Sat, May 4, 2024
[Archive]
Volume 16, Issue 12 (December 2018)
IJRM 2018, 16(12): 767-774 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Moradi F, Jahanian Sadatmahalleh S, Ziaei S. The effect of hormone replacement therapy
on cognitive function in postmenopausal
women: An RCT. IJRM 2018; 16 (12) :767-774
URL: http://ijrm.ir/article-1-1332-en.html
URL: http://ijrm.ir/article-1-1332-en.html
1- Department of Midwifery and Reproductive Health, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
2- Department of Midwifery and Reproductive Health, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran , Ziaei_sa@modares.ac.ir
2- Department of Midwifery and Reproductive Health, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran , Ziaei_sa@modares.ac.ir
Full-Text [PDF 4228 kb]
(814 Downloads)
| Abstract (HTML) (3292 Views)
Full-Text: (504 Views)
During the reproductive age, the human brain becomes a target for gonadal steroid hormones (1). Estrogens influence neural function through effects on neurons and effects indirectly on oxidative stress, inflammation, the cerebral vascular and the immune system (2-5). Forgetfulness is common during postmenopausal age, and many women complain of cognitive and emotional problems at times that are associated with decreasing in circu- lating levels of estrogens. Some researchers have suggested that declines in estrogen level may lead to deficits in the cognitive ability, including mem- ory, attention, language and visuospatial ability, of post-menopausal women (6–8). Mild cognitive impairment (MCI) is episodic memory loss without dementia. Approximately 15 to 20 percent of people age 65 or older have MCI. However, MCI does not always lead to dementia, the rate of transition from MCI to dementia is 10–20% per year (4, 9, 10).
Some evidence from clinical trials in post- menopausal women especially in older ones sug- gested that hormone replacement therapy (HRT) has no substantial cognitive effect (11–13). How- ever, for surgically menopausal women, Ratka and co-workers found that estrogen therapy could be of cognitive benefit (14). In women with menopausal symptoms during the early post- menopause, HRT may have specific cognitive effects, and future research should target these effects. Postmenopausal women who experience more hot flashes, particularly while sleeping, have a better cognitive function than postmenopausal women who did not experience hot flashes, accord- ing to a pilot study (15).
The objective of the present study was to evaluate the effect of the traditional HRT on the cognition of postmenopausal women through a randomized controlled study. Also, the authors
evaluate the relationship between cognitive func- tion and climacteric symptoms in postmenopausal women after the traditional HRT.
This randomized clinical trial was conducted on 140 postmenopausal women (Figure 1), who attended Gynecology Clinic in Arash Hospital in Tehran, Iran from November 2014 to February 2015.
The inclusion criteria were women whose men- struation had ceased at least 1 yr before and not more than 10 yr, age between 50 and 60 yr, and with serum Follicle-stimulating hormone concentrations over 40 Iu/ml. Volunteers had not undergone hormone therapy during the six months prior to the trial. All subjects were urban. Women suffering from chronic diseases such as diabetes, hypertension, cardiovascular diseases, psychiatric diseases, cancer and those who smoke were deemed ineligible. At the time of enrollment, the interviewer administered a questionnaire to collect baseline information on the sociodemographic sta- tus and medical history. Then, participants filled out Montreal Cognitive Assessment (MOCA) and Green Climacteric Scale (GCS) questionnaire for evaluation of cognitive function status and climac- teric symptoms, respectively.
Women were randomly divided into two groups. Randomization was performed through computer- generated list of random number groups. Each woman in the case group took traditional HRT (0.625 mg conjugated equine estrogens plus 2.5 mg medroxyprogesterone acetate daily) plus one Cal+D tablet (500 mg calcium+200IU vitamin D) daily for four months. Women in the control group received only one Cal+D tablet (500 mg calcium+200IU vitamin D) daily for four-months period. The MoCA and GCS were assessed after the intervention and compared between the two groups. Also, the relationship between cognitive
function and climacteric symptoms was evaluated after the interventions. The MoCA was created and validated by Nasreddine and colleagues (15).
