Mon, Feb 2, 2026
[Archive]
Volume 18, Issue 7 (July 2020)
IJRM 2020, 18(7): 491-500 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Teh W T, Polyakov A, Garrett C, Edgar D, Mcbain J, Rogers P. Reduced live birth rates in frozen versus fresh single cleavage stage embryo transfer cycles: A cross -sectional study. IJRM 2020; 18 (7) :491-500
URL: http://ijrm.ir/article-1-1429-en.html
URL: http://ijrm.ir/article-1-1429-en.html
1- Department of Obstetrics and Gynaecology, University of Melbourne, The Royal Women’s Hospital, Parkville, Victoria, Australia. Reproductive Services, The Royal Women’s Hospital, Parkville, Victoria, Australia. Melbourne IVF, East Melbourne, Victoria, Australia. , wantinn.teh@mivf.com.au
2- Reproductive Services, The Royal Women’s Hospital, Parkville, Victoria, Australia. Melbourne IVF, East Melbourne, Victoria, Australia.
3- Melbourne IVF, East Melbourne, Victoria, Australia.
4- Department of Obstetrics and Gynaecology, University of Melbourne, The Royal Women’s Hospital, Parkville, Victoria, Australia. Reproductive Services, The Royal Women’s Hospital, Parkville, Victoria, Australia. Melbourne IVF, East Melbourne, Victoria, Australia.
5- Department of Obstetrics and Gynaecology, University of Melbourne, The Royal Women’s Hospital, Parkville, Victoria, Australia.
2- Reproductive Services, The Royal Women’s Hospital, Parkville, Victoria, Australia. Melbourne IVF, East Melbourne, Victoria, Australia.
3- Melbourne IVF, East Melbourne, Victoria, Australia.
4- Department of Obstetrics and Gynaecology, University of Melbourne, The Royal Women’s Hospital, Parkville, Victoria, Australia. Reproductive Services, The Royal Women’s Hospital, Parkville, Victoria, Australia. Melbourne IVF, East Melbourne, Victoria, Australia.
5- Department of Obstetrics and Gynaecology, University of Melbourne, The Royal Women’s Hospital, Parkville, Victoria, Australia.
Full-Text [PDF 281 kb]
(1512 Downloads)
| Abstract (HTML) (3148 Views)
It is widely believed that controlled ovarian hyperstimulation (COH) used in IVF cycles has detrimental effects on the endometrium, as well as potentially disrupting normal synchronous development between the endometrium and the embryo (7-17). It has been shown that pregnancy is more likely when there are fewer endometrial histological alterations after COH (18). No pregnancy was reported if development of the endometrium was greater than 3 days more advanced than the embryos (13, 14, 19).
Evidence that synchronous development between the embryo and the endometrium is important for successful implantation comes from work comparing normal-growing and slow-growing embryos on implantation rates and pregnancy rates (PR) between fresh autologous and FET cycles. As expected, the clinical PR was higher for normal-growing than the slow-growing embryos in fresh cycles (51% vs. 33.3%). However, if the slower blastocyst growth rates are compensated for by transferring on developmental age (day 5) rather than chronological age (day 6), then there was no significant difference in The PR between the normal- and slow-growing cryopreserved blastocysts following FET cycles (63.6% vs. 58.9%). Slow embryos were also associated with a significantly greater PR in FET cycles than in fresh autologous cycles (58.9% vs. 33.3%). (20) This study supports the hypothesis that embryo-endometrial developmental asynchrony caused by slow-growing embryos can be corrected by freezing the embryo and transferring it back a day earlier in a subsequent cycle.
Further support for FET giving improved results to fresh transfers comes from a recent randomized multicenter trial involving women with polycystic ovarian syndrome (PCOS) (21). This study demonstrated a higher live birth rate (LBR; 49.3% vs 42.0%) with FET than fresh embryo transfer cycles.
The primary aim of this retrospective study of 10,744 single embryo transfers from the Melbourne IVF (MIVF) database was to compare pregnancy outcomes between fresh versus frozen autologous transfer cycles. We hypothesized that live birth and clinical pregnancy rates (CPR) would be higher in FET compared with fresh embryo transfers due to a combination of improved endometrial receptivity and improved embryo-endometrial synchrony in the FET cycles. Given that this is the largest such dataset ever published, we also hypothesized that the analysis would also provide important insights into factors that influence implantation rates in IVF and FET cycles.
According to the MIVF laboratory protocol, each embryo was evaluated twice before transfer. The first evaluation was performed 23-24 hr post-insemination/ICSI; referred to henceforth as the syngamy check. During this evaluation, embryos were assessed for the presence and number of cells (early cleavage (EC), nuclear envelope breakdown (NEBD), or 2 pronuclei (2PN)). The second evaluation was done on the morning of fresh embryo transfer (or before cryopreservation) on day 2 post-insemination/ICSI. Number of cells, degree of fragmentation, and multinucleation were assessed at the day 2 check. All embryos were cryopreserved using the slow freeze method (routine practice at the time of study) (22). The MIVF freeze-thaw protocols use post-thaw embryo culture to confirm resumed embryo development. Embryos for transfer in FET cycles are thawed the afternoon before the day of embryo transfer. These embryos are again evaluated twice; immediately after thawing for the number of surviving cells and a second time just prior to transfer in order to assess the resumption of mitosis and the total number of cells. Embryos are deemed suitable for transfer only if they survive the freeze-thaw process, defined as survival of ≥ 50% of the cells.
