Fri, Feb 20, 2026
[Archive]
Volume 18, Issue 8 (August 2020)
IJRM 2020, 18(8): 651-666 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
mehrabianfar P, dehghani F, karbalaei N, mesbah F. The effects of metformin on stereological and ultrastructural features of the ovary in streptozotocin -induced diabetes adult rats: An experimental study. IJRM 2020; 18 (8) :651-666
URL: http://ijrm.ir/article-1-1344-en.html
URL: http://ijrm.ir/article-1-1344-en.html
1- Department of Anatomical Sciences School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
2- professor of histology and embryology
3- Histomorphometry and Stereology Research Center, Shiraz University of Medical Science, Shiraz, Iran
4- Department of Anatomical Sciences School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran. ,mesbahf@sums.ac.ir
2- professor of histology and embryology
3- Histomorphometry and Stereology Research Center, Shiraz University of Medical Science, Shiraz, Iran
4- Department of Anatomical Sciences School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran. ,
Full-Text [PDF 2520 kb]
(1315 Downloads)
| Abstract (HTML) (2407 Views)
Previous studies show that diabetes has negative effects on the growth of the ovarian follicles and decreases the rate of fertility, but the exact mechanisms are unclear (4, 5). Alterations of glucose concentration can greatly affect some functions of reproductive system (6). For example, in streptozotocin (STZ)-induced diabetic rats, high level of glucose reduces the production of progesterone and estradiol in granulosa cells (GC) and causes reproductive disorders due to changes in folliculogenesis and steroidogenesis (7). Given the rapid incidence of diabetes and increase in the number of women with diabetes, it is essential to develop the animal models of diabetes to understand the effects of diabetes on the ovary and to identify ovarian dysfunctions, as well as to achieve a new treatment approach (8).
STZ is a preferred chemical agent that can be used to create an animal model of diabetes (9). It is a glucosamine-nitrosourea compound that has specific toxic effects on pancreatic beta cells. The mechanism of diabetes mellitus in experimental animals is not entirely clear, but it seems that the production of free radicals such as superoxide-hydrogen, peroxide, and nitric oxide has destructive effects of STZ on DNA strand of pancreatic beta cells (10).
In recent years, significant progress has been made in understanding and controlling diabetes, and in many cases we have been able to delay or prevent the complications of the disease (11). Metformin is an insulin-sensitizing drug and helps ovulation. This drug can help regulate the sexual cycle in about half of women with polycystic ovary syndrome. It can also reduce insulin levels by lowering appetite and helping in weight loss. Regular use of metformin along with other therapeutic proceedings including exercise, diet, and weight control can be more effective (12). Despite the long-term experimental and clinical experience with metformin in animal models and human, the exact mechanism of its function is still unclear (13). However, metformin is still used in the clinic without a perfect understanding of its mechanism. Therefore, more research is needed to investigate the effect of metformin on the reproductive system of diabetic patients (14).
To distinguish ovarian dysfunction, the ethical restrictions of human studies lead us to use animal models to investigate the effect of diabetes on accurate morphological (ultrastructure) and exact morphometric (stereological) features of ovary. So, this study was designed to investigate the preventive and therapeutic roles of metformin on ultrastructural and stereological features of ovary in the STZ-induced diabetes adult female rats.
Seventy female Sprague-Dawley rats aged 8 to 10 weeks with a weight of 180-200 gr were used in this experimental study.
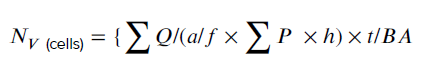
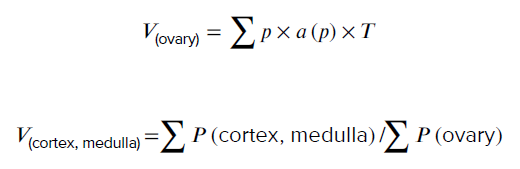
Using stereological software, the diameters of the nucleus and the cytoplasm were measured in oocytes.
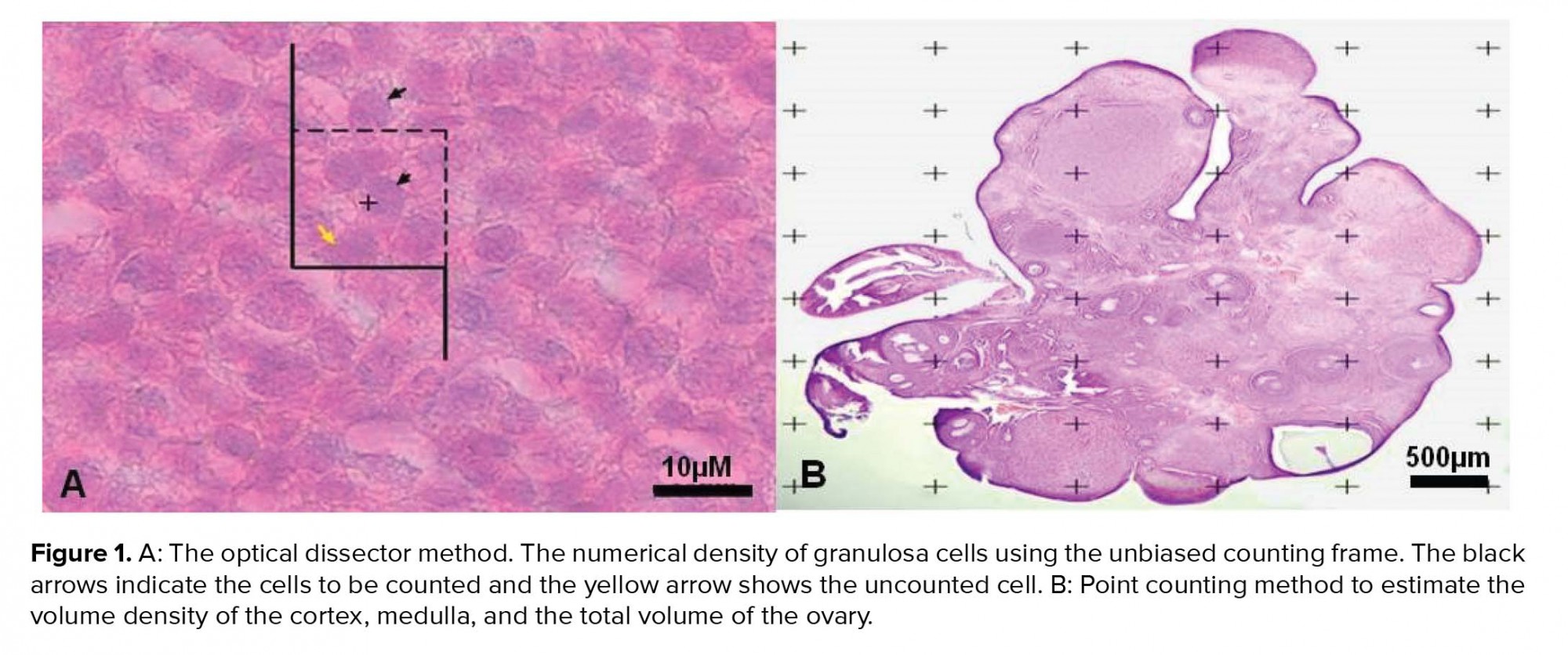
2.12. Ethical consideration
The animals were housed under controlled conditions (12:12 hr light-dark cycle at 23 ± 2ºC), with ad libitum access to food and tap water for 8 wk. Also, animal handling and all other procedures were done following the ethical principles and the national norms and standards for conducting medical research in Iran, which was approved by the Ethics committee of Shiraz University of Medical Sciences, Shiraz, Iran (Approval ID: IR.SUMS.REC.1397.659).
2.13.Statistical analysis
We used commercial scientific statistic software; graph pad prism version 7.05, (Prism Inc, San Diego, California, USA) in order to analyze my scientific data and present graph, Data are reported as means and standard deviation and analyzed using One-way ANOVA test. In addition, for statistical comparisons between the groups, we used Tukey’s multiple comparisons test as a post-hoc test. The mean difference was considered significant if p < 0.05.
3. Results
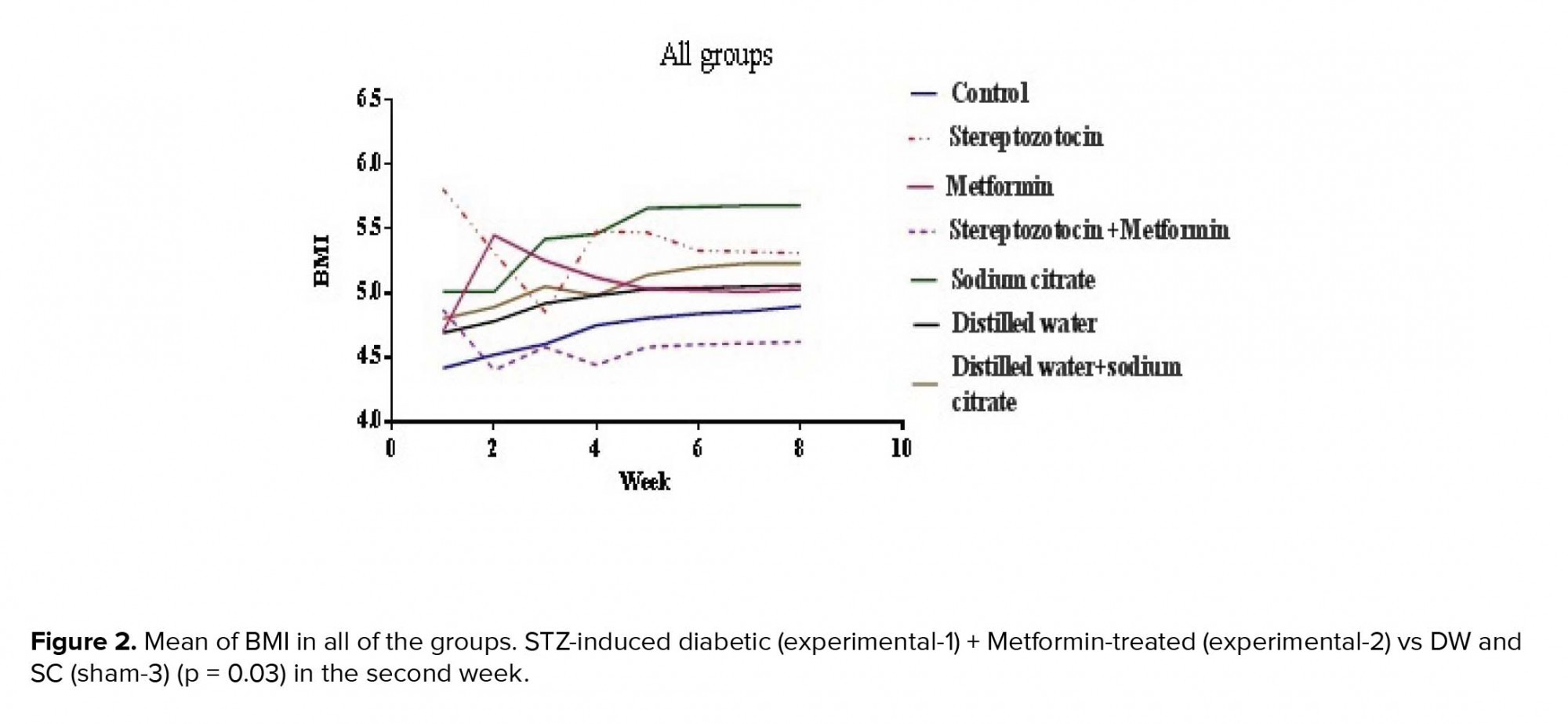
3.1.Body mass index
The BMI was significantly reduced in the experimental-1 and experimental-3 groups in the second week of experiment (p < 0.01, p = 0.03 respectively) (Figure 2).