The MoCA is a brief 30-question test that takes around 10–12 minutes to complete. Scores on the MoCA range 0–30, with a score of 26 and higher generally considered normal, while scores less than 26 are abnormal and suggestive of developing mild cognitive impairment (MCI) The MoCA assesses multiple cognitive domains including visuospatial and executive functioning (5 points), animal naming (3 points), attention (6 points), language (3 points), abstraction (2 points), delayed recall (short-term memory) (5 points), orientation (6 points), educa- tion level (1 point is added to the test-taker’s score if he or she has 12 years or less of formal education) (15).
Psychometric properties of this scale have been studied in several studies and its validity and reliability have been confirmed (16). The GCS is a self-report measure for menopausal symptoms. The GCS contains 21 items divided into various clusters with individual values. The clusters are psycho- logical (11 symptoms) subdivided into anxiety and depression, somatic (7 symptoms), vasomotor (2 symptoms) and sexual (1 symptom). Each symptom is rated according to its severity using a four- point Likert scale (0, not at all; 1, a little; 2, quite a bit; 3, extremely). The GCS is the sum of all 21 scores ranging from 0 to 63. A higher total score corresponds with more menopausal symptoms (17).
𝛼 = 0.05 and 𝛽 = 0.2, the sample size for
two independent samples was calculated as 65
for each arm. Thus, to allow for loss to follow up, 70 consecutive postmenopausal women who had inclusion criteria were eligible in this trial. The data of normality in distribution was examined by K-
S. As distribution was assumed to be normal in all variables, independent T -test, Paired T -test, Chi-square test and the Pearson Correlation test
was applied; 𝑝 < 0.05 was considered statisti-
