BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijrm.ir/article-1-1471-en.html


 , Farzaneh Darbeheshti2
, Farzaneh Darbeheshti2 

 , Seyed Mehdi Kalantar3
, Seyed Mehdi Kalantar3 

 , Atiyeh Javaheri4
, Atiyeh Javaheri4 

 , Seyed Hamidreza Mirabutalebi5
, Seyed Hamidreza Mirabutalebi5 

 , Mohammad Hasan Sheikhha *6
, Mohammad Hasan Sheikhha *6 


2- Department of Medical Genetics, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran. Breast Cancer Association (BrCA), Universal Scientific Education and Research Network (USERN), Tehran, Iran.
3- Abortion Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
4- Department of Obstetrics and Gynecology, Faculty of Medicine, Shahid Sadoughi Hospital, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
5- Abortion Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. Student Research Committee, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
6- Abortion Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. ,
-
Introduction
Endometriosis is a common estrogen-dependent benign disease, characterized by pelvic pain, dysmenorrhea, and infertility (1). It occurs when endometrial tissue exists outside the uterus, and affects 10-15% of women in the reproductive age (2). Although the underlying pathogenesis of endometriosis is still not fully understood, the available body of evidence indicate that both genetics and environmental factors contribute to the susceptibility and progression of endometriosis. While endometriosis is considered as a benign disorder, some cases may represent risk factors for developing estrogen-related malignancies such as ovarian, endometrial, and breast cancers (3-5). However, other types of endometriosis-associated cancers have been rarely reported (6, 7).
Molecular studies have shown a large number of dysregulated transcripts (both coding and noncoding) in the ectopic (Ec-p) and eutopic (Eu-p) endometrial samples from the women with endometriosis (8-11). miRNAs are highly conserved 21-nucleotide single-stranded noncoding RNAs that can bind to target mRNAs and regulate their expression (12). These small noncoding RNAs have a regulatory function in various cellular processes such as proliferation, migration, cell cycle, and apoptosis (13-15). Remarkable dysregulation of miRNAs in diseases results in using them as biomarkers for early diagnosis (16). In addition, miRNAs and their targets can be used for therapeutic purposes (17).
The dysregulation of miR-125 family, as either repressors or promoters, has been seen in several diseases (18). Moreover, a significant upregulation of miR-125b has been found in endometriosis and different cancers (19). In one study, mir-125b in serum samples of women with endometriosis showed more than 10-fold upregulation compared with women without endometriosis (20). It has been suggested that mir-125b plays pivotal role in molecular pathways driving cell proliferation and migration that are the crucial steps for initiating endometriosis and related malignancies (21, 22).
In this study, the co-expression meta-analysis of miRNA targets (CoMeTa) database was used to identify the potential miR-125b targets. After that, the protein-protein interaction (PPI) network of co-expressed miR-125b targets revealed TP53 gene as a hub gene, and showed its involvement in the functional module. These pieces of evidence clearly suggest that among potential miR-125b targets, TP53 has noticeable roles in cellular processes (Figure 1). It was reported that miR-125b caused downregulation of TP53 by binding to a microRNA element in the 3’ untranslated region of TP53 mRNA (23). For example, it was shown that the overexpression of miR-125b in human neuroblastoma suppresses apoptosis by downregulation of TP53. However, in the contrary, reducing the miR-125b level in human lung fibroblasts causes overexpression of TP53 which in turn induces apoptosis (23).
The most prominent roles of TP53 are cell-cycle and apoptosis regulation. Previous studies have shown dysregulation of TP53 in endometriosis and endometrial cancer (24, 25). In addition, two associated studies have revealed the link between TP53 polymorphism and the risk of endometriosis (26, 27). Interestingly, TP53 has been validated as a miR-125b target in several investigations by different methods, including reporter assay, western blot, and qPCR (23). However, the dysregulation of miR-125b and TP53 between ectopic and eutopic endometrial samples has rarely been investigated (28).
Taken together, the relationship between endometriosis, cancer risk, miR-125b, and TP53 encouraged us to compare the expression of both miR-125b and TP53 in three kinds of samples with each other (Ec-p, Eu-p, and Normal). In addition, we used the bioinformatics evidence to investigate that if TP53, among all the potential miR-125b targets, has noticeable roles in cellular processes or not?