3.2.Blood sugar levels
Mean ± SD of blood sugar levels of animals in the experimental-1 (p < 0.01) and experimental-3 groups (p < 0.01) showed a significant increase compared to the sham-1 and sham-3 groups on 10th day of experiment respectively. Also in the experimental-1 and experimental-3 rats, a significant increase was seen compared to the control group (p < 0.01 and p < 0.01, respectively).
In addition, on the 28th day of experiment, blood sugar was significantly higher in the the experimental-1 (p = 0.01) and experimental-3 groups (p = 0.01) compared to the control group.
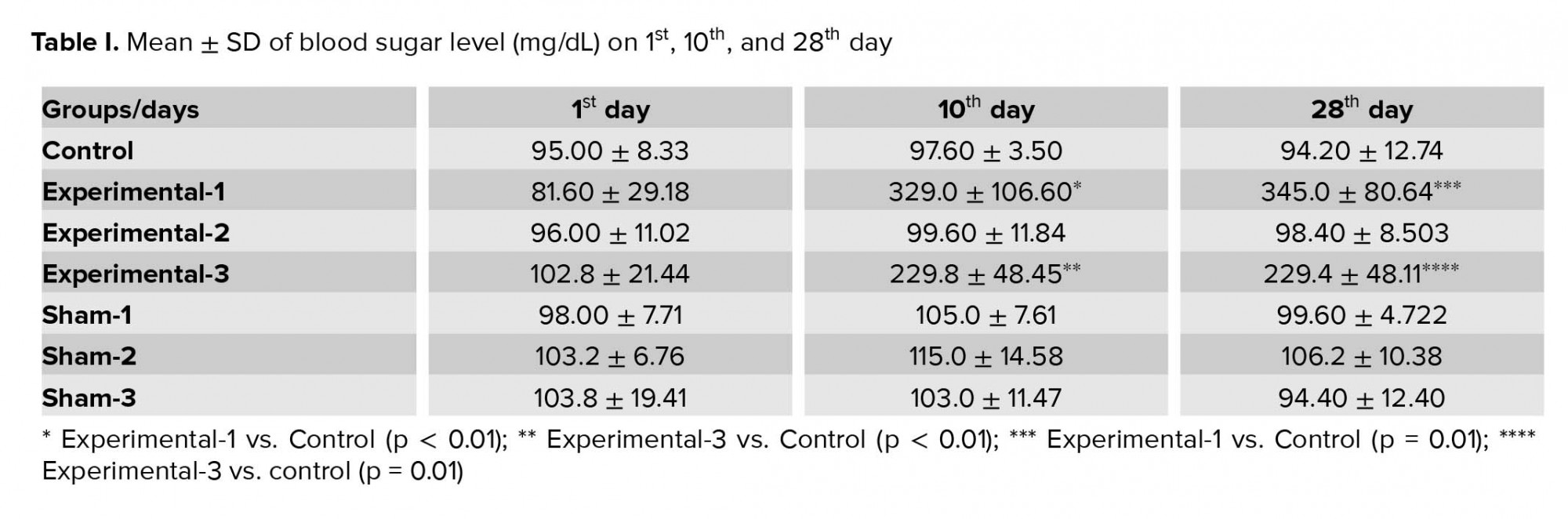
There were no significant differences in the blood sugar levels of animals between groups on the first day of experiment (Table I).
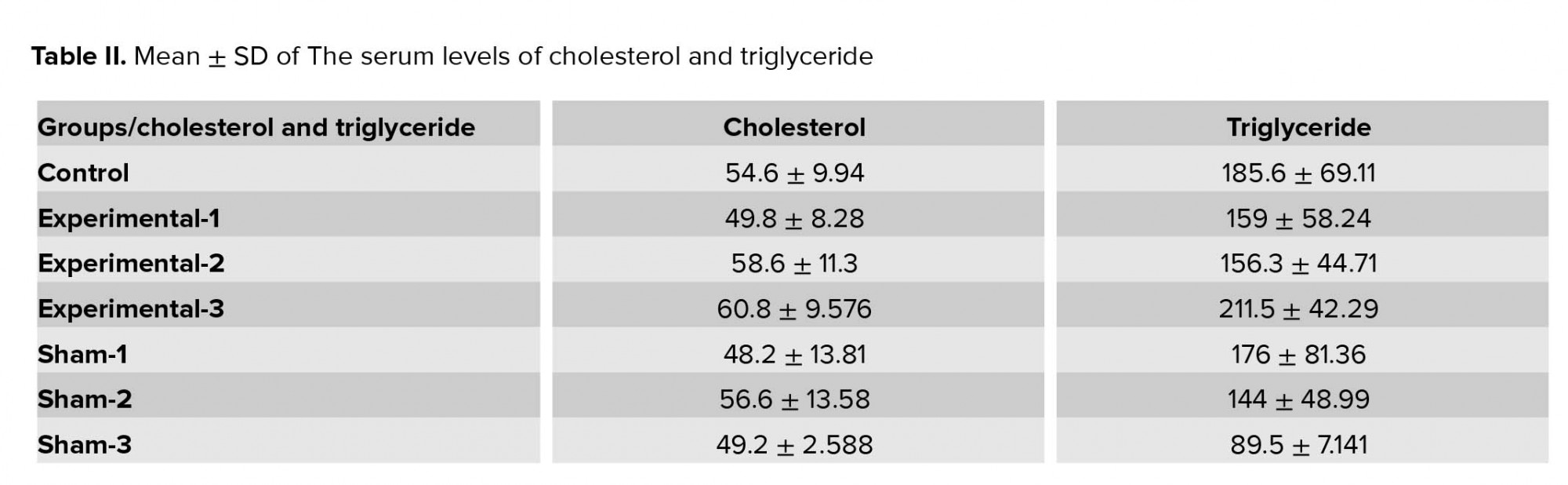
3.3.The serum levels of cholesterol and triglyceride
There were no significant differences in the serum levels of cholesterol and triglyceride between the groups (Table II).
3.4. Weight of ovaries
Despite the results of this study that show 18% decrease in the ovarian weight in experimental-1 group and 30% increase in ovarian weight in experimental-3 group compared to the control group, there were no significant differences in the ovarian weight between the studied groups.
3.5. Stereological study
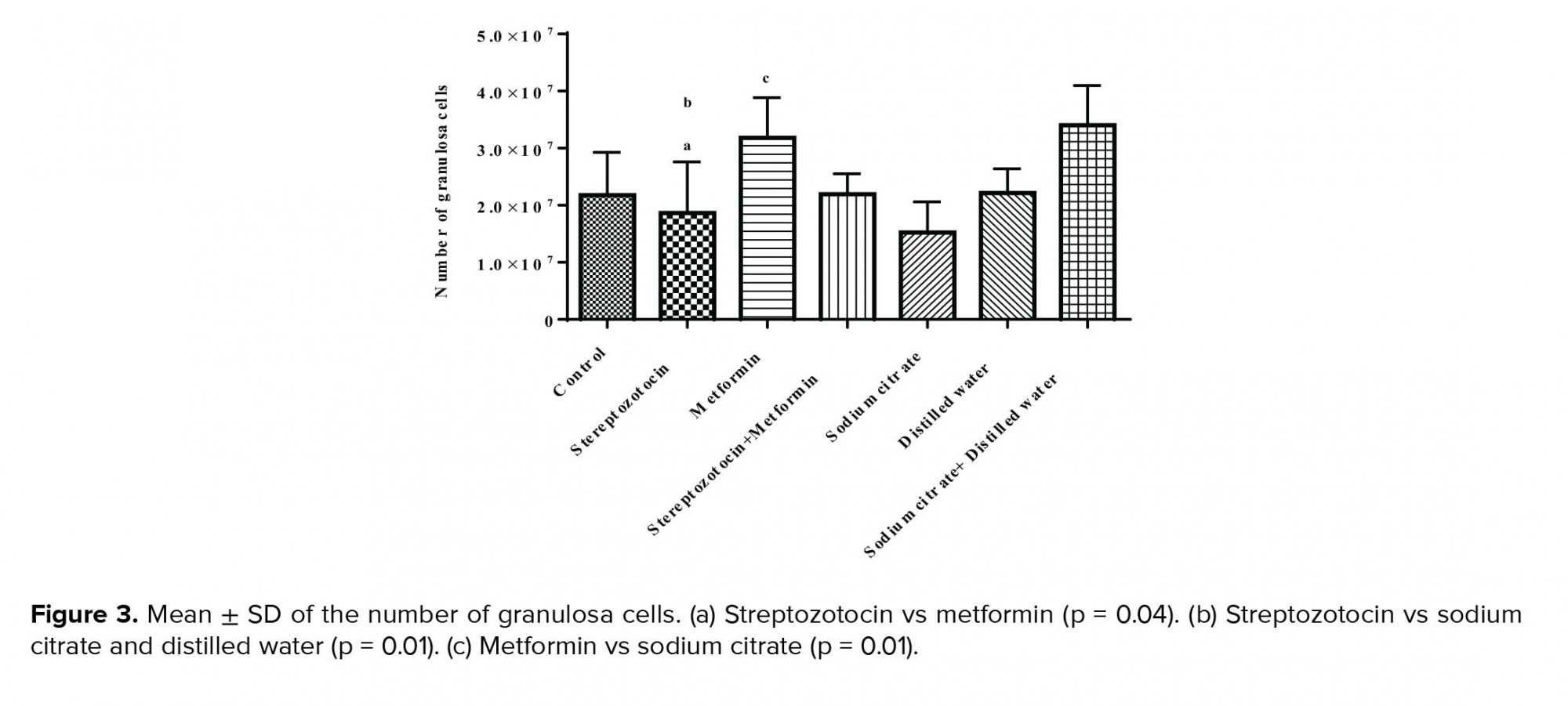
3.5.1. Estimation of the number of GCs
The mean ± SD of the number of GC was significantly decreased (p = 0.04) in the experimental-1 group as compared with the experimental-2 group and also sham-3 group (p = 0.01). In addition, there was a significant increase in experimental-2 group compared to the sham-1 group (p = 0.01; Figure 3).
