Thu, Apr 25, 2024
[Archive]
Volume 18, Issue 12 (December 2020)
IJRM 2020, 18(12): 1029-1038 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Aslfalah H, Jamilian M, Ansarihadipour H, Abdollahi M, Khosrowbeygi A. Effect of alpha-lipoic acid supplementation on the lipid profile and lipid ratios in women with gestational diabetes mellitus: A clinical trial study. IJRM 2020; 18 (12) :1029-1038
URL: http://ijrm.ir/article-1-1473-en.html
URL: http://ijrm.ir/article-1-1473-en.html
1- Students Research Committee, Arak University of Medical Sciences, Arak, Iran.
2- Department of Gynecology and Obstetrics, Endocrinology and Metabolism Research Center, School of Medicine, Arak University of Medical Sciences, Arak, Iran.
3- Department of Biochemistry and Genetics, Endocrinology and Metabolism Research Center, School of Medicine, Arak University of Medical Sciences, Arak, Iran.
4- Decorative and Hygienic Products, Control Laboratory of Food, Beverage, Food and Drug Administration, Arak University of Medical Sciences, Arak, Iran.
5- Department of Biochemistry and Genetics, Endocrinology and Metabolism Research Center, School of Medicine, Arak University of Medical Sciences, Arak, Iran. Traditional and Complementary Medicine Research Center (TCMRC), Arak University of Medical Sciences, Arak, Iran. , khosrowbeygi@yahoo.com
2- Department of Gynecology and Obstetrics, Endocrinology and Metabolism Research Center, School of Medicine, Arak University of Medical Sciences, Arak, Iran.
3- Department of Biochemistry and Genetics, Endocrinology and Metabolism Research Center, School of Medicine, Arak University of Medical Sciences, Arak, Iran.
4- Decorative and Hygienic Products, Control Laboratory of Food, Beverage, Food and Drug Administration, Arak University of Medical Sciences, Arak, Iran.
5- Department of Biochemistry and Genetics, Endocrinology and Metabolism Research Center, School of Medicine, Arak University of Medical Sciences, Arak, Iran. Traditional and Complementary Medicine Research Center (TCMRC), Arak University of Medical Sciences, Arak, Iran. , khosrowbeygi@yahoo.com
Full-Text [PDF 306 kb]
(912 Downloads)
| Abstract (HTML) (2045 Views)
Full-Text: (381 Views)
1. Introduction
Gestational diabetes mellitus (GDM) is a condition that is initiated or diagnosed for the first time in pregnancy, which is defined as glucose intolerance. This type of diabetes increases insulin resistance in body. Mothers suffering from GDM are at a high risk for type 2 diabetes in future. Clinical studies have shown that oxidative stress plays a role in the pathophysiology of numerous diseases (1). Normal pregnancy is regarded as a condition associated with increased oxidative stress. However, despite the increasing oxidative stress, there is a balance between antioxidants and oxidants in normal pregnancy. Bodies of evidence suggest that oxidative stress plays an important role in pregnancy complications such as GDM, preeclampsia, and hydatidiform mole (2, 3). Alpha-lipoic acid (ALA) (6, 8-thioctic acid or 1,2-dithiolane-3-pentanoic acid) is a sulfur-containing fatty acid (C8H14O2S2; MW 206.32) that plays a basic role as an antioxidant and is required for mitochondrial α-ketoacid dehydrogenase complex. ALA is produced by plants and animals. However, its synthesis in human is very low (4). Its antioxidant properties are attributed to several reasons including its capacity for direct scavenging of reactive oxygen species (ROS), regeneration of endogenous antioxidants such as glutathione, vitamins E and C, and its metal-chelating activity (5). Short-term treatment with ALA in patients with type 2 diabetes mellitus (T2DM) has been suggested to improve lipid profile by improving oxidative stress and inflammatory responses (6). A systematic review has shown that supplementation with ALA can improve lipid profile except HDL-C in patients with metabolic diseases (7).
A review study has suggested that ALA not only has no side effects but also has protective effects against embryopathy, fetal mortality, and placental ultrastructural changes due to diabetes during pregnancy (8). Women with a history of GDM are at an increased risk for complications such as endothelial dysfunction and cardiovascular diseases (9). It has been shown that lipid profile controlling during GDM can prevent impairment of the feto-placental endothelial function (10). On the other hand, women who use insulin during GDM can face some cardiovascular complications compared to those who undergo nutritional therapy for controlling the disease (11). However, little data are available about the effects of ALA supplementation on the values of lipid profile and lipid ratios in women with GDM. Therefore, the current clinical trial study was designed to investigate into this matter.
