Sun, Jul 13, 2025
[Archive]
Volume 18, Issue 6 (June 2020)
IJRM 2020, 18(6): 415-424 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Zal F, Ahmadi P, Davari M, Khademi F, Akbarzadeh Jahromi M, Anvar Z et al . Glutathione-dependent enzymes in the follicular fluid of the first-retrieved oocyte and their impact on oocyte and embryos in polycystic ovary syndrome: A cross-sectional study. IJRM 2020; 18 (6) :415-424
URL: http://ijrm.ir/article-1-1464-en.html
URL: http://ijrm.ir/article-1-1464-en.html
Fatemeh Zal1 

 , Pardis Ahmadi2
, Pardis Ahmadi2 

 , Maryam Davari3
, Maryam Davari3 

 , Fatemeh Khademi4
, Fatemeh Khademi4 

 , Mojgan Akbarzadeh Jahromi5
, Mojgan Akbarzadeh Jahromi5 

 , Zahra Anvar *6
, Zahra Anvar *6 

 , Bahia Namavar Jahromi7
, Bahia Namavar Jahromi7 




 , Pardis Ahmadi2
, Pardis Ahmadi2 

 , Maryam Davari3
, Maryam Davari3 

 , Fatemeh Khademi4
, Fatemeh Khademi4 

 , Mojgan Akbarzadeh Jahromi5
, Mojgan Akbarzadeh Jahromi5 

 , Zahra Anvar *6
, Zahra Anvar *6 

 , Bahia Namavar Jahromi7
, Bahia Namavar Jahromi7 


1- Department of Biochemistry, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran. Infertility Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
2- Department of Obstetrics and Gynecology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
3- Department of Obstetrics and Gynecology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran. IVF Section, Ghadir-Mother and Child Hospital of Shiraz, Shiraz, Iran.
4- Department of Biochemistry, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
5- Department of Pathology, Maternal-Fetal Medicine Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
6- Infertility Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. Department of Obstetrics and Gynecology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran. ,zahraanvar2000@yahoo.com
7- Infertility Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. Department of Obstetrics and Gynecology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
2- Department of Obstetrics and Gynecology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
3- Department of Obstetrics and Gynecology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran. IVF Section, Ghadir-Mother and Child Hospital of Shiraz, Shiraz, Iran.
4- Department of Biochemistry, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
5- Department of Pathology, Maternal-Fetal Medicine Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
6- Infertility Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. Department of Obstetrics and Gynecology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran. ,
7- Infertility Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. Department of Obstetrics and Gynecology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
Full-Text [PDF 382 kb]
(1012 Downloads)
| Abstract (HTML) (2754 Views)
Oxidative stress (OS) refers to an imbalance between produced ROS and detoxifying capacity of antioxidants (4). It is suggested that cellular ROS play an important role in several diseases including atherosclerosis, diabetes, metabolic syndrome, obesity, and impaired reproductive disorders like PCOS (5). How PCOS is related to OS could be explained by the role of antioxidants in folliculogenesis, ovulation, and some other ovarian features. Any disequilibrium in these mechanisms may impair the female reproductive system. Moreover, OS may affect the results of assisted reproductive technologies (ART) (6).
Glutathione (GSH) is an important intracellular antioxidant. It can be converted to its oxidized form (GSSG) by glutathione peroxidase (GPx) when functioning as a scavenger; it is converted back to its reduced form by glutathione reductase (GR) protecting the cell against excessive generation of damaging ROS (7). It is involved in oocyte activation and maturation and development of preimplantation embryos during ART (8).