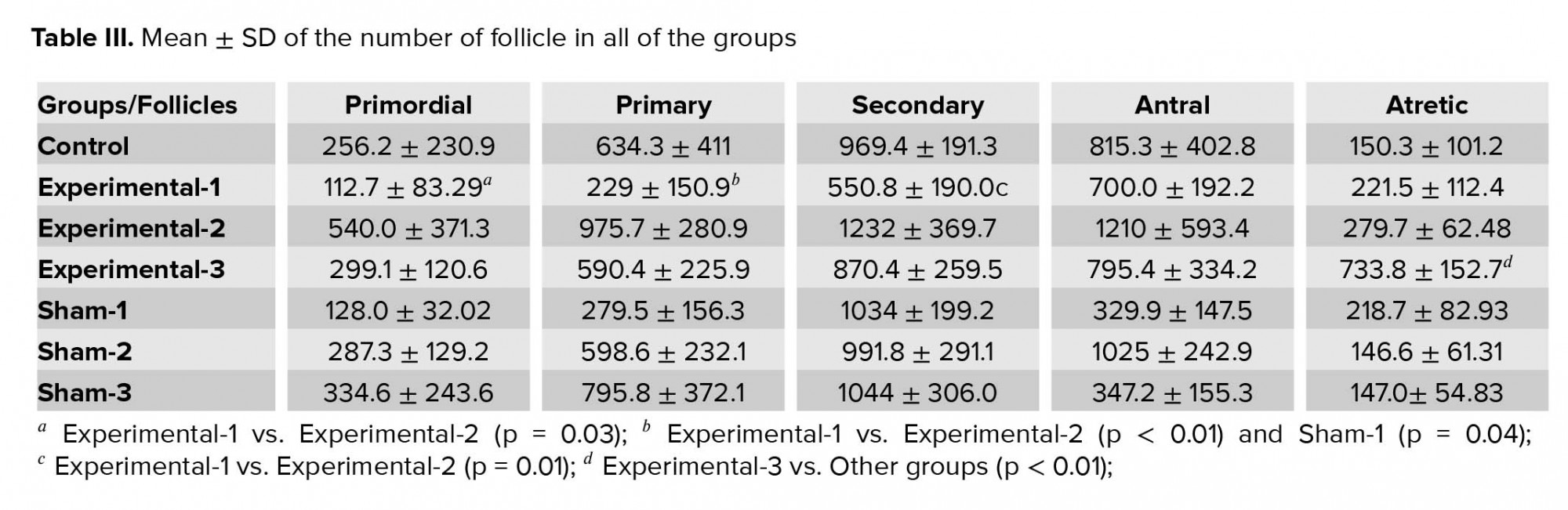
3.5.2. Number of follicles
The results of this study indicated that the mean ± SD of the number of primordial follicles were significantly decreased in the experimental-1 group compared with experimental-2 (p = 0.03), and the primary follicles were significantly decreased (p < 0.01) in the experimental-1 groups as compared with the experimental-2 and sham-3 group (p = 0.04). The secondary follicles were significantly decreased (p = 0.01) in the experimental-1 as compared with the experimental-2 group.
No significant differences were observed between the mean ± SD of the numbers of antral follicle in all of the groups. The mean ± SD of the number of atretic follicles in the experimental-3 rats was significantly higher than the other rats (p < 0.01) (Table III).
3.5.3. Total volume of the ovary, cortex, and medulla
The mean ± SD of the total volume of ovary was significantly increased in experimental-2 group compared to the experimental-1 (p < 0.01) and control groups (p = 0.01). Also, in the total volume of ovary, a significant increase was seen in the experimental-3 group as compared with the experiment-1 (p = 0.01).
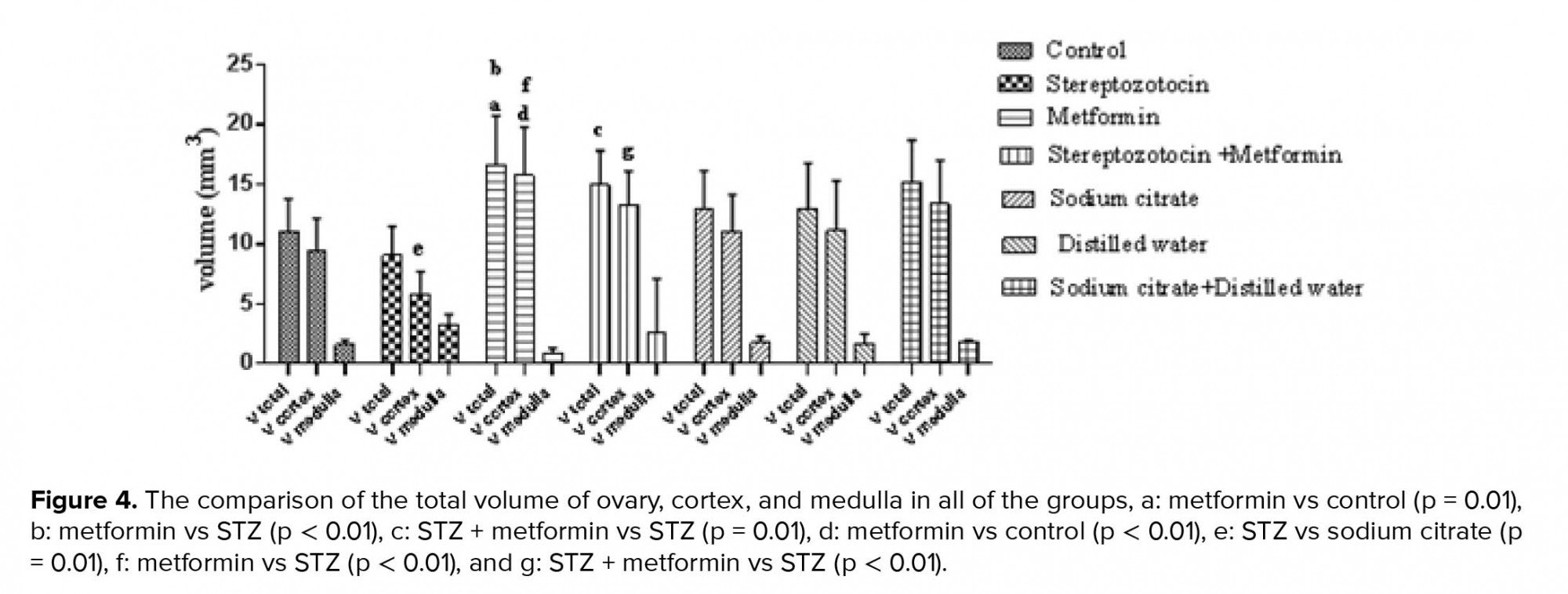
The mean ± SD of the volume of cortex significantly increased in the experimental-2 group compared to the control group (p <0.01). Further, in experimental-1 rats, a significant decrease in the volume of cortex was seen compared to the sham-1 group (p = 0.01). Besides, the volume of cortex was significantly increased in the experimental-2 group than the experimental-1 group (p < 0.01). Moreover, there was a significant increase in experimental-3 group compared to the experimental-1 group (p < 0.01). There were no significant differences in the mean ± SD of the volume of medulla between all of the groups (Figure 4).
3.5.4. Ratio of the diameter of nucleus to ooplasm
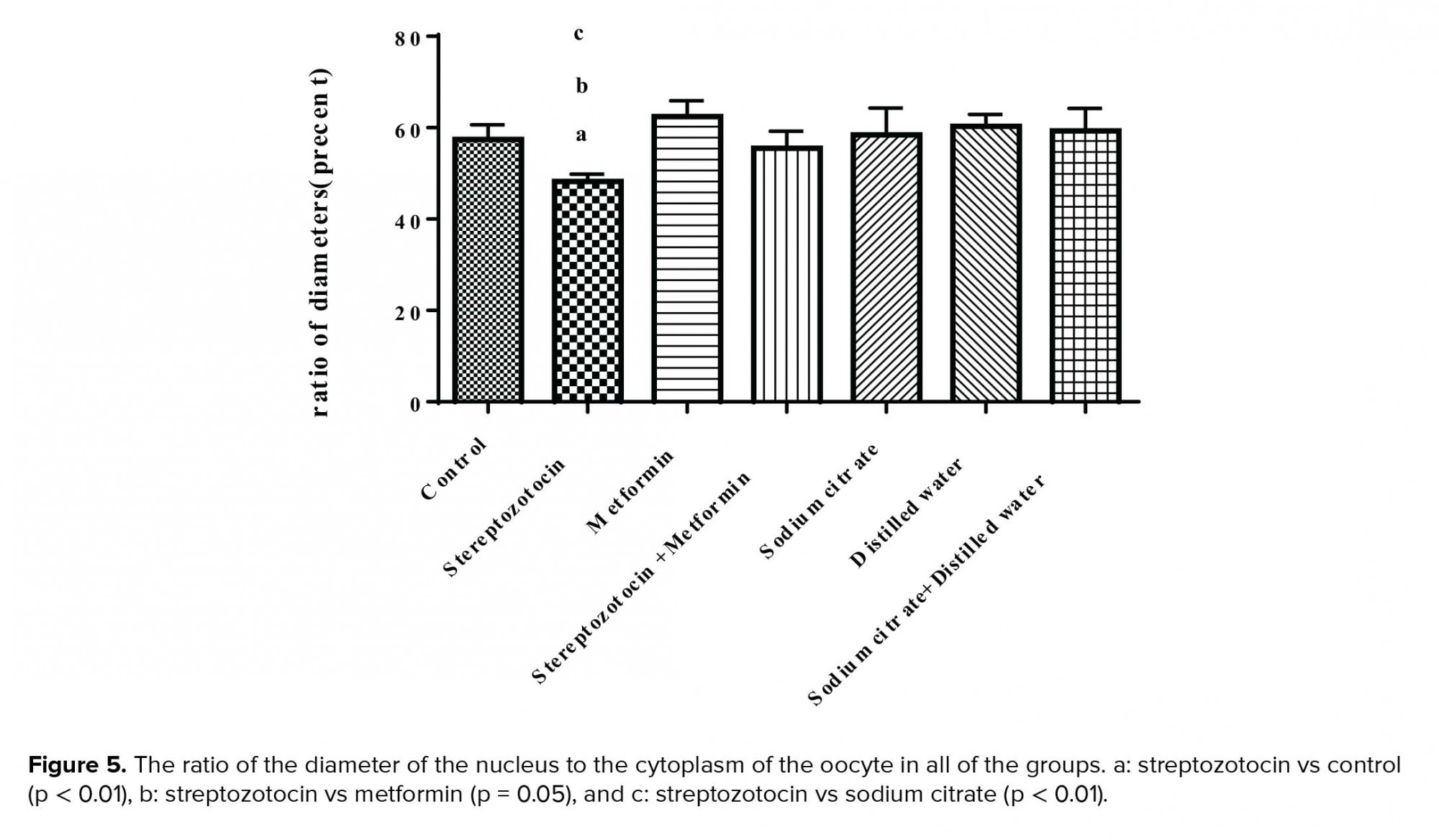
The mean ± SD of the ratio of the diameter of the nucleus to the ooplasm was decreased significantly in the experimental-1 group compared to the control (p < 0.01), experimental-2 (p = 0.05), and sham-1 groups (p < 0.01) (Figure 5).
