Sat, Feb 21, 2026
[Archive]
Volume 19, Issue 1 (January 2021)
IJRM 2021, 19(1): 47-56 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ansari A S, Sevliya K, Badar: A, Lohiya N K. Reversible inhibition of sperm under guidance as an intratubular and reversible contraception in female rats: An experimental study. IJRM 2021; 19 (1) :47-56
URL: http://ijrm.ir/article-1-1434-en.html
URL: http://ijrm.ir/article-1-1434-en.html
1- Department of Zoology, Center for Advanced Studies, University of Rajasthan, Jaipur, Rajasthan, India.
2- Department of Zoology, Center for Advanced Studies, University of Rajasthan, Jaipur, Rajasthan, India. ,badrayesha@gmail.com
2- Department of Zoology, Center for Advanced Studies, University of Rajasthan, Jaipur, Rajasthan, India. ,
Full-Text [PDF 787 kb]
(1264 Downloads)
| Abstract (HTML) (2473 Views)
1. Introduction
Family planning issues are often a significant cause of concern for global population. Unmet need for family planning is crucial to securing the well-being and sovereignty of women. The unprecedented population growth has also been considered as the primary drivers of various ecological, societal threats and mass species extinction (1). Hence, the concept of contraception has a paramount impact in regulating overpopulation (2). Males are typically restricted to condoms and vasectomy; consequently, females are often burdened with the responsibility of population regulation (3). The approaches for females are usually achieved through barrier methods, hormonal, non-hormonal, chemicals such as quinacrine, intrauterine devices, tubectomy, and transcervical sterilization. In India, sterilization methods dominate over all other contraceptives in females. Adolescents have their reproductive careers ahead of them, they also require to postpone or space pregnancies, which requires reversible and non-invasive methods. Female sterilization or tubectomy is meant to be permanently associated with health risk without preventing sexually transmitted diseases. It is an irreversible method, which can be reversed by canalization, but this is not always successful. If pregnancy occurs after the procedure, there is an increased risk for an ectopic pregnancy (4). Thus, limitations are still associated with tubectomy, and therefore, further research on efficacy and reversibility is needed to achieve better contraceptive modalities. So, in the light of extensive use of tubectomy along with drawbacks, there is still a lapse in an ideally accepted contraceptive method in terms of efficacy, safety, and reversibility for females.
A polymer-based contraceptive, Reversible Inhibition of Sperm Under Guidance (RISUG®) is currently in advanced phase III clinical trials, which could be an effective male contraceptive with the potential of reversibility (5, 6). The contraceptive activity of RISUG® as a potential molecule along with safety, efficacy, non-toxicity, and reversibility has been successfully confirmed in animal models, viz., rats, rabbits, and langur monkeys (5-12). Non-invasive approaches have been applied successfully in monkeys for the vas occlusion reversal (7, 12). Reversal with DMSO and/or NaHCO3 has also been studied in rats and rabbits proving its feasibility and safety up to F1 generation with restoration of complete fertility (6, 9-12). RISUG® exerts contraceptive effects by occlusion at delivery site, lowers pH, charges disturbance in gamete and excessive generation of reactive oxygen species. Its in vitro ovicidal properties also suggest RISUG® as a potential female fertility-regulating agent (13, 14).
Thus, this experimental study has been designed with an objective to develop a concept of non-invasive, transcervical, single intervention, and reversible female contraceptive using RISUG® as a fallopian tube implant. Therefore, in the present investigation, the contraceptive efficacy of RISUG®-induced tubal occlusion and functional reversal with dimethyl sulphoxide (DMSO) and NaHCO3 was carried out following a short-term reversal in female albino rats.
2. Materials and Methods
2.1. Drug
RISUG®, a copolymer of styrene and maleic anhydride (1:1 ratio), was prepared through cobalt 60 gamma irradiation of styrene and maleic anhydride monomer in the presence of nitrogen in ethyl acetate at a dose of 0.2-0.24 mega rad for every 40 g of polymer at a dose rate of 30-40 rad/sec. After that, it was precipitated, dried, and stored in a vacuum desiccator, dissolved in DMSO solvent (15). The drug was provided by Prof. S. K. Guha, School of Medical Science and Technology, Indian Institute of Technology, New Delhi, India.
2.2. Animals
Adult Wistar albino female rats (Rattus norvegicus), weighing 150-155 gr, 3-4 months old were used for the study. The animals were hygienically maintained at the Departmental Experimental Animal facility, with well-regulated photoperiod (12 hr light: 12 hr dark), in individual polypropylene cages (size 43 × 27 × 15 cm). The temperature in animal house was adjusted at 23±2ºC, and the relative humidity ranged between 32 and 70%. Animals were fed twice a day with rat pellet diet (Ashirwad Industries Limited, Chandigarh, India) and safe drinking water ad libitum.