2. Materials and Methods
2.1. Study registration and approval
The current clinical trial study was conducted at the Taleghani Hospital and Diabetes Clinic of Arak University of Medical Sciences between February 2017 and January 2018. The study is a double-blind clinical trial in which the participants and the statistician were not aware of the type of drug to be administered and the group classification, respectively. GDM diagnosis was carried out according to the 75-gr oral glucose tolerance test when at least one or more of the following glucose values were exceeded: fasting ≥ 92 mg/dl; 1 h ≥ 180 mg/dL; and 2 h ≥ 153 mg/dL (12). The inclusion criteria of the study were: diagnosis of GDM, 18-40 yr of age, and being resident. The exclusion criteria, on the other hand, included: receiving antioxidant supplements at six-month intervals before the trial, preterm delivery, preeclampsia and other complications during the intervention stage, liver diseases, the need for insulin or medication during the intervention stage, absolute rest, smoking, and previous GDM history. Accordingly, 70 women, who were diagnosed with GDM by a specialist physician during 24 and 28 weeks of pregnancy, were selected using the convenience sampling method. Of them, 10 were excluded based on the inclusion criteria. Thus, 60 of them were enrolled in the current study (Figure 1).
2.2. Randomization
Participants were divided into ALA and placebo groups using simple randomization method by the random number table. While 30 participants were included in the ALA group and received ALA (Puritan's Pride, Oakdale, NY, USA) (1 capsule 100 mg/day), the remaining 30 were included in the placebo group and received manually prepared capsule containing cellulose acetate (Sigma Aldrich, St. Louis, USA) (1 capsule 100 mg/day). The intervention lasted for eight weeks. Studies have shown that a single dosage administration (50-600 mg) of ALA is completely absorbed within 30-60 min (8). Since receiving 100 mg/day of ALA has been shown to have no significant side effects on mothers and newborns during pregnancy (8), this dosage was used in the current study. All subjects were under the same diet plan for controlling blood glucose levels. Participants were asked to follow the provided diet plan and not to change their ordinary daily physical activity during the trial. As a reminder, every two weeks, the patients were phone called for taking their capsules appropriately.
2.3. Assessment of variables
Demographic data including participants’ height, weight (light clothing without shoes weight), body mass index (BMI), blood pressure (right arm in sitting position) were measured at the beginning and end of the trial. BMI was determined as weight (kg)/height (m2). Venous blood samples were taken after 12 hr of fasting at the beginning and end of the intervention. Blood samples were centrifuged at 10,000 g for 15 min. Serum samples were stored at -80ºC before analysis. To measure hemoglobin A1C (HbA1c), 1.5 ml of total blood was poured into EDTA-containing tubes that were assayed by chromatography kit (Biosystems, Barcelona, Spain).
Moreover, fasting blood sugar (FBS), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), and total cholesterol (TC) (ZiestChem Diagnostic, Tehran, Iran) were measured using a chemical analyzer (BT3500, Italy). Low-density lipoprotein cholesterol (LDL-C) levels were calculated using the Friedewald equation (13). The atherogenic index of plasma (AIP) (14), the triglyceride-glucose (TyG) index as Ln [(FBS × TG)/2] (15), the non-HDL-C, and the lipid ratios including TG/HDL-C, TC/HDL-C, LDL-C/HDL-C, and TG/TC were calculated (16). Levels of thiobarbituric acid-reactive substances (TBARS), a measure of lipid peroxidation, were determined for evaluating oxidative stress status (17). Serum ALA levels were measured by high-performance liquid chromatography method (KNAUER, Berlin, Germany) (18).

2.4. Ethical consideration
The current study was approval by the Ethics Committee (IR.ARAKMU.REC.1395.343) of the Taleghani Hospital and Diabetes Clinic of Arak University of Medical Sciences.
2.5. Statistical analysis
Qualitative data were evaluated using the Chi-square test. The normal distribution of variables was explored by the Kolmogorov-Smirnov test. Comparisons of quantitative parameters were performed using paired and independent t tests. Interactions between the corresponding biochemical variables and the ALA group were explored using the Analysis of Covariance (ANCOVA). Data with p-values < 0.05 were considered as significant. All data are expressed as mean ± SEM. All statistical analyses were conducted using the SPSS, version 21 for windows (SPSS Inc., Chicago, IL, USA).
3. Results
In this study, data of the participants in two groups (n = 30/each) were analyzed. No significant differences were observed in the demographic and biochemical data of the two groups at the baseline (Tables I and II). In addition, no significant changes were observed in the weight, systolic blood pressure, and BMI in each group following the intervention.