3.6.Histological observation of resin embedded sections
3.6.1. Control group
The germinal epithelium (GE) that contained cuboidal cells with distinct boundaries and underlying tunica albuginea (TA) were observed. The ovarian stroma contains normal cells and blood vessels. Some primordial and primary follicles with normal GCs with dividing nuclei and oocyte with typical distribution of organelles in the cortex were observed. Outside the basement membrane, theca interna, and externa cells with long and spindle shape were seen. The thickness of zona pellucida (ZP) was normal and perivitelline space (PVS) wasn't observed (the figure is not shown).
3.6.2. Experimental groups
Experimental-1
The ovaries of diabetic rats were covered by squamous epithelium and the TA was unclear. The number of degenerative oocytes increased. The cytoplasm of the GCs and theca cells (TCs) contained many vacuoles (VC). There were many degenerated GCs and TCs with pyknotic nuclei. The ZP had normal thickness and the ooplasm had homogenous distribution of organelles (Figure 6).
Experimental-2
The intact GE with cuboidal and squamous cells and distinct boundaries and underlying TA was observed. The GCs and TCs and also stromal cells were full of VC. The ZP with regular thickness and normal distribution of ooplasmic organelles were observed (Figure 6).
Experimental-3
The GE had cuboidal and also squamous cells. The underlying TA was unclear. The GCs had normal appearance but there were some degenerated cells. The TCs with foamy cytoplasm were observed and also there was no clear boundary between interna and externa. The ZP had regular thickness. The normal distribution of ooplasmic organelles was observed. Some primordial follicles with normal GCs and normal oocytes were seen (Figure 6).
3.6.3. Sham groups
Sham-1
Germinal epithelium with normal and cuboidal cells were observed. A number of follicles were seen within the cortex. The ovarian stromal cells were normal. There were no pyknotic nuclei in GCs. The ooplasmic organelles had regular distribution (the figure is not shown).
Sham-2
The cuboidal and squamous cells were observed in GE. The underlying TA was distinct. Some of the stromal cells contained VC. The GCs were completely normal without the pyknotic nuclei. The ZP with regular thickness was observed. The normal ooplasmic distribution of organelles was detected.
Sham-3
The cuboidal and columnar epithelial cells covered the ovaries and also stromal cells had normal appearance. The blood vessels that contained RBC were noticed in ovary cortex. The primordial, primary and antral follicles with normal GCs and regular ZP thickness were observed (Figure 6).
3.7. Ultrastructural observation
3.7.1. Control group
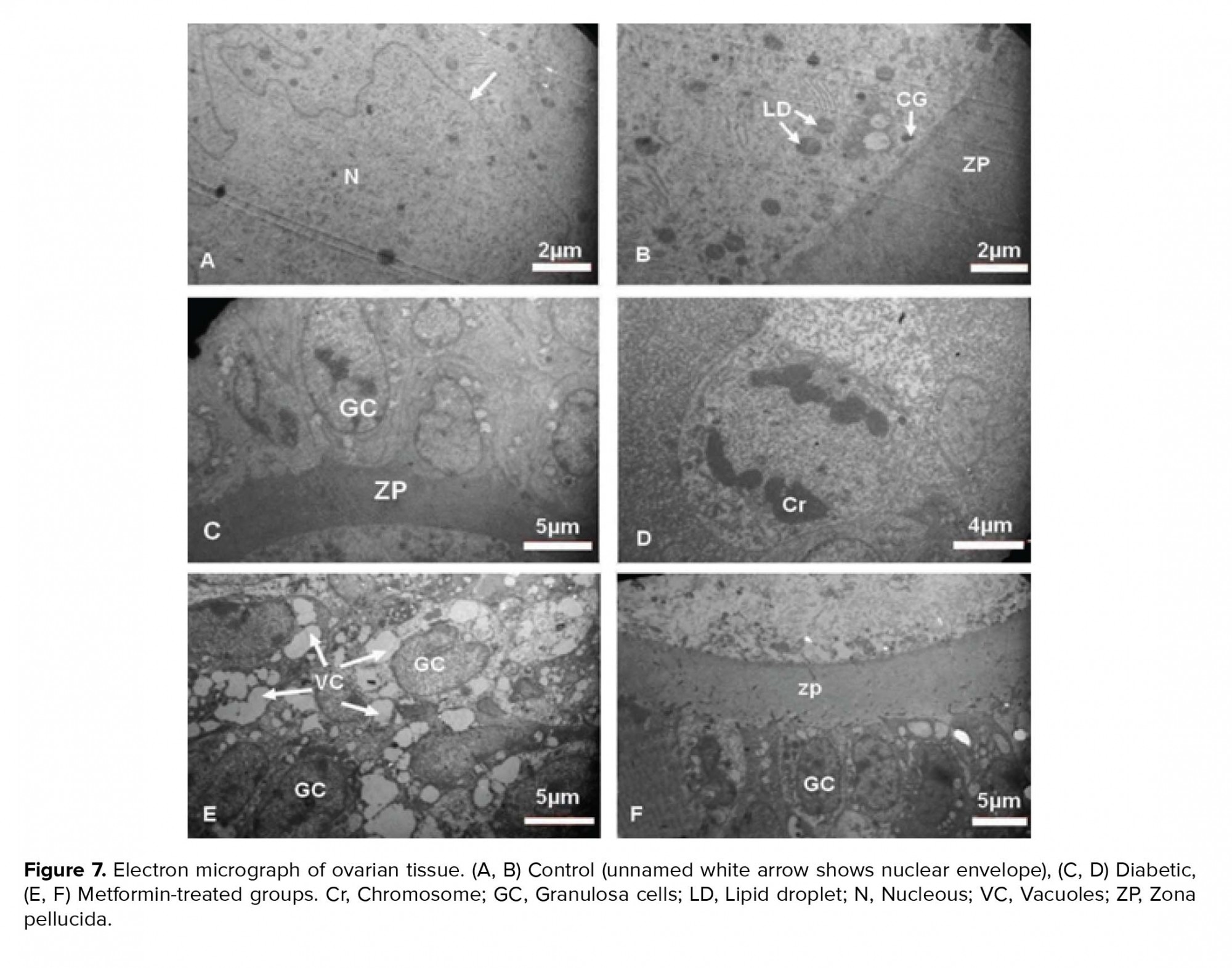
The GCs were normal with distinct cytoplasmic membrane. The normal and regular distribution of ooplasmic organelles such as endoplasmic reticulum and mitochondria (Mt) were observed. The ZP had regular thickness and contained microvilli. There were some cortical granules (CGs) and lipid droplet (LD) in close contact with oolemma. The double membrane of nucleus with nuclear envelope pores and reticular nucleolus were distinct (Figure 7).
The nuclei of the GCs were observed with the dense chromatin. The cytoplasm of TCs and GCs contained many VCs. The GCs degenerated and TCs became inflamed with foamy cytoplasm. In spite of the large number of degenerated GCs, a number of mitotic cells were also observed. The irregular ZP with less thickness was observed and did not contain microvilli and cumulus cells process endings. The oolemma was not clearly defined and perivitelline space was not seen. The distribution of oocyte organelles was uniform. The two-layer membrane of the nuclear envelope with its pores and reticular nucleolus was observed in the oocyte (Figure 7).
Experimental-2
The ovaries of metformin-treated rats contained GCs with pyknotic nuclei and cytoplasmic VC. The TCs layers were normal with some VC. The ZP with normal thickness was observed and no perivitelline space was seen. The ooplasmic organelles had regular distribution. Also, in the ooplasm a few Mt and annulate lamella were observed (Figure 7).
Experimental-3
The few GCs were destroyed; however, the basement membrane remained intact and separated the TI and GCs layers. There was some VC in GCs. The cytoplasmic organelles of GCs, especially Mt, had regular distribution. The cell junctions between the GCs were not observed.