Information about embryo transfer cycles using cleavage-stage embryos was retrieved from the MIVF database. Given the large size of the database, we were able to enforce strict inclusion and exclusion criteria, but still retained a large dataset to analyze. A maximum of two stimulated cycles (cycle involving egg collection, embryo transfer of the best embryo, and freezing of remaining embryos for future use during thaw embryo cycles) were included in the analysis for each patient. Loss of a blastomere that is evident immediately post thaw is known to be associated with reduced embryo implantation potential (23). Therefore, we excluded those cycles using embryos where cell loss occurred during post-thaw embryo culture and evaluation. We also excluded cycles involving transfer of more than one embryo, use of donor gametes, or embryos with pre-implantation genetic testing. Women who had been pregnant from a previous IVF treatment were also excluded from the analysis.
We compared the clinical outcomes between fresh embryo transfer and FET cycles. The main outcome was LBR. A ‘live birth’ is defined by the World Health Organization to be ‘the complete expulsion or extraction from its mother of a baby, irrespective of the duration of the pregnancy, which, after such separation, breathes or shows any other evidence of life’. The secondary outcome of this study was CPR. Clinical pregnancy is defined as the presence of fetal heart beat at first viability ultrasound (typically performed at gestational week 6 to 7 according to the MIVF protocol).
Stratification of cycles into fast- and slow-growing embryos was also performed. Slow cleavage-stage embryos were defined as those embryos that had 2PN during the syngamy check and were still at the 2-cell stage at the day 2 check. For the FET cycles, all embryos were at the 2-cells stage when frozen, and both cells survived the thawing process. Due to the post-thaw embryo culture protocol at MIVF for FET cycles, extra time allowed for development of frozen embryos, these embryos were half a day more advanced chronologically than their counterparts in fresh cycles. With the assumption that endometrial development is slightly advanced by COH, transferring frozen-thawed embryos that are half a day more advanced chronologically should improve embryo-endometrial developmental synchrony, leading to better CPR and LBR outcomes.
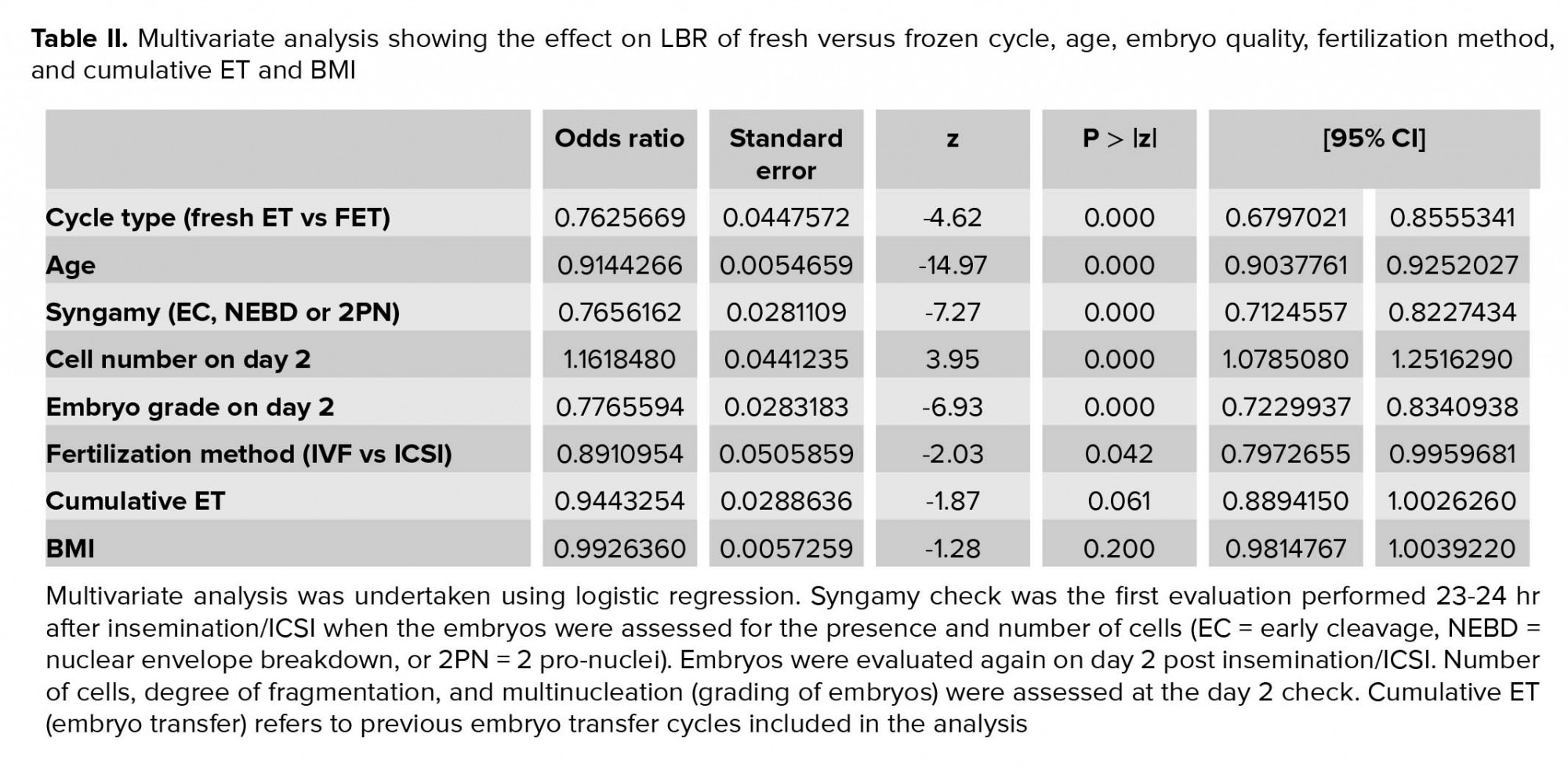
Multivariate analysis shows that women receiving a frozen-thawed embryo had a significantly lower LBR rate compared to those receiving a fresh embryo (OR 0.76, 95% CI 0.68-0.86, p < 0.00), after correcting for potential confounding factors - age, embryo quality (syngamy, cell number, embryo grade), fertilization method, cumulative ET and BMI (Table II). As expected, a higher pregnancy rate was observed in younger women (OR 0.91, 95% CI 0.90-0.93, p < 0.00) and those who had a better-quality embryo transferred (syngamy: OR 0.77, 95% CI 0.71-0.82, p < 0.00; cell number on day 2: OR 1.17, 95% CI 1.08-1.25, p < 0.00; embryo grade on day 2: OR 0.77, 95% CI 0.72-0.84, p < 0.00). Those who had embryo fertilized through ICSI had lower LBR compared to IVF (OR 0.89, 95% CI 0.80-1.0, p = 0.04). Previous cumulative embryo transfer number (OR 0.94, 95% CI 0.89-1.0, p = 0.06) and BMI of the women (OR 0.99, 95% CI 0.98-1.1, p = 0.2) did not have a statistically significant effect on LBR (Table II).