The developing oocyte is surrounded by complex microenvironment consisting of granulosa, theca cells, and follicular fluid (FF) to form its microenvironment. FF is formed from plasma proteins and through the secretory function of the granulosa and theca cells (9, 10). It is suspected that biochemical specifications of the FF and its ROS threshold affect the oocyte quality, fertilization, embryo development, and pregnancy rate (11, 12). Moreover, the compositions of FF and blood are reciprocally affected by each other (13, 14). It is also suggested that FF is naturally supplied by powerful antioxidant systems to protect the oocyte from OS. Therefore, elevated levels of antioxidants in FF or serum are accompanied by increased chances of high-quality oocytes-retrieved ART and high-quality embryo production (14, 15). Vignini and colleagues showed that the free radical nitric oxide concentrations in FF is significantly higher in in vitro fertilization (IVF)-produced embryos with severe fragmentations, which shows its detrimental impact on embryo quality (16). In a recent study, the negative effect of total non-enzymatic antioxidant capacity and 8-hydroxy-2'-deoxyguanosine in the FF on the number of good-quality embryos was reported (17).
To the best of our knowledge, all relevant studies carried out so far have collected and examined the entire bulk of FF drawn upon ovum pick-up. In the present study, however, only the FF of the first aspirated follicle was examined in order to achieve more precise observations and results on the oocyte maturation stages and their correlations with enzyme levels and activities. Our aim was to show the correlation between the maturation stages of oocytes/embryos and GSH detoxification capacity in FF and serum separately. Moreover, due to difficult FF accessibility, we investigated whether the GSH levels in the serum represented those in the FF and therefore if serum could be used as an indicator of antioxidant activities in the FF.
The first-retrieved FF samples that contained more than one oocyte, had no oocyte, or those that became bloody were excluded from the study. This process was carried on until 80 acceptable FF samples were collected. The first aspirated oocytes were graded as GV, MI, or MII according to the standard classifications (19) were placed in standard culture media and monitored for their ongoing development after conventional IVF. Conventional IVF represents standard insemination, which involves combining sperm and egg outside the body in the laboratory and transferring the resulting embryo(s) back into the uterus. Three days post-oocyte retrieval, the resulting embryos were assessed as I, II, and III, where I indicated the highest and III showed the lowest embryo quality (20).
"GR activity was assayed by a previously described method (21). It was performed in a cuvette in a total volume of 1 ml that included 60 μM buffer, 5 mM EDTA (pH 8.0), 0.033 M GSSG, 2 mM NADPH, and the sample. The decrease in absorbance, which reflects the oxidation of NADPH during reduction of GSSG by the sample’s GR, was monitored spectrophotometrically at 340 nm for 3 min. Results were based on a molar extinction coefficient for NADPH of 6.22 × 106M-1cm-1. One unit of GR is defined as mU/mg cell protein".
The mean GPx and GR activity and GSH levels were significantly higher in the FF of the MII oocytes. The mean GPX activity and GSH levels in the serum samples did not show significant statistical difference among different oocyte grades but the mean serum GR activity was significantly higher for MII oocytes compared to GVs (Table II).
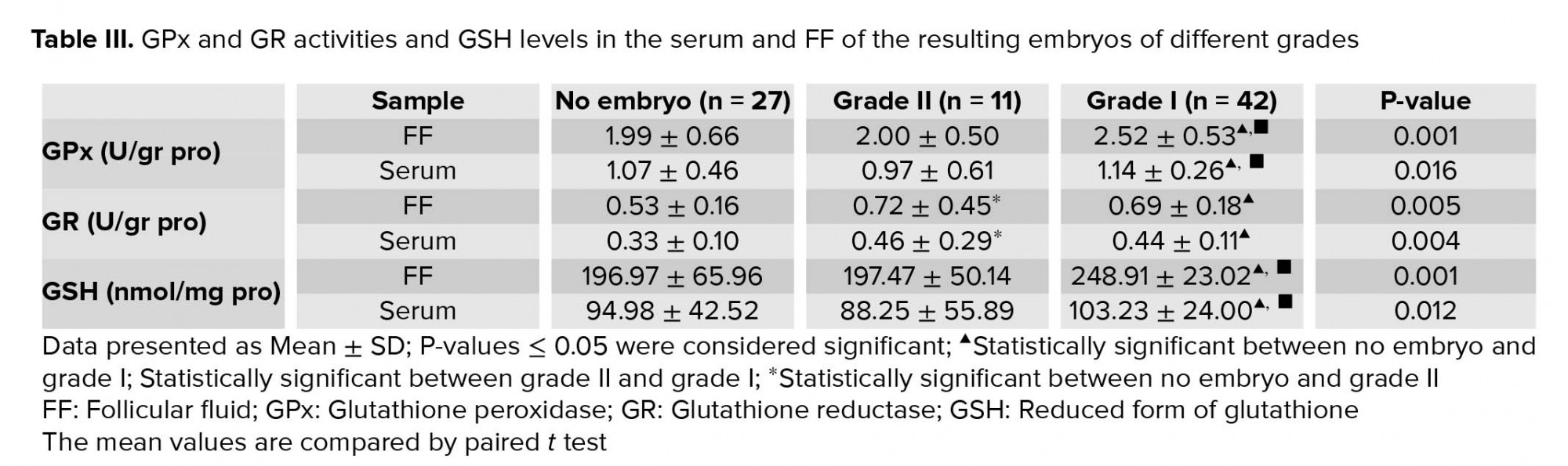
GPX activity and GSH levels were significantly higher in the serum and FF of the high-quality embryos with grade I compared to grade II embryo or no embryo group (Table III). Moreover, the mean of GR activity was significantly higher in the serum and FF of grades I and II embryos compared to no embryo group. Examination of serum and FF of grades I and II embryos revealed no significant difference in their GR activities (Table III).

