Tue, Feb 3, 2026
[Archive]
Volume 18, Issue 6 (June 2020)
IJRM 2020, 18(6): 395-406 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hadizadeh-Talasaz Z, Taghipour A, Mosavi-Vahed S H, Latifnejad Roudsari R. Predictive value of pregnancy-associated plasma protein-A in relation to fetal loss: A systematic review and meta-analysis. IJRM 2020; 18 (6) :395-406
URL: http://ijrm.ir/article-1-1461-en.html
URL: http://ijrm.ir/article-1-1461-en.html
Zahra Hadizadeh-Talasaz1 

 , Ali Taghipour *2
, Ali Taghipour *2 

 , Seyede Hoora Mosavi-Vahed3
, Seyede Hoora Mosavi-Vahed3 

 , Robab Latifnejad Roudsari4
, Robab Latifnejad Roudsari4 




 , Ali Taghipour *2
, Ali Taghipour *2 

 , Seyede Hoora Mosavi-Vahed3
, Seyede Hoora Mosavi-Vahed3 

 , Robab Latifnejad Roudsari4
, Robab Latifnejad Roudsari4 


1- Student Research Committee, Mashhad University of Medical Sciences, Mashhad, Iran.
2- Social Determinants of Health Research Center, Mashhad University of Medical Sciences, Mashhad, Iran. Department of Epidemiology, School of Health, Mashhad University of Medical Sciences, Mashhad, Iran. ,Taghipoura@mums.ac.ir
3- Department of Obstetrics and Gynecology, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
4- Nursing and Midwifery Care Research Centre, Mashhad University of Medical Sciences, Mashhad, Iran. Department of Midwifery, School of Nursing and Midwifery, Mashhad University of Medical Sciences, Mashhad, Iran.
2- Social Determinants of Health Research Center, Mashhad University of Medical Sciences, Mashhad, Iran. Department of Epidemiology, School of Health, Mashhad University of Medical Sciences, Mashhad, Iran. ,
3- Department of Obstetrics and Gynecology, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
4- Nursing and Midwifery Care Research Centre, Mashhad University of Medical Sciences, Mashhad, Iran. Department of Midwifery, School of Nursing and Midwifery, Mashhad University of Medical Sciences, Mashhad, Iran.
Full-Text [PDF 835 kb]
(1770 Downloads)
| Abstract (HTML) (2989 Views)
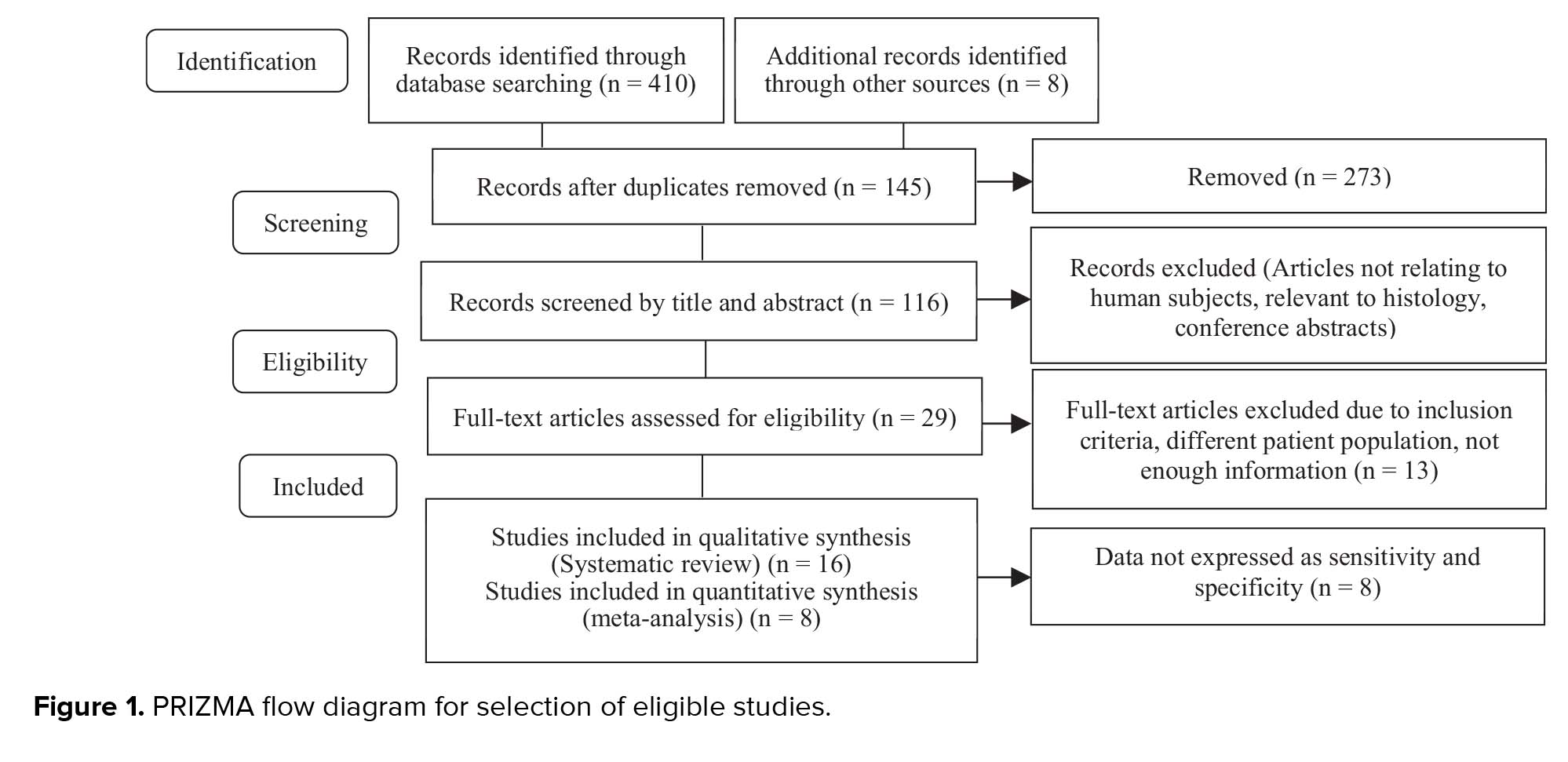
First-trimester screening is done for all pregnant women at 11-13 + 6 wk, and it is a non-invasive evaluation to determine the risk of chromosomal abnormalities, such as Down syndrome (10). Since this marker is routinely measured in all women, and it has a relation with pregnancy outcomes, this cost-effective method can be used for determining the prognosis of threatened abortion (1). Identifying patients at risk lead to increased monitoring of pregnant women who are at high risk for pregnancy complications (11). Many studies consider this marker as an important marker for abortion (12, 13). Several studies have examined the association between PAPP-A and fetal loss; however, they reported different sensitivity, specificity, and critical points and there is no same agreement among researchers; therefore, the present study discusses the results of various studies (1, 2, 8). Also, one limitation of many studies was the small sample size, which is controlled through systematic reviews. Systematic review and meta-analysis are essential tools for summarizing the evidence in an accurate, correct, and reliable way. So, researchers felt a need to conduct a systematic review to get a clear and uniform result and a comprehensive guide to clinical use.
This systematic review and meta-analysis aimed to determine the predictive value of PAPP-A for fetal loss.