cally significant. All statistical tests were 2-tailed.
All statistical analyses were performed using the SPSS software (Statistical Package for the Social Sciences, version 20.0, SPSS Inc, Chicago, IL, USA).
correlated after the intervention (𝑟 = −0.235 𝑝 = 0.006).
Table I: Comparison of demographic characteristics between the two groups.
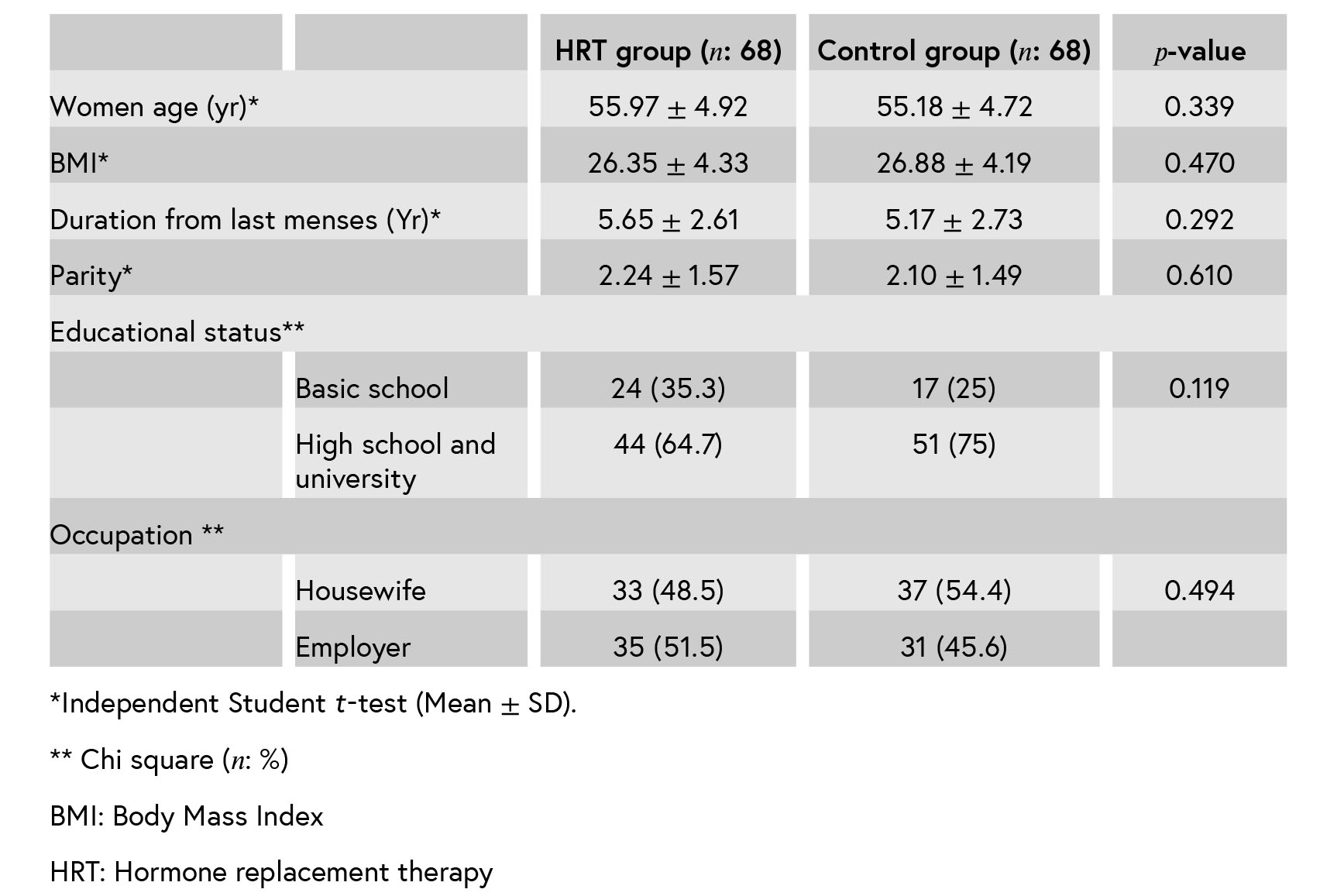
Table II: Comparison between the two groups in MoCA points and GCS scores before and after the treatment (Mean ± SD).
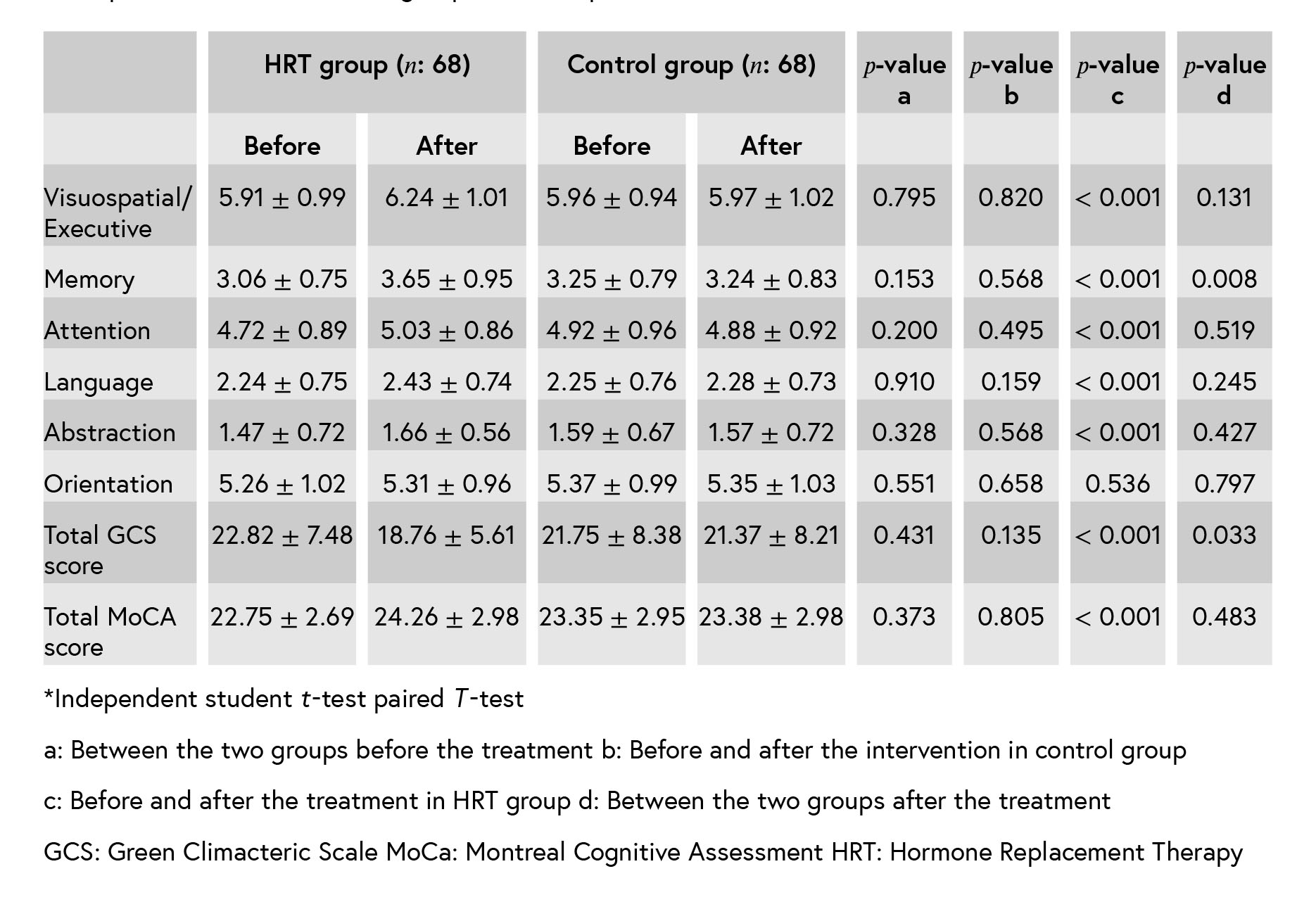
Table III: Correlation of MoCA points and GCS scores after the intervention (n: 138).*

Figure 1: The consort flowchart.
4. Discussion
The mean points of the MoCA after the inter- vention indicate that all MoCA domains, except for the orientation improved in the case group. There was a significant difference in the memory domain after the treatment between the two groups. MoCA domains and Greene climacteric scales were negatively correlated after the inter- vention.
A majority of menopausal women report cog- nitive dysfunction. Two pathways may under- line menopause-associated alternation in cogni- tive function. 1) A symptom associated with the
menopause may cause poorer cognitive perfor- mance 2) estrogen may directly benefit neural tissue; estrogen augments hippocampal and pre- frontal cortical function, potentially enhancing ver- bal memory and executive function. Reduction in estrogen levels during menopause could there- fore negatively affect cognitive function. Then, hormone therapy with amelioration of vasomo- tor symptoms may improve cognitive impairment (3).