2. Materials and Methods
2.1. PPI network construction
In order to provide a comprehensive, genome-scale analysis of miR-125b regulatory networks based on its co-expressed targets; co-expression meta-analysis of miRNA Targets (CoMeTa) database was used (26). It is hypothesized that the targets of miR-125b are co-expressed with each other, and they belong to the same gene-regulatory network. In this database, three sequence-based prediction tools including miRanda, PicTar, and TargetScan are used. We have kept the top 100 ranks of co-expressed target genes. Then, STRING database v10.5 (functional protein association networks) was run to identify the protein interactions between them (29). In the next step, Cytoscape v3.6.1 was applied to visualize the PPI networks (30). Finally, the Molecular Complex Detection (MCODE), a Cytoscape app, was used to identify the module (31).
2.2. Functional enrichment analysis
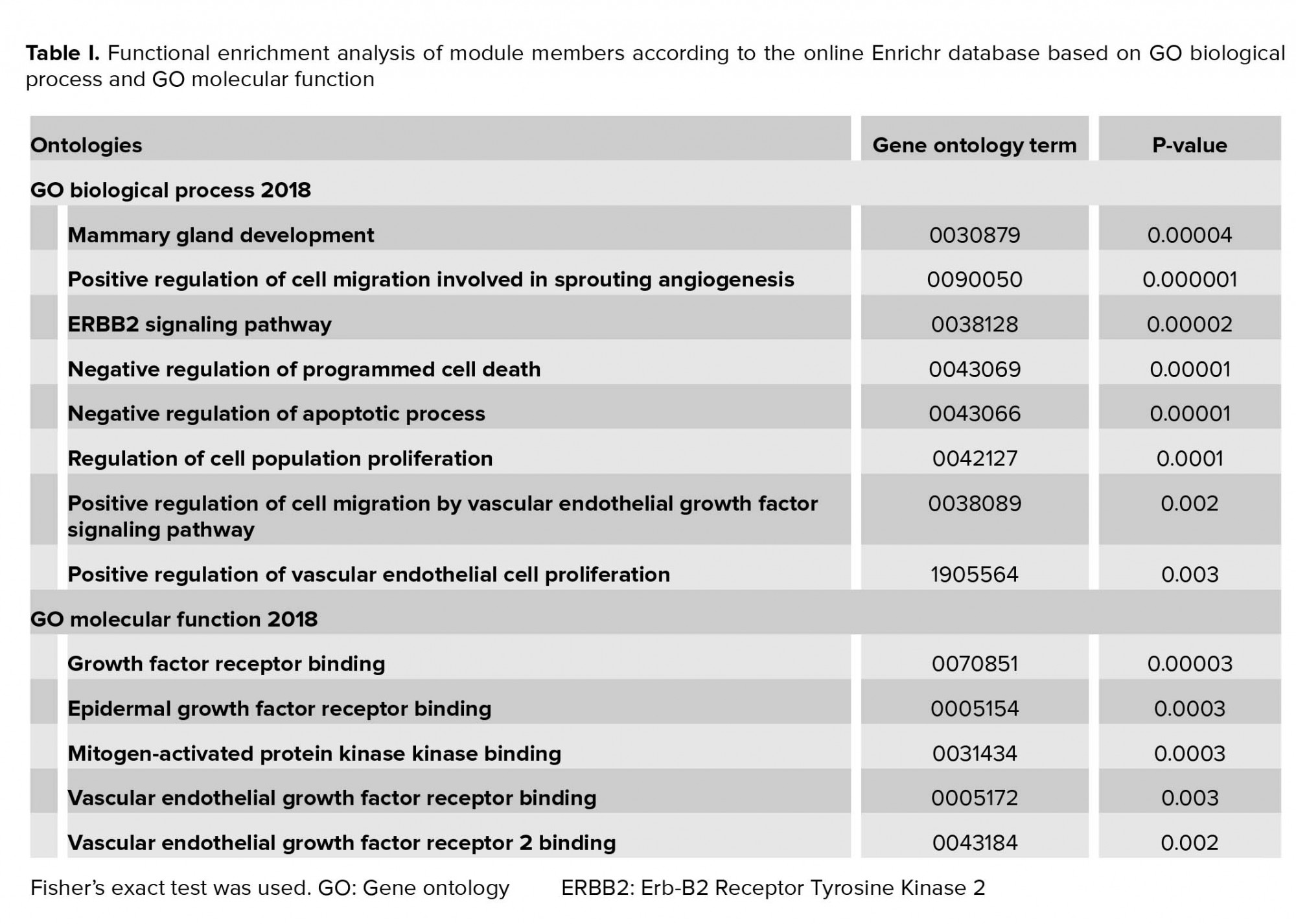
The Enrichr web server was applied for functional annotation of module members based on GO biological process and GO molecular function (32). P-value < 0.05 was considered to be significant enrichment.