Newcastle-Ottawa tool (NOS) was used to assess the quality for cohort and case-control studies. This tool consists of three main parts: 1) selection of participants, 2) comparability, and 3) ascertainment of exposure or the outcome. Each study can obtain a maximum of nine stars. We can allocate “four stars” for part one, “two stars” for part two, and “three stars” for part three (14). The face/content validity and inter-rater reliability of the NOS has been established based on a critical review of the items by several experts (15). Scores 9, 8, and 7 were assigned to the high-quality papers 6and 5 to the moderate ones, and below 4 were assigned to the low-quality group.
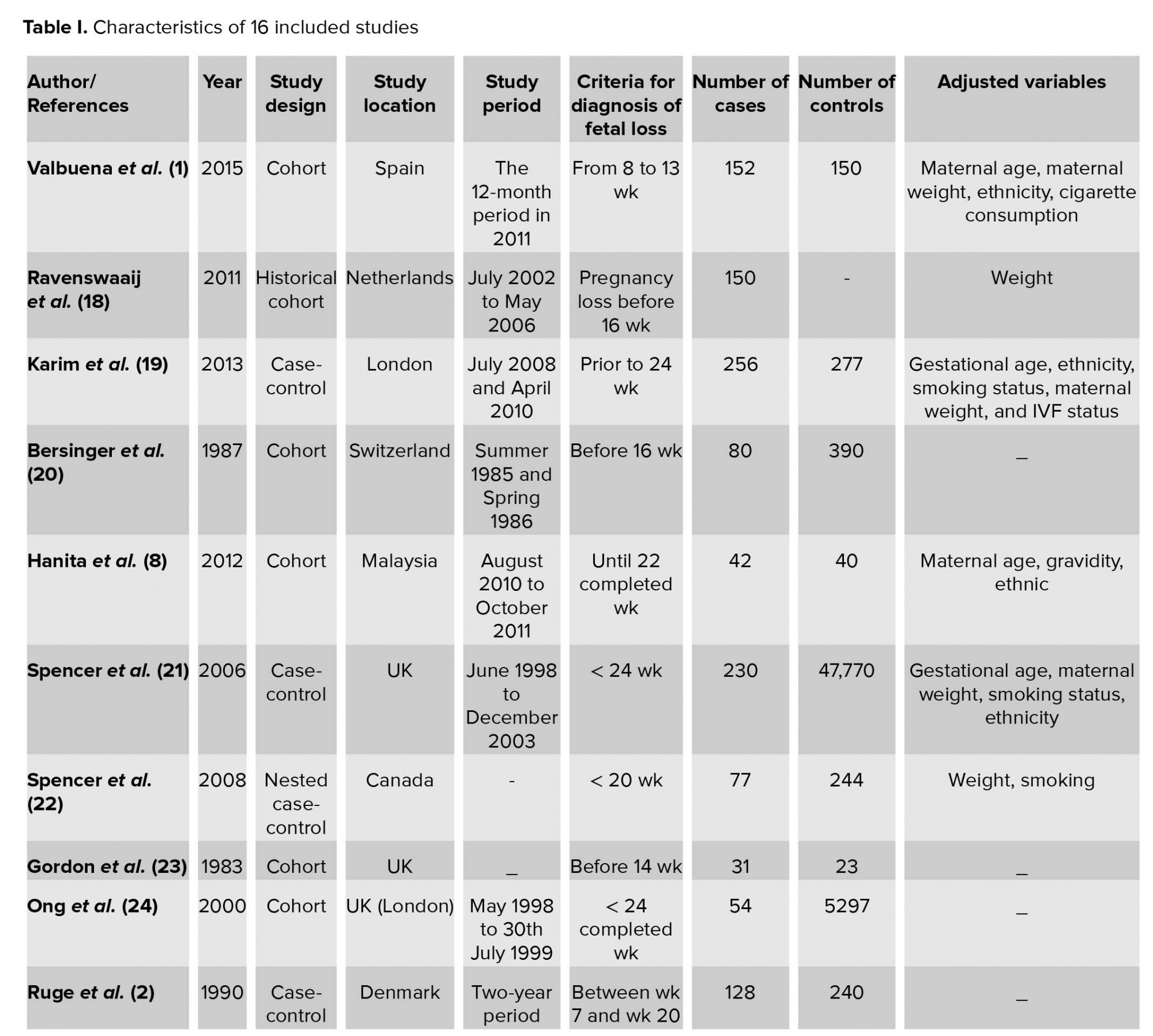
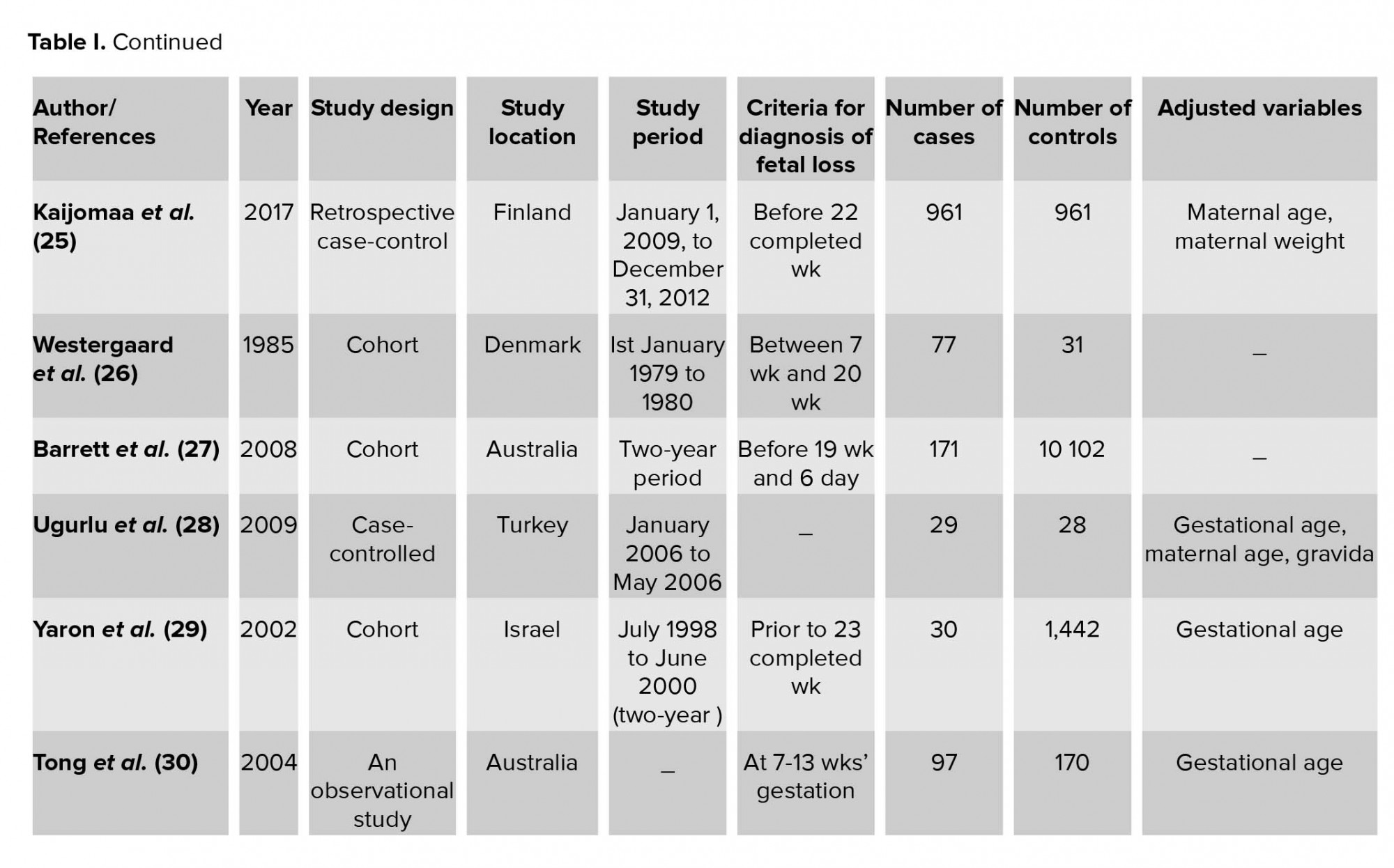
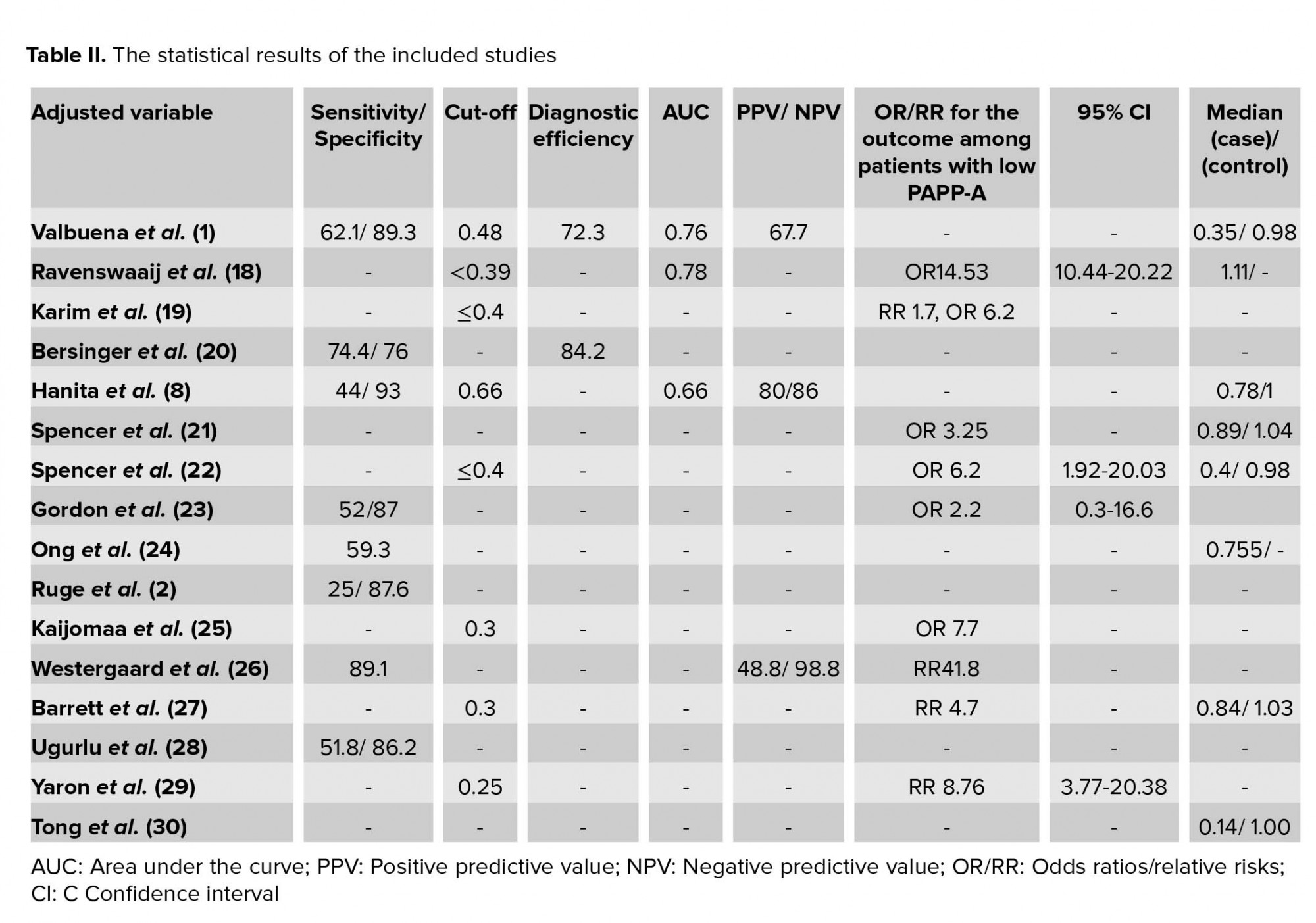
The characteristics of 16 included studies are shown in Table I. All selected studies were case-control and cohort studies. They had a variety of definitions of fetal loss that ranged from 6 to 24 wk, and also had different matched variable. The statistical results extracted from each study have been demonstrated in Table II. In all articles, the mean or median of PAPP-A was lower in a group with fetal loss versus group without fetal loss. None of the studies found significant relationships between high levels of PAPP-A and adverse obstetric outcomes. Cut-offs used in various studies ranged from 0.25 to 0.66.
The quality assessment of selected articles for evaluating publication bias showed that nine studies had high quality, six had medium quality, and no study had poor quality. In total, most studies had a strong methodology. In selection domain, some articles did not have adequate case or control definition. For the comparability domain, articles that got zero had no matched variables, and if articles had adjusted variable for either in study designs or in the statistical analysis were awarded one star. In the ascertainment of exposure or assessment of outcome domain, most articles did not report non-response rate or subjects lost to follow and also used medical record only or written self-report for evaluation of exposure (Table III).