This study had two aims. The first was to investigate whether HRT improves cognitive perfor- mance in postmenopausal women. The second was to consider the relationship between menopause- associated symptoms with cognitive function. The results of this study show improvement in cognitive function, memory, visuospatial/executive, atten- tion, language and abstraction domains after the HRT treatment. However, in comparison with the control group, only memory domain improves after HRT. These findings have been replicated in Ratca and colleagues’ study (14). Although the current finding is not consistent with other reports (12, 13, 18, 19), Fischer et al. showed an equivocal regarding the benefits of HRT to cognition and affect (20). The other results suggest that HRT when taken early postmenopause may have a beneficial effect on cognitive control prefrontal mechanisms (21).
The WHI study showed that HRT (CEE/MPA) worsened verbal memory and dementia risk in women aged 65 yr and above, but not the risk of mild cognitive impairment (12). Differences in the test used to assess cognitive function, assessing dementia and/or mild cognitive impairment, as well as the difference in mean age might explain these discrepancies. Considering these conflicting results in the literature, further research based on well-controlled clinical trials with large sam- ple is necessary to yield a consistent conclu- sion. The authors used MoCA for evaluation of cognitive function. The MoCA has been tested and validated in the setting of MCI and has subsequently been adopted in numerous other settings clinically. It was completed in 10 min. This test was recently proposed as a cognitive screening test for MCI, having surpassed the well-known limitations of the Mini-Mental State Examination (MMSE). The MMSE had a sensitiv- ity of 18% to detect MCI, whereas the MoCA detected 90% of MCI subjects. Specificity was excellent for both MMSE and MoCA (100% and 87%, respectively).The authors found that MOCA domains negatively correlated with Greene climac- teric scales.