2.3. Sample collection
In this case-control study, the eutopic and ectopic samples were collected from women with regular menstrual cycles in the late luteal phase and referred to the Shahid Sadoughi Hospital, Yazd, Iran. Case samples were obtained from endometriosis patients who underwent laparoscopic surgery. Two biopsies were randomly removed from the endometriosis tissues (Ec-p, N = 20), and the endometrium inside the uterus (Eu-p, N = 20). The diagnosis of endometriosis in case samples was confirmed by histology in all women with the disease. The control subjects (Normal, N = 20) consisted of those women who had no evidence of endometriosis but were referred to the hospital for reasons such as fallopian tube blockage and pelvic pain and in whom no evidence of pelvic inflammation or any reproductive system diseases were found after laparoscopy. The mean age in the case group was 32 ± 2 yr (20-45 yr) and in the control group was 36 ± 2 yr (22-43 yr). Women aged 20-45 yr with regular menstrual cycles (28 to 32 days) were included in the study. All samples were stored in RNase free microtubes containing RNAlater™ Stabilization Solution (Thermo Fisher Scientific) at -80°C for later use. The exclusion criteria, on the other hand, were hormone therapy usage within 3 months before surgery, pregnancy, cancer, and contamination with common DNA viruses and human papilloma virus as well. Cancer patients were excluded because we wanted to find the relation with endometriosis as a cancer-like features and the genes under study and not the cancer itself.
2.4. RNA extraction and cDNA synthesis
The total RNA was extracted from samples using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. The ratio of absorbance at 260 nm and 280 nm (A260/280) was used to assess RNA purity and quantity by spectrophotometer. Revert Aid First Strand cDNA Synthesis Kit (Fermentase, USA) was applied to synthesize cDNA of mRNA. Additionally, the Bon-Mir RT kit (Bonyakhteh, Tehran, Iran) was used to synthesize cDNA of miRNA based on the manufacturer’s instructions. The resultant cDNA mixtures were stored at -20ºC.
2.5.Gene expression study
Real-time PCR was performed using the commercial master mix (Takara, Japan). The sequences of primers were: SNORD forward 5´-ATCACTGTAAAACCGTTCCA-3´, miR-125b forward primer as well as universal reverse primers for both miR-125b and SNORD were obtained from Bonyakhteh (Bonyakhteh, Tehran, Iran), TP53 forward 5´-GCTCAGATAGCGATGGTCTGG-3´, TP53 reverse 5´-CTGTCATCCAAATACTCCACACG-3´, GAPDH forward 5´-CTCATTTCCTGGTATGACAACGA-3´, GAPDH reverse 5´-TCTTCCTCTTGTGCTCTTGCTG-3´. Moreover, the miR-125b and TP53 expression levels were normalized by SNORD and GAPDH expression, respectively. Reaction mixture included 2 μl cDNA templates, 0.5 μl forward and reverse primers, 7 μl ribonuclease-free water, and 10 μl commercial master mix for a final reaction volume of 20 μl. The thermal cycling conditions were initiated with denaturation at 95ºC for 10 min, and then 40 cycles at 95ºC for 15 sec, and annealing at 60ºC for 20 sec. For miR-125b detection, the real-time PCR profile was as described earlier, except for a melting temperature of 57ºC. Each reaction was performed in duplicate, and expression levels were evaluated using two (-∆ct).