2.3. Experimental design
A total of 60 animals were included in this experimental study. Group I (Sham-operated control) contained 30 animals (Sub-groups Ia, Ib, and Ic (n= 10/each)), while groups II, III, and IV contained 10 animals each. Animals in the sub-groups served as parallel controls for groups II-IV to determine the fertility among the experimental groups. In group I, the animals were subjected to bilateral tubal occlusion, but no drug was injected and served as control; group II: the animals were subjected to tubal occlusion with 5-7 µL of RISUG®, bilaterally, and euthanized after 90 days; group III: the animals were subjected to tubal occlusion reversal with DMSO after 90 days of occlusion; group IV: the animals were subjected to tubal occlusion reversal with 5% NaHCO3 after 90 days of occlusion. Same animals were repeated at every mating schedule. The repeated mating ensured the fertility at given interval. Following the last mating schedule, animals were sacrificed.
2.4. Methodology
2.4.1. Tubal occlusion
Animals of groups II-IV were subjected to bilateral fallopian tube occlusion, after an overnight fasting, under sodium thiopentone anesthesia (20 mg/kg body weight, intraperitoneal; Thiosol Sodium: Neon Laboratories Ltd., Mumbai, India). Skin was disinfected and lateral incision was made to locate the uterus. Using the microsyringe fitted with blunt needle (26 G), a 5-7 µL of therapeutic standardized dose of RISUG® was injected into each fallopian tube to block the median to distal segment (antegrade). RISUG® polymerization was catalyzed by application of normal saline and solidified on contact with luminal fluid. The tube was placed in its original position and incision was closed by catgut suture in the inner and by silken suture in the outer skin. Postoperative care was acquired through antibiotic Ceftriaxone injection 0.025 mg/ml i.p. (Cefoat, A to Z Pharmaceuticals Ltd., Gujarat, India), anti-inflammatory Meloxicam 0.025 ml i.p. (Melonex, Intas Pharmaceuticals Ltd., Gujarat, India), and topical HealexPlus Spray (Shreya Life Sciences Pvt. Ltd., Mumbai, India). Until healing, the animals were supervised and provided proper bedding, feed, and water. The fallopian tubes of group I animals (sham-operated control) were exposed in a similar way, but no drug was injected.
2.4.2. Tubal occlusion reversal
The reversal was performed following 90 days of tubal occlusion. The tubes were exposed in a similar way as that of tubal occlusion and injected bilaterally with 250-500 µL of DMSO in group III and 500-700 µL of 5% NaHCO3 in group IV animals to liquefy RISUG® (11).The dissolution of RISUG® was confirmed by free flow of the DMSO and 5% NaHCO3 through the entire length of tube and DMSO and 5% NaHCO3 swill out due to the positive pressure emanating from the tube through the vagina via uterus. The tube was returned to original position and postoperative care was provided as described previously.
2.5. Parameters
2.5.1. Monitoring of estrous cycle
Estrous cycle was screened by taking vaginal swabs (16). Vaginal secretion was collected with the sterile glass dropper filled with 10-20μL of normal saline (0.9% NaCl) by inserting the tip smoothly into the vagina and gently flushing the saline in and drawing it back into the glass dropper. The vaginal fluid was uniformly spread on the marked slides and observed under the light microscope (Model: DM1000, Leica Wetziar, Germany).
2.5.2. Fertility test
Periodical fertility tests were conducted in sham operation, tubal occlusion, and reversal groups as per following schedule, viz., 30, 60, and 90 days of tubal occlusion and 45, 105, 150, and 195 days of tubal occlusion reversal by cohabitating the experimental females with the proven fertile male rats at 1:1 ratio. Mating success was confirmed by the appearance of vaginal plug and presence of spermatozoa in the vaginal smear examined under light microscope (Model: DM1000, Leica Wetziar, Germany). The number of females that delivered and the litter size was recorded for calculating fertility.
2.5.3. Histology of fallopian tube
Fallopian tube from all animals was removed surgically at scheduled sacrification and fixed overnight in 4% paraformaldehyde, dehydrated in ethanol, cleared in xylene, and embedded in paraffin wax. Next, 5-µm thin sections were cut, fixed, spreaded on Mayer’s albumin-coated slides and processed for hematoxylin and eosin staining for light microscopic observations (Model: DM1000, Leica Wetziar, Germany).
2.6. Ethical considerations
The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) under certificate no. UDZ/IAEC/I/02, dated 03.07.2015, Department of Zoology, University of Rajasthan, Jaipur, India. Animals were maintained under perfect veterinary supervision, and the guidelines of “Committee for the Purpose of Control and Supervision of Experiments on Animals” (17) were strictly followed.
2.7. Statistical analysis
Data are represented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) test followed by post-hoc (Duncan’s new multiple range test) procedure was applied for statistical comparisons, wherever applicable. Data were analyzed using the Holm-Sidak multiple comparison test to detect the inter-group difference using the statistical software SPSS, version 10.0 (SPSS Inc., Chicago, IL, USA). P <0.05 was considered as significant.
3. Results
3.1. Characteristics of estrous cycle
The estrous cycle phases, that is, proestrus, estrus, metestrus, and diestrus were found to be comparable to the control. Thus, the estrous cycle length was normal, 4-5 days long in the animals of groups II-IV when compared with group I animals (Figure 1).