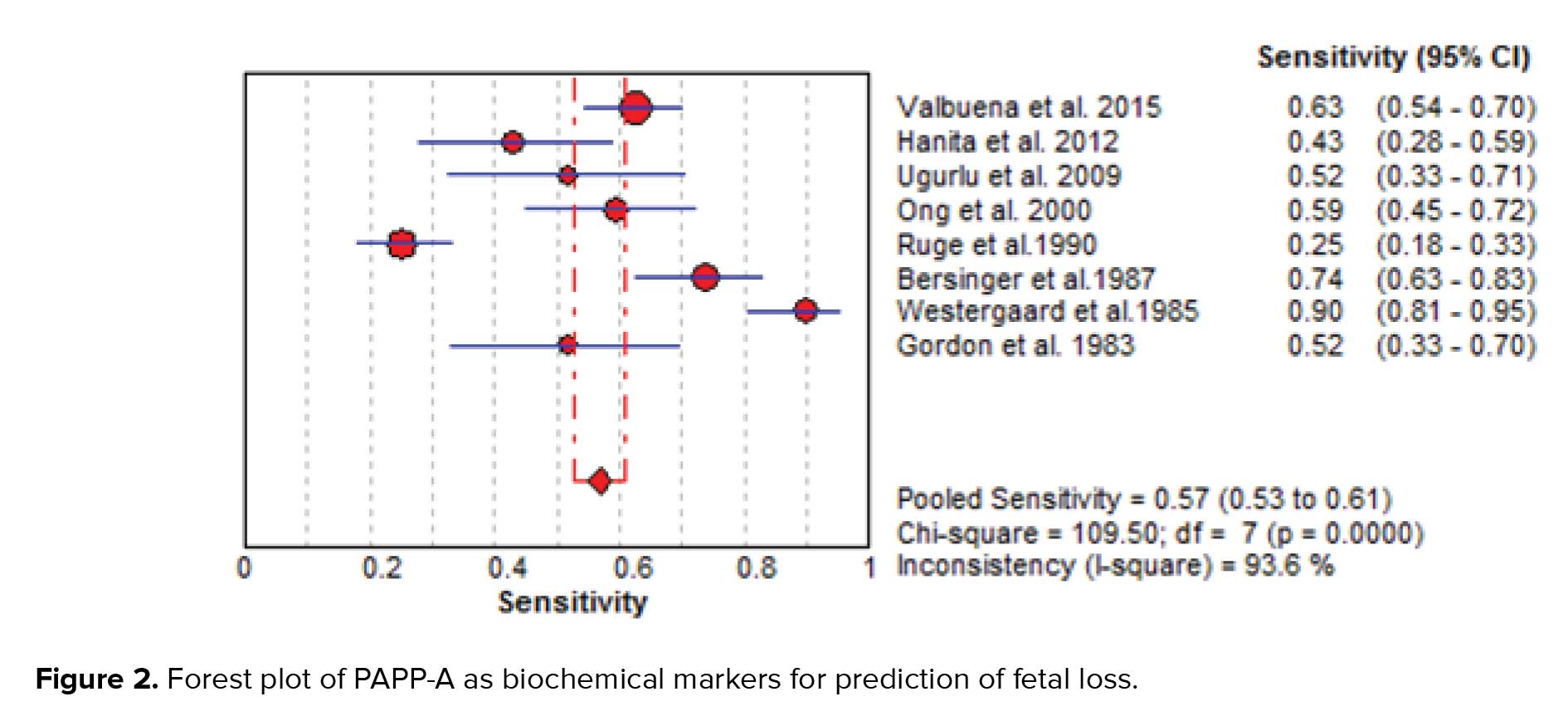
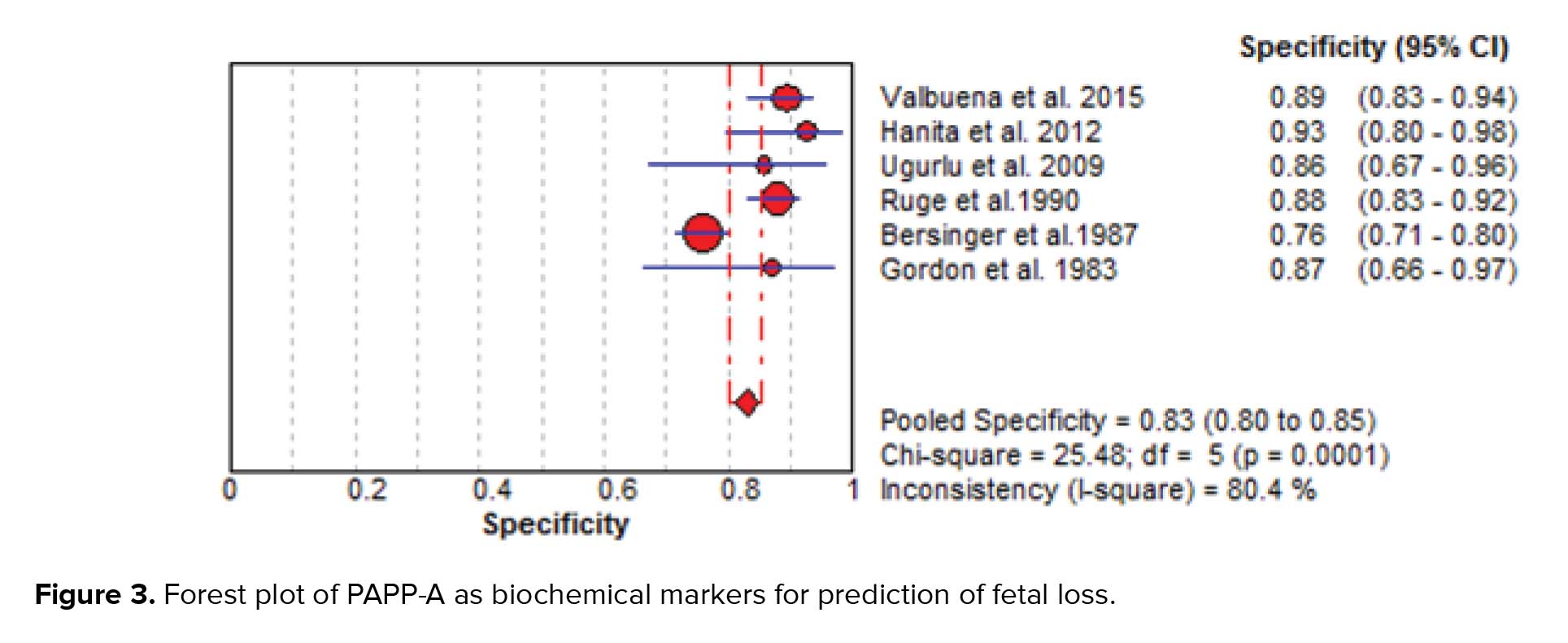
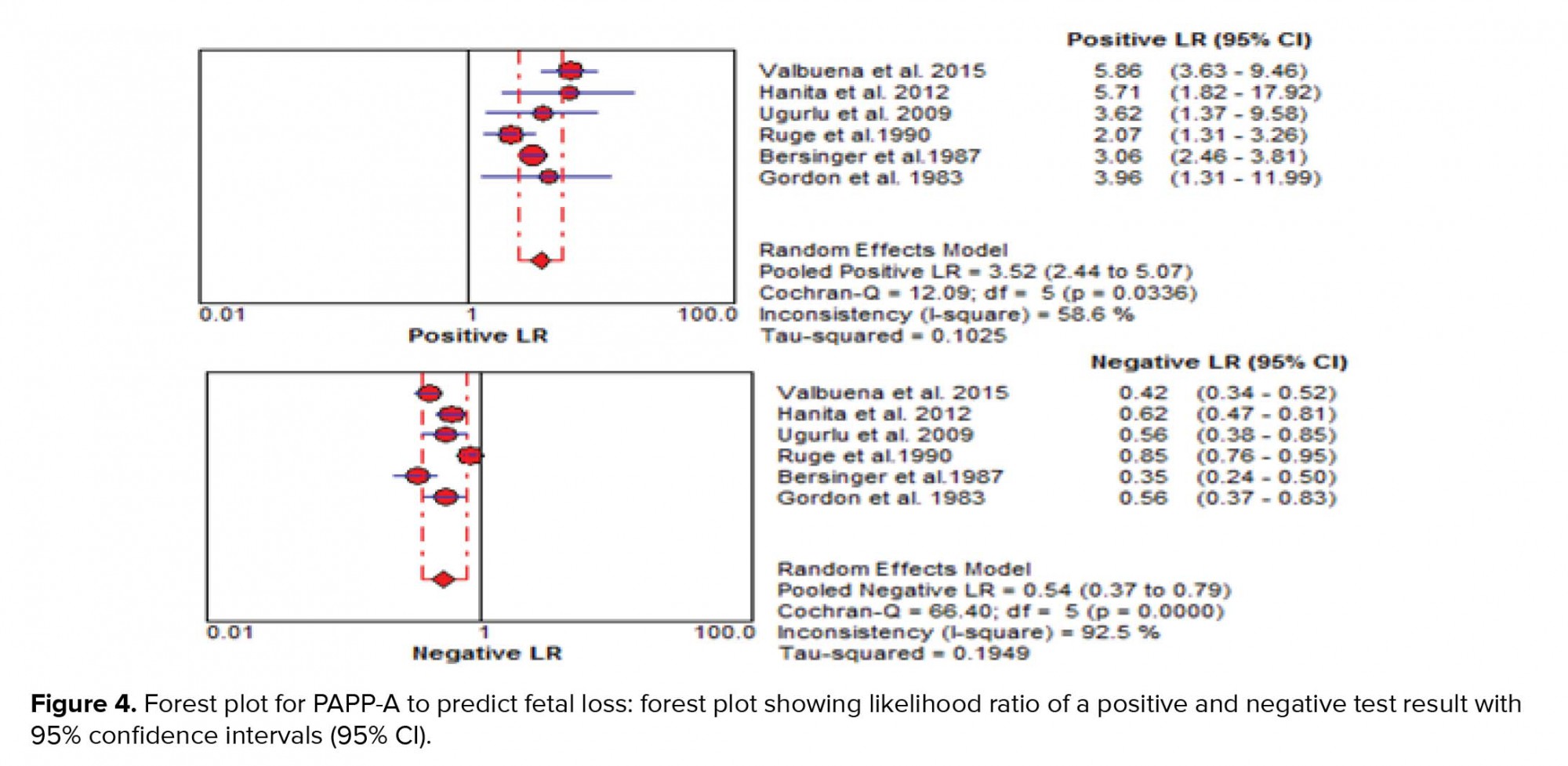
The results of the meta-analysis for PAPPA as biochemical markers to predict fetal loss are summarized in Figures 2-5. We tabulated results in a 2 × 2 contingency tables and forest plots created for the sensitivity and specificity of PAPP-A with their CI. The forest plots analysis showed a sensitivity of 57% (95% CI: 53-63%) for eight studies (1, 2, 8, 20, 24, 26, 28, 31), a specificity of 83% (95% CI: 80-85%) for six studies (1, 2, 8, 20, 28, 31), an LR+ of 3.52 (95% CI: 2.44-5.07), an LR- of 0.54 (95% CI: 0.37-0.79), and a diagnostic odds ratio of 6.95 (95% CI: 3.58-13.50).
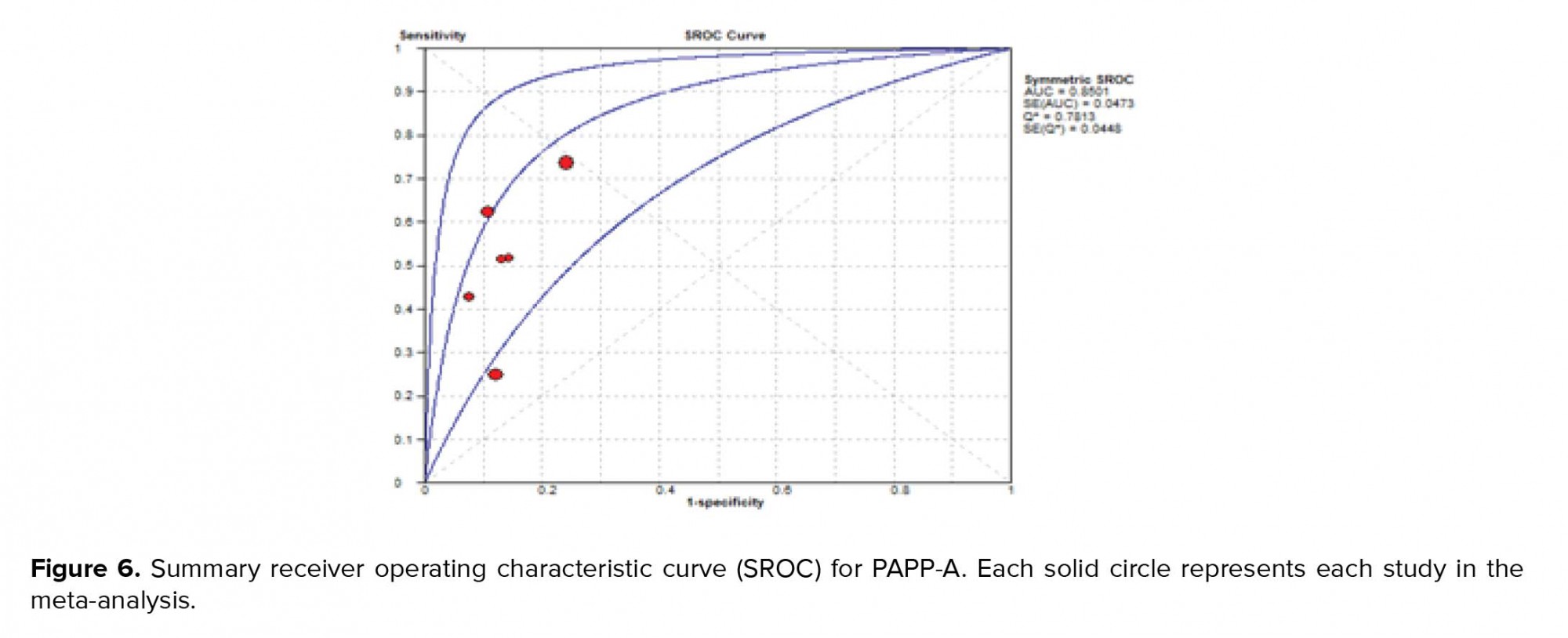
"The SROC curve presents a global summary of test performance and shows the tradeoff between specificity and sensitivity. The AUC and an index Q value are discussed as useful summaries of the curve. SROC curve is shown in Figure 6. Our data showed that AUC = 0.85 and Q* = 0.78".
Funnel plots of pooling sensitivity and specificity are demonstrated in Figure 7. The asymmetrical scattering of the points for sensitivity and specificity suggests possible publication bias which was confirmed by Egger's test intercept.



4. Discussion
Clinicians use diagnostic and predictive tests to identify the absence or presence of a condition in patients for the purpose of conducting an appropriate treatment plan. New diagnostic tools should be developed for improvements in ease of performance, speed, cost, patient safety, and accuracy. Systematic review of diagnostic test studies provides a summary of the accuracy of the test based on all available evidence (31). In women with pain and bleeding in the early stages of pregnancy, ultrasound alone cannot provide a definitive result for fetal loss, so it is necessary to provide an auxiliary test. This review has highlighted the predictive value of PAPP-A as biochemical markers to predict fetal loss. It is clinically important to predict the outcome of patients with bleeding and pain in the early stage of pregnancy. The prognostic value of PAPP-A was investigated in different studies and they reported different sensitivity and specificity. Our study combined the results of these studies. The result of the present study showed low predictive accuracy overall. The low predictive value may be described because of the heterogeneity between studies. Pillai and colleagues in their systematic review titled “role of serum biomarkers in the prediction of outcome in women with threatened miscarriage” found that PAPP-A had a poor and wide sensitivity ranging from 25 to 64% but a high specificity ranging from 88 to 94%. Their result about sensitivity was different from ours because our findings did not show the wide range and had 57% sensitivity (53-63), but their specificity was almost near our result of 83% (80-85). This difference may be due to the difference in the number of articles reviewed; their evaluation consisted of just three articles. Dugoff and colleagues in the FaSTER trial study showed that although a low level of PAPP-A alone associated with adverse pregnancy outcomes, it is a poor predictor of such outcomes (11).
