental stages of oocytes. Furthermore, ER can be the site of calcium storage needed for future events of oocytes such as fertilization and cortical reaction.
The cytoplasmic inclusions of mature oocytes of different mammalian species are characterized by the presence of multivesicular bodies, lipid droplets and myelin figures. They may play a role during the maturation of oocytes and in early embryo development (33-35). In electron micrograph of oocyte of co-cultured follicles, there were prominent multivesicular bodies and myelin-like lamellar bodies; however, there were a few fat droplets in the ooplasm of all groups. It seems that the lipid content of oocytes in preantral follicles was lower than that of mature oocytes which was reported earlier (35). However, the fate of lipid droplets during the oogenesis and embryogenesis in mammals is not clear so far.
Myelin figures, as described in follicles of other species (33) are thought to represent digestive vesicles responsible for degradation of aged and non-functional cellular structures. It seems that the lamellar bodies or myelin like structure are observed in the oocytes since oocyte is a long lived cell. The granulosa and theca cells are the main sources of steroid hormones. The electron micrograph of granulosa cells in our study showed a large number of round or cylindrical mitochondria with lamellar or tubular cristae as well as well-developed smooth endoplasmic reticulum tubules especially in co-cultured samples. Also, it was shown that the granulosa cells used for co-culture system gained ultrastructure characteristics typical of metabolically active and steroidogenic cells (32, 36). In conclusion, the oocyte and granulosa cells in co-cultured system showed more remarkable maturation features than that of control and this system could be effective to improve the maturation of isolated follicles, although it needs further studies.
Acknowledgements
This work was supported by a grant from Tarbiat Modares University.
Type of Study: Original Article |
References
1. Adam AA, Takahashi Y, Katagiri S, Nagano M. In vitro culture of mouse preantral follicles using membrane inserts and developmental competence of in vitro ovulated oocytes. J Reprod Dev 2004; 50:579-586. [DOI:10.1262/jrd.50.579]
2. Bishonga C, Takashi Y, Katagiri S, Nagano M, Ishikawa A. In vitro growth of ovarian peantral follicles and the capacity of their oocytes to develop to the blastocyst stage. J Vet Med Sci 2001; 63:619-624. [DOI:10.1292/jvms.63.619]
3. Cortvrindt R, Smitz J. Early preantral mouse follicle in vitro maturation: oocytes growth, meiotic maturation and granulose-cell proliferation. Theriogenology 1998; 49:845-859. [DOI:10.1016/S0093-691X(98)00034-X]
4. Yamanaka K, Aono N, Yoshida H, Sato E. Cryopreservation and in vitro maturation of germinal vesicle stage oocytes of animals for application in assisted reproductive technology. Reprod Med Biol 2007; 6:61-68. [DOI:10.1111/j.1447-0578.2007.00167.x]
5. Hasegawa A, Koyama K. In vitro growth and maturation of mouse oocyte-granulosa cell complex from cryopreserved ovaries and achievement of pup birth. Reprod Med Biol 2007; 6:77-83. [DOI:10.1111/j.1447-0578.2007.00169.x]
6. Cecconi S, Rossi G, Palmerini MG. Mouse oocyte differentiation during antral follicle development. Microsc Res Tech 2006; 69:408-414. [DOI:10.1002/jemt.20300]
7. Boland NI, Gosden RG. Effects of epidermal growth factor on the growth and differentiation of cultured mouse ovarian follicles. J Reprod Fertil 1994; 101:369-374. [DOI:10.1530/jrf.0.1010369]
8. Demeestere I, Gervy C, Centner J, Devreker F, Englert Y, Delbaere A. Effect of insulin-like growth factor-I during preantral follicular culture on steroidogenesis, in vitro oocyte maturation, and embryo development in mice. Biol Reprod 2004; 70:1664-1669. [DOI:10.1095/biolreprod.103.023317]
9. Demeestere I, Centner J, Gervy C, Englert Y, Delbaere A. Impact of various endocrine and paracrine factors on in vitro culture of preantral follicles in rodents. Reproduction 2005; 130:147-156. [DOI:10.1530/rep.1.00648]
10. Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update 2005; 1:461-471. [DOI:10.1093/humupd/dmi020]
11. Hilton DJ, Gough NM. Leukemia inhibitory factor: a biological perspective. J Cell Biochem 1991; 46:21-26. [DOI:10.1002/jcb.240460105]
12. Senturk LM, Arici A. Leukemia inhibitory factor in human reproduction. Am J Reprod Immunol 1998; 39:144- 151. [DOI:10.1111/j.1600-0897.1998.tb00346.x]
13. Tsai HD, Chang CC, Hsieh YY, Hsu LW, Chang SC, Lo HY. Effect of different concentrations of recombinant leukemia inhibitory factor (LIF) on different developmental stage of mouse embryo in vitro. J Assist Reprod Genet 2000; 17:352-355. [DOI:10.1023/A:1009413329977]
14. Ptak G, Lopes F, Matsukawa K, Tischner M, Loi P. Leukaemia inhibitory factor enhances sheep fertilization in vitro via an influence on the oocyte. Theriogenology 2006; 65:1891-1899. [DOI:10.1016/j.theriogenology.2005.10.018]
15. Morita Y, Manganaro TF, Tao XJ, Martimbeau S, Donahoe PK, Tilly JL. Requirement for phosphatidylinositol-3'-kinase in cytokine-mediated germ cell survival during fetal oogenesis in the mouse. Endocrinol 1999; 140: 941-949. [DOI:10.1210/endo.140.2.6539]
16. Gearing DP, Thut CJ, Van de Bos T, Gimpel SD, Delaney PB, King J, et al. leukemia inhibitory factor receptor is structurally related to the IL-6 signal transducer, gp 130. EMBO J 1991; 6: 2839-2848. [DOI:10.1002/j.1460-2075.1991.tb07833.x]
17. van Eijk MJ, Mandelbaum J, Salat-Baroux J, Belaisch-Allart J, Plachot M, Junca AM, et al. Expression of leukaemia inhibitory factor receptor subunits LIFR beta and gp130 in human oocytes and preimplantation embryos. Mol Hum Reprod 1996; 2:355-360. [DOI:10.1093/molehr/2.5.355]
18. Nicholas J, Davidson D, Taga T, Yoshida K, Chambers I, Smith A. Complementary tissue specific expression of LIF and LIF receptor mRNA in early mouse embryogenesis. Mech Dev 1996; 57: 123-131. [DOI:10.1016/0925-4773(96)00531-X]
19. Sirisathien S, Hernandez-Fonseca HJ, Bosch P, Hollet BR, Lott JD, Brackett BG. Effect of leukemia inhibitory factor on bovine embryos produced in vitro under chemically defined conditions. Theriogenology 2003; 59: 1751-1763. [DOI:10.1016/S0093-691X(02)01258-X]
20. Nilsson EE, Kezele P, Skinner MK. Leukemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries. Mol Cell Endocrinol 2002; 188: 65-73. [DOI:10.1016/S0303-7207(01)00746-8]
21. Haidari K, Salehnia M, Rezazadeh Valojerdi M. The effects of different concentrations of leukemia inhibitory factor on development of isolated preantral follicles from fresh and vitrified ovaries. Iranian Biomed J 2006; 10: 185-190.