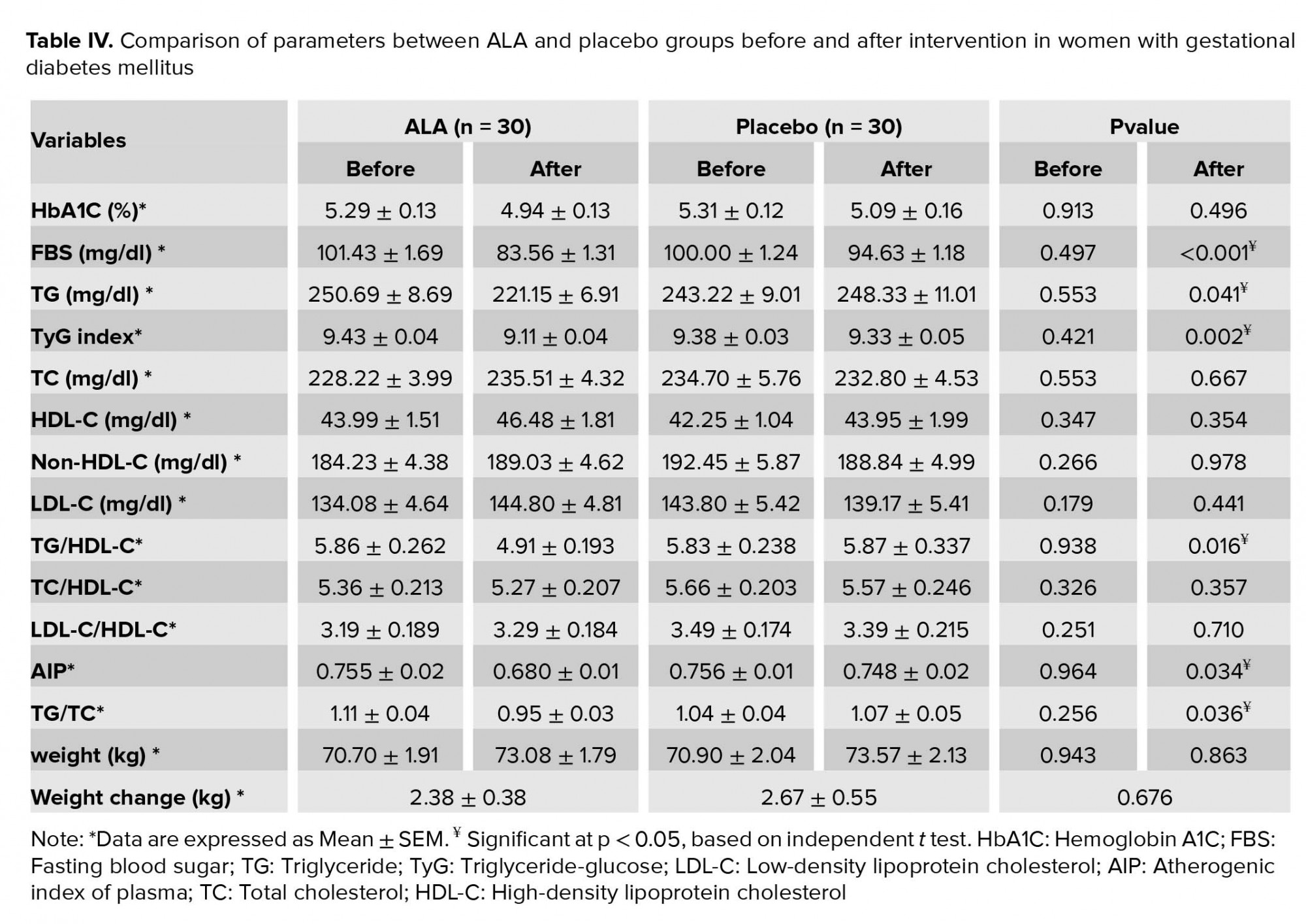
Intragroup and intergroup comparisons of the variables are given in Tables III and IV, respectively. At the end of the intervention, values of FBS (p < 0.001), TyG index (p < 0.001), TG (p = 0.006), TG/HDL-C (p = 0.003), TG/TC (p = 0.004), AIP (p = 0.005), and TBARS (p < 0.001) significantly decreased in the ALA group compared with the baseline. Values of HbA1C decreased marginally in the ALA group (p = 0.059). No significant changes were observed in the values of TC (p = 0.189), LDL-C (p = 0.104), HDL-C (p = 0.328), non-HDL-C (p = 0.750), LDL-C/HDL-C (p = 0.727), and TC/HDL-C (p = 0.750) compared with the baseline in the ALA group. A comparison between the two groups showed a significant reduction in the serum levels of FBS (p < 0.001), TyG index (p = 0.022), TG (p = 0.041), TG/HDL-C (p = 0.016), TG/TC (p = 0.036), and AIP (p = 0.034) in the ALA group compared with the placebo group. However, no significant changes were observed in the values of HbA1C (p = 0.496), TC (p = 0.667), LDL-C (p = 0.441), HDL-C (p = 0.354), non-HDL-C (p = 0.978), LDL-C/HDL-C (p = 0.710), and TC/HDL-C (p = 0.357) between the two groups after the supplementation.