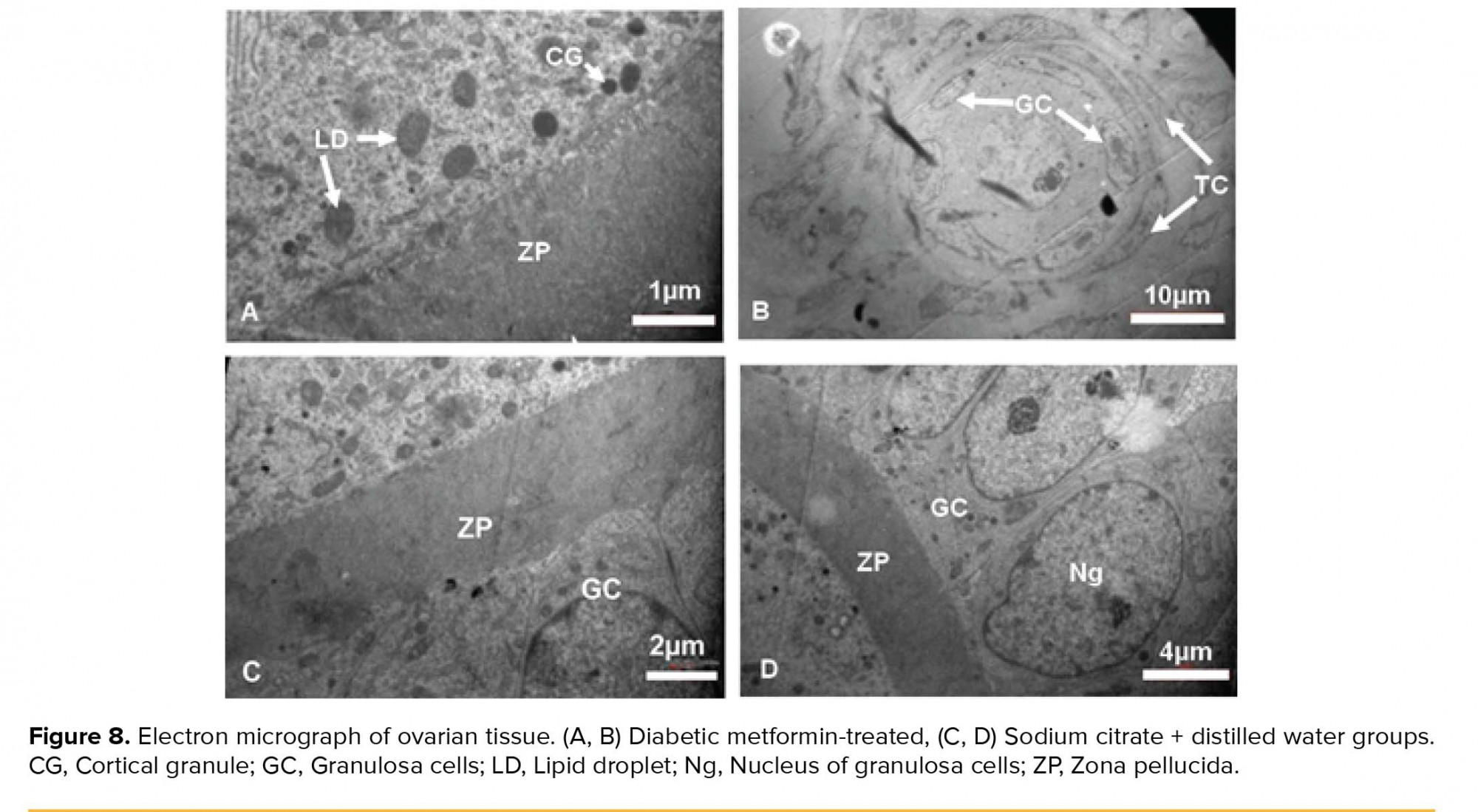
The ZP had regular thickness and contained microvilli. The organelles were scattered throughout ooplasm. Some LDs and CGs were in close contact with oolemma. A lot of AL was seen in ooplasm. The double layer of nuclear envelope with nuclear pores was noticed. A primordial follicle with normal oocyte and normal distribution of ooplasmic organelles and also normal GCs and TCs were observed (Figure 8).
3.7.3. Sham groups
Sham-1
Most GCs were normal and the TI and granulose layer were also separated by intact basement membrane. The few TCs were destroyed. The ZP had regular thickness. Some ooplasmic organelles like AL and Mt with regular distribution were observed. There were some CGs and LDs under the oolemma (not shown here).
Sham-2
The GCs and TCs were normal. The ZP had regular thickness and the ooplasmic organelles were distributed regularly. There were some CGs and LDs in close contact with the oolemma (not shown here).
Sham-3
The GCs were columnar and distinct membrane and cell junctions were noticed. Also, the GCs with distinct nucleolus and nuclear membrane were seen. ZP had regular thickness. The CGs were seen under oolemma. The theca layers with spindle-shaped cells were seen outside of the GCs (Figure 8).

4. Discussion
The results of our study show that, STZ induces the appropriate diabetic rat model; it is confirmed by high levels of blood sugar and reduction of body weight. STZ-induced diabetes decreases the number of dynamic follicles and increases atretic follicles, and has destructive effects on ovarian tissue. Therefore, the weight and volume of ovary is reduced. Also, the numbers of GCs and crucial organelles of oocyte and GCs, which are involved in the growth and development of the follicles are affected by diabetes.
The results of present study show a significant BMI reduction in STZ-induced diabetic rats, same as the body weight reduction in patient with diabetes (17). This is confirmed by the animal model studies that show a reduction in the body weight of the STZ-induced diabetic rats (18). In spite of this, after about three weeks, the BMI reduction remains constant until the end of the experiment, which is similar to the result of Courteix and Eschaliera (19). In our study, the metformin also cannot inhibit the weight decline in STZ-induced diabetic rats.
One of the important indexes in the evaluation of ovarian functions is the change in the weight and volume of ovary, which is the result of alteration in ovarian structures, like the change in the number and nature of follicles. In this study, there is a rational reduction in the weight of ovary in STZ-induced diabetic rats and also a non-statistically significant weight gains in STZ-induced diabetic + metformin-treated rats. Tesone and colleagues observed the ovarian weight reduction in diabetic animal model (20). Their results indicate that ovarian tissue is damaged by diabetes and metformin has somewhat prevented this impairment.
The size and volume of ovary affect the ovarian functions and the destructive effects of diabetes on the growth and development of follicle can reduce the volume of cortex and subsequently the total volume of ovary. In this study, the cortical and total volume of ovary decreased in STZ-induced diabetic rats and metformin was partly able to recover this reduction; on the other hand, in the non-diabetic rats, metformin also increased the volume of ovaries. A clinical study showed that 25% of the ovarian volume decreased in diabetic patients per year versus 13% in healthy people (21). The reduction of cortical volume in diabetic rats indicates damage to the cortex and reduces the number of follicles and GCs. An estimation of the exact number of ovarian follicles at different stages of development is an important index of folliculogenesis and shows the maturation of oocyte and supporting cells. In this research, the number of granulose cells and of course the primordial, primary and secondary, but not antral, follicles were reduced and atretic follicles in diabetic rats increased, so the diabetes can increase the degeneration of GCs and follicular atresia. In 2005, a study on STZ-diabetic model reported that hyperglycemia decreases the expression of connexin 43. This reduction can affect the communication between the oocyte and GCs, so the degeneration of GCs increased, also the reduction of connexin43 expression causes abnormal oocyte and weak folliculogenesis (4). The number of GCs increased in metformin-treated rats, but there was no difference between the STZ-induced diabetic + metformin-treated and STZ-induced diabetic rats without any treatment, so that, metformin cannot prevent the reduction of GCs in diabetes.
The supportive roles of GCs is established through the gap junction between GCs and oocytes, which is important to transport the essential nutrients to the oocyte (22). Therefore, due to damage of GCs in STZ-induced diabetic rats, it is not unexpected that the damage in primordial, primary and secondary, but not antral follicles will increase, because these types of follicles need GCs for growth and development process. So, in this research there was no significant difference in the number of antral follicles between the STZ-induced diabetic and control groups. Similar conclusions were obtained by Erbas and colleagues in 2014; this study was conducted in diabetic rats and showed that diabetes affects ovarian follicles, especially in primordial, primary and secondary follicles, but does not affect the number of antral follicles. They believed that the cause of the increased follicular atresia is a result of damage to the GCs (23), which may be due to the less biochemical needs of the oocytes.
The growth and development of an oocyte simultaneously requires both nuclear and cytoplasmic maturation. Although, oocytes with nuclear maturation can be fertilized, they may fail due to the deficiency of cytoplasmic factors that are essential to complete the maturation process (24).
The reduction in the ratio of the diameter of the nucleus to the cytoplasm can be caused by the reduction of oocyte diameter in diabetic rats. Previous research on STZ-induced diabetic models suggests that the size of oocytes in the antral follicle is less than other nondiabetic groups (4). Also, Afrough and colleagues reported that diabetes causes a delay in the arrival of oocyte into metaphase II and decreases oocyte diameter and increases the perivitelline space (25). The results further show that the ratio of the diameter of the nucleus to the ooplasm increases significantly in the metformin-treated compared to the STZ-induced diabetic rats. Metformin has not been able to recover this reduction in the STZ-induced diabetic rats, because the results indicate that there is no significant difference in the STZ-induced diabetic + metformin-treated rats compared to the STZ-induced diabetic rats.
Both the histological and ultrastructural aspects of ovary in this study revealed serious injury in the follicles of STZ-induced diabetic rats. These are in agreement with the morphometric finding, because the results of this study show that the dynamic types of follicles were reduced and degenerated follicles and oocytes increased. Nevertheless, as a result of this damage and the reduction in the number of supportive cells, intercellular junction between the GCs and oocyte was not clearly observed. The supportive roles of GCs and cell junctions between GCs and oocytes are of particular importance for the transmitting of growth factors and nutrient materials for cytoplasmic and nuclear maturation. Even bidirectional communication between GCs and oocytes is essential for cumulus cells expansion, as the third aspect of follicular development (26). In STZ-induced diabetic rats, the TCs were not spindle shaped and became swollen. It is well-known that the endocrine roles of the TCs are essential for the production of estrogen, the function of TCs is like a double-edged blade, both over and under activity of these cells lead to infertility due to hyperandrogenism and lack of estrogen, respectively (27). We also observed the irregular thicknesses of ZP in STZ-induced diabetic rats. The ZP ensures the major roles in fertilization and communication between oocytes, and also as a non-cellular structure, it has a protective role for oocytes, eggs, and embryos (28). The thickness of ZP seems to be a major factor in sperm penetration. In the clinic, a thick ZP (≥ 22 µm) is used as a major indicator in the microinjection procedure (29). Generally, it can be concluded that the diabetes has introduced the irreversible damages to ovarian tissue, which can cause infertility.