Lower perfusion in specific areas of the cerebral cortex correlated significantly with rapid dete- rioration in the MMSE. The decline in gonadal steroids hormones during menopause gives rise to a wide range of physiological and psychological As a result of treatment with estrogens, there was an increased global blood flow (2–4). One pilot study reported that the cognitive function of women who had hot flash was better than in women who did not (15). It is hypothesized that hot flashes in postmenopausal women a counter- regulatory response to the impaired of glucose delivery to the brain and may have a beneficial effect on general cognitive function. This hypoth- esis was discussed by Ratka (14) who proposed two possible pathways of immediate and delayed responses. ”In the first pathway, in the absence of a counter-regulatory mechanism and lack of hot flashes, a cascade of neuropathological reactions evoked by glucose deprivation in the brain is initiated. It is likely that a reduced carbohydrate metabolism in the brain forms a basis for other processes known to cause neuronal injury and pos- sible cognition impairment. In the second possible pathway, in response to glucose deprivation in the brain, a hot flash occurs that triggers a counter- regulatory mechanism aimed at the delivery of glucose to the brain and also a critical signal initiating a brain-defense mechanism to counter- regulate the cascade of detrimental neurological processes.”
The HRT has affected some of the MoCA factors. The relationship between climacteric symptoms and cognitive function should be studied in a large prospective study in a group of women in their early and late menopausal ages with periodic assessment of their cognitive function during these follow-up years.
The authors would like to thank all the partic- ipating women. This study was funded by Tarbiat Modares University, Tehran, Iran.
The authors have no conflicts of interest.
Some evidence from clinical trials in post- menopausal women especially in older ones sug- gested that hormone replacement therapy (HRT) has no substantial cognitive effect (11–13). How- ever, for surgically menopausal women, Ratka and co-workers found that estrogen therapy could be of cognitive benefit (14). In women with menopausal symptoms during the early post- menopause, HRT may have specific cognitive effects, and future research should target these effects. Postmenopausal women who experience more hot flashes, particularly while sleeping, have a better cognitive function than postmenopausal women who did not experience hot flashes, accord- ing to a pilot study (15).
The objective of the present study was to evaluate the effect of the traditional HRT on the cognition of postmenopausal women through a randomized controlled study. Also, the authors
evaluate the relationship between cognitive func- tion and climacteric symptoms in postmenopausal women after the traditional HRT.
1. Materials and Methods
This randomized clinical trial was conducted on 140 postmenopausal women (Figure 1), who attended Gynecology Clinic in Arash Hospital in Tehran, Iran from November 2014 to February 2015.
The inclusion criteria were women whose men- struation had ceased at least 1 yr before and not more than 10 yr, age between 50 and 60 yr, and with serum Follicle-stimulating hormone concentrations over 40 Iu/ml. Volunteers had not undergone hormone therapy during the six months prior to the trial. All subjects were urban. Women suffering from chronic diseases such as diabetes, hypertension, cardiovascular diseases, psychiatric diseases, cancer and those who smoke were deemed ineligible. At the time of enrollment, the interviewer administered a questionnaire to collect baseline information on the sociodemographic sta- tus and medical history. Then, participants filled out Montreal Cognitive Assessment (MOCA) and Green Climacteric Scale (GCS) questionnaire for evaluation of cognitive function status and climac- teric symptoms, respectively.
Women were randomly divided into two groups. Randomization was performed through computer- generated list of random number groups. Each woman in the case group took traditional HRT (0.625 mg conjugated equine estrogens plus 2.5 mg medroxyprogesterone acetate daily) plus one Cal+D tablet (500 mg calcium+200IU vitamin D) daily for four months. Women in the control group received only one Cal+D tablet (500 mg calcium+200IU vitamin D) daily for four-months period. The MoCA and GCS were assessed after the intervention and compared between the two groups. Also, the relationship between cognitive
function and climacteric symptoms was evaluated after the interventions. The MoCA was created and validated by Nasreddine and colleagues (15).