3. Discussion
The microenvironment of the oocyte determines its developmental potential. In this study, we measured GPx and GR activities and GSH levels in the serum and FF of the first-retrieved oocytes of PCOS women who underwent IVF. Subsequently, the oocytes and resulting embryos were graded according to their quality.
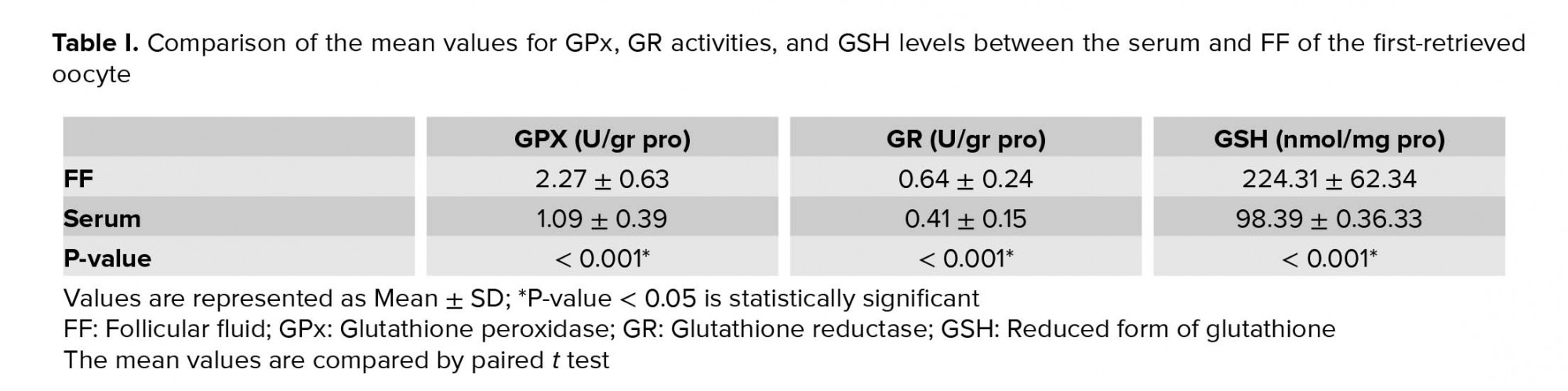
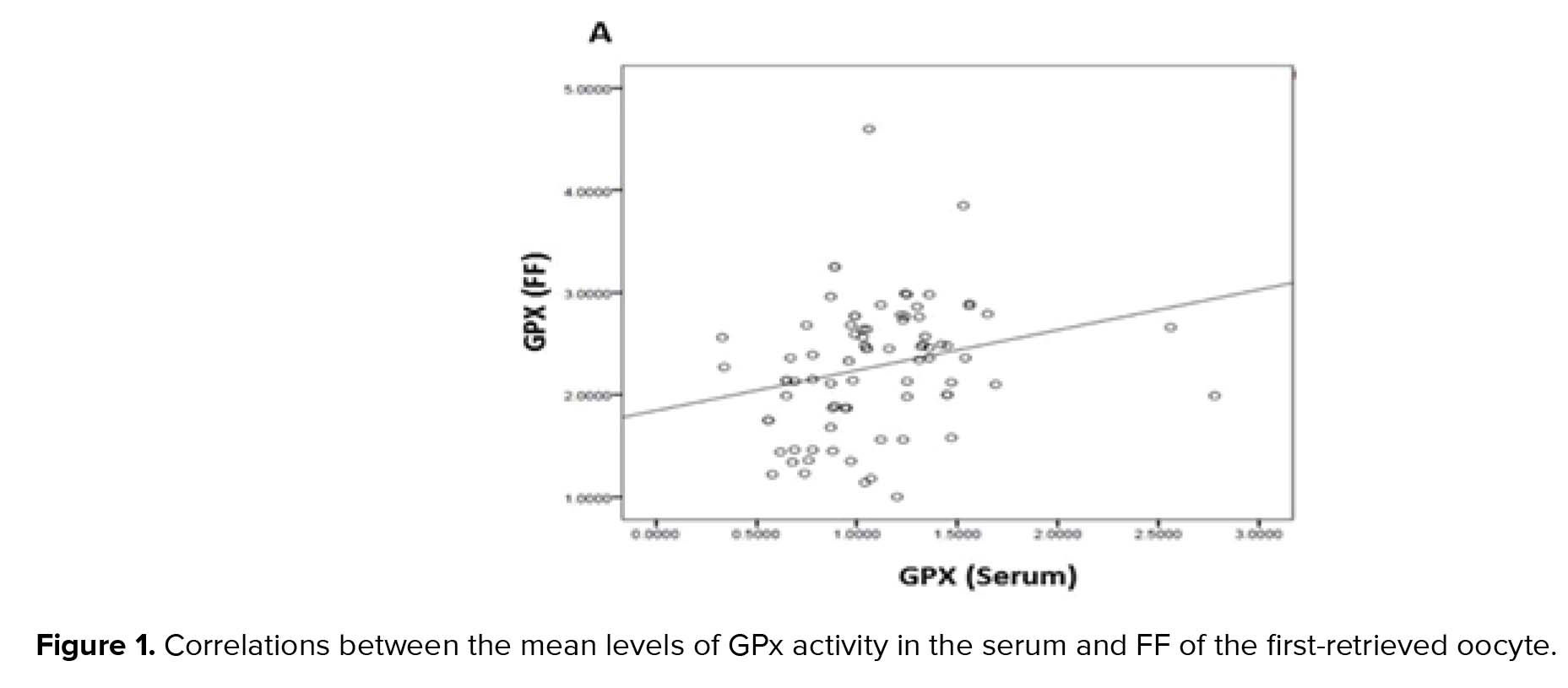
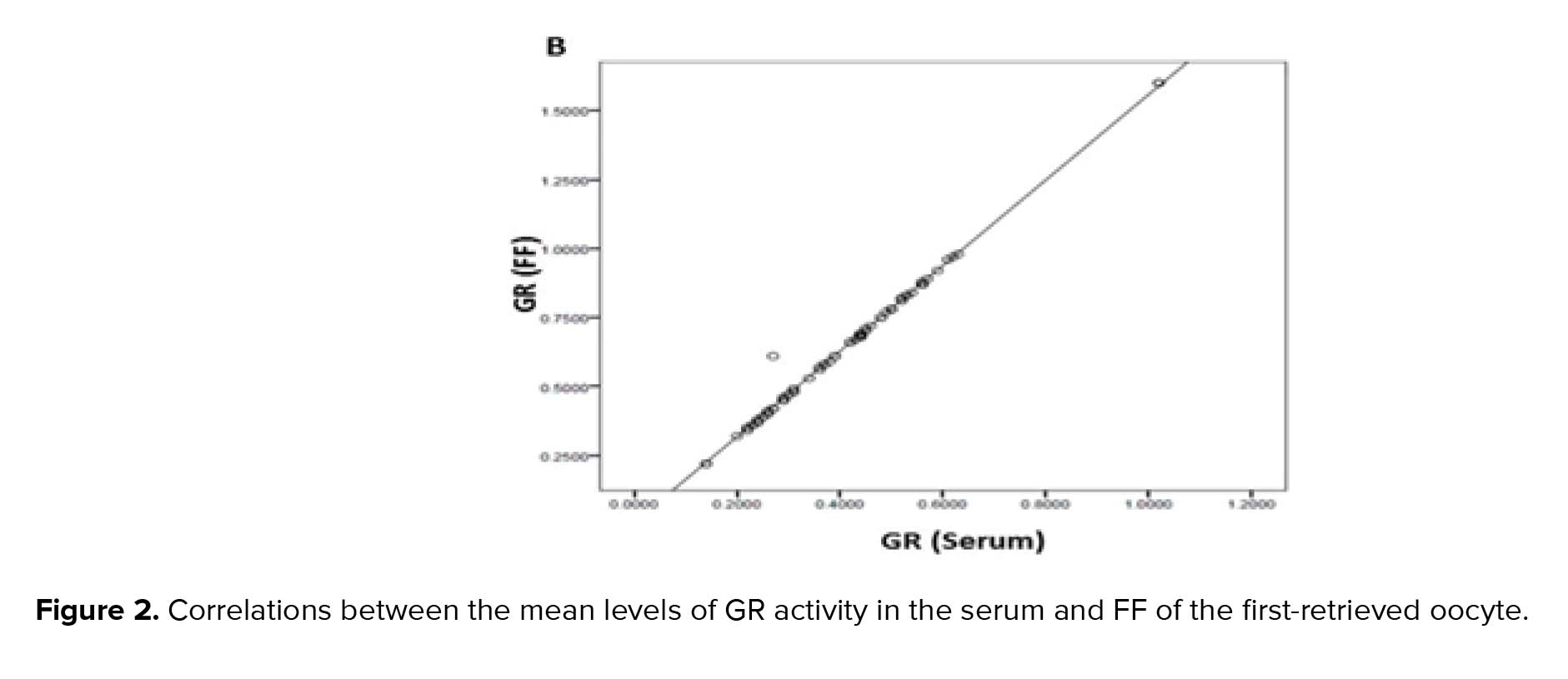
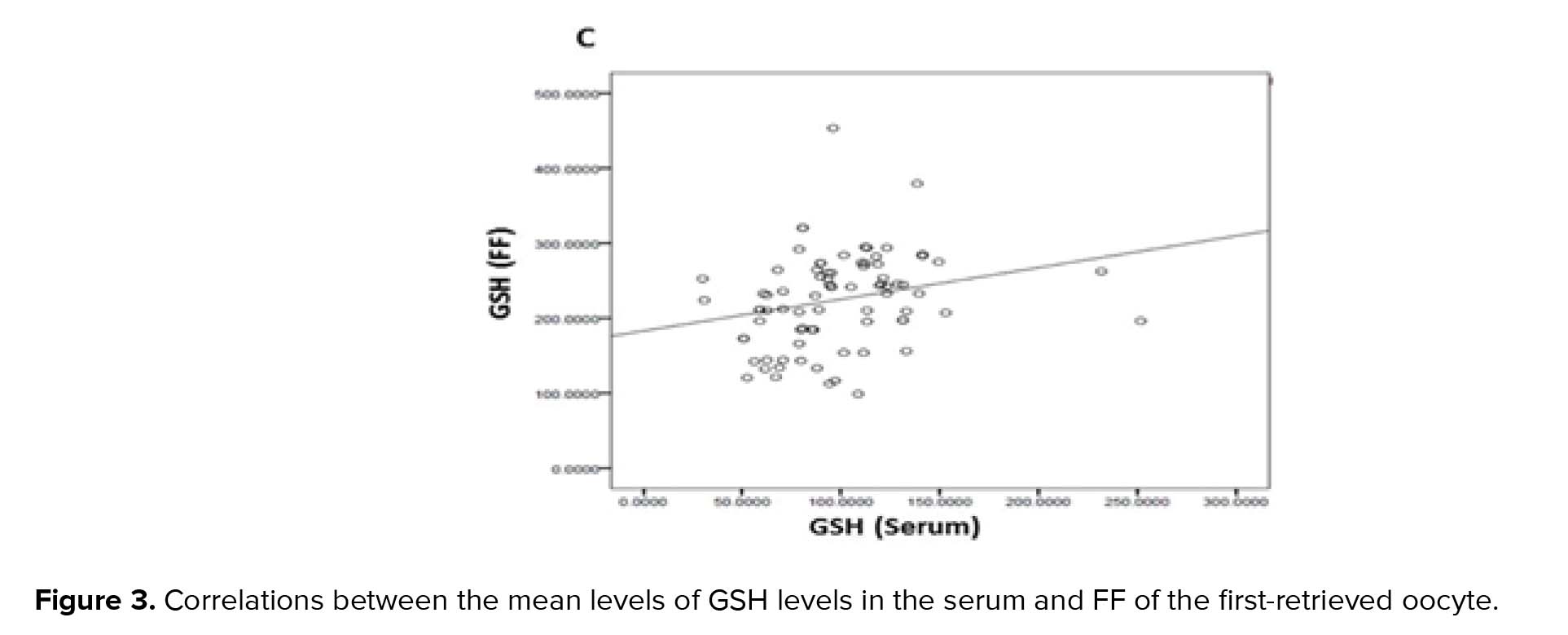
GPx, GR, and GSH are antioxidants that scavenge the free radicals and lipid peroxides to maintain the intracellular balance (23). Our results indicated that the mean levels of all of the antioxidants were higher in the FF compared to serum with a positive correlation between these two biological samples. Similarly, Leroy and colleagues demonstrated that changes of all metabolites in the serum of dairy cows were accompanied by similar changes in their FF especially for glucose and urea (13). However, to the best of our knowledge, this is the first time that the correlation between the serum changes of the GSH-related antioxidants and first-retrieved FF is evaluated.
The biochemical composition of FF has a direct effect on the maturation ability of oocyte and evidence shows that the GSH antioxidant system in FF plays an important role in oocyte maturation and subsequent IVF outcome (12, 24). For instance, reduced OS when the ovarian stimulation was associated with micronutrients supplementation improved the number of good-quality oocytes (25). Kish and colleagues showed that the transcript levels of thioredoxin (a regulating protein of reduction-oxidation reactions) in FF of oocyte-containing follicles was higher compared to empty follicles (26). In agreement with these studies, our study showed that higher GPx and GR activities and GSH levels in the FF were associated with higher oocyte quality, and there was an increasing trend correlating with better oocyte quality.
However, despite an increasing trend in the presence of more developed first-retrieved oocytes, no significant difference was observed in their corresponding serum GPx and GSH levels, except for GR activity which recorded statistically significant values in the serum of MII oocytes when compared to that of GVs.