4. Discussion
This cross-sectional analysis of outcomes from fresh vs. FETs is the first to only include single embryo transfer cycles; and with 7,014 fresh cycles and 3,730 FET cycles, it is also the largest study of this type to be published. The primary finding from our analysis was a significantly lower LBR and CPR in FET cycles compared to fresh cycles, using single autologous cleavage-stage embryos. Multivariate analysis identified six variables that impacted significantly on LBR (Table II). Controlling for these confounding factors confirmed the finding of lower LBR in FET cycles. These results do not support our hypothesis that LBR and CPR would be higher in FET compared with fresh embryo transfers due to a combination of improved endometrial receptivity and improved embryo-endometrial synchrony in the FET cycles. Rather, they suggest that any potential gains in LBR due to improved endometrial receptivity and improved embryo-endometrial synchrony are lost with FET, presumably due to embryo damage caused during the freeze-thaw process.
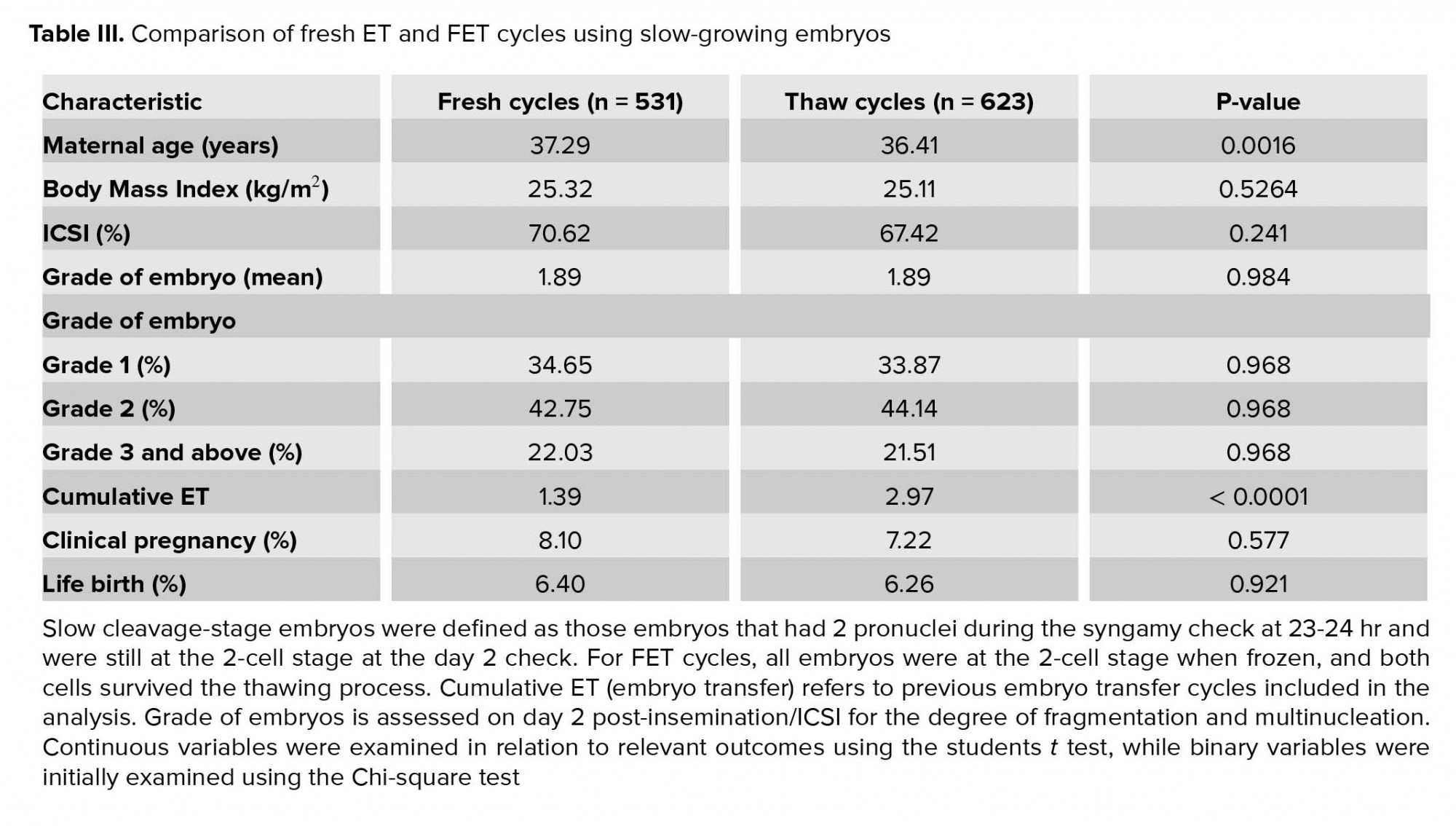
A secondary finding from this study was a sub-analysis of cycles with slow-growing embryos. We hypothesized that embryo-endometrial developmental synchrony would be improved in FET compared to fresh cycles using slow embryos due to the post-thaw embryo culture protocol. In our analysis of 1,154 cycles using slow embryos, there was no statistical difference in LBR or CPR between the two groups. We interpret this result as showing that the reduction in LBR due to freeze-thawing damage to the embryo seen in fast-growing embryos is fully compensated for by the improved synchrony in the slow-growing embryos. Slower-growing embryos typically result in lower PR in fresh cycles, potentially due to reduced embryo viability and increased embryo-endometrial asynchrony. Taken together, the results from this study confirm the positive influence of younger maternal age, better embryo quality, faster embryo development and improved embryo-endometrial developmental synchrony on LBR, while also demonstrating that embryo freezing has a negative impact on LBR.