3.2. Fertility test
The presence of spermatozoa in the vaginal smear along with vaginal plug confirmed successful mating in all groups. Animals of group I showed 100% fertility during all mating schedules of pre-injection, tubal occlusion, and reversal periods. Animals of group II- IV indicated positive mating, but 0% fertility was evident following 30, 60, and 90 days of tubal occlusion. In groups III and IV, animals exhibited 0% fertility following tubal occlusion and gradual increase in fertility following reversal was observed. However, group III animals exhibited gradual increase in fertility (50% at 45 days, 80% at 105 days, 100% at 150 days, and 100% at 195 days) following tubal occlusion reversal with DMSO. Group IV animals indicated a gradual enhancement in fertility (70% at 45 days, 70% at 105 days, 100% at 150 days, and 100% at 195 days) following tubal occlusion reversal with NaHCO3 during all mating schedules, respectively. No remarkable changes were observed in the mean litter size in all experimental groups as compared with group I (Table I).
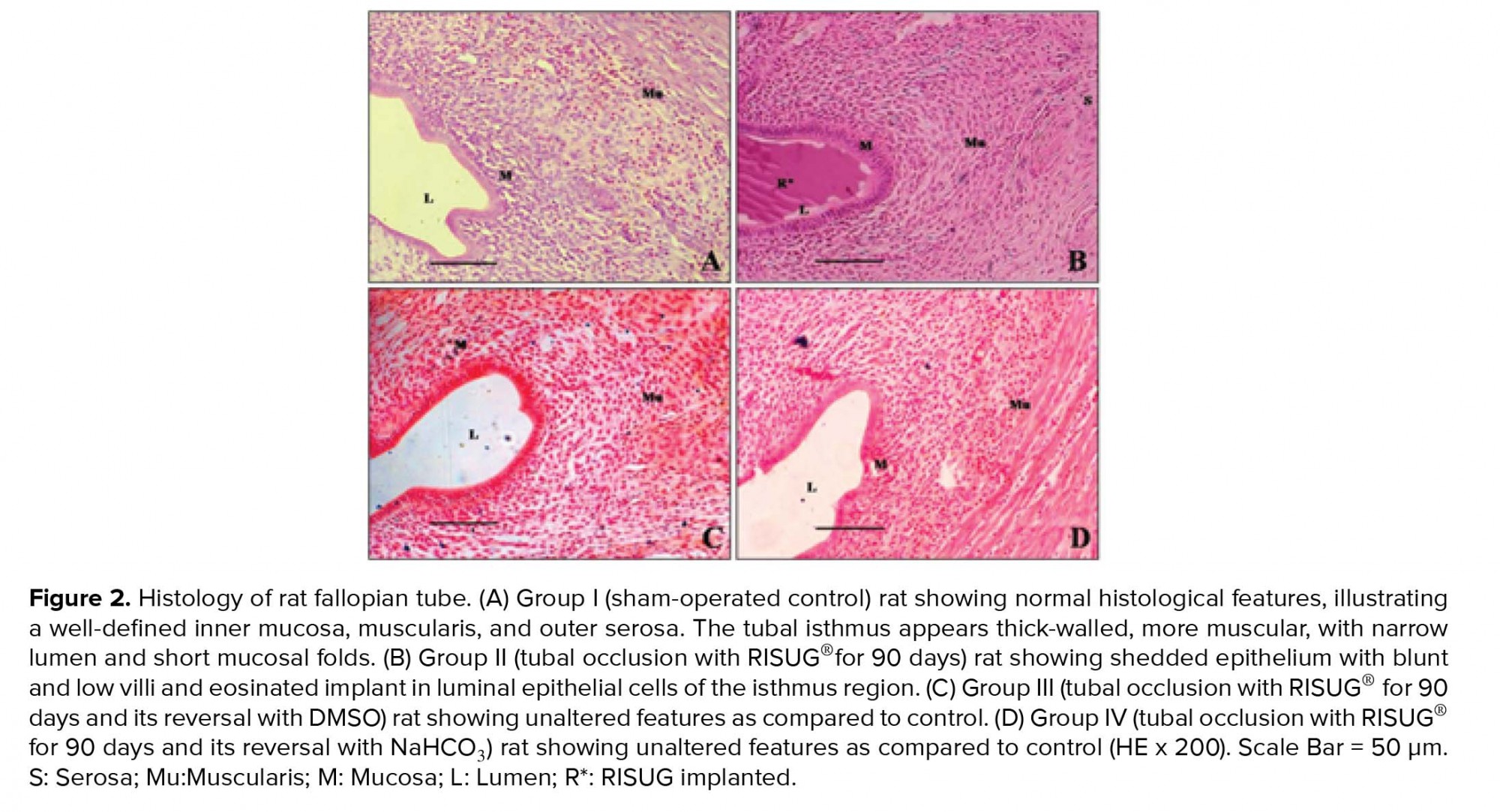
3.3. Histology of fallopian tube
The fallopian tubes of group I animals revealed normal histological features, illustrating a definite inner mucosa, circular and longitudinal muscularis, and outer serosa. The tubal isthmus was noticed with thick wall, more muscular with narrow lumen and short mucosal folds (Figure 2A). The fallopian tubes of group II animals revealed distorted cellular configuration along with disordered epithelial cells at certain places in the implanted region; nonetheless, normal histological architecture of mucosa, muscularis, and serosa were observed. In the implanted isthmus, mucosal folds were irregular with disturbed structural pattern. The accumulation of shedded and detached epithelial tissues from mucosal folds and the isthmic lumen filled with eosinated RISUG® implant were observed (Figure 2B). The fallopian tubes of reversal groups (III and IV) animals revealed complete regeneration of the epithelial linings when compared with the controls (Figures 2C and 2D).