2.6. Cancer-related pathway analysis of miR-125b
DIANA miRPath was run to miR-125b pathway analysis based on experimentally validated miRNA interactions derived from TarBase database (33). Cancer-related KEGG pathways were extracted and visualized by Cytoscape software.
2.7. Ethical consideration
This study was approved by the ethics committee of Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Science, Yazd, Iran (code: IR.SSU.MEDICIN.REC.1396.151) and written informed consents were obtained from all individuals.
2.8. Statistical analysis
Statistical calculations were performed using the SPSS (Statistical Package for the Social Sciences, version 21.0, SPSS Inc, Chicago, Illinois, USA) software. Data were presented with mean and 95% CI. Kruskal-Wallis ANOVA was applied to analyze miR-125b or TP53 expression among case-eutopic, case-ectopic, and control tissues. Mann-Whitney U-test was used to analyze expression between two groups. Data were considered significant when p < 0.05 was obtained. The Pearson’s correlation statistic was used to evaluate the linear correlation between TP53 and miR-125b expression. The linear regression model was applied to evaluate the linear effect of miR-125b expression on TP53 expression.
3. Results
3.1. PPI network of predicted miR-125b targets
CoMeTa data were used to provide miR-125b regulatory network based on its co-expressed targets. PPI network has been constructed for potential miR-125b targets, which are co-expressed by STRING database. STRING outputs were further visualized using Cytoscape (Figure 1a). TP53 with the highest degree (the number of incoming and outgoing edges) and between centrality (a measure of centrality in a graph based on shortest paths) was selected as a hub gene. After that, the top module was extracted by MCODE. The results showed that TP53 gene is included in the top module (Figure 1b).
3.2. Functional enrichment analysis of module