Also, ultrastructural observations on the ovary of non-diabetic metformin-treated rats showed degeneration and the large numbers of VC in the cytoplasm and of GCs, while the stereological study did not show any reductions in the number of GCs. The VC can be residues of the LDs that are used to produce energy for normal cell activity (30). However, there were no VCs in the STZ-induced diabetic + metformin-treated rats and also the degeneration of GCs was decreased. This study has shown that metformin in non-diabetic rats has a deleterious effect on ovarian structures. Previous studies have shown that metformin does not affect ovarian functions in human with normal or low levels of testosterone, while in patients with high testosterone levels, metformin increases the ovulation (31). Previous research has shown that testosterone levels in diabetics are reduced (32), as we find in our study (not shown in the result section).
5. Conclusion
STZ induces the appropriate diabetic rat model; it is confirmed by high levels of blood sugar on the 10th day of injection. The STZ-induced diabetes reduces body and ovarian weights, decreases the number of dynamic follicles and increases atretic follicle, and has destructive effects on ovarian tissue. Therefore, the total and cortical volume of ovary is reduced. Also, the fertility criteria such as the size of follicle and oocyte, numbers of GCs and crucial organelles of oocyte and GCs, which are involved in the growth and development of the follicles are affected by diabetes. Hence, it seems that diabetes can lead to reduced fertility and metformin has no preventive effects.
Acknowledgments
The authors would like to thank the Vice Chancellery of Research, Shiraz University of Medical Sciences, Shiraz, Iran for financial support, Mr. Izad Noori for performing the histological procedure, and Dr. Ghaem Maghami for statistical consulting.
Conflict of Interest
The authors declare no conflict of interest.
Full-Text: (460 Views)
- Introduction
Previous studies show that diabetes has negative effects on the growth of the ovarian follicles and decreases the rate of fertility, but the exact mechanisms are unclear (4, 5). Alterations of glucose concentration can greatly affect some functions of reproductive system (6). For example, in streptozotocin (STZ)-induced diabetic rats, high level of glucose reduces the production of progesterone and estradiol in granulosa cells (GC) and causes reproductive disorders due to changes in folliculogenesis and steroidogenesis (7). Given the rapid incidence of diabetes and increase in the number of women with diabetes, it is essential to develop the animal models of diabetes to understand the effects of diabetes on the ovary and to identify ovarian dysfunctions, as well as to achieve a new treatment approach (8).
STZ is a preferred chemical agent that can be used to create an animal model of diabetes (9). It is a glucosamine-nitrosourea compound that has specific toxic effects on pancreatic beta cells. The mechanism of diabetes mellitus in experimental animals is not entirely clear, but it seems that the production of free radicals such as superoxide-hydrogen, peroxide, and nitric oxide has destructive effects of STZ on DNA strand of pancreatic beta cells (10).
In recent years, significant progress has been made in understanding and controlling diabetes, and in many cases we have been able to delay or prevent the complications of the disease (11). Metformin is an insulin-sensitizing drug and helps ovulation. This drug can help regulate the sexual cycle in about half of women with polycystic ovary syndrome. It can also reduce insulin levels by lowering appetite and helping in weight loss. Regular use of metformin along with other therapeutic proceedings including exercise, diet, and weight control can be more effective (12). Despite the long-term experimental and clinical experience with metformin in animal models and human, the exact mechanism of its function is still unclear (13). However, metformin is still used in the clinic without a perfect understanding of its mechanism. Therefore, more research is needed to investigate the effect of metformin on the reproductive system of diabetic patients (14).
To distinguish ovarian dysfunction, the ethical restrictions of human studies lead us to use animal models to investigate the effect of diabetes on accurate morphological (ultrastructure) and exact morphometric (stereological) features of ovary. So, this study was designed to investigate the preventive and therapeutic roles of metformin on ultrastructural and stereological features of ovary in the STZ-induced diabetes adult female rats.
- Materials and Methods
- 1. Animals
Seventy female Sprague-Dawley rats aged 8 to 10 weeks with a weight of 180-200 gr were used in this experimental study.
- 2. Experimental design
- Control group, received no drugs.
- Experimental-1 (STZ-induced diabetes) group, received a single dose of 65 mg/kg STZ in sodium citrate (SC) (29.41 mg/1 ml normal saline) intraperitoneal (IP) on the first day of the first week.
- Sham-1 (SC-treated) group, received SC, the same as the experimental-1 group.
- Experimental-2 (Metformin-treated) group, received 50 mg/100 gr of body weight daily per os in 0.5 mL distilled water (DW) on the first day of the fifth week for three weeks (15).
- Sham-2 (DW-treated) group, received DW, the same as the experimental-2 group.
- Experimental-3 (STZ-induced diabetic + Metformin-treated) group, the induced-diabetic rats received metformin the same as the experimental-2 group.
- Sham-3 (SC & DW-treated) group, received SC and DW, the same as the experimental-1 and 2 groups, respectively.
- 3. Induction of diabetes
- 4. Measurement of the body weight, height, and body mass index (BMI)
- 5. Measurement of blood sugar levels
- 6. Measurement of blood level of cholesterol and triglyceride
- 7. Measurement of ovary weight
- 8. Stereological study
- 9. Estimation of the number of granulose cells and follicles
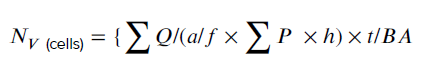
- 10. Total volume of the ovary, cortex, and medulla
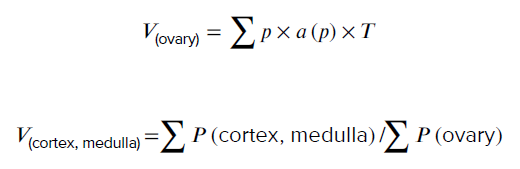
Using stereological software, the diameters of the nucleus and the cytoplasm were measured in oocytes.
- 11. Tissue preparation for transmission electron microscopy

2.12. Ethical consideration
The animals were housed under controlled conditions (12:12 hr light-dark cycle at 23 ± 2ºC), with ad libitum access to food and tap water for 8 wk. Also, animal handling and all other procedures were done following the ethical principles and the national norms and standards for conducting medical research in Iran, which was approved by the Ethics committee of Shiraz University of Medical Sciences, Shiraz, Iran (Approval ID: IR.SUMS.REC.1397.659).
2.13.Statistical analysis
We used commercial scientific statistic software; graph pad prism version 7.05, (Prism Inc, San Diego, California, USA) in order to analyze my scientific data and present graph, Data are reported as means and standard deviation and analyzed using One-way ANOVA test. In addition, for statistical comparisons between the groups, we used Tukey’s multiple comparisons test as a post-hoc test. The mean difference was considered significant if p < 0.05.
3. Results
3.1.Body mass index
The BMI was significantly reduced in the experimental-1 and experimental-3 groups in the second week of experiment (p < 0.01, p = 0.03 respectively) (Figure 2).
3.2.Blood sugar levels
Mean ± SD of blood sugar levels of animals in the experimental-1 (p < 0.01) and experimental-3 groups (p < 0.01) showed a significant increase compared to the sham-1 and sham-3 groups on 10th day of experiment respectively. Also in the experimental-1 and experimental-3 rats, a significant increase was seen compared to the control group (p < 0.01 and p < 0.01, respectively).
In addition, on the 28th day of experiment, blood sugar was significantly higher in the the experimental-1 (p = 0.01) and experimental-3 groups (p = 0.01) compared to the control group.
There were no significant differences in the blood sugar levels of animals between groups on the first day of experiment (Table I).
3.3.The serum levels of cholesterol and triglyceride
There were no significant differences in the serum levels of cholesterol and triglyceride between the groups (Table II).
3.4. Weight of ovaries
Despite the results of this study that show 18% decrease in the ovarian weight in experimental-1 group and 30% increase in ovarian weight in experimental-3 group compared to the control group, there were no significant differences in the ovarian weight between the studied groups.
3.5. Stereological study
3.5.1. Estimation of the number of GCs
The mean ± SD of the number of GC was significantly decreased (p = 0.04) in the experimental-1 group as compared with the experimental-2 group and also sham-3 group (p = 0.01). In addition, there was a significant increase in experimental-2 group compared to the sham-1 group (p = 0.01; Figure 3).
3.5.2. Number of follicles
The results of this study indicated that the mean ± SD of the number of primordial follicles were significantly decreased in the experimental-1 group compared with experimental-2 (p = 0.03), and the primary follicles were significantly decreased (p < 0.01) in the experimental-1 groups as compared with the experimental-2 and sham-3 group (p = 0.04). The secondary follicles were significantly decreased (p = 0.01) in the experimental-1 as compared with the experimental-2 group.
No significant differences were observed between the mean ± SD of the numbers of antral follicle in all of the groups. The mean ± SD of the number of atretic follicles in the experimental-3 rats was significantly higher than the other rats (p < 0.01) (Table III).
3.5.3. Total volume of the ovary, cortex, and medulla
The mean ± SD of the total volume of ovary was significantly increased in experimental-2 group compared to the experimental-1 (p < 0.01) and control groups (p = 0.01). Also, in the total volume of ovary, a significant increase was seen in the experimental-3 group as compared with the experiment-1 (p = 0.01).
The mean ± SD of the volume of cortex significantly increased in the experimental-2 group compared to the control group (p <0.01). Further, in experimental-1 rats, a significant decrease in the volume of cortex was seen compared to the sham-1 group (p = 0.01). Besides, the volume of cortex was significantly increased in the experimental-2 group than the experimental-1 group (p < 0.01). Moreover, there was a significant increase in experimental-3 group compared to the experimental-1 group (p < 0.01). There were no significant differences in the mean ± SD of the volume of medulla between all of the groups (Figure 4).
3.5.4. Ratio of the diameter of nucleus to ooplasm
The mean ± SD of the ratio of the diameter of the nucleus to the ooplasm was decreased significantly in the experimental-1 group compared to the control (p < 0.01), experimental-2 (p = 0.05), and sham-1 groups (p < 0.01) (Figure 5).
3.6.Histological observation of resin embedded sections
3.6.1. Control group
The germinal epithelium (GE) that contained cuboidal cells with distinct boundaries and underlying tunica albuginea (TA) were observed. The ovarian stroma contains normal cells and blood vessels. Some primordial and primary follicles with normal GCs with dividing nuclei and oocyte with typical distribution of organelles in the cortex were observed. Outside the basement membrane, theca interna, and externa cells with long and spindle shape were seen. The thickness of zona pellucida (ZP) was normal and perivitelline space (PVS) wasn't observed (the figure is not shown).