4. Discussion
The results of the present investigation demonstrate that the RISUG®-induced complete sterility following 90 days of tubal occlusion and reversibility with both DMSO and NaHCO3 without any changes in estrous cyclicity. Histology of fallopian tube revealed distortion of epithelial cell configuration in the implanted site in tubal occluded animals and complete regeneration of the epithelial linings following reversal. The alarming population growth demands better contraceptive modalities to expand the contraceptive selections for both sexes. Tubectomy is an extensively exercised surgical contraceptive, yet post-tubectomy pregnancies and abnormalities have been reported (18). The sale and use of Essure®, the first US Food and Drug Administration (FDA)-approved standard transcervical procedure was restricted due to Essure problems (19). The second FDA-approved Adiana® has also been associated with menstruation cramping, intractable pain, tubal perforations, and ectopic pregnancies (4, 20-22). Quinacrine sterilization is less acceptable as it was proved as a mutagen (23, 24). Recently discovered polidocanol foam (PF) also resulted in nonsurgical female contraception through tubal occlusion via multiple treatments in baboons (25) and rhesus (26). An ex vivo macaque fallopian tube has also been employed for assessing the PF outcomes (27). Conversely, further research has been warranted to induce permanent tubal occlusion with a single transcervical intervention and successful reversibility. These reports exceedingly warrant substitute contraceptives with minimal invasive approach.
In the present study, the RISUG® has been investigated as a single intervention approach with a possibility of reversal. The drug was injected through micro-syringe via distal section (infundibulum with fimbriae) occluding the tubes and consequently hindering capacitation. The fimbriae have crucial role in sweeping the oocyte into the fallopian tubes for fertilization. If they no longer function then the oocyte never makes it to its destination for fertilization (28). RISUG® implants in fallopian tubes appear to block the tubes on solidification, alter the pH and osmolarity, thus, generating unfavorable conditions for fertilization. As reproductive tract, pH is the chief determinant in the sperm-ova interaction; an elevated pH can adversely affect cervical mucus and sperm cell motility leading to infertility (29). The implants may adversely affect the ova morphology on interaction, making it non-viable by generating reactive oxygen species. In order to achieve reversal, the RISUG® blockage was flushed out favorably through DMSO and NaHCO3, normalizing the fallopian tubes as well as oocyte movement, subsequently resuming fertility with normal F1 progeny. Simultaneously, various parameters proved safety, efficacy, and reversibility of the procedure (9, 10, 13). Further investigations in a suitable non-human primate model will open avenues for clinical trials to adjudge suitability of RISUG® as a non-invasive female contraceptive.
5. Conclusion
RISUG® proved to be suitable for single intervention, intratubular, reversible contraception in female rats.
Acknowledgements
This work has been financially supported by the Indian Council of Medical Research (ICMR), New Delhi. The Department of Science and Technology (DST), Ministry of Science and Technology, Govt. of India, New Delhi is acknowledged for INSPIRE Fellowship to Kiran Sevliya. N. K. Lohiya is thankful to the National Academy of Sciences, India, for NASI Senior Scientist’s assignment. The infrastructural facilities provided by the Head of the Department are gratefully acknowledged. The authors are also thankful to Prof. Sujoy K. Guha, School of Medical Science and Technology, Indian Institute of Technology, Kharagpur, India, for providing ‘RISUG®’, and for his keen interest and periodical guidance in the study.
Conflict of Interest
The authors declare that there is no conflict of interest.
Full-Text: (645 Views)
1. Introduction
Family planning issues are often a significant cause of concern for global population. Unmet need for family planning is crucial to securing the well-being and sovereignty of women. The unprecedented population growth has also been considered as the primary drivers of various ecological, societal threats and mass species extinction (1). Hence, the concept of contraception has a paramount impact in regulating overpopulation (2). Males are typically restricted to condoms and vasectomy; consequently, females are often burdened with the responsibility of population regulation (3). The approaches for females are usually achieved through barrier methods, hormonal, non-hormonal, chemicals such as quinacrine, intrauterine devices, tubectomy, and transcervical sterilization. In India, sterilization methods dominate over all other contraceptives in females. Adolescents have their reproductive careers ahead of them, they also require to postpone or space pregnancies, which requires reversible and non-invasive methods. Female sterilization or tubectomy is meant to be permanently associated with health risk without preventing sexually transmitted diseases. It is an irreversible method, which can be reversed by canalization, but this is not always successful. If pregnancy occurs after the procedure, there is an increased risk for an ectopic pregnancy (4). Thus, limitations are still associated with tubectomy, and therefore, further research on efficacy and reversibility is needed to achieve better contraceptive modalities. So, in the light of extensive use of tubectomy along with drawbacks, there is still a lapse in an ideally accepted contraceptive method in terms of efficacy, safety, and reversibility for females.