At the end of the trial, serum levels of ALA were increased in the ALA group (1.10 ± 0.10 vs 4.51 ± 0.24 µg/ml, p < 0.001), while no changes were observed in the placebo group (1.14 ± 0.07 vs 1.12 ± 0.07 µg/ml, p = 0.134).
Results of the ANCOVA test were significant for the TyG index (p = 0.001), TG (p < 0.001), TG/HDL-C (p = 0.006), AIP (p = 0.012), and TBARS (p = 0.007). However, it was not significant for the TG/TC (p = 0.308).



4. Discussion
The current study showed that the maternal circulating values of TyG index, TG, TG/HDL-C, and AIP decreased after an eight-week supplementation with ALA in womenwith GDM. ALA supplementation at a dosage of 100 mg/day in women with GDM showed no significant changes in weight, systolic blood pressure, and BMI in both groups.
De Cicco and colleagues (19) found that the combination therapy with ALA and myo-inositol significantly reduces BMI in women with polycystic ovary syndrome. Noori and colleagues (20) found that consumption of 800 mg of ALA in diabetic nephropathy significantly decreases systolic blood pressure in the supplement group compared with the placebo group.
Orally consumed ALA is absorbed almost entirely from intestine (21). In the current study, values of ALA increased significantly in the treatment group compared with the placebo group after the intervention. Therefore, this finding can be accounted for ALA consumption by participants in the ALA group.
In addition, in the current study, HbA1C levels decreased marginally in the ALA group after supplementation with ALA, albeit the absence of statistical significance according to the results of ANCOVA. Accordingly, marginally reduced values of HbA1C may not be attributed to the intervention effects. Bao and co-workers (22) found that the daily consumption of 600 mg of ALA in patients with T2DM does not change HbA1C levels significantly. Chang and co-workers (23) observed that using 600 mg of ALA for 12 weeks does not affect HbA1c levels in individuals with end-stage renal disease. Porasuphatana and co-workers (24) observed that taking different doses of 300, 600, 900, and 1200 mg/day of ALA for six months causes a significant reduction in the HbA1C levels in individuals with T2DM. Derosa and co-workers (5) found that taking 600 mg of ALA supplementation in individuals with T2DM reduces HbA1C levels significantly.
An in vitro study conducted by Di Fulvio and co-workers (9) has shown that vascular cells obtained from umbilical cords of women with GDM maintain certain dysfunctions such as increased ROS and reduced nitric oxide (NO) bioavailability even after culturing for several times. Another in vitro study conducted by Di Tomo and colleagues (25) has shown that ALA significantly improves detrimental effects of hyperglycemia on umbilical vein endothelial cells obtained from mothers with GDM, concluding that this may point to new strategies for prevention of diabetes-related vascular complications.
A systematic review study has shown that supplementation with ALA can improve lipid profile except HDL-C in individuals with metabolic diseases (7). However, little data are available in this matter in GDM. In the current study, the mean TC/HDL and LDL/HDL level changes in the ALA and placebo groups did not show a statistically significant difference. However, the mean TyG index, TG, TG/TC, TG/HDL, and AIP levels in the ALA group significantly decreased after the intervention. It has been reported that the increased values of TyG index, TG, and TG/HDL-C in early pregnancy are associated with high incidence of GDM development (15). The TyG index is the best indicator of insulin resistance compared with other lipid parameters as a noninvasive, effective, and simple tool. On the other hand, TyG index is a better predictor of atherosclerosis than homeostatic model assessment of insulin resistance (HOMA-IR) (15, 26). AIP value has been shown to be a good biomarker for the risk of atherosclerosis and cardiovascular diseases. In addition, the calculation of AIP, especially when lipid profile is in normal range, has been proposed, which can show more useful data than traditional lipid analysis about patient's situation (14, 27). Results from the ANCOVA did not point to a statistically significant difference for TG/TC. Therefore, reduced TG/TC values may not be attributed to the intervention effects. An increase in the TG/HDL-C ratio was reported in GDM compared with healthy pregnancy (28). The TC/HDL-C, LDL-C/HDL-C, and TG/HDL ratios showed significant positive correlations with HOMA-IR. Therefore, these ratios could be used as simple alternative markers for assessing insulin resistance during pregnancy and predicting women at a high risk for GDM (29). It has been shown that ALA can increase adenosine monophosphate-activated protein kinase (AMPK) activity which causes inhibition of fatty acid synthesis, while simultaneously fatty acids oxidation is activated. On the other hand, expression of enzymes acetyl-CoA carboxylase and fatty acid synthase, two rate-limiting enzymes in fatty acid synthesis, are depressed in subjects supplemented with ALA (7).