3.6.2. Experimental groups
Experimental-1
The ovaries of diabetic rats were covered by squamous epithelium and the TA was unclear. The number of degenerative oocytes increased. The cytoplasm of the GCs and theca cells (TCs) contained many vacuoles (VC). There were many degenerated GCs and TCs with pyknotic nuclei. The ZP had normal thickness and the ooplasm had homogenous distribution of organelles (Figure 6).
Experimental-2
The intact GE with cuboidal and squamous cells and distinct boundaries and underlying TA was observed. The GCs and TCs and also stromal cells were full of VC. The ZP with regular thickness and normal distribution of ooplasmic organelles were observed (Figure 6).
Experimental-3
The GE had cuboidal and also squamous cells. The underlying TA was unclear. The GCs had normal appearance but there were some degenerated cells. The TCs with foamy cytoplasm were observed and also there was no clear boundary between interna and externa. The ZP had regular thickness. The normal distribution of ooplasmic organelles was observed. Some primordial follicles with normal GCs and normal oocytes were seen (Figure 6).
3.6.3. Sham groups
Sham-1
Germinal epithelium with normal and cuboidal cells were observed. A number of follicles were seen within the cortex. The ovarian stromal cells were normal. There were no pyknotic nuclei in GCs. The ooplasmic organelles had regular distribution (the figure is not shown).
Sham-2
The cuboidal and squamous cells were observed in GE. The underlying TA was distinct. Some of the stromal cells contained VC. The GCs were completely normal without the pyknotic nuclei. The ZP with regular thickness was observed. The normal ooplasmic distribution of organelles was detected.
Sham-3
The cuboidal and columnar epithelial cells covered the ovaries and also stromal cells had normal appearance. The blood vessels that contained RBC were noticed in ovary cortex. The primordial, primary and antral follicles with normal GCs and regular ZP thickness were observed (Figure 6).
3.7. Ultrastructural observation
3.7.1. Control group
The GCs were normal with distinct cytoplasmic membrane. The normal and regular distribution of ooplasmic organelles such as endoplasmic reticulum and mitochondria (Mt) were observed. The ZP had regular thickness and contained microvilli. There were some cortical granules (CGs) and lipid droplet (LD) in close contact with oolemma. The double membrane of nucleus with nuclear envelope pores and reticular nucleolus were distinct (Figure 7).
3.7.2Experimental groups
Experimental-1The nuclei of the GCs were observed with the dense chromatin. The cytoplasm of TCs and GCs contained many VCs. The GCs degenerated and TCs became inflamed with foamy cytoplasm. In spite of the large number of degenerated GCs, a number of mitotic cells were also observed. The irregular ZP with less thickness was observed and did not contain microvilli and cumulus cells process endings. The oolemma was not clearly defined and perivitelline space was not seen. The distribution of oocyte organelles was uniform. The two-layer membrane of the nuclear envelope with its pores and reticular nucleolus was observed in the oocyte (Figure 7).
Experimental-2
The ovaries of metformin-treated rats contained GCs with pyknotic nuclei and cytoplasmic VC. The TCs layers were normal with some VC. The ZP with normal thickness was observed and no perivitelline space was seen. The ooplasmic organelles had regular distribution. Also, in the ooplasm a few Mt and annulate lamella were observed (Figure 7).
Experimental-3
The few GCs were destroyed; however, the basement membrane remained intact and separated the TI and GCs layers. There was some VC in GCs. The cytoplasmic organelles of GCs, especially Mt, had regular distribution. The cell junctions between the GCs were not observed.
The ZP had regular thickness and contained microvilli. The organelles were scattered throughout ooplasm. Some LDs and CGs were in close contact with oolemma. A lot of AL was seen in ooplasm. The double layer of nuclear envelope with nuclear pores was noticed. A primordial follicle with normal oocyte and normal distribution of ooplasmic organelles and also normal GCs and TCs were observed (Figure 8).
3.7.3. Sham groups
Sham-1
Most GCs were normal and the TI and granulose layer were also separated by intact basement membrane. The few TCs were destroyed. The ZP had regular thickness. Some ooplasmic organelles like AL and Mt with regular distribution were observed. There were some CGs and LDs under the oolemma (not shown here).
Sham-2
The GCs and TCs were normal. The ZP had regular thickness and the ooplasmic organelles were distributed regularly. There were some CGs and LDs in close contact with the oolemma (not shown here).
Sham-3
The GCs were columnar and distinct membrane and cell junctions were noticed. Also, the GCs with distinct nucleolus and nuclear membrane were seen. ZP had regular thickness. The CGs were seen under oolemma. The theca layers with spindle-shaped cells were seen outside of the GCs (Figure 8).










4. Discussion
The results of our study show that, STZ induces the appropriate diabetic rat model; it is confirmed by high levels of blood sugar and reduction of body weight. STZ-induced diabetes decreases the number of dynamic follicles and increases atretic follicles, and has destructive effects on ovarian tissue. Therefore, the weight and volume of ovary is reduced. Also, the numbers of GCs and crucial organelles of oocyte and GCs, which are involved in the growth and development of the follicles are affected by diabetes.
The results of present study show a significant BMI reduction in STZ-induced diabetic rats, same as the body weight reduction in patient with diabetes (17). This is confirmed by the animal model studies that show a reduction in the body weight of the STZ-induced diabetic rats (18). In spite of this, after about three weeks, the BMI reduction remains constant until the end of the experiment, which is similar to the result of Courteix and Eschaliera (19). In our study, the metformin also cannot inhibit the weight decline in STZ-induced diabetic rats.
One of the important indexes in the evaluation of ovarian functions is the change in the weight and volume of ovary, which is the result of alteration in ovarian structures, like the change in the number and nature of follicles. In this study, there is a rational reduction in the weight of ovary in STZ-induced diabetic rats and also a non-statistically significant weight gains in STZ-induced diabetic + metformin-treated rats. Tesone and colleagues observed the ovarian weight reduction in diabetic animal model (20). Their results indicate that ovarian tissue is damaged by diabetes and metformin has somewhat prevented this impairment.
The size and volume of ovary affect the ovarian functions and the destructive effects of diabetes on the growth and development of follicle can reduce the volume of cortex and subsequently the total volume of ovary. In this study, the cortical and total volume of ovary decreased in STZ-induced diabetic rats and metformin was partly able to recover this reduction; on the other hand, in the non-diabetic rats, metformin also increased the volume of ovaries. A clinical study showed that 25% of the ovarian volume decreased in diabetic patients per year versus 13% in healthy people (21). The reduction of cortical volume in diabetic rats indicates damage to the cortex and reduces the number of follicles and GCs. An estimation of the exact number of ovarian follicles at different stages of development is an important index of folliculogenesis and shows the maturation of oocyte and supporting cells. In this research, the number of granulose cells and of course the primordial, primary and secondary, but not antral, follicles were reduced and atretic follicles in diabetic rats increased, so the diabetes can increase the degeneration of GCs and follicular atresia. In 2005, a study on STZ-diabetic model reported that hyperglycemia decreases the expression of connexin 43. This reduction can affect the communication between the oocyte and GCs, so the degeneration of GCs increased, also the reduction of connexin43 expression causes abnormal oocyte and weak folliculogenesis (4). The number of GCs increased in metformin-treated rats, but there was no difference between the STZ-induced diabetic + metformin-treated and STZ-induced diabetic rats without any treatment, so that, metformin cannot prevent the reduction of GCs in diabetes.
The supportive roles of GCs is established through the gap junction between GCs and oocytes, which is important to transport the essential nutrients to the oocyte (22). Therefore, due to damage of GCs in STZ-induced diabetic rats, it is not unexpected that the damage in primordial, primary and secondary, but not antral follicles will increase, because these types of follicles need GCs for growth and development process. So, in this research there was no significant difference in the number of antral follicles between the STZ-induced diabetic and control groups. Similar conclusions were obtained by Erbas and colleagues in 2014; this study was conducted in diabetic rats and showed that diabetes affects ovarian follicles, especially in primordial, primary and secondary follicles, but does not affect the number of antral follicles. They believed that the cause of the increased follicular atresia is a result of damage to the GCs (23), which may be due to the less biochemical needs of the oocytes.
The growth and development of an oocyte simultaneously requires both nuclear and cytoplasmic maturation. Although, oocytes with nuclear maturation can be fertilized, they may fail due to the deficiency of cytoplasmic factors that are essential to complete the maturation process (24).
The reduction in the ratio of the diameter of the nucleus to the cytoplasm can be caused by the reduction of oocyte diameter in diabetic rats. Previous research on STZ-induced diabetic models suggests that the size of oocytes in the antral follicle is less than other nondiabetic groups (4). Also, Afrough and colleagues reported that diabetes causes a delay in the arrival of oocyte into metaphase II and decreases oocyte diameter and increases the perivitelline space (25). The results further show that the ratio of the diameter of the nucleus to the ooplasm increases significantly in the metformin-treated compared to the STZ-induced diabetic rats. Metformin has not been able to recover this reduction in the STZ-induced diabetic rats, because the results indicate that there is no significant difference in the STZ-induced diabetic + metformin-treated rats compared to the STZ-induced diabetic rats.
Both the histological and ultrastructural aspects of ovary in this study revealed serious injury in the follicles of STZ-induced diabetic rats. These are in agreement with the morphometric finding, because the results of this study show that the dynamic types of follicles were reduced and degenerated follicles and oocytes increased. Nevertheless, as a result of this damage and the reduction in the number of supportive cells, intercellular junction between the GCs and oocyte was not clearly observed. The supportive roles of GCs and cell junctions between GCs and oocytes are of particular importance for the transmitting of growth factors and nutrient materials for cytoplasmic and nuclear maturation. Even bidirectional communication between GCs and oocytes is essential for cumulus cells expansion, as the third aspect of follicular development (26). In STZ-induced diabetic rats, the TCs were not spindle shaped and became swollen. It is well-known that the endocrine roles of the TCs are essential for the production of estrogen, the function of TCs is like a double-edged blade, both over and under activity of these cells lead to infertility due to hyperandrogenism and lack of estrogen, respectively (27). We also observed the irregular thicknesses of ZP in STZ-induced diabetic rats. The ZP ensures the major roles in fertilization and communication between oocytes, and also as a non-cellular structure, it has a protective role for oocytes, eggs, and embryos (28). The thickness of ZP seems to be a major factor in sperm penetration. In the clinic, a thick ZP (≥ 22 µm) is used as a major indicator in the microinjection procedure (29). Generally, it can be concluded that the diabetes has introduced the irreversible damages to ovarian tissue, which can cause infertility.