22. Haidari K, Salehnia M, Rezazadeh Valojerdi M. The effect of Leukemia inhibitory factor and co-culture on the in vitro maturation and ultrastructure of vitrified and non vitrified isolated mouse preantral follicles. Fertil Steril 2008; 90: 2389-2397. [DOI:10.1016/j.fertnstert.2007.10.052]
23. Mazoochi T, Salehnia M, Rezazadeh Valojerdi M, Mowla SJ. Morphologic, ultrastructural, and biochemical identification of apoptosis in vitrified-warmed mouse ovarian tissue. Fertil Steril 2008; 90: 1480-1486. [DOI:10.1016/j.fertnstert.2007.07.1384]
24. Quinn P, Margalit R. Beneficial effects of coculture with cumulus cells on blastocyst formation in a prospective trial with supernumerary human embryos. J Assist Reprod Genet 1996; 13: 9-14. [DOI:10.1007/BF02068862]
25. Kohata Y, Gupta PD, Yasuzumi F. Stereo-electron microscopy of the ovarian follicles of cat and mouse. Okajimas Folia Anat Jpn 2007; 83: 97-106. [DOI:10.2535/ofaj.83.97]
26. Makabe S, Naguro T, Stallone T. Oocyte-follicle cell interactions during ovarian follicle development, as seen by high resolution scanning and transmission electron microscopy in humans. Micros Res Tech 2006; 69: 436-449. [DOI:10.1002/jemt.20303]
27. Nishi Y, Takeshita T, Sato K, Araki T. Change of the mitochondrial distribution in mouse ooplasm during in vitro maturation. J Nippon Med Sch 2003; 70: 408-415. [DOI:10.1272/jnms.70.408]
28. Nilsson E, Parrott JA, Skinner MK. Basic fibroblast growth factor induces primordial follicle development and initiates folliculogenesis. Mol Cell Endocrinol 2001; 175: 123-130. [DOI:10.1016/S0303-7207(01)00391-4]
29. Santos RR, Rodrigues PR, Costa SHF, Silva JRV, Matos MHT, Lucci CM, et al. Histological and ultrastructural analysis of cryopreserved sheep preantral follicles. Anim Reprod Sci 2006; 91: 246-263. [DOI:10.1016/j.anireprosci.2005.04.013]
30. Silva JR, Bao SN, Lucci CM, Carvalho FC, Andrade ER, Ferreira MA, et al. Morphological and ultrastructural changes occurring during degeneration of goat preantral follicles preserved in vitro. Anim Reprod Sci 2001; 66: 209-223. [DOI:10.1016/S0378-4320(01)00102-6]
31. Wassarman PM, Josefowicz WJ. Oocyte development in the mouse: an ultrastructural comparison of oocytes isolated at various stage of growth and meiotic competence. J Morphol 1978; 156: 209-235. [DOI:10.1002/jmor.1051560206]
32. Motta PM, Nottola SA, Makabe S, Heyn R. Mitochondrial morphology in human fetal and adult female germ cell. Hum Reprod 2000; 15: 129-147. [DOI:10.1093/humrep/15.suppl_2.129]
33. Kacinskis MA, Lucci CM, Luque MCA, Bao SN. Morphometric and ultrastructural characterization of Bos indicus preantral follicles. Anim Reprod Sci 2005; 87: 45-57. [DOI:10.1016/j.anireprosci.2004.09.003]
34. Niimura S, Kawakami S, Takano H. Changes in the amount of cytoplasmic inclusions in mouse oocytes during meiotic maturation in vivo and in vitro. Reprod Med Biol 2004; 3: 231-236. [DOI:10.1111/j.1447-0578.2004.00075.x]
35. Valojerdi MR, Salehnia M. Developmental potential and ultrastructural injuries of metaphase II (MII) mouse oocytes after slow freezing or vitrification. J Assist Reprod Genet 2005; 22: 119-127. [DOI:10.1007/s10815-005-4876-8]
36. Nottola SA, Heyn R, Camboni A, Correr S, Macchiarelli G. Ultrastructural characteristics of human granulosa cells in a coculture system for in vitro fertilization. Microsc Res Tech 2006; 69: 508-516. [DOI:10.1002/jemt.20309]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