We hypothesized that GSH-related metabolites in serum played as direct indicators of oocyte quality, which obviously is much more available compared with FF. Nevertheless, our data did not affirm the whole idea. It is worth mentioning that oocyte is in direct contact with its own FF metabolites and therefore oocyte quality and their FF antioxidant levels are tightly related, while the serum antioxidant levels reflect the metabolic condition of the whole body. Moreover, in addition to serum and FF antioxidants capacity, oocyte and embryo are protected by other antioxidant systems (27).
The adequate antioxidant concentrations influence the fertilization rate and are essential for proper embryo development (12, 28, 29). There is a cut-off value for ROS in the FF. Values greater than the cut-off are considered toxic for the embryo formation and negatively impact the pregnancy outcomes (14, 15). It has been shown that in patients with low fertilization rates and low-quality blastocysts, total GSH levels were lower (12). In another study, Vignini and colleagues showed that the concentration of free radical nitric oxide in FF was significantly higher in IVF-produced embryos with severe fragmentations, confirming its detrimental role in embryo quality (16). In the present study, mean levels for serum and FF GPx activity and GSH were significantly higher in grade I embryos compared to grade II and no-embryo groups. The mean GR activity in serum and FF of patients undergone IVF cycle with resulting embryos were significantly higher than no embryo group. These data affirm the role of GSH antioxidant system in the production of good-quality embryos.
To the best of our knowledge, this study is the first to examine the FF of the first-retrieved follicle to demonstrate the association between elevated follicular GPx and GR activities and GSH levels with higher quality oocytes and embryos. In addition, higher serum antioxidant levels were associated with higher embryo qualities in IVF cycles.
Full-Text: (495 Views)
- Introduction
Oxidative stress (OS) refers to an imbalance between produced ROS and detoxifying capacity of antioxidants (4). It is suggested that cellular ROS play an important role in several diseases including atherosclerosis, diabetes, metabolic syndrome, obesity, and impaired reproductive disorders like PCOS (5). How PCOS is related to OS could be explained by the role of antioxidants in folliculogenesis, ovulation, and some other ovarian features. Any disequilibrium in these mechanisms may impair the female reproductive system. Moreover, OS may affect the results of assisted reproductive technologies (ART) (6).