A polymer-based contraceptive, Reversible Inhibition of Sperm Under Guidance (RISUG®) is currently in advanced phase III clinical trials, which could be an effective male contraceptive with the potential of reversibility (5, 6). The contraceptive activity of RISUG® as a potential molecule along with safety, efficacy, non-toxicity, and reversibility has been successfully confirmed in animal models, viz., rats, rabbits, and langur monkeys (5-12). Non-invasive approaches have been applied successfully in monkeys for the vas occlusion reversal (7, 12). Reversal with DMSO and/or NaHCO3 has also been studied in rats and rabbits proving its feasibility and safety up to F1 generation with restoration of complete fertility (6, 9-12). RISUG® exerts contraceptive effects by occlusion at delivery site, lowers pH, charges disturbance in gamete and excessive generation of reactive oxygen species. Its in vitro ovicidal properties also suggest RISUG® as a potential female fertility-regulating agent (13, 14).
Thus, this experimental study has been designed with an objective to develop a concept of non-invasive, transcervical, single intervention, and reversible female contraceptive using RISUG® as a fallopian tube implant. Therefore, in the present investigation, the contraceptive efficacy of RISUG®-induced tubal occlusion and functional reversal with dimethyl sulphoxide (DMSO) and NaHCO3 was carried out following a short-term reversal in female albino rats.
2. Materials and Methods
2.1. Drug
RISUG®, a copolymer of styrene and maleic anhydride (1:1 ratio), was prepared through cobalt 60 gamma irradiation of styrene and maleic anhydride monomer in the presence of nitrogen in ethyl acetate at a dose of 0.2-0.24 mega rad for every 40 g of polymer at a dose rate of 30-40 rad/sec. After that, it was precipitated, dried, and stored in a vacuum desiccator, dissolved in DMSO solvent (15). The drug was provided by Prof. S. K. Guha, School of Medical Science and Technology, Indian Institute of Technology, New Delhi, India.
2.2. Animals
Adult Wistar albino female rats (Rattus norvegicus), weighing 150-155 gr, 3-4 months old were used for the study. The animals were hygienically maintained at the Departmental Experimental Animal facility, with well-regulated photoperiod (12 hr light: 12 hr dark), in individual polypropylene cages (size 43 × 27 × 15 cm). The temperature in animal house was adjusted at 23±2ºC, and the relative humidity ranged between 32 and 70%. Animals were fed twice a day with rat pellet diet (Ashirwad Industries Limited, Chandigarh, India) and safe drinking water ad libitum.
2.3. Experimental design
A total of 60 animals were included in this experimental study. Group I (Sham-operated control) contained 30 animals (Sub-groups Ia, Ib, and Ic (n= 10/each)), while groups II, III, and IV contained 10 animals each. Animals in the sub-groups served as parallel controls for groups II-IV to determine the fertility among the experimental groups. In group I, the animals were subjected to bilateral tubal occlusion, but no drug was injected and served as control; group II: the animals were subjected to tubal occlusion with 5-7 µL of RISUG®, bilaterally, and euthanized after 90 days; group III: the animals were subjected to tubal occlusion reversal with DMSO after 90 days of occlusion; group IV: the animals were subjected to tubal occlusion reversal with 5% NaHCO3 after 90 days of occlusion. Same animals were repeated at every mating schedule. The repeated mating ensured the fertility at given interval. Following the last mating schedule, animals were sacrificed.
2.4. Methodology
2.4.1. Tubal occlusion
Animals of groups II-IV were subjected to bilateral fallopian tube occlusion, after an overnight fasting, under sodium thiopentone anesthesia (20 mg/kg body weight, intraperitoneal; Thiosol Sodium: Neon Laboratories Ltd., Mumbai, India). Skin was disinfected and lateral incision was made to locate the uterus. Using the microsyringe fitted with blunt needle (26 G), a 5-7 µL of therapeutic standardized dose of RISUG® was injected into each fallopian tube to block the median to distal segment (antegrade). RISUG® polymerization was catalyzed by application of normal saline and solidified on contact with luminal fluid. The tube was placed in its original position and incision was closed by catgut suture in the inner and by silken suture in the outer skin. Postoperative care was acquired through antibiotic Ceftriaxone injection 0.025 mg/ml i.p. (Cefoat, A to Z Pharmaceuticals Ltd., Gujarat, India), anti-inflammatory Meloxicam 0.025 ml i.p. (Melonex, Intas Pharmaceuticals Ltd., Gujarat, India), and topical HealexPlus Spray (Shreya Life Sciences Pvt. Ltd., Mumbai, India). Until healing, the animals were supervised and provided proper bedding, feed, and water. The fallopian tubes of group I animals (sham-operated control) were exposed in a similar way, but no drug was injected.
2.4.2. Tubal occlusion reversal
The reversal was performed following 90 days of tubal occlusion. The tubes were exposed in a similar way as that of tubal occlusion and injected bilaterally with 250-500 µL of DMSO in group III and 500-700 µL of 5% NaHCO3 in group IV animals to liquefy RISUG® (11).The dissolution of RISUG® was confirmed by free flow of the DMSO and 5% NaHCO3 through the entire length of tube and DMSO and 5% NaHCO3 swill out due to the positive pressure emanating from the tube through the vagina via uterus. The tube was returned to original position and postoperative care was provided as described previously.