A major limitation of the present study was the lack of a healthy control group to allow the comparison of the results obtained from the participants with them.
5. Conclusion
The results of the current study in women with GDM showed a significant decrease in the maternal circulating values of TyG index, TG, TG/HDL, AIP, and TBARS in the ALA group compared to the placebo group at the end of the intervention. More studies are required to evaluate the potential effects of ALA on the reduction of birth defects in women suffering from GDM.
Acknowledgements
The authors gratefully acknowledge the Research Council of Arak University of Medical Sciences (Grant Number: 808B/2951) for financially supporting the study. This work was performed in partial fulfillment of the requirements for an M.Sc. degree of Hadise Aslfalah, at the School of Medicine, Arak University of Medical Sciences, Arak, Iran. The authors also wish to thank Ms. Fateme Rafiei for her assistance in statistical analysis and all participants for their patience and time.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Gestational diabetes mellitus (GDM) is a condition that is initiated or diagnosed for the first time in pregnancy, which is defined as glucose intolerance. This type of diabetes increases insulin resistance in body. Mothers suffering from GDM are at a high risk for type 2 diabetes in future. Clinical studies have shown that oxidative stress plays a role in the pathophysiology of numerous diseases (1). Normal pregnancy is regarded as a condition associated with increased oxidative stress. However, despite the increasing oxidative stress, there is a balance between antioxidants and oxidants in normal pregnancy. Bodies of evidence suggest that oxidative stress plays an important role in pregnancy complications such as GDM, preeclampsia, and hydatidiform mole (2, 3). Alpha-lipoic acid (ALA) (6, 8-thioctic acid or 1,2-dithiolane-3-pentanoic acid) is a sulfur-containing fatty acid (C8H14O2S2; MW 206.32) that plays a basic role as an antioxidant and is required for mitochondrial α-ketoacid dehydrogenase complex. ALA is produced by plants and animals. However, its synthesis in human is very low (4). Its antioxidant properties are attributed to several reasons including its capacity for direct scavenging of reactive oxygen species (ROS), regeneration of endogenous antioxidants such as glutathione, vitamins E and C, and its metal-chelating activity (5). Short-term treatment with ALA in patients with type 2 diabetes mellitus (T2DM) has been suggested to improve lipid profile by improving oxidative stress and inflammatory responses (6). A systematic review has shown that supplementation with ALA can improve lipid profile except HDL-C in patients with metabolic diseases (7).
A review study has suggested that ALA not only has no side effects but also has protective effects against embryopathy, fetal mortality, and placental ultrastructural changes due to diabetes during pregnancy (8). Women with a history of GDM are at an increased risk for complications such as endothelial dysfunction and cardiovascular diseases (9). It has been shown that lipid profile controlling during GDM can prevent impairment of the feto-placental endothelial function (10). On the other hand, women who use insulin during GDM can face some cardiovascular complications compared to those who undergo nutritional therapy for controlling the disease (11). However, little data are available about the effects of ALA supplementation on the values of lipid profile and lipid ratios in women with GDM. Therefore, the current clinical trial study was designed to investigate into this matter.
2. Materials and Methods
2.1. Study registration and approval
The current clinical trial study was conducted at the Taleghani Hospital and Diabetes Clinic of Arak University of Medical Sciences between February 2017 and January 2018. The study is a double-blind clinical trial in which the participants and the statistician were not aware of the type of drug to be administered and the group classification, respectively. GDM diagnosis was carried out according to the 75-gr oral glucose tolerance test when at least one or more of the following glucose values were exceeded: fasting ≥ 92 mg/dl; 1 h ≥ 180 mg/dL; and 2 h ≥ 153 mg/dL (12). The inclusion criteria of the study were: diagnosis of GDM, 18-40 yr of age, and being resident. The exclusion criteria, on the other hand, included: receiving antioxidant supplements at six-month intervals before the trial, preterm delivery, preeclampsia and other complications during the intervention stage, liver diseases, the need for insulin or medication during the intervention stage, absolute rest, smoking, and previous GDM history. Accordingly, 70 women, who were diagnosed with GDM by a specialist physician during 24 and 28 weeks of pregnancy, were selected using the convenience sampling method. Of them, 10 were excluded based on the inclusion criteria. Thus, 60 of them were enrolled in the current study (Figure 1).