Glutathione (GSH) is an important intracellular antioxidant. It can be converted to its oxidized form (GSSG) by glutathione peroxidase (GPx) when functioning as a scavenger; it is converted back to its reduced form by glutathione reductase (GR) protecting the cell against excessive generation of damaging ROS (7). It is involved in oocyte activation and maturation and development of preimplantation embryos during ART (8).
The developing oocyte is surrounded by complex microenvironment consisting of granulosa, theca cells, and follicular fluid (FF) to form its microenvironment. FF is formed from plasma proteins and through the secretory function of the granulosa and theca cells (9, 10). It is suspected that biochemical specifications of the FF and its ROS threshold affect the oocyte quality, fertilization, embryo development, and pregnancy rate (11, 12). Moreover, the compositions of FF and blood are reciprocally affected by each other (13, 14). It is also suggested that FF is naturally supplied by powerful antioxidant systems to protect the oocyte from OS. Therefore, elevated levels of antioxidants in FF or serum are accompanied by increased chances of high-quality oocytes-retrieved ART and high-quality embryo production (14, 15). Vignini and colleagues showed that the free radical nitric oxide concentrations in FF is significantly higher in in vitro fertilization (IVF)-produced embryos with severe fragmentations, which shows its detrimental impact on embryo quality (16). In a recent study, the negative effect of total non-enzymatic antioxidant capacity and 8-hydroxy-2'-deoxyguanosine in the FF on the number of good-quality embryos was reported (17).
To the best of our knowledge, all relevant studies carried out so far have collected and examined the entire bulk of FF drawn upon ovum pick-up. In the present study, however, only the FF of the first aspirated follicle was examined in order to achieve more precise observations and results on the oocyte maturation stages and their correlations with enzyme levels and activities. Our aim was to show the correlation between the maturation stages of oocytes/embryos and GSH detoxification capacity in FF and serum separately. Moreover, due to difficult FF accessibility, we investigated whether the GSH levels in the serum represented those in the FF and therefore if serum could be used as an indicator of antioxidant activities in the FF.