2.5. Parameters
2.5.1. Monitoring of estrous cycle
Estrous cycle was screened by taking vaginal swabs (16). Vaginal secretion was collected with the sterile glass dropper filled with 10-20μL of normal saline (0.9% NaCl) by inserting the tip smoothly into the vagina and gently flushing the saline in and drawing it back into the glass dropper. The vaginal fluid was uniformly spread on the marked slides and observed under the light microscope (Model: DM1000, Leica Wetziar, Germany).
2.5.2. Fertility test
Periodical fertility tests were conducted in sham operation, tubal occlusion, and reversal groups as per following schedule, viz., 30, 60, and 90 days of tubal occlusion and 45, 105, 150, and 195 days of tubal occlusion reversal by cohabitating the experimental females with the proven fertile male rats at 1:1 ratio. Mating success was confirmed by the appearance of vaginal plug and presence of spermatozoa in the vaginal smear examined under light microscope (Model: DM1000, Leica Wetziar, Germany). The number of females that delivered and the litter size was recorded for calculating fertility.
2.5.3. Histology of fallopian tube
Fallopian tube from all animals was removed surgically at scheduled sacrification and fixed overnight in 4% paraformaldehyde, dehydrated in ethanol, cleared in xylene, and embedded in paraffin wax. Next, 5-µm thin sections were cut, fixed, spreaded on Mayer’s albumin-coated slides and processed for hematoxylin and eosin staining for light microscopic observations (Model: DM1000, Leica Wetziar, Germany).
2.6. Ethical considerations
The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) under certificate no. UDZ/IAEC/I/02, dated 03.07.2015, Department of Zoology, University of Rajasthan, Jaipur, India. Animals were maintained under perfect veterinary supervision, and the guidelines of “Committee for the Purpose of Control and Supervision of Experiments on Animals” (17) were strictly followed.
2.7. Statistical analysis
Data are represented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) test followed by post-hoc (Duncan’s new multiple range test) procedure was applied for statistical comparisons, wherever applicable. Data were analyzed using the Holm-Sidak multiple comparison test to detect the inter-group difference using the statistical software SPSS, version 10.0 (SPSS Inc., Chicago, IL, USA). P <0.05 was considered as significant.
3. Results
3.1. Characteristics of estrous cycle
The estrous cycle phases, that is, proestrus, estrus, metestrus, and diestrus were found to be comparable to the control. Thus, the estrous cycle length was normal, 4-5 days long in the animals of groups II-IV when compared with group I animals (Figure 1).
3.2. Fertility test
The presence of spermatozoa in the vaginal smear along with vaginal plug confirmed successful mating in all groups. Animals of group I showed 100% fertility during all mating schedules of pre-injection, tubal occlusion, and reversal periods. Animals of group II- IV indicated positive mating, but 0% fertility was evident following 30, 60, and 90 days of tubal occlusion. In groups III and IV, animals exhibited 0% fertility following tubal occlusion and gradual increase in fertility following reversal was observed. However, group III animals exhibited gradual increase in fertility (50% at 45 days, 80% at 105 days, 100% at 150 days, and 100% at 195 days) following tubal occlusion reversal with DMSO. Group IV animals indicated a gradual enhancement in fertility (70% at 45 days, 70% at 105 days, 100% at 150 days, and 100% at 195 days) following tubal occlusion reversal with NaHCO3 during all mating schedules, respectively. No remarkable changes were observed in the mean litter size in all experimental groups as compared with group I (Table I).
3.3. Histology of fallopian tube
The fallopian tubes of group I animals revealed normal histological features, illustrating a definite inner mucosa, circular and longitudinal muscularis, and outer serosa. The tubal isthmus was noticed with thick wall, more muscular with narrow lumen and short mucosal folds (Figure 2A). The fallopian tubes of group II animals revealed distorted cellular configuration along with disordered epithelial cells at certain places in the implanted region; nonetheless, normal histological architecture of mucosa, muscularis, and serosa were observed. In the implanted isthmus, mucosal folds were irregular with disturbed structural pattern. The accumulation of shedded and detached epithelial tissues from mucosal folds and the isthmic lumen filled with eosinated RISUG® implant were observed (Figure 2B). The fallopian tubes of reversal groups (III and IV) animals revealed complete regeneration of the epithelial linings when compared with the controls (Figures 2C and 2D).