The MoCA is a brief 30-question test that takes around 10–12 minutes to complete. Scores on the MoCA range 0–30, with a score of 26 and higher generally considered normal, while scores less than 26 are abnormal and suggestive of developing mild cognitive impairment (MCI) The MoCA assesses multiple cognitive domains including visuospatial and executive functioning (5 points), animal naming (3 points), attention (6 points), language (3 points), abstraction (2 points), delayed recall (short-term memory) (5 points), orientation (6 points), educa- tion level (1 point is added to the test-taker’s score if he or she has 12 years or less of formal education) (15).
Psychometric properties of this scale have been studied in several studies and its validity and reliability have been confirmed (16). The GCS is a self-report measure for menopausal symptoms. The GCS contains 21 items divided into various clusters with individual values. The clusters are psycho- logical (11 symptoms) subdivided into anxiety and depression, somatic (7 symptoms), vasomotor (2 symptoms) and sexual (1 symptom). Each symptom is rated according to its severity using a four- point Likert scale (0, not at all; 1, a little; 2, quite a bit; 3, extremely). The GCS is the sum of all 21 scores ranging from 0 to 63. A higher total score corresponds with more menopausal symptoms (17).
1.1.Ethical consideration
This study was conducted with the approval of the Ethics Committee of Tarbiat Modares Uni- versity of Medical Sciences (IRB#525000). All the women were informed about the project and had given a written consent before participating in the study.1.2.Statistical analysis
Based on the results of a pilot study, with𝛼 = 0.05 and 𝛽 = 0.2, the sample size for
two independent samples was calculated as 65
for each arm. Thus, to allow for loss to follow up, 70 consecutive postmenopausal women who had inclusion criteria were eligible in this trial. The data of normality in distribution was examined by K-
S. As distribution was assumed to be normal in all variables, independent T -test, Paired T -test, Chi-square test and the Pearson Correlation test
was applied; 𝑝 < 0.05 was considered statisti-
cally significant. All statistical tests were 2-tailed.
All statistical analyses were performed using the SPSS software (Statistical Package for the Social Sciences, version 20.0, SPSS Inc, Chicago, IL, USA).
2.Results
In a randomized, controlled trial, 140 non- surgically postmenopausal women were allocated into three groups. Four women were excluded from the initial analyses, fear of breast cancer in two women and abnormal vaginal bleeding in two other women. Finally, 68 cases and 68 controls completed the study and analyzed. (Figure 1). There were no significant differences in demographic and clinical characteristics between the two groups after the randomization (Table I). At baseline, there were no significant differences in all domains of MoCA and Greene climacteric scale between the two groups (Table II). The mean points of the MoCA after the intervention indicate that all MoCA domains except for the orientation improved in the case group and not in the control group. There was a significant difference in the memory domain after the treatment between the two groups (Table III). Also, as it is shown in Table III, MoCA domains and Greene climacteric scales were negativelycorrelated after the intervention (𝑟 = −0.235 𝑝 = 0.006).
Table I: Comparison of demographic characteristics between the two groups.

Table II: Comparison between the two groups in MoCA points and GCS scores before and after the treatment (Mean ± SD).

Table III: Correlation of MoCA points and GCS scores after the intervention (n: 138).*


Figure 1: The consort flowchart.
4. Discussion
The mean points of the MoCA after the inter- vention indicate that all MoCA domains, except for the orientation improved in the case group. There was a significant difference in the memory domain after the treatment between the two groups. MoCA domains and Greene climacteric scales were negatively correlated after the inter- vention.
A majority of menopausal women report cog- nitive dysfunction. Two pathways may under- line menopause-associated alternation in cogni- tive function. 1) A symptom associated with the
menopause may cause poorer cognitive perfor- mance 2) estrogen may directly benefit neural tissue; estrogen augments hippocampal and pre- frontal cortical function, potentially enhancing ver- bal memory and executive function. Reduction in estrogen levels during menopause could there- fore negatively affect cognitive function. Then, hormone therapy with amelioration of vasomo- tor symptoms may improve cognitive impairment (3).