Also, ultrastructural observations on the ovary of non-diabetic metformin-treated rats showed degeneration and the large numbers of VC in the cytoplasm and of GCs, while the stereological study did not show any reductions in the number of GCs. The VC can be residues of the LDs that are used to produce energy for normal cell activity (30). However, there were no VCs in the STZ-induced diabetic + metformin-treated rats and also the degeneration of GCs was decreased. This study has shown that metformin in non-diabetic rats has a deleterious effect on ovarian structures. Previous studies have shown that metformin does not affect ovarian functions in human with normal or low levels of testosterone, while in patients with high testosterone levels, metformin increases the ovulation (31). Previous research has shown that testosterone levels in diabetics are reduced (32), as we find in our study (not shown in the result section).
5. Conclusion
STZ induces the appropriate diabetic rat model; it is confirmed by high levels of blood sugar on the 10th day of injection. The STZ-induced diabetes reduces body and ovarian weights, decreases the number of dynamic follicles and increases atretic follicle, and has destructive effects on ovarian tissue. Therefore, the total and cortical volume of ovary is reduced. Also, the fertility criteria such as the size of follicle and oocyte, numbers of GCs and crucial organelles of oocyte and GCs, which are involved in the growth and development of the follicles are affected by diabetes. Hence, it seems that diabetes can lead to reduced fertility and metformin has no preventive effects.
Acknowledgments
The authors would like to thank the Vice Chancellery of Research, Shiraz University of Medical Sciences, Shiraz, Iran for financial support, Mr. Izad Noori for performing the histological procedure, and Dr. Ghaem Maghami for statistical consulting.
Conflict of Interest
The authors declare no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Anatomy
References
1. Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of teh prevalence of diabetes for 2011 and 2030. Diabetes research and clinical practice. 2011;94(3):311-21. [DOI:10.1016/j.diabres.2011.10.029] [PMID]
2. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for teh year 2000 and projections for 2030. Diabetes care. 2004;27(5):1047-53. [DOI:10.2337/diacare.27.5.1047] [PMID]
3. Steger RW, Rabe MB. Teh effect of diabetes mellitus on endocrine and reproductive function. Proceedings of teh Society for Experimental Biology and Medicine. 1997;214(1):1-10. [DOI:10.3181/00379727-214-44064] [PMID]
4. Chang AS, Dale AN, Moley KH. Maternal Diabetes Adversely Effects Preovulatory Oocyte Maturation, Development, and Granulosa Cell Apoptosis. Endocrinology. 2005;146(5):2445-53. [DOI:10.1210/en.2004-1472] [PMID]
5. Wu Y, Zhang Z, Liao X, Wang Z. High fat diet triggers cell cycle arrest and excessive apoptosis of granulosa cells during teh follicular development. Biochemical and biophysical research communications. 2015;466(3):599-605. [DOI:10.1016/j.bbrc.2015.09.096] [PMID]
6. Colton SA, Pieper GM, Downs SM. Altered meiotic regulation in oocytes from diabetic mice. Biology of reproduction. 2002;67(1):220-31. [DOI:10.1095/biolreprod67.1.220] [PMID]
7. Chabrolle C, JeanPierre E, Tosca L, Ramé C, Dupont J. Effects of high levels of glucose on teh steroidogenesis and teh expression of adiponectin receptors in rat ovarian cells. Reproductive Biology and Endocrinology. 2008;6(1):11. [DOI:10.1186/1477-7827-6-11] [PMID] [PMCID]
8. Wu Y, Li Y, Liao X, Wang Z, Li R, Zou S, et al. Diabetes Induces Abnormal Ovarian Function via Triggering Apoptosis of Granulosa Cells and Suppressing Ovarian Angiogenesis. International journal of biological sciences. 2017;13(10):1297. [DOI:10.7150/ijbs.21172] [PMID] [PMCID]
9. King AJ. Teh use of animal models in diabetes research. British journal of pharmacology. 2012;166(3):877-94. [DOI:10.1111/j.1476-5381.2012.01911.x] [PMID] [PMCID]
10. Kruszyńska A, Słowińska-Srzednicka J, Jeske W, Zgliczyński W. Proinsulin, adiponectin and hsCRP in reproductive age women wif polycystic ovary syndrome (PCOS)-teh effect of metformin treatment. Endokrynologia Polska. 2014;65(1):2-10. [DOI:10.5603/EP.2014.0001] [PMID]
11. Kennedy M, Dunning P. Diabetes education: Essential but underfunded in Australia. Diabetes and primary care Australia. 2017;2(1):10-4.
12. Diamanti‐Kandarakis E, Economou F, Palimeri S, Christakou C. Metformin in polycystic ovary syndrome. Annals of teh New York Academy of Sciences. 2010;1205(1):192-8. [DOI:10.1111/j.1749-6632.2010.05679.x] [PMID]
13. Todd JN, Florez JC. An update on teh pharmacogenomics of metformin: progress, problems and potential. Pharmacogenomics. 2014;15(4):529-39. [DOI:10.2217/pgs.14.21] [PMID] [PMCID]
14. Elia E, Sander V, Luchetti C, Solano M, Di Girolamo G, Gonzalez C, et al. Teh mechanisms involved in teh action of metformin in regulating ovarian function in hyperandrogenized mice. MHR: Basic science of reproductive medicine. 2006;12(8):475-81. [DOI:10.1093/molehr/gal057] [PMID]
15. Shirwaikar A, Rajendran K, Barik R. Effect of aqueous bark extract of Garuga pinnata Roxb. in streptozotocin-nicotinamide induced type-II diabetes mellitus. Journal of ethnopharmacology. 2006;107(2):285-90. [DOI:10.1016/j.jep.2006.03.012] [PMID]
16. Feldman M, Schiller LR. Disorders of gastrointestinal motility associated wif diabetes mellitus. Annals of Internal Medicine. 1983;98(3):378-84. [DOI:10.7326/0003-4819-98-3-378] [PMID]
17. Akbarzadeh A, Norouzian D, Mehrabi M, Jamshidi S, Farhangi A, Verdi AA, et al. Induction of diabetes by streptozotocin in rats. Indian Journal of Clinical Biochemistry. 2007;22(2):60-4. [DOI:10.1007/BF02913315] [PMID] [PMCID]
18. Courteix C, Eschalier A, Lavarenne J. Streptozocin-induced diabetic rats: behavioural evidence for a model of chronic pain. Pain. 1993;53(1):81-8. [DOI:10.1016/0304-3959(93)90059-X]
19. Tesone M, Ladenheim RG, Oliveira-Filho RM, Chiauzzi VA, Foglia VG, Charreau EH. Ovarian Dysfunction in Streptozotocin-lnduced Diabetic Rats. Proceedings of teh Society for Experimental Biology and Medicine. 1983;174(1):123-30. [DOI:10.3181/00379727-174-41714] [PMID]
20. Oppermann K, Fuchs SC, Spritzer PM. Ovarian volume in pre-and perimenopausal women: a population-based study. Menopause. 2003;10(3):209-13. [DOI:10.1097/00042192-200310030-00006] [PMID]
21. Eppig JJ. Mouse oocyte development in vitro wif various culture systems. Developmental biology. 1977;60(2):371-88. [DOI:10.1016/0012-1606(77)90135-X]
22. Erbas O, Pala HG, Pala EE, Oltulu F, Aktug H, Yavasoglu A, et al. Ovarian failure in diabetic rat model: Nuclear factor-kappaB, oxidative stress, and pentraxin-3. Taiwanese Journal of Obstetrics and Gynecology. 2014;53(4):498-503. [DOI:10.1016/j.tjog.2013.11.008] [PMID]
23. Sun Q-Y, Nagai T. Molecular mechanisms underlying pig oocyte maturation and fertilization. Journal of Reproduction and Development. 2003;49(5):347-59. [DOI:10.1262/jrd.49.347] [PMID]
24. Afrough M, Majd NE, Najafzadeh H. Protective Effects of Aloe Vera on Superovulated Oocytes and Folliculogenesis in Diabetic Mice. Zahedan Journal of Research in Medical Sciences. 2016;18(8). [DOI:10.17795/zjrms-7939]
25. Huang Z, Wells D. Teh human oocyte and cumulus cells relationship: new insights from teh cumulus cell transcriptome. Molecular human reproduction. 2010;16(10):715-25. [DOI:10.1093/molehr/gaq031] [PMID]
26. Magoffin DA. Ovarian theca cell. Teh international journal of biochemistry & cell biology. 2005;37(7):1344-9. [DOI:10.1016/j.biocel.2005.01.016] [PMID]
27. Wassarman P, Chen J, Cohen N, Litscher E, Liu C, Qi H, et al. Structure and function of teh mammalian egg zona pellucida. Journal of Experimental Zoology. 1999;285(3):251-8.
https://doi.org/10.1002/(SICI)1097-010X(19991015)285:3<251::AID-JEZ8>3.0.CO;2-1 [DOI:10.1002/(SICI)1097-010X(19991015)285:33.0.CO;2-1]
28. Bertrand E, Van den Bergh M, Englert Y. Fertilization and early embryology: does zona pellucida thickness influence teh fertilization rate? Human reproduction. 1995;10(5):1189-93. [DOI:10.1093/oxfordjournals.humrep.a136116] [PMID]
29. Szymanski KM, Binns D, Bartz R, Grishin NV, Li W-P, Agarwal AK, et al. Teh lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proceedings of teh National Academy of Sciences. 2007;104(52):20890-5. [DOI:10.1073/pnas.0704154104] [PMID] [PMCID]
30. Pirwany I, Yates R, Cameron I, Fleming R. Effects of teh insulin sensitizing drug metformin on ovarian function, follicular growth and ovulation rate in obese women wif oligomenorrhoea. Human reproduction. 1999;14(12):2963-8. [DOI:10.1093/humrep/14.12.2963] [PMID]
31. Barrett-Connor E, Khaw KT, Yen SSC. ENDOGENOUS SEX HORMONE LEVELS IN OLDER ADULT MEN WITH DIABETES MELLITUS. American Journal of Epidemiology. 1990;132(5):895-901. [DOI:10.1093/oxfordjournals.aje.a115732] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