4. Discussion
The results of the present investigation demonstrate that the RISUG®-induced complete sterility following 90 days of tubal occlusion and reversibility with both DMSO and NaHCO3 without any changes in estrous cyclicity. Histology of fallopian tube revealed distortion of epithelial cell configuration in the implanted site in tubal occluded animals and complete regeneration of the epithelial linings following reversal. The alarming population growth demands better contraceptive modalities to expand the contraceptive selections for both sexes. Tubectomy is an extensively exercised surgical contraceptive, yet post-tubectomy pregnancies and abnormalities have been reported (18). The sale and use of Essure®, the first US Food and Drug Administration (FDA)-approved standard transcervical procedure was restricted due to Essure problems (19). The second FDA-approved Adiana® has also been associated with menstruation cramping, intractable pain, tubal perforations, and ectopic pregnancies (4, 20-22). Quinacrine sterilization is less acceptable as it was proved as a mutagen (23, 24). Recently discovered polidocanol foam (PF) also resulted in nonsurgical female contraception through tubal occlusion via multiple treatments in baboons (25) and rhesus (26). An ex vivo macaque fallopian tube has also been employed for assessing the PF outcomes (27). Conversely, further research has been warranted to induce permanent tubal occlusion with a single transcervical intervention and successful reversibility. These reports exceedingly warrant substitute contraceptives with minimal invasive approach.
In the present study, the RISUG® has been investigated as a single intervention approach with a possibility of reversal. The drug was injected through micro-syringe via distal section (infundibulum with fimbriae) occluding the tubes and consequently hindering capacitation. The fimbriae have crucial role in sweeping the oocyte into the fallopian tubes for fertilization. If they no longer function then the oocyte never makes it to its destination for fertilization (28). RISUG® implants in fallopian tubes appear to block the tubes on solidification, alter the pH and osmolarity, thus, generating unfavorable conditions for fertilization. As reproductive tract, pH is the chief determinant in the sperm-ova interaction; an elevated pH can adversely affect cervical mucus and sperm cell motility leading to infertility (29). The implants may adversely affect the ova morphology on interaction, making it non-viable by generating reactive oxygen species. In order to achieve reversal, the RISUG® blockage was flushed out favorably through DMSO and NaHCO3, normalizing the fallopian tubes as well as oocyte movement, subsequently resuming fertility with normal F1 progeny. Simultaneously, various parameters proved safety, efficacy, and reversibility of the procedure (9, 10, 13). Further investigations in a suitable non-human primate model will open avenues for clinical trials to adjudge suitability of RISUG® as a non-invasive female contraceptive.
5. Conclusion
RISUG® proved to be suitable for single intervention, intratubular, reversible contraception in female rats.
Acknowledgements
This work has been financially supported by the Indian Council of Medical Research (ICMR), New Delhi. The Department of Science and Technology (DST), Ministry of Science and Technology, Govt. of India, New Delhi is acknowledged for INSPIRE Fellowship to Kiran Sevliya. N. K. Lohiya is thankful to the National Academy of Sciences, India, for NASI Senior Scientist’s assignment. The infrastructural facilities provided by the Head of the Department are gratefully acknowledged. The authors are also thankful to Prof. Sujoy K. Guha, School of Medical Science and Technology, Indian Institute of Technology, Kharagpur, India, for providing ‘RISUG®’, and for his keen interest and periodical guidance in the study.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Biology
References
1. Ceballos G, Ehrlich PR, Dirzo R. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc Natl Acad Sci USA 2017; 114: E6089-E6096. [DOI:10.1073/pnas.1704949114] [PMID] [PMCID]
2. Bavel JV. The world population explosion: causes, backgrounds and projections for the future. Facts Views Vis Obgyn 2013; 5: 281-291.
3. Jain R, Muralidhar S. Contraceptive methods: Needs, options and utilization. J Obstet Gynecol India 2011; 61: 626-634. [DOI:10.1007/s13224-011-0107-7] [PMID] [PMCID]
4. Jokinen E, Heino A, Karipohja T, Gissler M, Hurskainen R. Safety and effectiveness of female tubal sterilisation by hysteroscopy, laparoscopy or laparotomy: a register based study. An Int J of Obstet Gynaecol 2017; 124: 1851-1857. [DOI:10.1111/1471-0528.14719] [PMID]
5. Sharma RS, Rajanna A, Singh BK, Mathur AK, Mukherjee AK. Current Status of development of RISUG: An intravasal injectable male contraceptive. In: Sharma RS, Rajanna A, Rajalakshmi M (Eds.). Recent advances and challenges in Reproductive Health Research. New Delhi: Indian Council of Medical Research; 2008. pp: 135-149.
6. Lohiya NK, Manivannan B, Mishra PK, Pathak N, Balasubramanian SPA. Intravasal contraception with styrene maleic anhydride and its non-invasive reversal in langur monkeys (Presbytis entellus entellus). Contraception 1998; 58: 119-128. [DOI:10.1016/S0010-7824(98)00073-0]
7. Lohiya NK, Suthar R, Khandelwal A, Goyal S, Ansari AS, Manivannan B. Sperm characteristics and teratology in rats following vas occlusion with RISUG and its reversal. Int J Androl 2010; 33: e198-e206. [DOI:10.1111/j.1365-2605.2009.00992.x] [PMID]
8. Lohiya NK, Alam I, Hussain M, Khan SR, Ansari AS. RISUG: An intravasal injectable male contraceptive. Indian J Med Res 2014; 140 (Suppl.): 63-72.