This study had two aims. The first was to investigate whether HRT improves cognitive perfor- mance in postmenopausal women. The second was to consider the relationship between menopause- associated symptoms with cognitive function. The results of this study show improvement in cognitive function, memory, visuospatial/executive, atten- tion, language and abstraction domains after the HRT treatment. However, in comparison with the control group, only memory domain improves after HRT. These findings have been replicated in Ratca and colleagues’ study (14). Although the current finding is not consistent with other reports (12, 13, 18, 19), Fischer et al. showed an equivocal regarding the benefits of HRT to cognition and affect (20). The other results suggest that HRT when taken early postmenopause may have a beneficial effect on cognitive control prefrontal mechanisms (21).
The WHI study showed that HRT (CEE/MPA) worsened verbal memory and dementia risk in women aged 65 yr and above, but not the risk of mild cognitive impairment (12). Differences in the test used to assess cognitive function, assessing dementia and/or mild cognitive impairment, as well as the difference in mean age might explain these discrepancies. Considering these conflicting results in the literature, further research based on well-controlled clinical trials with large sam- ple is necessary to yield a consistent conclu- sion. The authors used MoCA for evaluation of cognitive function. The MoCA has been tested and validated in the setting of MCI and has subsequently been adopted in numerous other settings clinically. It was completed in 10 min. This test was recently proposed as a cognitive screening test for MCI, having surpassed the well-known limitations of the Mini-Mental State Examination (MMSE). The MMSE had a sensitiv- ity of 18% to detect MCI, whereas the MoCA detected 90% of MCI subjects. Specificity was excellent for both MMSE and MoCA (100% and 87%, respectively).The authors found that MOCA domains negatively correlated with Greene climac- teric scales.
Lower perfusion in specific areas of the cerebral cortex correlated significantly with rapid dete- rioration in the MMSE. The decline in gonadal steroids hormones during menopause gives rise to a wide range of physiological and psychological As a result of treatment with estrogens, there was an increased global blood flow (2–4). One pilot study reported that the cognitive function of women who had hot flash was better than in women who did not (15). It is hypothesized that hot flashes in postmenopausal women a counter- regulatory response to the impaired of glucose delivery to the brain and may have a beneficial effect on general cognitive function. This hypoth- esis was discussed by Ratka (14) who proposed two possible pathways of immediate and delayed responses. ”In the first pathway, in the absence of a counter-regulatory mechanism and lack of hot flashes, a cascade of neuropathological reactions evoked by glucose deprivation in the brain is initiated. It is likely that a reduced carbohydrate metabolism in the brain forms a basis for other processes known to cause neuronal injury and pos- sible cognition impairment. In the second possible pathway, in response to glucose deprivation in the brain, a hot flash occurs that triggers a counter- regulatory mechanism aimed at the delivery of glucose to the brain and also a critical signal initiating a brain-defense mechanism to counter- regulate the cascade of detrimental neurological processes.”
1.Conclusion
The HRT has affected some of the MoCA factors. The relationship between climacteric symptoms and cognitive function should be studied in a large prospective study in a group of women in their early and late menopausal ages with periodic assessment of their cognitive function during these follow-up years.
Acknowledgments
The authors would like to thank all the partic- ipating women. This study was funded by Tarbiat Modares University, Tehran, Iran.
Conflict of Interest
The authors have no conflicts of interest.