All the module members were uploaded into the online Enrichr database to investigate the GO categories. The results revealed that module members are significantly enriched in cell proliferation, apoptosis, cell migration, and cell signaling (Table I).
3.3. miR125b and TP53 expression in endometriosis
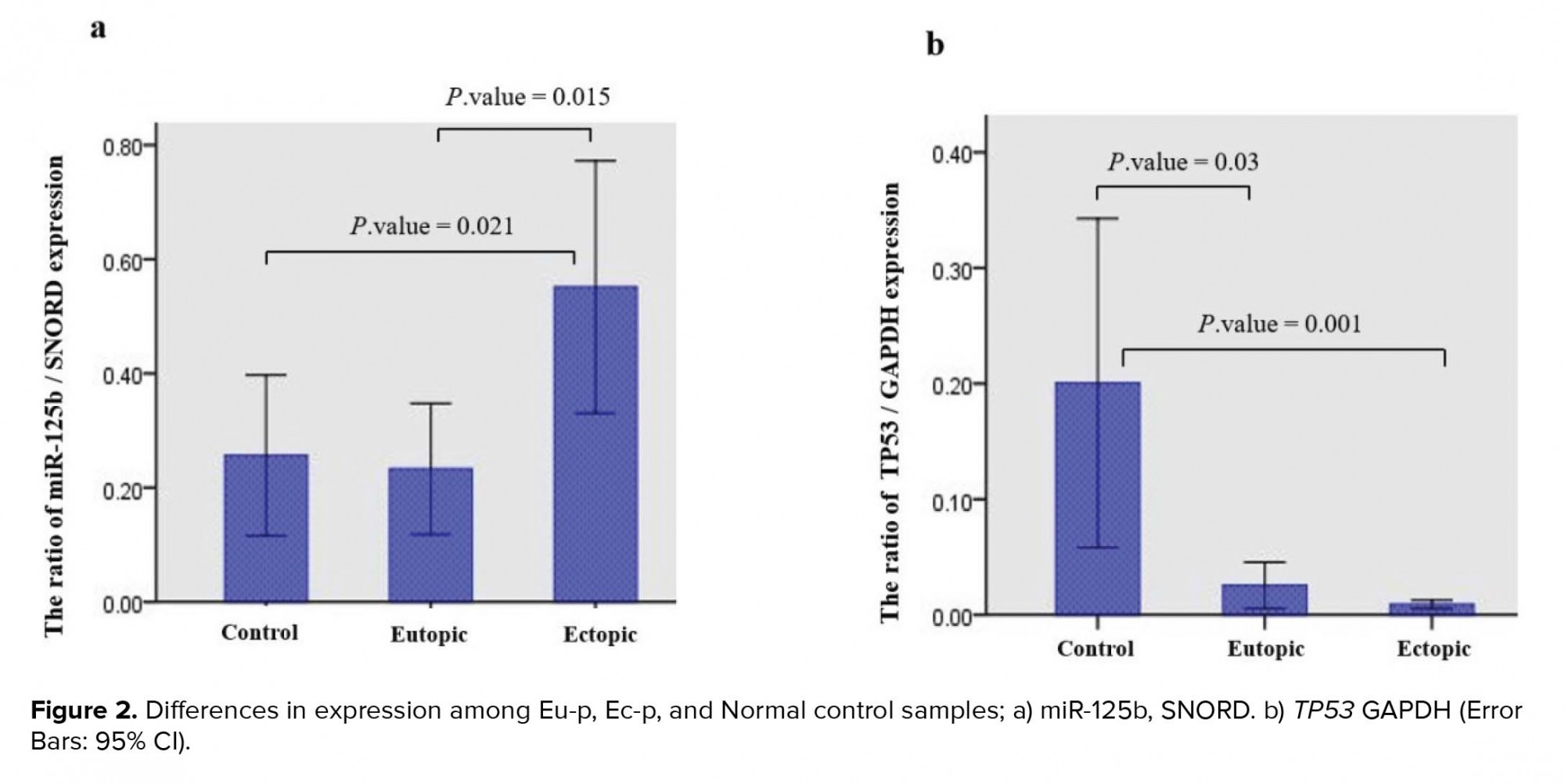
The analysis of miR125b and TP53 expression in Eu-p and Ec-p as well as Normal tissues by Kruskal-Wallis test indicated that both miR125b (p = 0.024) and TP53 (p = 0.003) expression were significantly different between cases (Eu-p and Ec-p) and Normal control samples. miR-125b in the Ec-p and TP53 in the Normal control tissues had the highest expression levels. In addition, Mann-Whitney test outputs revealed that miR-125b expression was significantly different between the Eu-p and Ec-p tissues (p = 0.015) as well as between the Ec-p and Normal control tissues (p = 0.021). Concerning TP53 expression, Mann-Whitney test indicated that its expression was significantly different between the Eu-p and Normal control tissues (P-value = 0.03) as well as between the Ec-p and Normal control tissues (p = 0.001) (Figure 2).
3.4. Correlation between TP53 and miR-125b expression
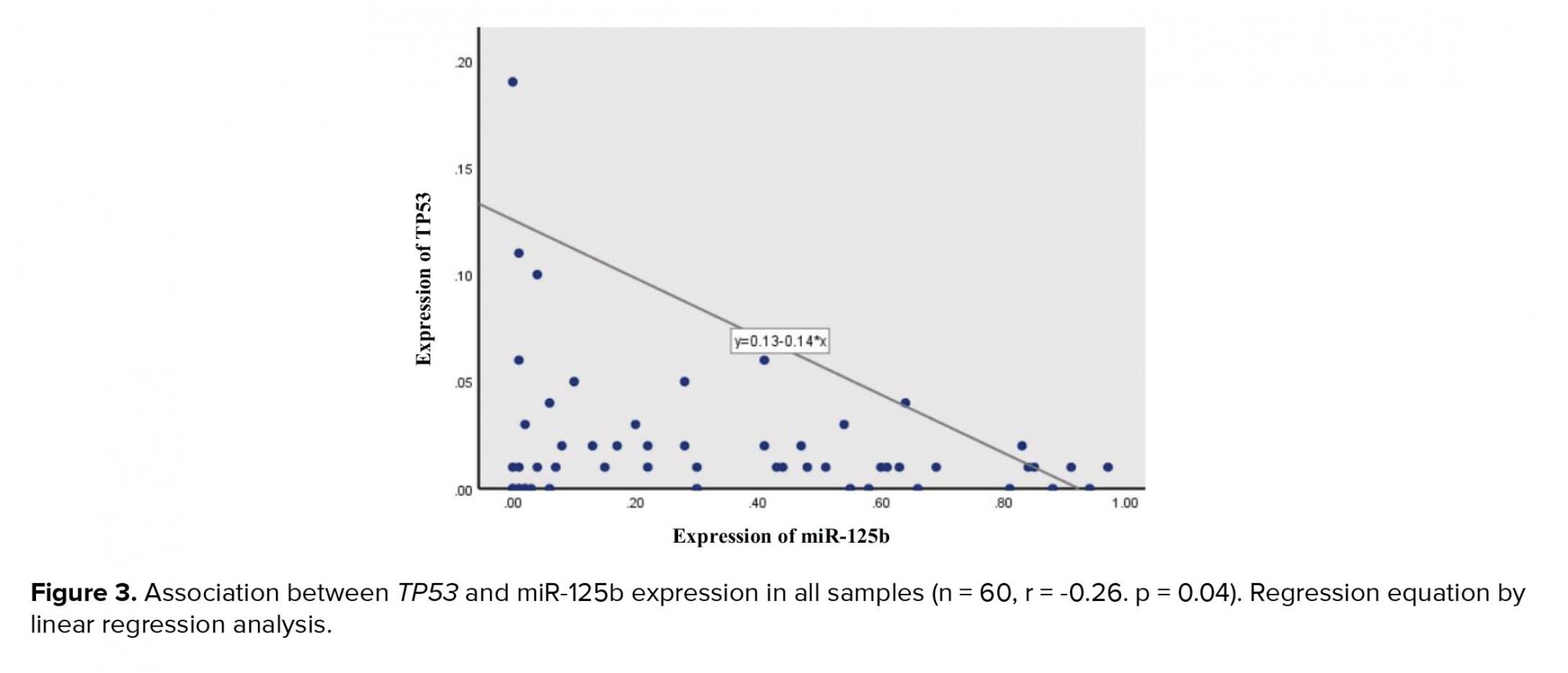
A negative correlation was seen between the expression of the two targeted genes (r = -0.2, p = 0.04) among all samples (Figure 3). The linear regression model revealed that for every 1 unit additional miR-125b expression in samples, we would expect to see a 0.14-unit decreseas in the TP53 expression (Figure 2).
3.5. Cancer-related KEGG pathways of miR-125b
DIANA miRPath database revealed that miR-125b has several experimentally validated target genes, which are involved in cancer-related KEGG pathways, such as endometrial and ovarian cancers. Among the miR-125b-validated targets, TP53 is associated with more cancer-related pathways (Figure 3).
4. Discussion