- Materials and Methods
- 1. Materials
- 2. Subject selection
- 3. Ovarian stimulation protocol
The first-retrieved FF samples that contained more than one oocyte, had no oocyte, or those that became bloody were excluded from the study. This process was carried on until 80 acceptable FF samples were collected. The first aspirated oocytes were graded as GV, MI, or MII according to the standard classifications (19) were placed in standard culture media and monitored for their ongoing development after conventional IVF. Conventional IVF represents standard insemination, which involves combining sperm and egg outside the body in the laboratory and transferring the resulting embryo(s) back into the uterus. Three days post-oocyte retrieval, the resulting embryos were assessed as I, II, and III, where I indicated the highest and III showed the lowest embryo quality (20).
- 4. Collection and preparation of FF
- 5. Determination of total protein concentration
- 6. Determination of GPx and GR activity
"GR activity was assayed by a previously described method (21). It was performed in a cuvette in a total volume of 1 ml that included 60 μM buffer, 5 mM EDTA (pH 8.0), 0.033 M GSSG, 2 mM NADPH, and the sample. The decrease in absorbance, which reflects the oxidation of NADPH during reduction of GSSG by the sample’s GR, was monitored spectrophotometrically at 340 nm for 3 min. Results were based on a molar extinction coefficient for NADPH of 6.22 × 106M-1cm-1. One unit of GR is defined as mU/mg cell protein".
- 7. Determination of the total intracellular reduced GSH
- 8. Ethical consideration
- 9. Statistical analysis
- Results
The mean GPx and GR activity and GSH levels were significantly higher in the FF of the MII oocytes. The mean GPX activity and GSH levels in the serum samples did not show significant statistical difference among different oocyte grades but the mean serum GR activity was significantly higher for MII oocytes compared to GVs (Table II).
GPX activity and GSH levels were significantly higher in the serum and FF of the high-quality embryos with grade I compared to grade II embryo or no embryo group (Table III). Moreover, the mean of GR activity was significantly higher in the serum and FF of grades I and II embryos compared to no embryo group. Examination of serum and FF of grades I and II embryos revealed no significant difference in their GR activities (Table III).