There have been a limited number of published studies involving smaller sample sizes comparing clinical outcomes between fresh and FET cycles. While each of these provides some insight into the relative contributions of embryo viability, uterine receptivity, and embryo-endometrial synchrony to LBR, the individual studies are not all directly comparable and their findings not in complete agreement.
The study published by Shapiro and colleagues mentioned earlier in this paper, found increased CPR in the FET cycle cohorts. It is important to note that the best blastocysts of the cohort were transferred in fresh cycles, while only good-quality embryos (expanded supernumerary blastocysts) were selected for cryopreservation and subsequent use in FET cycles. Embryo selection was a potential confounder in this study, which was acknowledged by the authors in their article (20). Two subsequent prospective randomized studies by the same group comparing fresh blastocyst transfer and oocyte cryopreservation (with subsequent blastocyst transfer grown from the frozen-thawed oocytes) has shown a significantly greater CPR in the oocyte cryopreservation group in normal responders, but no statistical difference in high responders (24, 25). Overall, these studies support the hypothesis that FET improves CPR, presumably through improving uterine receptivity and/or embryo-endometrial synchrony. There is no evidence from these studies for a negative effect from freeze-thawing on embryo viability, possibly due to factors such as reduced blastomere size in the blastocyst compared to a cleavage-stage embryo. An alternative explanation is that by selecting embryos that have reached the expanded blastocyst stage, it is possible to eliminate all the nonviable embryos that appeared “normal” at the cleavage stage.
In two prospective cohort studies involving use of cleavage-stage embryos and large number of cycles, LBR was lower in FET cycles when compared to fresh cycles (26, 27). Four other randomized controlled trials using cleavage-stage embryos have also found no difference in LBR between fresh and FET cycles (28-31). The first trial involved 2,157 young (20-35 year old) women undergoing IVF/ICSI treatment due to tubal and/or male factors infertility (28). In the second trial, 782 infertile women were randomly assigned to fresh transfer or frozen embryos on day 3. In the FET group, only good-quality embryos (grade 1 or 2) were used (29). The last two studies involved women at risk of OHSS (30, 31). All studies involved the transfer of multiple embryos (28-31). Differences between the above studies and our study include patient population, number of embryos transferred, and embryo freezing method. Any or all of these factors could have contributed to the different clinical outcomes between these studies (no difference in LBR between fresh and FET cycles) and ours (higher LBR in fresh cycles).
While studies involving blastocyst transfer have suggested a better LBR with FET cycles (20, 24), our results and other studies using cleavage-stage embryos have not found the same (28-30). A major difference between protocols is that cleavage-stage embryos are cryopreserved according to their chronological age (day 2), while blastocysts are only cryopreserved if they reached the desired developmental stage of expanded blastocyst. The developmental stage of embryos is an important factor when considering embryo-endometrial synchrony. The cleavage-stage embryo freezing protocol has not been designed to correct for any potential asynchrony between developmental stage for the embryo and endometrium. There may be clinical benefit in the future to considering developmental stage rather than chronological age when thawing cleavage-stage embryos for transfer.
In conclusion, in this retrospective study of 10,744 IVF cycles, we were not able to support our hypothesis that LBR and CPR would be higher in FET compared with fresh embryo transfers. Multivariate analysis with correction for five significant variables showed a significantly higher LBR rate in cycles using fresh embryos compared to frozen-thawed embryos. In our sub-analysis of cycles involving slow-growing embryos, we interpreted the fact that there was no statistical difference in LBR between the fresh and FET groups as evidence for improved embryo-endometrial synchrony in the FET cycles. The large size of our dataset coupled with the strict inclusion and exclusion criteria have allowed us to identify six variables that significantly impact LBR, in addition to the results from our sub-analysis on slow-growing embryos which provides data to support the view that embryo-endometrial developmental synchrony is important. While most IVF units have moved to blastocysts transfer and freezing, the findings from this study on two-day old embryos remain highly relevant for understanding and optimizing LBR in today’s IVF clinic.