Endometriosis is a common chronic disease and affects about 10-15% of women of reproductive age. This disease is characterized by the growth of endometriotic tissue outside the uterine. Although endometriosis is generally considered as a benign condition, it shows a risk for transformation and becoming cancerous (34). miRNAs, as a non-coding regulatory RNAs, are involved in post-transcriptional gene regulation. Dysregulation of miRNAs in endometriosis and cancers have been reported in the previous studies (35, 36). Hawkins and colleagues have shown several upregulated and downregulated miRNAs in endometriomas compared with the normal endometrium (37). Also, Ohlsson Teague and co-workers have found dysregulated miRNAs between pairs of ectopic and eutopic endometrial tissues (19). These investigations indicate the roles of miRNAs in the development of endometriosis.
Phenotypic resemblance between endometriotic and malignant cells such as uncontrolled cell growth and decreased apoptosis indicates their common molecular features. In addition, it is suggested that miRNAs and their targets can be considered as effectors in both endometriosis and endometriosis-associated cancers. The identification of dysregulated miRNAs and their targets in the eutopic and ectopic endometrium is an essential step toward understanding molecular links between endometriosis and cancers. Moreover, this information can be useful for developing therapeutic strategies. In the current study, in the first step, we selected miR-125b and investigated its potential targets with bioinformatic approach. In the previous studies, miR-125b had shown a significant upregulation in both endometriotic tissues and serum samples of women with endometriosis (19, 20). In addition, the role of this microRNA as an oncomiR have been reported in cancers. Zhou and co-authors indicated that mir-125b acts as an apoptosis suppressor in breast cancers (38). Furthermore, Bousquet and colleagues suggested that miR-125b confers a proliferative advantage to the leukemic cells (39). Similarly, Xia et al demonstrated that overexpression of miR-125b promotes human glioma cell proliferation (40).