The SROC curve summarizes the predictive power to distinguish the samples with the disease from those without the disease. It is a plot of sensitivity against specificity. The AUC is obtained from operating curve (ROC) analysis. The power considers it good if AUC is closed to 1. In the present study, the AUC was 0.85 and it shows that more than 35% of women at risk for fetal loss can be correctly classified by the predictive model (Figure 6). As seen in Table II, the results of other studies are approximately near our results, but our data showed more powerful differentiation. The diagnostic odds ratio measures the effectiveness of a diagnostic test and is less likely to change with the disease prevalence. It ranges from zero to infinity. In the present meta-analysis, we found that the mean DOR of 6.95 indicate a low level of accuracy.
LRs allow interpretation of the findings for use in clinical practice by showing how much a given test results in boost or reduce the probability of having the condition. It shows how many times sample with target disorders are more likely to receive a particular test result than those without target disorders. Our meta-analysis showed an LR+ of 3.52 (95% CI: 2.44-5.07) and an LR- of 0.54 (95% CI: 0.37- 0.79). A PLR means low maternal serum PAPP-A would be three times as likely to be seen in someone with fetal loss as opposed to someone without fetal loss. An NLR 0.5 suggests PAPP-A alone cannot detect patients with fetal loss (Figure 4).
Table I shows that the median or means values for PAPP-A was lower in fetal loss group. Zhang et al. reported that the level of PAPP-A mRNA in basal decidual tissue was decreased in the group who had a recurrent spontaneous abortion (32). Suzuki and colleagues said that genetic factor such as the PAPPA polymorphism may increase the risk of some types of recurrent pregnancy loss (RPL) (13). In a group of patients, Santolaya-Forgas and colleagues declared that low levels of PAPP-A concentrations can cause a down-regulation of IGF-II accessibility in fetal and placental development and that this may cause spontaneous abortions (33). Dumps and colleagues suggested that PAPP-A concentrations decrease in pregnancy failure, and circulating PAPP-A concentrations in extra uterine pregnancy (EUP) and abnormal intrauterine pregnancy (abIUP) were significantly lower in comparison to normal intrauterine pregnancy (nIUP) (p= 0.02) (17). Bischof and colleagues demonstrated that PAPP-A levels were consistently decreased or even undetectable in established ectopic pregnancies, and also after IVF when threatened abortions happen (16). The possible reason for these findings is impaired placentation because the low level of PAPP-A in maternal serum is the consequence of poor placental function that can cause fetal loss (24).
We faced some limitations in our study that must be considered. One limitation of our study was substantial heterogeneity among studies, and the sources of heterogeneity may be definition of fetal loss, the study design, adjusted variable, and gestational age at testing. Also, the publication bias could be a key concern so that our results should be interpreted with more caution (34). The other limitation was the difference in reporting statistics, which led to exclusion of some articles from being included in the meta-analysis; therefore, they made our meta-analysis limited. The other limitation was our search process. We did not search for unpublished works (including unpublished dissertations) and were limited to the Internet search. So, it is better to include unpublished papers for future studies. Finally, it should be noticed that this review was conducted on studies with singleton pregnancies and should not be generalized to all pregnant women. Also, considering the definition of fetal loss under 24 wk in this study, the results would be irrelevant to the abortions before 8-9 wk.
5. Conclusion
In conclusion, PAPP-A cannot be recommended for predicting fetal loss on a routine basis and still further research is required with a combination of other biomarkers. Fetal loss may be the result of a variety of etiologies and not a single disorder; therefore, a single test cannot predict all causes of fetal loss. Further studies should be conducted with a combination of different tests such as biophysical and biochemical tests to predict fetal loss with more precision.
Acknowledgments
This study was supported by the Mashhad University of Medical Sciences under code 960488 and Ethical code of IR.MUMS.REC.1397.130. The authors hereby express their gratitude to the Mashhad University of Medical Sciences for funding this study.
Conflict of Interest
The authors declare that there is no conflict of interest.
Full-Text: (600 Views)
- Introduction
First-trimester screening is done for all pregnant women at 11-13 + 6 wk, and it is a non-invasive evaluation to determine the risk of chromosomal abnormalities, such as Down syndrome (10). Since this marker is routinely measured in all women, and it has a relation with pregnancy outcomes, this cost-effective method can be used for determining the prognosis of threatened abortion (1). Identifying patients at risk lead to increased monitoring of pregnant women who are at high risk for pregnancy complications (11). Many studies consider this marker as an important marker for abortion (12, 13). Several studies have examined the association between PAPP-A and fetal loss; however, they reported different sensitivity, specificity, and critical points and there is no same agreement among researchers; therefore, the present study discusses the results of various studies (1, 2, 8). Also, one limitation of many studies was the small sample size, which is controlled through systematic reviews. Systematic review and meta-analysis are essential tools for summarizing the evidence in an accurate, correct, and reliable way. So, researchers felt a need to conduct a systematic review to get a clear and uniform result and a comprehensive guide to clinical use.
This systematic review and meta-analysis aimed to determine the predictive value of PAPP-A for fetal loss.
- Materials and Methods
- 1. Literature search strategy and study selection
- 2. Definition of fetal loss
- 3. Data extraction and risk of bias assessment
Newcastle-Ottawa tool (NOS) was used to assess the quality for cohort and case-control studies. This tool consists of three main parts: 1) selection of participants, 2) comparability, and 3) ascertainment of exposure or the outcome. Each study can obtain a maximum of nine stars. We can allocate “four stars” for part one, “two stars” for part two, and “three stars” for part three (14). The face/content validity and inter-rater reliability of the NOS has been established based on a critical review of the items by several experts (15). Scores 9, 8, and 7 were assigned to the high-quality papers 6and 5 to the moderate ones, and below 4 were assigned to the low-quality group.
- 4. Statistical analysis
- Results
The characteristics of 16 included studies are shown in Table I. All selected studies were case-control and cohort studies. They had a variety of definitions of fetal loss that ranged from 6 to 24 wk, and also had different matched variable. The statistical results extracted from each study have been demonstrated in Table II. In all articles, the mean or median of PAPP-A was lower in a group with fetal loss versus group without fetal loss. None of the studies found significant relationships between high levels of PAPP-A and adverse obstetric outcomes. Cut-offs used in various studies ranged from 0.25 to 0.66.
The quality assessment of selected articles for evaluating publication bias showed that nine studies had high quality, six had medium quality, and no study had poor quality. In total, most studies had a strong methodology. In selection domain, some articles did not have adequate case or control definition. For the comparability domain, articles that got zero had no matched variables, and if articles had adjusted variable for either in study designs or in the statistical analysis were awarded one star. In the ascertainment of exposure or assessment of outcome domain, most articles did not report non-response rate or subjects lost to follow and also used medical record only or written self-report for evaluation of exposure (Table III).
The results of the meta-analysis for PAPPA as biochemical markers to predict fetal loss are summarized in Figures 2-5. We tabulated results in a 2 × 2 contingency tables and forest plots created for the sensitivity and specificity of PAPP-A with their CI. The forest plots analysis showed a sensitivity of 57% (95% CI: 53-63%) for eight studies (1, 2, 8, 20, 24, 26, 28, 31), a specificity of 83% (95% CI: 80-85%) for six studies (1, 2, 8, 20, 28, 31), an LR+ of 3.52 (95% CI: 2.44-5.07), an LR- of 0.54 (95% CI: 0.37-0.79), and a diagnostic odds ratio of 6.95 (95% CI: 3.58-13.50).