2.2. Randomization
Participants were divided into ALA and placebo groups using simple randomization method by the random number table. While 30 participants were included in the ALA group and received ALA (Puritan's Pride, Oakdale, NY, USA) (1 capsule 100 mg/day), the remaining 30 were included in the placebo group and received manually prepared capsule containing cellulose acetate (Sigma Aldrich, St. Louis, USA) (1 capsule 100 mg/day). The intervention lasted for eight weeks. Studies have shown that a single dosage administration (50-600 mg) of ALA is completely absorbed within 30-60 min (8). Since receiving 100 mg/day of ALA has been shown to have no significant side effects on mothers and newborns during pregnancy (8), this dosage was used in the current study. All subjects were under the same diet plan for controlling blood glucose levels. Participants were asked to follow the provided diet plan and not to change their ordinary daily physical activity during the trial. As a reminder, every two weeks, the patients were phone called for taking their capsules appropriately.
2.3. Assessment of variables
Demographic data including participants’ height, weight (light clothing without shoes weight), body mass index (BMI), blood pressure (right arm in sitting position) were measured at the beginning and end of the trial. BMI was determined as weight (kg)/height (m2). Venous blood samples were taken after 12 hr of fasting at the beginning and end of the intervention. Blood samples were centrifuged at 10,000 g for 15 min. Serum samples were stored at -80ºC before analysis. To measure hemoglobin A1C (HbA1c), 1.5 ml of total blood was poured into EDTA-containing tubes that were assayed by chromatography kit (Biosystems, Barcelona, Spain).
Moreover, fasting blood sugar (FBS), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), and total cholesterol (TC) (ZiestChem Diagnostic, Tehran, Iran) were measured using a chemical analyzer (BT3500, Italy). Low-density lipoprotein cholesterol (LDL-C) levels were calculated using the Friedewald equation (13). The atherogenic index of plasma (AIP) (14), the triglyceride-glucose (TyG) index as Ln [(FBS × TG)/2] (15), the non-HDL-C, and the lipid ratios including TG/HDL-C, TC/HDL-C, LDL-C/HDL-C, and TG/TC were calculated (16). Levels of thiobarbituric acid-reactive substances (TBARS), a measure of lipid peroxidation, were determined for evaluating oxidative stress status (17). Serum ALA levels were measured by high-performance liquid chromatography method (KNAUER, Berlin, Germany) (18).

2.4. Ethical consideration
The current study was approval by the Ethics Committee (IR.ARAKMU.REC.1395.343) of the Taleghani Hospital and Diabetes Clinic of Arak University of Medical Sciences.
2.5. Statistical analysis
Qualitative data were evaluated using the Chi-square test. The normal distribution of variables was explored by the Kolmogorov-Smirnov test. Comparisons of quantitative parameters were performed using paired and independent t tests. Interactions between the corresponding biochemical variables and the ALA group were explored using the Analysis of Covariance (ANCOVA). Data with p-values < 0.05 were considered as significant. All data are expressed as mean ± SEM. All statistical analyses were conducted using the SPSS, version 21 for windows (SPSS Inc., Chicago, IL, USA).
3. Results
In this study, data of the participants in two groups (n = 30/each) were analyzed. No significant differences were observed in the demographic and biochemical data of the two groups at the baseline (Tables I and II). In addition, no significant changes were observed in the weight, systolic blood pressure, and BMI in each group following the intervention.
Intragroup and intergroup comparisons of the variables are given in Tables III and IV, respectively. At the end of the intervention, values of FBS (p < 0.001), TyG index (p < 0.001), TG (p = 0.006), TG/HDL-C (p = 0.003), TG/TC (p = 0.004), AIP (p = 0.005), and TBARS (p < 0.001) significantly decreased in the ALA group compared with the baseline. Values of HbA1C decreased marginally in the ALA group (p = 0.059). No significant changes were observed in the values of TC (p = 0.189), LDL-C (p = 0.104), HDL-C (p = 0.328), non-HDL-C (p = 0.750), LDL-C/HDL-C (p = 0.727), and TC/HDL-C (p = 0.750) compared with the baseline in the ALA group. A comparison between the two groups showed a significant reduction in the serum levels of FBS (p < 0.001), TyG index (p = 0.022), TG (p = 0.041), TG/HDL-C (p = 0.016), TG/TC (p = 0.036), and AIP (p = 0.034) in the ALA group compared with the placebo group. However, no significant changes were observed in the values of HbA1C (p = 0.496), TC (p = 0.667), LDL-C (p = 0.441), HDL-C (p = 0.354), non-HDL-C (p = 0.978), LDL-C/HDL-C (p = 0.710), and TC/HDL-C (p = 0.357) between the two groups after the supplementation.
At the end of the trial, serum levels of ALA were increased in the ALA group (1.10 ± 0.10 vs 4.51 ± 0.24 µg/ml, p < 0.001), while no changes were observed in the placebo group (1.14 ± 0.07 vs 1.12 ± 0.07 µg/ml, p = 0.134).