Acknowledgements
The authors would like to thank Professor Gordon Baker and Doctor Franca Agresta for their intellectual contribution. No financial support was received for this work.
Conflict of Interests
None declared.
Full-Text: (672 Views)
- Introduction
It is widely believed that controlled ovarian hyperstimulation (COH) used in IVF cycles has detrimental effects on the endometrium, as well as potentially disrupting normal synchronous development between the endometrium and the embryo (7-17). It has been shown that pregnancy is more likely when there are fewer endometrial histological alterations after COH (18). No pregnancy was reported if development of the endometrium was greater than 3 days more advanced than the embryos (13, 14, 19).
Evidence that synchronous development between the embryo and the endometrium is important for successful implantation comes from work comparing normal-growing and slow-growing embryos on implantation rates and pregnancy rates (PR) between fresh autologous and FET cycles. As expected, the clinical PR was higher for normal-growing than the slow-growing embryos in fresh cycles (51% vs. 33.3%). However, if the slower blastocyst growth rates are compensated for by transferring on developmental age (day 5) rather than chronological age (day 6), then there was no significant difference in The PR between the normal- and slow-growing cryopreserved blastocysts following FET cycles (63.6% vs. 58.9%). Slow embryos were also associated with a significantly greater PR in FET cycles than in fresh autologous cycles (58.9% vs. 33.3%). (20) This study supports the hypothesis that embryo-endometrial developmental asynchrony caused by slow-growing embryos can be corrected by freezing the embryo and transferring it back a day earlier in a subsequent cycle.
Further support for FET giving improved results to fresh transfers comes from a recent randomized multicenter trial involving women with polycystic ovarian syndrome (PCOS) (21). This study demonstrated a higher live birth rate (LBR; 49.3% vs 42.0%) with FET than fresh embryo transfer cycles.
The primary aim of this retrospective study of 10,744 single embryo transfers from the Melbourne IVF (MIVF) database was to compare pregnancy outcomes between fresh versus frozen autologous transfer cycles. We hypothesized that live birth and clinical pregnancy rates (CPR) would be higher in FET compared with fresh embryo transfers due to a combination of improved endometrial receptivity and improved embryo-endometrial synchrony in the FET cycles. Given that this is the largest such dataset ever published, we also hypothesized that the analysis would also provide important insights into factors that influence implantation rates in IVF and FET cycles.
- Materials and Methods
According to the MIVF laboratory protocol, each embryo was evaluated twice before transfer. The first evaluation was performed 23-24 hr post-insemination/ICSI; referred to henceforth as the syngamy check. During this evaluation, embryos were assessed for the presence and number of cells (early cleavage (EC), nuclear envelope breakdown (NEBD), or 2 pronuclei (2PN)). The second evaluation was done on the morning of fresh embryo transfer (or before cryopreservation) on day 2 post-insemination/ICSI. Number of cells, degree of fragmentation, and multinucleation were assessed at the day 2 check. All embryos were cryopreserved using the slow freeze method (routine practice at the time of study) (22). The MIVF freeze-thaw protocols use post-thaw embryo culture to confirm resumed embryo development. Embryos for transfer in FET cycles are thawed the afternoon before the day of embryo transfer. These embryos are again evaluated twice; immediately after thawing for the number of surviving cells and a second time just prior to transfer in order to assess the resumption of mitosis and the total number of cells. Embryos are deemed suitable for transfer only if they survive the freeze-thaw process, defined as survival of ≥ 50% of the cells.
Information about embryo transfer cycles using cleavage-stage embryos was retrieved from the MIVF database. Given the large size of the database, we were able to enforce strict inclusion and exclusion criteria, but still retained a large dataset to analyze. A maximum of two stimulated cycles (cycle involving egg collection, embryo transfer of the best embryo, and freezing of remaining embryos for future use during thaw embryo cycles) were included in the analysis for each patient. Loss of a blastomere that is evident immediately post thaw is known to be associated with reduced embryo implantation potential (23). Therefore, we excluded those cycles using embryos where cell loss occurred during post-thaw embryo culture and evaluation. We also excluded cycles involving transfer of more than one embryo, use of donor gametes, or embryos with pre-implantation genetic testing. Women who had been pregnant from a previous IVF treatment were also excluded from the analysis.
We compared the clinical outcomes between fresh embryo transfer and FET cycles. The main outcome was LBR. A ‘live birth’ is defined by the World Health Organization to be ‘the complete expulsion or extraction from its mother of a baby, irrespective of the duration of the pregnancy, which, after such separation, breathes or shows any other evidence of life’. The secondary outcome of this study was CPR. Clinical pregnancy is defined as the presence of fetal heart beat at first viability ultrasound (typically performed at gestational week 6 to 7 according to the MIVF protocol).
Stratification of cycles into fast- and slow-growing embryos was also performed. Slow cleavage-stage embryos were defined as those embryos that had 2PN during the syngamy check and were still at the 2-cell stage at the day 2 check. For the FET cycles, all embryos were at the 2-cells stage when frozen, and both cells survived the thawing process. Due to the post-thaw embryo culture protocol at MIVF for FET cycles, extra time allowed for development of frozen embryos, these embryos were half a day more advanced chronologically than their counterparts in fresh cycles. With the assumption that endometrial development is slightly advanced by COH, transferring frozen-thawed embryos that are half a day more advanced chronologically should improve embryo-endometrial developmental synchrony, leading to better CPR and LBR outcomes.