"The SROC curve presents a global summary of test performance and shows the tradeoff between specificity and sensitivity. The AUC and an index Q value are discussed as useful summaries of the curve. SROC curve is shown in Figure 6. Our data showed that AUC = 0.85 and Q* = 0.78".
Funnel plots of pooling sensitivity and specificity are demonstrated in Figure 7. The asymmetrical scattering of the points for sensitivity and specificity suggests possible publication bias which was confirmed by Egger's test intercept.











4. Discussion
Clinicians use diagnostic and predictive tests to identify the absence or presence of a condition in patients for the purpose of conducting an appropriate treatment plan. New diagnostic tools should be developed for improvements in ease of performance, speed, cost, patient safety, and accuracy. Systematic review of diagnostic test studies provides a summary of the accuracy of the test based on all available evidence (31). In women with pain and bleeding in the early stages of pregnancy, ultrasound alone cannot provide a definitive result for fetal loss, so it is necessary to provide an auxiliary test. This review has highlighted the predictive value of PAPP-A as biochemical markers to predict fetal loss. It is clinically important to predict the outcome of patients with bleeding and pain in the early stage of pregnancy. The prognostic value of PAPP-A was investigated in different studies and they reported different sensitivity and specificity. Our study combined the results of these studies. The result of the present study showed low predictive accuracy overall. The low predictive value may be described because of the heterogeneity between studies. Pillai and colleagues in their systematic review titled “role of serum biomarkers in the prediction of outcome in women with threatened miscarriage” found that PAPP-A had a poor and wide sensitivity ranging from 25 to 64% but a high specificity ranging from 88 to 94%. Their result about sensitivity was different from ours because our findings did not show the wide range and had 57% sensitivity (53-63), but their specificity was almost near our result of 83% (80-85). This difference may be due to the difference in the number of articles reviewed; their evaluation consisted of just three articles. Dugoff and colleagues in the FaSTER trial study showed that although a low level of PAPP-A alone associated with adverse pregnancy outcomes, it is a poor predictor of such outcomes (11).
The SROC curve summarizes the predictive power to distinguish the samples with the disease from those without the disease. It is a plot of sensitivity against specificity. The AUC is obtained from operating curve (ROC) analysis. The power considers it good if AUC is closed to 1. In the present study, the AUC was 0.85 and it shows that more than 35% of women at risk for fetal loss can be correctly classified by the predictive model (Figure 6). As seen in Table II, the results of other studies are approximately near our results, but our data showed more powerful differentiation. The diagnostic odds ratio measures the effectiveness of a diagnostic test and is less likely to change with the disease prevalence. It ranges from zero to infinity. In the present meta-analysis, we found that the mean DOR of 6.95 indicate a low level of accuracy.
LRs allow interpretation of the findings for use in clinical practice by showing how much a given test results in boost or reduce the probability of having the condition. It shows how many times sample with target disorders are more likely to receive a particular test result than those without target disorders. Our meta-analysis showed an LR+ of 3.52 (95% CI: 2.44-5.07) and an LR- of 0.54 (95% CI: 0.37- 0.79). A PLR means low maternal serum PAPP-A would be three times as likely to be seen in someone with fetal loss as opposed to someone without fetal loss. An NLR 0.5 suggests PAPP-A alone cannot detect patients with fetal loss (Figure 4).
Table I shows that the median or means values for PAPP-A was lower in fetal loss group. Zhang et al. reported that the level of PAPP-A mRNA in basal decidual tissue was decreased in the group who had a recurrent spontaneous abortion (32). Suzuki and colleagues said that genetic factor such as the PAPPA polymorphism may increase the risk of some types of recurrent pregnancy loss (RPL) (13). In a group of patients, Santolaya-Forgas and colleagues declared that low levels of PAPP-A concentrations can cause a down-regulation of IGF-II accessibility in fetal and placental development and that this may cause spontaneous abortions (33). Dumps and colleagues suggested that PAPP-A concentrations decrease in pregnancy failure, and circulating PAPP-A concentrations in extra uterine pregnancy (EUP) and abnormal intrauterine pregnancy (abIUP) were significantly lower in comparison to normal intrauterine pregnancy (nIUP) (p= 0.02) (17). Bischof and colleagues demonstrated that PAPP-A levels were consistently decreased or even undetectable in established ectopic pregnancies, and also after IVF when threatened abortions happen (16). The possible reason for these findings is impaired placentation because the low level of PAPP-A in maternal serum is the consequence of poor placental function that can cause fetal loss (24).
We faced some limitations in our study that must be considered. One limitation of our study was substantial heterogeneity among studies, and the sources of heterogeneity may be definition of fetal loss, the study design, adjusted variable, and gestational age at testing. Also, the publication bias could be a key concern so that our results should be interpreted with more caution (34). The other limitation was the difference in reporting statistics, which led to exclusion of some articles from being included in the meta-analysis; therefore, they made our meta-analysis limited. The other limitation was our search process. We did not search for unpublished works (including unpublished dissertations) and were limited to the Internet search. So, it is better to include unpublished papers for future studies. Finally, it should be noticed that this review was conducted on studies with singleton pregnancies and should not be generalized to all pregnant women. Also, considering the definition of fetal loss under 24 wk in this study, the results would be irrelevant to the abortions before 8-9 wk.
5. Conclusion
In conclusion, PAPP-A cannot be recommended for predicting fetal loss on a routine basis and still further research is required with a combination of other biomarkers. Fetal loss may be the result of a variety of etiologies and not a single disorder; therefore, a single test cannot predict all causes of fetal loss. Further studies should be conducted with a combination of different tests such as biophysical and biochemical tests to predict fetal loss with more precision.
Acknowledgments
This study was supported by the Mashhad University of Medical Sciences under code 960488 and Ethical code of IR.MUMS.REC.1397.130. The authors hereby express their gratitude to the Mashhad University of Medical Sciences for funding this study.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Review Article |
Subject:
Reproductive Biology
References
1. Valbuena H, Ramis J, Sagala J, Sanchez MA, Aulesa C. First-trimester screening biochemical markers (free beta-subunit human chorionic gonadotropin, pregnancy-associated plasma protein-A) and risk of early fetal loss. J Obstet Gynaecol Res 2015; 41: 69-76.
2. Ruge S, Pedersen JF, Sorensen S, Lange AP. Can pregnancy-associated plasma protein A (PAPP-A) predict the outcome of pregnancy in women with threatened abortion and confirmed fetal viability? Acta Obstet Gynecol Scand 1990; 69: 589-595.
3. Lin TM, Galbert SP, Kiefer D, Spellacy WN, Gall S. Characterization of four human pregnancy-associated plasma proteins. Am J Obstet Gynecol 1974; 118: 223-236.
4. Zhong Y, Zhu F, Ding Y. Serum screening in first trimester to predict pre-eclampsia, small for gestational age and preterm delivery: systematic review and meta-analysis. BMC Pregnancy Childbirth 2015; 15: 191-201.
5. Smith GC, Stenhouse EJ, Crossley JA, Aitken DA, Cameron AD, Connor JM. Early pregnancy levels of pregnancy-associated plasma protein a and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J Clin Endocrinol Metab 2002; 87: 1762-1767.