9. Ansari AS, Alam I, Hussain M, Khan SR, Lohiya NK. Evaluation of genotoxicity in leukocytes and testis following intra-vasal contraception with RISUG and its reversal by DMSO and NaHCO3 in Wistar albino rats. Reprod Toxicol 2013; 36: 53-59. [DOI:10.1016/j.reprotox.2012.11.005] [PMID]
10. Ansari AS, Hussain M, Khan SR, Lohiya NK. Relative suitability of DMSO and NaHCO3 for reversal of RISUG® induced long-term contraception. Andrology 2016; 4: 306-313. [DOI:10.1111/andr.12155] [PMID]
11. Ansari AS, Badar A, Balasubramanian K, Lohiya NK. Contraception with RISUG® and functional reversal through DMSO and NaHCO3 in male rabbits. Asian J Androl 2017; 19: 389-395. [DOI:10.4103/1008-682X.185000] [PMID] [PMCID]
12. Lohiya NK, Manivannan B, Mishra PK, Sriram S, Bhande SS, Panneerdoss S. Preclinical evaluation for non-invasive reversal following long-term vas occlusion with styrene maleic anhydride in langur monkeys. Contraception 2005; 71: 214-226. [DOI:10.1016/j.contraception.2004.08.016] [PMID]
13. Chaube SK, Misro MM. Effect of styrene maleic anhydride (SMA) on viability and integrity of isolated oocytes in vitro. Contraception 2002; 66: 469-472. [DOI:10.1016/S0010-7824(02)00419-5]
14. Jha RK, Jha PK, Rana SV, Guha SK. Spermicidal action of styrene maleic anhydride polyelectrolyte in combination with magnetic and electrically conductive particle. Int J Pharmacol 2009; 5: 1-12. [DOI:10.3923/ijp.2009.1.12]
15. Guha SK. Contraceptive for use by a male. US: Patent; 54880705; 1996.
16. Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Research 2007; 80: 84-97. [DOI:10.1002/bdrb.20106] [PMID]
17. CPCSEA guidelines. Guidelines on the regulation of scientific experiments on animals. Available at: http://cpcsea.nic.in/WriteReadData/userfiles/file/SOP_CPCSEA_inner_page.pdf.
18. Lin ChM, Ku YL, Cheng YT, Giin NY, Ou YCh, Lee MCh, et al. An uncommon spontaneous right distal tubal pregnancy post bilateral laparoscopic sterilization. A case report. Medicine 2019; 98: e14193-e14195. [DOI:10.1097/MD.0000000000014193] [PMID] [PMCID]
19. FDA medical devices. FDA activities: Essure. page last updated 04/09/2018. Available at: https://www.fda.gov/news-events/press-announcements/fda-restricts-sale-and-distribution-essure-protect-women-and-require-patients-receive-risk.
20. Hologic. Instructions for use and radiofrequency (RF) generator operator are manual. Available at: www.adiana.com/pdf/info/adiana-patient-information-booklet.pdf.
21. Frietze G, Leyser-Whalen O, Rahman M, Rouhani M, Berenson AB. A meta-analysis of bilateral essure® procedural placement success rates on first attempt. J Gynecol Surg 2015; 31: 308-317. [DOI:10.1089/gyn.2015.0054] [PMID] [PMCID]
22. Mao J, Pfeifer S, Schlegel P, Sedrakyan A. Safety and efficacy of hysteroscopic sterilization compared with laparoscopic sterilization: an observational cohort study. Br Med J 2015; 31: 351. [DOI:10.1136/bmj.h5162] [PMID] [PMCID]
23. Clarke JJ, Sokal DC, Cancel AM, Campen DB, Gudi R, Wagner VO, et al. Re-evaluation of the mutagenic potential of quinacrine dihydrochloride dihydrate. Mutat Res 2001; 494: 41-53. [DOI:10.1016/S1383-5718(01)00178-4]
24. Lippes J. Quinacrine sterilization (QS): Time for reconsideration. Contraception 2015; 92: 91-95. [DOI:10.1016/j.contraception.2015.06.005] [PMID]
25. Jensen JT, Hanna C, Yao Sh, Micks E, Edelman A, Holden L, et al. Blockade of tubal patency following transcervical administration of polidocanol foam: initial studies in rhesus macaques. Contraception 2014; 89: 540-549. [DOI:10.1016/j.contraception.2013.11.017] [PMID] [PMCID]
26. Jensen JT, Hanna C, Yao Sh, Bauer C, Morgan TK, Slayden OD. Characterization of tubal occlusion after transcervical polidocanol foam (PF) infusion in baboons. Contraception 2015; 92: 96-102. [DOI:10.1016/j.contraception.2015.06.002] [PMID] [PMCID]
27. Yao S, Hanna C, Slayden OD, Jensen J. An ex vivo model for assessing acute effects of transcervical polidocanol foam in the macaque fallopian tube. Fertil Steril 2016; 106: e113. [DOI:10.1016/j.fertnstert.2016.07.338]
28. Briceag I, Costache A, Purcarea VL, Cergan R, Dumitru M, Briceag I, et al. Fallopian tubes: Literature review of anatomy and etiology in female infertility. J Med Life 2015; 8: 129-131.
29. Eggert-Kruse W, Kohler A, Rohr G, Runnebaum B. The pH as an important determinant of sperm mucus interaction. Fertil Steril 1993; 59: 617-628 [DOI:10.1016/S0015-0282(16)55810-5]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