Type of Study: Original Article |
References
1. [1] Nguyen TV, Ducharme S, Karama S. Effects of sex steroids in the human brain. Mol Neurobiol 2017; 54: 7507–-7519. [DOI:10.1007/s12035-016-0198-3]
2. [2] Sturdee DW, Pines A, International Menopause Society Writing Group, Archer DF, Baber RJ, Barlow D, et al. Updated IMS recommendations on postmenopausal hor- mone therapy and preventive strategies for midlife health. Climacteric 2011; 14: 302–320. [DOI:10.3109/13697137.2011.570590]
3. [3] Greendale GA, Wight RG, Huang MH, Avis N, Gold EB, Joffe H, et al. Menopause-associated symptoms and cognitive performance: results from the study of women's health across the nation. Am J Epidemiol 2010; 171: 1214–1224. [DOI:10.1093/aje/kwq067]
4. [4] Etgen T, Sander D, Bickel H, Forstl H. Mild cognitive impairment and dementia: the importance of modifiable risk factors. Dtsch Arztebl Int 2011; 108: 743–-750.
5. [5] Karlamangla AS, Lachman ME, Han W, Huang M, Greendale GA. Evidence for cognitive aging in midlife women: study of women's health across the nation. PLoS One 2017; 12: e0169008. [DOI:10.1371/journal.pone.0169008]
6. [6] Pimenta F, Leal I, Maroco J, Ramos C. Menopausal symp- toms: do life events predict severity of symptoms in peri- and post-menopause? Maturitas 2012; 72: 324–-331. [DOI:10.1016/j.maturitas.2012.04.006]
7. [7] Goveas JS, Espeland MA, Hogan P, Dotson V, Tarima S, Coker LH, et al. Depressive symptoms, brain volumes and subclinical cerebrovascular disease in postmenopausal women: the Women's Health Initiative MRI Study. J Affect Disord 2011; 132: 275–-284. [DOI:10.1016/j.jad.2011.01.020]
8. [8] Goveas JS, Espeland MA, Woods NF, Wassertheil-Smoller S, Kotchen JM. Depressive symptoms and incidence of mild cognitive impairment and probable dementia in elderly women: the Women's Health Initiative Memory Study. J Am Geriatr Soc 2011; 59: 57–-66. [DOI:10.1111/j.1532-5415.2010.03233.x]
9. [9] Rapp SR, Legault C, Henderson VW, Brunner RL, Masaki K, Jones B, et al. Subtypes of mild cognitive impairment in older postmenopausal women: the Women's Health Initiative Memory Study. Alzheimer Dis Assoc Disord 2010; 24: 248–-255. [DOI:10.1097/WAD.0b013e3181d715d5]
10. [10] Mitchell ES, Woods NF. Cognitive symptoms during the menopausal transition and early postmenopause. Climac- teric 2011; 14: 252–-261.
11. [11] Etgen T, Bickel H, Forstl H. Metabolic and endocrine factors in mild cognitive impairment. Ageing Res Rev 2010; 9: 280– [DOI:10.1016/j.arr.2010.01.003]
12. [12] Maki PM, Henderson VW. Hormone therapy, dementia, and cognition: the Women's Health Initiative 10 years on. Climacteric 2012; 15: 256–-262. [DOI:10.3109/13697137.2012.660613]
13. [13] Scali J, Ryan J, Carriere I, Dartigues JF, Tavernier B, Ritchie K, et al. A prospective study of hormone therapy and depression in community-dwelling elderly women: the Three City Study. J Clin Psychiatry 2010; 71: 1673–-1679. [DOI:10.4088/JCP.09m05188blu]
14. [14] Ratka A. Menopausal hot flashes and development of cognitive impairment. Ann N Y Acad Sci 2005; 1052: 11–-26. [DOI:10.1196/annals.1347.002]
15. [15] Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assess- ment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–-699. [DOI:10.1111/j.1532-5415.2005.53221.x]
16. [16] Emsaki G, Molavi H, Chitsaz A, Movahed Abtahi M, Asgari K. [Psychometric properties of the montreal cognitive assessment (MoCA) in parkinson's disease patients in Isfahan]. J Isfahan Med Sch 2011; 29: 1391–-1400 (in Persian).
17. [17] Greene JG. A factor analytic study of climacteric symp- toms. J Psychosom Res 1976; 20: 425–-430. [DOI:10.1016/0022-3999(76)90005-2]
18. [18] Henderson VW, St John JA, Hodis HN, McCleary CA, Stanczyk FZ, Shoupe D, et al. Cognitive effects of estradiol
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