Here, miR-125b regulatory network was investigated and PPI network was constructed based on its co-expressed potential targets. These analyses indicate that TP53 is a remarkable potential target for miR-125b. Moreover, the top module in the PPI network includes TP53 protein (Figure 1). Module-based functional pathway enrichment analysis has revealed roles of TP53 and other members of module in different cancer-related pathways such as regulations of cell migration, of vascular endothelial cell proliferation, and of apoptotic process (Table I). As far as the association between endometriosis and cancer is concerned, our bioinformatic analyses point out that TP53 is a potential appropriate target for miR-125b. Interestingly, the interaction between miR-125b and TP53 miRNA has been validated in human neuroblastoma, myeloid, and cardiac fibroblasts cells (23, 41, 42). It is shown that the loss of miR-125b during zebrafish embryogenesis leads to aberrant apoptosis due to overexpression of TP53. Jiang and colleagues proposed that miR-125b inhibits TP53 network activity by regulating the dose of both proliferative and apoptotic regulators (43). In the present study, the expression of miR-125b and TP53 in eutopic and ectopic endometrium of women with endometriosis as well as normal control tissues were explored. Our results revealed a negative correlation between miR-125b and TP53 expression in the three types of samples. These findings suggest that miR-125b regulates TP53 expression in endometrium. The ectopic tissues showed the highest expression of miR-125b and the lowest expression of TP53.
Furthermore, several studies have reported inhibitory roles of TP53 in cell invasion and metastasis (44, 45). This finding can explain the characteristics of ectopic endometrium such as greater ability of migration and invasion compared with eutopic endometrium (46). Our data demonstrated that expression of both miR-125b and TP53 is significantly different between ectopic endometrium and normal control samples. Moreover, while there was no overexpression of miR-125b in eutopic samples, it significantly showed low expression of TP53 compared with normal samples. This could be due to an overexpression of miR-125b in Ec-P samples and its effect on all of the patient’s tissues including Eu-p tissues. Consistently, Allavena and co-workers showed that the expression of TP53 decreases in the ectopic endometrium of patients compared with eutopic and normal samples (25).
Our results, consistent with previous studies, revealed that TP53 expression decreases in benign endometriotic cysts compared with normal controls. This observation can suggest that alterations in the TP53 expression, as a tumor suppressor gene, may be involved in the same molecular features between endometriosis and cancers. Our results, according to previous studies, demonstrates the existence of negative correlation between the expression of miR-125b and TP53 in both the case and control samples (Figure 3).
Finally, in order to investigate the potential roles of miR-125b in endometriosis-associated cancers, we constructed cancer-related KEGG pathways of validated targets of miR-125b. Among the miR-125b-validated targets, such as TP53, STAT3, E2F3, CDKN2A, AKT1, and ERBB2, TP53 shows association with more cancer-related pathways (Figure 4). A combination of this analysis and our experimental data indicates multiple roles of miR-125b, which may underlie endometriosis-associated cancers. However, since both tumor suppressor genes and oncogenes are seen among validated targets of miR-125b, it is proposed that miR-125b has an opposing function as an oncogene and a tumor suppressor in different cell contexts. Further studies are necessary for investigating the association between miR125b expression and developing endometriosis-associated cancers in women with endometriosis.

5. Conclusion
In conclusion, the negative correlation between miR-125b and TP53 as well as the noticeable decreased expression of TP53 in both eutopic and ectopic samples compared with Normal controls may be interpreted in roles of miR-125b/TP53 axis in the pathogenesis of endometriosis. In addition, these finding and bioinformatics analyses imply a possible role of miR-125b in the connection between endometriosis and malignant transformation.
Acknowledgments
This study was supported by the Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. Samples were provided by the Yazd Reproductive Sciences Institute, Yazd, Iran.
Conflict of Interest
The authors declare that there is no conflict of interest that could be perceived as prejudicial to the impartiality of the reported research.
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