- 1. Ethical consideration
- 2. Statistical analysis
- Results
Multivariate analysis shows that women receiving a frozen-thawed embryo had a significantly lower LBR rate compared to those receiving a fresh embryo (OR 0.76, 95% CI 0.68-0.86, p < 0.00), after correcting for potential confounding factors - age, embryo quality (syngamy, cell number, embryo grade), fertilization method, cumulative ET and BMI (Table II). As expected, a higher pregnancy rate was observed in younger women (OR 0.91, 95% CI 0.90-0.93, p < 0.00) and those who had a better-quality embryo transferred (syngamy: OR 0.77, 95% CI 0.71-0.82, p < 0.00; cell number on day 2: OR 1.17, 95% CI 1.08-1.25, p < 0.00; embryo grade on day 2: OR 0.77, 95% CI 0.72-0.84, p < 0.00). Those who had embryo fertilized through ICSI had lower LBR compared to IVF (OR 0.89, 95% CI 0.80-1.0, p = 0.04). Previous cumulative embryo transfer number (OR 0.94, 95% CI 0.89-1.0, p = 0.06) and BMI of the women (OR 0.99, 95% CI 0.98-1.1, p = 0.2) did not have a statistically significant effect on LBR (Table II).
- 1. Sub-analysis using slow-growing embryos



4. Discussion
This cross-sectional analysis of outcomes from fresh vs. FETs is the first to only include single embryo transfer cycles; and with 7,014 fresh cycles and 3,730 FET cycles, it is also the largest study of this type to be published. The primary finding from our analysis was a significantly lower LBR and CPR in FET cycles compared to fresh cycles, using single autologous cleavage-stage embryos. Multivariate analysis identified six variables that impacted significantly on LBR (Table II). Controlling for these confounding factors confirmed the finding of lower LBR in FET cycles. These results do not support our hypothesis that LBR and CPR would be higher in FET compared with fresh embryo transfers due to a combination of improved endometrial receptivity and improved embryo-endometrial synchrony in the FET cycles. Rather, they suggest that any potential gains in LBR due to improved endometrial receptivity and improved embryo-endometrial synchrony are lost with FET, presumably due to embryo damage caused during the freeze-thaw process.
A secondary finding from this study was a sub-analysis of cycles with slow-growing embryos. We hypothesized that embryo-endometrial developmental synchrony would be improved in FET compared to fresh cycles using slow embryos due to the post-thaw embryo culture protocol. In our analysis of 1,154 cycles using slow embryos, there was no statistical difference in LBR or CPR between the two groups. We interpret this result as showing that the reduction in LBR due to freeze-thawing damage to the embryo seen in fast-growing embryos is fully compensated for by the improved synchrony in the slow-growing embryos. Slower-growing embryos typically result in lower PR in fresh cycles, potentially due to reduced embryo viability and increased embryo-endometrial asynchrony. Taken together, the results from this study confirm the positive influence of younger maternal age, better embryo quality, faster embryo development and improved embryo-endometrial developmental synchrony on LBR, while also demonstrating that embryo freezing has a negative impact on LBR.
There have been a limited number of published studies involving smaller sample sizes comparing clinical outcomes between fresh and FET cycles. While each of these provides some insight into the relative contributions of embryo viability, uterine receptivity, and embryo-endometrial synchrony to LBR, the individual studies are not all directly comparable and their findings not in complete agreement.
The study published by Shapiro and colleagues mentioned earlier in this paper, found increased CPR in the FET cycle cohorts. It is important to note that the best blastocysts of the cohort were transferred in fresh cycles, while only good-quality embryos (expanded supernumerary blastocysts) were selected for cryopreservation and subsequent use in FET cycles. Embryo selection was a potential confounder in this study, which was acknowledged by the authors in their article (20). Two subsequent prospective randomized studies by the same group comparing fresh blastocyst transfer and oocyte cryopreservation (with subsequent blastocyst transfer grown from the frozen-thawed oocytes) has shown a significantly greater CPR in the oocyte cryopreservation group in normal responders, but no statistical difference in high responders (24, 25). Overall, these studies support the hypothesis that FET improves CPR, presumably through improving uterine receptivity and/or embryo-endometrial synchrony. There is no evidence from these studies for a negative effect from freeze-thawing on embryo viability, possibly due to factors such as reduced blastomere size in the blastocyst compared to a cleavage-stage embryo. An alternative explanation is that by selecting embryos that have reached the expanded blastocyst stage, it is possible to eliminate all the nonviable embryos that appeared “normal” at the cleavage stage.
In two prospective cohort studies involving use of cleavage-stage embryos and large number of cycles, LBR was lower in FET cycles when compared to fresh cycles (26, 27). Four other randomized controlled trials using cleavage-stage embryos have also found no difference in LBR between fresh and FET cycles (28-31). The first trial involved 2,157 young (20-35 year old) women undergoing IVF/ICSI treatment due to tubal and/or male factors infertility (28). In the second trial, 782 infertile women were randomly assigned to fresh transfer or frozen embryos on day 3. In the FET group, only good-quality embryos (grade 1 or 2) were used (29). The last two studies involved women at risk of OHSS (30, 31). All studies involved the transfer of multiple embryos (28-31). Differences between the above studies and our study include patient population, number of embryos transferred, and embryo freezing method. Any or all of these factors could have contributed to the different clinical outcomes between these studies (no difference in LBR between fresh and FET cycles) and ours (higher LBR in fresh cycles).