Results of the ANCOVA test were significant for the TyG index (p = 0.001), TG (p < 0.001), TG/HDL-C (p = 0.006), AIP (p = 0.012), and TBARS (p = 0.007). However, it was not significant for the TG/TC (p = 0.308).




4. Discussion
The current study showed that the maternal circulating values of TyG index, TG, TG/HDL-C, and AIP decreased after an eight-week supplementation with ALA in womenwith GDM. ALA supplementation at a dosage of 100 mg/day in women with GDM showed no significant changes in weight, systolic blood pressure, and BMI in both groups.
De Cicco and colleagues (19) found that the combination therapy with ALA and myo-inositol significantly reduces BMI in women with polycystic ovary syndrome. Noori and colleagues (20) found that consumption of 800 mg of ALA in diabetic nephropathy significantly decreases systolic blood pressure in the supplement group compared with the placebo group.
Orally consumed ALA is absorbed almost entirely from intestine (21). In the current study, values of ALA increased significantly in the treatment group compared with the placebo group after the intervention. Therefore, this finding can be accounted for ALA consumption by participants in the ALA group.
In addition, in the current study, HbA1C levels decreased marginally in the ALA group after supplementation with ALA, albeit the absence of statistical significance according to the results of ANCOVA. Accordingly, marginally reduced values of HbA1C may not be attributed to the intervention effects. Bao and co-workers (22) found that the daily consumption of 600 mg of ALA in patients with T2DM does not change HbA1C levels significantly. Chang and co-workers (23) observed that using 600 mg of ALA for 12 weeks does not affect HbA1c levels in individuals with end-stage renal disease. Porasuphatana and co-workers (24) observed that taking different doses of 300, 600, 900, and 1200 mg/day of ALA for six months causes a significant reduction in the HbA1C levels in individuals with T2DM. Derosa and co-workers (5) found that taking 600 mg of ALA supplementation in individuals with T2DM reduces HbA1C levels significantly.
An in vitro study conducted by Di Fulvio and co-workers (9) has shown that vascular cells obtained from umbilical cords of women with GDM maintain certain dysfunctions such as increased ROS and reduced nitric oxide (NO) bioavailability even after culturing for several times. Another in vitro study conducted by Di Tomo and colleagues (25) has shown that ALA significantly improves detrimental effects of hyperglycemia on umbilical vein endothelial cells obtained from mothers with GDM, concluding that this may point to new strategies for prevention of diabetes-related vascular complications.
A systematic review study has shown that supplementation with ALA can improve lipid profile except HDL-C in individuals with metabolic diseases (7). However, little data are available in this matter in GDM. In the current study, the mean TC/HDL and LDL/HDL level changes in the ALA and placebo groups did not show a statistically significant difference. However, the mean TyG index, TG, TG/TC, TG/HDL, and AIP levels in the ALA group significantly decreased after the intervention. It has been reported that the increased values of TyG index, TG, and TG/HDL-C in early pregnancy are associated with high incidence of GDM development (15). The TyG index is the best indicator of insulin resistance compared with other lipid parameters as a noninvasive, effective, and simple tool. On the other hand, TyG index is a better predictor of atherosclerosis than homeostatic model assessment of insulin resistance (HOMA-IR) (15, 26). AIP value has been shown to be a good biomarker for the risk of atherosclerosis and cardiovascular diseases. In addition, the calculation of AIP, especially when lipid profile is in normal range, has been proposed, which can show more useful data than traditional lipid analysis about patient's situation (14, 27). Results from the ANCOVA did not point to a statistically significant difference for TG/TC. Therefore, reduced TG/TC values may not be attributed to the intervention effects. An increase in the TG/HDL-C ratio was reported in GDM compared with healthy pregnancy (28). The TC/HDL-C, LDL-C/HDL-C, and TG/HDL ratios showed significant positive correlations with HOMA-IR. Therefore, these ratios could be used as simple alternative markers for assessing insulin resistance during pregnancy and predicting women at a high risk for GDM (29). It has been shown that ALA can increase adenosine monophosphate-activated protein kinase (AMPK) activity which causes inhibition of fatty acid synthesis, while simultaneously fatty acids oxidation is activated. On the other hand, expression of enzymes acetyl-CoA carboxylase and fatty acid synthase, two rate-limiting enzymes in fatty acid synthesis, are depressed in subjects supplemented with ALA (7).
A major limitation of the present study was the lack of a healthy control group to allow the comparison of the results obtained from the participants with them.
5. Conclusion
The results of the current study in women with GDM showed a significant decrease in the maternal circulating values of TyG index, TG, TG/HDL, AIP, and TBARS in the ALA group compared to the placebo group at the end of the intervention. More studies are required to evaluate the potential effects of ALA on the reduction of birth defects in women suffering from GDM.