3. Discussion
The microenvironment of the oocyte determines its developmental potential. In this study, we measured GPx and GR activities and GSH levels in the serum and FF of the first-retrieved oocytes of PCOS women who underwent IVF. Subsequently, the oocytes and resulting embryos were graded according to their quality.
GPx, GR, and GSH are antioxidants that scavenge the free radicals and lipid peroxides to maintain the intracellular balance (23). Our results indicated that the mean levels of all of the antioxidants were higher in the FF compared to serum with a positive correlation between these two biological samples. Similarly, Leroy and colleagues demonstrated that changes of all metabolites in the serum of dairy cows were accompanied by similar changes in their FF especially for glucose and urea (13). However, to the best of our knowledge, this is the first time that the correlation between the serum changes of the GSH-related antioxidants and first-retrieved FF is evaluated.
The biochemical composition of FF has a direct effect on the maturation ability of oocyte and evidence shows that the GSH antioxidant system in FF plays an important role in oocyte maturation and subsequent IVF outcome (12, 24). For instance, reduced OS when the ovarian stimulation was associated with micronutrients supplementation improved the number of good-quality oocytes (25). Kish and colleagues showed that the transcript levels of thioredoxin (a regulating protein of reduction-oxidation reactions) in FF of oocyte-containing follicles was higher compared to empty follicles (26). In agreement with these studies, our study showed that higher GPx and GR activities and GSH levels in the FF were associated with higher oocyte quality, and there was an increasing trend correlating with better oocyte quality.
However, despite an increasing trend in the presence of more developed first-retrieved oocytes, no significant difference was observed in their corresponding serum GPx and GSH levels, except for GR activity which recorded statistically significant values in the serum of MII oocytes when compared to that of GVs.
We hypothesized that GSH-related metabolites in serum played as direct indicators of oocyte quality, which obviously is much more available compared with FF. Nevertheless, our data did not affirm the whole idea. It is worth mentioning that oocyte is in direct contact with its own FF metabolites and therefore oocyte quality and their FF antioxidant levels are tightly related, while the serum antioxidant levels reflect the metabolic condition of the whole body. Moreover, in addition to serum and FF antioxidants capacity, oocyte and embryo are protected by other antioxidant systems (27).
The adequate antioxidant concentrations influence the fertilization rate and are essential for proper embryo development (12, 28, 29). There is a cut-off value for ROS in the FF. Values greater than the cut-off are considered toxic for the embryo formation and negatively impact the pregnancy outcomes (14, 15). It has been shown that in patients with low fertilization rates and low-quality blastocysts, total GSH levels were lower (12). In another study, Vignini and colleagues showed that the concentration of free radical nitric oxide in FF was significantly higher in IVF-produced embryos with severe fragmentations, confirming its detrimental role in embryo quality (16). In the present study, mean levels for serum and FF GPx activity and GSH were significantly higher in grade I embryos compared to grade II and no-embryo groups. The mean GR activity in serum and FF of patients undergone IVF cycle with resulting embryos were significantly higher than no embryo group. These data affirm the role of GSH antioxidant system in the production of good-quality embryos.
To the best of our knowledge, this study is the first to examine the FF of the first-retrieved follicle to demonstrate the association between elevated follicular GPx and GR activities and GSH levels with higher quality oocytes and embryos. In addition, higher serum antioxidant levels were associated with higher embryo qualities in IVF cycles.
4. Conclusion
According to our results, GSH -dependent antioxidant system acts more efficiently in the advanced maturation stages of oocytes and embryos and the antioxidants quantities in the FF can be potential predictors of oocyte and embryo quality.
Acknowledgments
This study was partly extracted from a thesis written by Dr. Pardis Ahmadi and was financially supported by Shiraz University of Medical Sciences (Grant number: 91-01-01-4622). The authors wish to extend their special thanks to Dr. Najmeh Moein Vaziri for her contribution to the manuscript edition.
Conflict of Interest
The authors declare that they have no conflict of interest.
According to our results, GSH -dependent antioxidant system acts more efficiently in the advanced maturation stages of oocytes and embryos and the antioxidants quantities in the FF can be potential predictors of oocyte and embryo quality.
Acknowledgments
This study was partly extracted from a thesis written by Dr. Pardis Ahmadi and was financially supported by Shiraz University of Medical Sciences (Grant number: 91-01-01-4622). The authors wish to extend their special thanks to Dr. Najmeh Moein Vaziri for her contribution to the manuscript edition.
Conflict of Interest
The authors declare that they have no conflict of interest.
Type of Study: Original Article |
Subject:
Fertility & Infertility
References
1. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004; 81: 19-25. [DOI:10.1016/j.fertnstert.2003.10.004]
2. Wei D, Xie J, Yin B, Hao H, Song X, Liu Q, et al. Significantly lengthened telomere in granulosa cells from women with polycystic ovarian syndrome (PCOS). J Assist Reprod Genet 2017; 34: 861-866. [DOI:10.1007/s10815-017-0945-z] [PMID] [PMCID]
3. Papalou O, Victor VM, Diamanti-Kandarakis E. Oxidative stress in polycystic ovary syndrome. Curr Pharm Des 2016; 22: 2709-2722. [DOI:10.2174/1381612822666160216151852] [PMID]
4. Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol 2010; 42: 1634-1650. [DOI:10.1016/j.biocel.2010.06.001] [PMID]
5. Banuls C, Rovira-LIopis S, Martinez de Maranon A, Veses S, Jover A, Gomez M, et al. Metabolic syndrome enhances endoplasmic reticulum, oxidative stress and leukocyte-endothelium interactions in PCOS. Metabolism 2017; 71: 153-162. [DOI:10.1016/j.metabol.2017.02.012] [PMID]
6. de Melo AS, Rodrigues JK, Junior AA, Ferriani RA, Navarro PA. Oxidative stress and polycystic ovary syndrome: an evaluation during ovarian stimulation for intracytoplasmic sperm injection. Reproduction 2017; 153: 97-105. [DOI:10.1530/REP-16-0084] [PMID]
7. Rani V, Deep G, Singh RK, Palle K, Yadav UC. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci 2016; 148: 183-193. [DOI:10.1016/j.lfs.2016.02.002] [PMID]
8. Ashibe S, Miyamoto R, Kato Y, Nagao Y. Detrimental effects of oxidative stress in bovine oocytes during intracytoplasmic sperm injection (ICSI). Theriogenology 2019; 133: 71-78. [DOI:10.1016/j.theriogenology.2019.04.012] [PMID]
9. Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: From single biochemical markers to metabolomics. Reprod Biol Endocrinol 2009; 7: 40-52. [DOI:10.1186/1477-7827-7-40] [PMID] [PMCID]
10. Ambekar AS, Kelkar DS, Pinto SM, Sharma R, Hinduja I, Zaveri K, et al. Proteomics of follicular fluid from women with polycystic ovary syndrome suggests molecular defects in follicular development. J Clin Endocrinol Metab 2015; 100: 744-753. [DOI:10.1210/jc.2014-2086] [PMID] [PMCID]
11. Seleem AK, El Refaeey AA, Shaalan D, Sherbiny Y, Badway A. Superoxide dismutase in polycystic ovary syndrome patients undergoing intracytoplasmic sperm injection. J Assist Reprod Genet 2014; 31: 499-504. [DOI:10.1007/s10815-014-0190-7] [PMID] [PMCID]
12. Nishihara T, Matsumoto K, Hosoi Y, Morimoto Y. Evaluation of antioxidant status and oxidative stress markers in follicular fluid for human in vitro fertilization outcome. Reprod Med Biol 2018; 17: 481-486. [DOI:10.1002/rmb2.12229] [PMID] [PMCID]
13. Leroy JL, Vanholder T, Delanghe JR, Opsomer G, Van Soom A, Bols PE, et al. Metabolic changes in follicular fluid of the dominant follicle in high-yielding dairy cows early post partum. Theriogenology 2004; 62: 1131-1143. [DOI:10.1016/j.theriogenology.2003.12.017] [PMID]
14. Jana SK, Narendra Babu K, Chattopadhyay R, Chakravarty B, Chaudhury K. Upper control limit of reactive oxygen species in follicular fluid beyond which viable embryo formation is not favorable. Reprod Toxicol 2010; 29: 447-451. [DOI:10.1016/j.reprotox.2010.04.002] [PMID]
15. Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: A review. Reprod Biol Endocrinol 2012; 10: 49-79. [DOI:10.1186/1477-7827-10-49] [PMID] [PMCID]
16. Vignini A, Turi A, Giannubilo SR, Pescosolido D, Scognamiglio P, Zanconi S, et al. Follicular fluid nitric oxide (NO) concentrations in stimulated cycles: the relationship to embryo grading. Arch Gynecol Obstet 2008; 277: 229-232. [DOI:10.1007/s00404-007-0445-y] [PMID]
17. Vàrnagy A, Koszegi T, Gyorgyi E, Szegedi S, Sulyok E, Prèmusz V, et al. Levels of total antioxidant capacity and 8-hydroxy-2'-deoxyguanosine of serum and follicular fluid in women undergoing in vitro fertilization: focusing on endometriosis. Hum Fertil 2018; 13: 1-9. [DOI:10.1080/14647273.2018.1535719] [PMID]
18. Lu JC, Huang YF, Lu NQ. [WHO laboratory manual for the examination and processing of human semen: Its applicability to andrology laboratories in china]. Zhonghua Nan Ke Xue 2010; 16: 867-871. (in China)
19. Veeck LL. Oocyte assessment and biological performance. Ann N Y Acad Sci 1988; 541: 259-274. [DOI:10.1111/j.1749-6632.1988.tb22263.x] [PMID]
20. Veeck LL. An atlas of human gametes and conceptuses: All illustrated reference for assisted reproductive technology (the encyclopedia of visual medicine series). 1st Ed. CRC Press, USA; 1999. [DOI:10.1201/b14639]
21. Mostafavi-Pour Z, Khademi F, Zal F, Sardarian AR, Amini F. In vitro analysis of csa-induced hepatotoxicity in hepg2 cell line: Oxidative stress and α2 and β1 integrin subunits expression. Hepat Mon 2013; 13: e11447. [DOI:10.5812/hepatmon.11447]
22. Mashhoody T, Rastegar K, Zal F. Perindopril may improve the hippocampal reduced glutathione content in rats. Adv Pharm Bull 2014; 4: 155-159.
23. Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol 2005; 3: 28-48. [DOI:10.1186/1477-7827-3-28] [PMID] [PMCID]
24. O'Gorman A, Wallace M, Cottell E, Gibney MJ, McAuliffe FM, Wingfield M, et al. Metabolic profiling of human follicular fluid identifies potential biomarkers of oocyte developmental competence. Reproduction 2013; 146: 389-395. [DOI:10.1530/REP-13-0184] [PMID]
25. Luddi A, Capaldo A, Focarelli R, Gori M, Morgante G, Piomboni P, et al. Antioxidants reduce oxidative stress in follicular fluid of aged women undergoing IVF. Reprod Biol Endocrinol 2016; 14: 57-63. [DOI:10.1186/s12958-016-0184-7] [PMID] [PMCID]
26. Kish I, Ohishi M, Akiba Y, Asada H, Konishi Y, Nakano M, et al. Thioredoxin, an antioxidant redox protein, in ovarian follicles of women undergoing in vitro fertilization. Endocr J 2016; 63: 9-20. [DOI:10.1507/endocrj.EJ15-0210] [PMID]
27. Zuelke KA, Jeffay SC, Zucker RM, Perreault SD. Glutathione (GSH) concentrations vary with the cell cycle in maturing hamster oocytes, zygotes, and pre-implantation stage embryos. Mol Reprod Dev 2003; 64: 106-112. [DOI:10.1002/mrd.10214] [PMID]
28. Pasqualotto EB, Lara LV, Salvador M, Sobreiro BP, Borges E, Pasqualotto FF. The role of enzymatic antioxidants detected in the follicular fluid and semen of infertile couples undergoing assisted reproduction. Hum Fertil 2009; 12: 166-171. [DOI:10.1080/14647270903207941] [PMID]
29. Agarwal A, Majzoub A. Role of antioxidants in assisted reproductive techniques. World J Mens Health 2017; 35: 77-93. [DOI:10.5534/wjmh.2017.35.2.77] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