While studies involving blastocyst transfer have suggested a better LBR with FET cycles (20, 24), our results and other studies using cleavage-stage embryos have not found the same (28-30). A major difference between protocols is that cleavage-stage embryos are cryopreserved according to their chronological age (day 2), while blastocysts are only cryopreserved if they reached the desired developmental stage of expanded blastocyst. The developmental stage of embryos is an important factor when considering embryo-endometrial synchrony. The cleavage-stage embryo freezing protocol has not been designed to correct for any potential asynchrony between developmental stage for the embryo and endometrium. There may be clinical benefit in the future to considering developmental stage rather than chronological age when thawing cleavage-stage embryos for transfer.
In conclusion, in this retrospective study of 10,744 IVF cycles, we were not able to support our hypothesis that LBR and CPR would be higher in FET compared with fresh embryo transfers. Multivariate analysis with correction for five significant variables showed a significantly higher LBR rate in cycles using fresh embryos compared to frozen-thawed embryos. In our sub-analysis of cycles involving slow-growing embryos, we interpreted the fact that there was no statistical difference in LBR between the fresh and FET groups as evidence for improved embryo-endometrial synchrony in the FET cycles. The large size of our dataset coupled with the strict inclusion and exclusion criteria have allowed us to identify six variables that significantly impact LBR, in addition to the results from our sub-analysis on slow-growing embryos which provides data to support the view that embryo-endometrial developmental synchrony is important. While most IVF units have moved to blastocysts transfer and freezing, the findings from this study on two-day old embryos remain highly relevant for understanding and optimizing LBR in today’s IVF clinic.
Acknowledgements
The authors would like to thank Professor Gordon Baker and Doctor Franca Agresta for their intellectual contribution. No financial support was received for this work.
Conflict of Interests
None declared.
Type of Study: Original Article |
Subject:
Fertility & Infertility
References
1. Mandelbaum J, Junca AM, Plachot M, Cohen J, Alvarez S, Cornet D, et al. The implantation window in humans after fresh or frozen-thawed embryo transfers. Adv Assist Reprod Technol 1990: 729-735. [DOI:10.1007/978-1-4613-0645-0_77]
2. Navot D, Scott RT, Droesch K, Veeck LL, Liu HC, Rosenwaks Z. The window of embryo transfer and the efficiency of human conception in vitro. Fertil Steril 1991; 55: 114-118. [DOI:10.1016/S0015-0282(16)54069-2]
3. Teh WT, McBain J, Rogers P. What is the contribution of embryo-endometrial asynchrony to implantation failure? J Assist Reprod Genet 2016; 33: 1419-1430. [DOI:10.1007/s10815-016-0773-6] [PMID] [PMCID]
4. Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med 1999; 340: 1796-1799. [DOI:10.1056/NEJM199906103402304] [PMID]
5. Dukic V, Hogan JW. A hierarchical Bayesian approach to modeling embryo implantation following in vitro fertilization. Biostatistics 2002; 3: 361-377. [DOI:10.1093/biostatistics/3.3.361] [PMID]
6. Rogers PA, Milne BJ, Trounson AO. A model to show human uterine receptivity and embryo viability following ovarian stimulation for in vitro fertilization. J In Vitro Fert Embryo Transf 1986; 3: 93-98. [DOI:10.1007/BF01139353] [PMID]
7. Bourgain C, Ubaldi F, Tavaniotou A, Smitz J, Van Steirteghem AC, Devroey P. Endometrial hormone receptors and proliferation index in the periovulatory phase of stimulated embryo transfer cycles in comparison with natural cycles and relation to clinical pregnancy outcome. Fertil Steril 2002; 78: 237-244. [DOI:10.1016/S0015-0282(02)03228-4]
8. Noci I, Borri P, Coccia ME, Criscuoli L, Scarselli G, Messeri G, et al. Hormonal patterns, steroid receptors and morphological pictures of endometrium in hyperstimulated IVF cycles. Eur J Obstet Gynecol Reprod Biol 1997; 75: 215-220. [DOI:10.1016/S0301-2115(97)00126-7]
9. Meyer WR, Novotny DB, Fritz MA, Beyler SA, Wolf LJ, Lessey BA. Effect of exogenous gonadotropins on endometrial maturation in oocyte donors. Fertil Steril 1999; 71: 109-114. [DOI:10.1016/S0015-0282(98)00390-2]
10. Seif MW, Pearson JM, Ibrahim ZH, Buckley CH, Aplin JD, Buck P, et al. Endometrium in in-vitro fertilization cycles: morphological and functional differentiation in the implantation phase. Hum Reprod 1992; 7: 6-11. [DOI:10.1093/oxfordjournals.humrep.a137559] [PMID]
11. Kolibianakis EM, Devroey P. The luteal phase after ovarian stimulation. Reprod BioMed Online 2002; 5 (Suppl.): 26-35. [DOI:10.1016/S1472-6483(11)60214-9]
12. Lass A, Peat D, Avery S, Brinsden P. Histological evaluation of endometrium on the day of oocyte retrieval after gonadotrophin-releasing hormone agonist-follicle stimulating hormone ovulation induction for in-vitro fertilization. Hum Reprod 1998; 13: 3203-3205. [DOI:10.1093/humrep/13.11.3203] [PMID]