6. Gupta S, Goyal M, Verma D, Sharma A, Bharadwaj N, Kabra M, et al. Adverse pregnancy outcome in patients with low pregnancy-associated plasma protein-A: The Indian Experience. J Obstet Gynaecol Res 2015; 41: 1003-1008.
7. Talasaz ZH, Sadeghi R, Askari F, Dadgar S, Vatanchi A. First trimesters pregnancy-associated plasma protein-a levels value to predict gestational diabetes mellitus: A systematic review and meta-analysis of the literature. Taiwan J Obstet Gynecol 2018; 57: 181-189.
8. Hanita O, Roslina O, Azlin MI. Maternal level of pregnancy-associated plasma protein A as a predictor of pregnancy failure in threatened abortion. Malays J Pathol 2012; 34: 145-151
9. Goetzl L, Krantz D, Simpson JL, Silver RK, Zachary JM, Pergament E, et al. Pregnancy-associated plasma protein A, free beta-hCG, nuchal translucency, and risk of pregnancy loss. Obstet Gynecol 2004; 104: 30-36.
10. Kagan KO, Maier V, Sonek J, Abele H, Lüthgens K, Schmid M, et al. False-positive rate in first-trimester screening based on ultrasound and cell-free DNA versus first-trimester combined screening with additional ultrasound markers. Fetal Diagn Ther 2019; 45: 317-324.
11. Dugoff L, Hobbins JC, Malone FD, Porter TF, Luthy D, Comstock CH, et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population-based screening study (the FASTER Trial). Am J Obstet Gynecol 2004; 191: 1446-1451.
12. Dugoff L, Cuckle HS, Hobbins JC, Malone FD, Belfort MA, Nyberg DA, et al. Prediction of patient-specific risk for fetal loss using maternal characteristics and first- and second-trimester maternal serum Down syndrome markers. Am J Obstet Gynecol 2008; 199: 290. e1-6.
13. Suzuki K, Sata F, Yamada H, Saijo Y, Tsuruga N, Minakami H, et al. Pregnancy-associated plasma protein-A polymorphism and the risk of recurrent pregnancy loss. J Reprod Immunol 2006; 70: 99-108.
14. Wells GA, Shea B, O’connell D, Peterson J, Welch V, Losos M, et al. The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, Hospital Research Institute; 2011.
15. Peterson J, Welch V, Losos M, Tugwell P. The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Liverpool, UK. 2011.
16. Bischof P, Mignot TM, Cédard L. Are pregnancy-associated plasma protein-A (PAPP-A) and CA 125 measurements after IVF-ET possible predictors of early pregnancy wastage? Hum Reprod 1989; 4: 843-847.
17. Dumps P, Meisser A, Pons D, Morales MA, Anguenot JL, Campana A, et al. Accuracy of single measurements of pregnancy-associated plasma protein-A, human chorionic gonadotropin and progesterone in the diagnosis of early pregnancy failure. Eur J Obstet Gynecol Reprod Biol 2002; 100: 174-180.
18. Van Ravenswaaij R, Tesselaar‐Van der Goot M, de Wolf S, van Leeuwen‐Spruijt M, Visser GH, Schielen PC. First‐trimester serum PAPP‐A and fβ‐hCG concentrations and other maternal characteristics to establish logistic regression‐based predictive rules for adverse pregnancy outcome. Prenat Diagn 2011; 31: 50-57.
19. Karim JN, Sau A. Low pregnancy associated plasma protein-A in the 1st trimester: is it a predictor of poor perinatal outcome? J Obstet Gynaecol 2013; 33: 351-354.
20. Bersinger NA, Keller PJ, Naiem A, Fischer M, Schneider H. Pregnancy-specific and pregnancy-associated proteins in threatened abortion. Gynecol Endocrinol 1987; 1: 379-384.
21. Spencer K, Cowans NJ, Avgidou K, Nicolaides KH. First‐trimester ultrasound and biochemical markers of aneuploidy and the prediction of impending fetal death. Ultrasound Obstet Gynecol 2006; 28: 637-643.
22. Spencer CA, Allen VM, Flowerdew G, Dooley K, Dodds L. Low levels of maternal serum PAPP-A in early pregnancy and the risk of adverse outcomes. Prenat Diagn 2008; 28: 1029-1036.
23. Masson GM, Anthony F, Wilson MS. Value of Schwangerschaftsprotein 1 (SP1) and pregnancy‐associated plasma protein‐A (PAPP‐A) in the clinical management of threatened abortion. Br J Obstet Gynecol 1983; 90: 146-149.
24. Ong CY, Liao AW, Spencer K, Munim S, Nicolaides KH. First trimester maternal serum free beta human chorionic gonadotrophin and pregnancy associated plasma protein A as predictors of pregnancy complications. BJOG 2000; 107: 1265-1270.
25. Kaijomaa M, Rahkonen L, Ulander VM, Hämäläinen E, Alfthan H, Markkanen H, et al. Low maternal pregnancy-associated plasma protein A during the first trimester of pregnancy and pregnancy outcomes. Int J Gynecol Obstet 2017; 136: 76-82.
26. Westergaard JG, Teisner B, Sinosich MJ, Madsen LT, Grudzinskas JG. Does ultrasound examination render biochemical tests obsolete in the prediction of early pregnancy failure? Br J Obstet Gynaecol 1985; 92: 77-83.
27. Barrett SL, Bower C, Hadlow NC. Use of the combined first‐trimester screen result and low PAPP‐A to predict risk of adverse fetal outcomes. Prenat Diagn 2008; 28: 28-35.
28. Ugurlu EN, Ozaksit G, Karaer A, Zulfikaroglu E, Atalay A, Ugur M. The value of vascular endothelial growth factor, pregnancy-associated plasma protein-A, and progesterone for early differentiation of ectopic pregnancies, normal intrauterine pregnancies, and spontaneous miscarriages. Fertil Steril 2009; 91: 1657-1661.
29. Yaron Y, Heifetz S, Ochshorn Y, Lehavi O, Orr-Urtreger A. Decreased first trimester PAPP-A is a predictor of adverse pregnancy outcome. Prenat Diagn 2002; 22: 778-782.
30. Tong S, Marjono B, Mulvey S, Wallace EM. Low levels of pregnancy-associated plasma protein-A in asymptomatic women destined for miscarriage. Fertil Steril 2004; 82: 1468-1470.
31. Campbell J, Klugar M, Ding S, Carmody D, Hakonsen S, Jadotte Y. The systematic review of studies of diagnostic test accuracy. Joanna Briggs Institute Reviewers’ Manual 2015: 1-46.
32. Zhang Y, Zhao Q, Xie Y, Su K, Yang J, Yang L. A correlation analysis between the expression of pregnancy-associated plasma protein A in basal decidual cells and recurrent spontaneous abortion. Exp Ther Med 2013; 6: 485-488.
33. Santolaya-Forgas J, De Leon JA, Cullen Hopkins R, Castracane VD, Kauffman RP, Sifuentes GA. Low pregnancy-associated plasma protein-a at 10(+1) to 14(+6) weeks of gestation and a possible mechanism leading to miscarriage. Fetal Diagn Ther 2004; 19: 456-461.
34. Ghazanfarpour M, Sadeghi R, Latifnejad Roudsari R. The application of soy isoflavones for subjective symptoms and objective signs of vaginal atrophy in menopause: A systematic review of randomized controlled trials, J Obstet Gynaecol 2016; 36: 160-171.
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