13. Ubaldi F, Bourgain C, Tournaye H, Smitz J, Van Steirteghem A, Devroey P. Endometrial evaluation by aspiration biopsy on the day of oocyte retrieval in the embryo transfer cycles in patients with serum progesterone rise during the follicular phase. Fertil Steril 1997; 67: 521-526. [DOI:10.1016/S0015-0282(97)80080-5]
14. Van Vaerenbergh I, Van Lommel L, Ghislain V, In't Veld P, Schuit F, Fatemi HM, et al. In GnRH antagonist/rec-FSH stimulated cycles, advanced endometrial maturation on the day of oocyte retrieval correlates with altered gene expression. Hum Reprod 2009; 24: 1085-1091. [DOI:10.1093/humrep/den501] [PMID]
15. Chai J, Lee KF, Ng EH, Yeung WS, Ho PC. Ovarian stimulation modulates steroid receptor expression and spheroid attachment in peri-implantation endometria: studies on natural and stimulated cycles. Fertil Steril 2011; 96: 764-768. [DOI:10.1016/j.fertnstert.2011.06.015] [PMID]
16. Mirkin S, Nikas G, Hsiu JG, Diaz J, Oehninger S. Gene expression profiles and structural/functional features of the peri-implantation endometrium in natural and gonadotropin-stimulated cycles. J Clin Endocrinol Metab 2004; 89: 5742-5752. [DOI:10.1210/jc.2004-0605] [PMID]
17. Papanikolaou EG, Bourgain C, Kolibianakis E, Tournaye H, Devroey P. Steroid receptor expression in late follicular phase endometrium in GnRH antagonist IVF cycles is already altered, indicating initiation of early luteal phase transformation in the absence of secretory changes. Hum Reprod 2005; 20: 1541-1547. [DOI:10.1093/humrep/deh793] [PMID]
18. Evans J, Hannan NJ, Hincks C, Rombauts LJ, Salamonsen LA. Defective soil for a fertile seed? altered endometrial development is detrimental to pregnancy success. PLoS One 2012; 7: e53098. [DOI:10.1371/journal.pone.0053098] [PMID] [PMCID]
19. Kolibianakis E, Bourgain C, Albano C, Osmanagaoglu K, Smitz J, Van Steirteghem A, et al. Effect of ovarian stimulation with recombinant follicle-stimulating hormone, gonadotropin releasing hormone antagonists, and human chorionic gonadotropin on endometrial maturation on the day of oocyte pick-up. Fertil Steril 2002; 78: 1025-1029. [DOI:10.1016/S0015-0282(02)03323-X]
20. Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Ross R. Contrasting patterns in in vitro fertilization pregnancy rates among fresh autologous, fresh oocyte donor, and cryopreserved cycles with the use of day 5 or day 6 blastocysts may reflect differences in embryo-endometrium synchrony. Fertil Steril 2008; 89: 20-26. [DOI:10.1016/j.fertnstert.2006.08.092] [PMID]
21. Chen ZJ, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med 2016; 375: 523-533. [DOI:10.1056/NEJMoa1513873] [PMID]
22. Edgar DH, Karani J, Gook DA. Increasing dehydration of human cleavage-stage embryos prior to slow cooling significantly increases cryosurvival. Reprod Biomed Online 2009; 19: 521-525. [DOI:10.1016/j.rbmo.2009.06.002] [PMID]
23. Edgar DH, Bourne H, Speirs AL, McBain JC. A quantitative analysis of the impact of cryopreservation on the implantation potential of human early cleavage stage embryos. Hum Reprod 2000; 15: 175-179. [DOI:10.1093/humrep/15.1.175] [PMID]
24. Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril 2011; 96: 344-348.
https://doi.org/10.1016/j.fertnstert.2011.02.059 [DOI:10.1016/j.fertnstert.2011.05.050]
25. Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfers in high responders. Fertil Steril 2011; 96: 516-518.
https://doi.org/10.1016/j.fertnstert.2011.02.059 [DOI:10.1016/j.fertnstert.2011.05.050] [PMID]
26. Aflatoonian A, Karimzadeh Maybodi MA, Aflatoonian N, Tabibnejad N, Amir-Arjmand MH, Soleimani M, et al. Perinatal outcome in fresh versus frozen embryo transfer in ART cycles. Int J Reprod Biomed 2016; 14: 167-172. [DOI:10.29252/ijrm.14.3.167]
27. Aflatoonian A, Mansoori Moghaddam F, Mashayekhy M, Mohamadian F. Comparison of early pregnancy and neonatal outcomes after frozen and fresh embryo transfer in ART cycles. J Assist Reprod Genet 2010; 27: 695-700. [DOI:10.1007/s10815-010-9470-z] [PMID] [PMCID]
28. Shi Y, Sun Y, Hao C, Zhang H, Wei D, Zhang Y, et al. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med 2018; 378: 126-136. [DOI:10.1056/NEJMoa1705334] [PMID]
29. Vuong LN, Dang VQ, Ho TM, Huynh BG, Ha DT, Pham TD, et al. IVF transfer of fresh or frozen embryos in women without polycystic ovaries. N Engl J Med 2018; 378: 137-147. [DOI:10.1056/NEJMoa1703768] [PMID]
30. Ferraretti AP, Gianaroli L, Magli C, Fortini D, Selman HA, Feliciani E. Elective cryopreservation of all pronucleate embryos in women at risk of ovarian hyperstimulation syndrome: efficiency and safety. Hum Reprod 1999; 14: 1457-1460. [DOI:10.1093/humrep/14.6.1457] [PMID]
31. Aflatoonian A, Mansoori-Torshizi M, Farid Mojtahedi M, Aflatoonian B, Khalili MA, Amir-Arjmand MH, et al. Fresh versus frozen embryo transfer after gonadotropin-releasing hormone agonist trigger in gonadotropin-releasing hormone antagonist cycles among high responder women: A randomized, multi-center study. Int J Reprod Biomed 2018; 16: 9-18. [DOI:10.29252/ijrm.16.1.9]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