Acknowledgements
The authors gratefully acknowledge the Research Council of Arak University of Medical Sciences (Grant Number: 808B/2951) for financially supporting the study. This work was performed in partial fulfillment of the requirements for an M.Sc. degree of Hadise Aslfalah, at the School of Medicine, Arak University of Medical Sciences, Arak, Iran. The authors also wish to thank Ms. Fateme Rafiei for her assistance in statistical analysis and all participants for their patience and time.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Type of Study: Original Article |
Subject:
Pregnancy Health
References
1. Abbasi M, Mazloum Khorasani Z, Etminani K, Rahmanvand R. Determination of the most important risk factors of gestational diabetes in Iran by group analytical hierarchy process. Int J Reprod Biomed 2017; 15: 109-114. [DOI:10.29252/ijrm.15.2.109] [PMID] [PMCID]
2. Lappas M, Hiden U, Desoye G, Froehlich J, Hauguel-de Mouzon S, Jawerbaum A. The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid Redox Signal 2011; 15: 3061-3100. [DOI:10.1089/ars.2010.3765] [PMID]
3. Ghate J, Choudhari AR, Ghugare B, Ramji S. Antioxidant role of Vitamin C in normal pregnancy. Biomed Res 2011; 22: 49-51.
4. Monastra G, De Grazia S, Cilaker Micili S, Goker A, Unfer V. Immunomodulatory activities of alpha lipoic acid with a special focus on its efficacy in preventing miscarriage. Expert Opin Drug Deliv 2016; 13: 1695-1708. [DOI:10.1080/17425247.2016.1200556] [PMID]
5. Derosa G, D'Angelo A, Romano D, Maffioli P. A clinical trial about a food supplement 6containing α-lipoic acid on oxidative stress markers in type 2 diabetic patients. Int J Mol Sci 2016; 17: 1802-1809. [DOI:10.3390/ijms17111802] [PMID] [PMCID]
6. Zhang Y, Han P, Wu N, He B, Lu Y, Li Sh, et al. Amelioration of lipid abnormalities by α‐Lipoic acid through antioxidative and anti‐Inflammatory effects. Obesity 2011; 19: 1647-1653. [DOI:10.1038/oby.2011.121] [PMID]
7. Akbari M, Ostadmohammadi V, Lankarani KB, Tabrizi R, Kolahdooz F, Khatibi SR, et al. The effects of alpha-lipoic acid supplementation on glucose control and lipid profiles among patients with metabolic diseases: A systematic review and meta-analysis of randomized controlled trials. Metabolism 2018; 87: 56-69. [DOI:10.1016/j.metabol.2018.07.002] [PMID]
8. Di Tucci Ch, Di Feliciantonio M, Vena F, Capone C, Schiavi MC, Pietrangeli D, et al. Alpha lipoic acid in obstetrics and gynecology. Gynecol Endocrinol 2018; 34: 729-733. [DOI:10.1080/09513590.2018.1462320] [PMID]
9. Di Fulvio P, Pandolfi A, Formoso G, Di Silvestre S, Di Tomo P, Giardinelli A, et al. Features of endothelial dysfunction in umbilical cord vessels of women with gestational diabetes. Nutr Metab Cardiovasc Dis 2014; 24: 1337-1345. [DOI:10.1016/j.numecd.2014.06.005] [PMID]
10. Contreras-Duarte S, Carvajal L, Garchitorena MJ, Subiabre M, Fuenzalida B, Cantin C, et al. Gestational diabetes mellitus treatment schemes modify maternal plasma cholesterol levels dependent to women' s weight: Possible impact on feto-placental vascular function. Nutrients 2020; 12: 506-527. [DOI:10.3390/nu12020506] [PMID] [PMCID]
11. Jasper R, Skelding K. Cardiovascular disease risk unmasked by pregnancy complications. Eur J Intern Med 2018; 57: 1-6. [DOI:10.1016/j.ejim.2018.07.020] [PMID]
12. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014; 37 (Suppl.): S81-S90. [DOI:10.2337/dc14-S081] [PMID]
13. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry 1972; 18: 499-502. [DOI:10.1093/clinchem/18.6.499] [PMID]
14. Niroumand Sh, Khajedaluee M, Khadem-Rezaiyan M, Abrishami M, Juya M, Khodaee G, et al. Atherogenic index of plasma (AIP): A marker of cardiovascular disease. Med J Islam Repub Iran 2015; 29: 240-248.
15. Pazhohan A, Rezaee Moradali M, Pazhohan N. Association of first-trimester maternal lipid profiles and triglyceride-glucose index with the risk of gestational diabetes mellitus and large for gestational age newborn. J Matern Fetal Neonatal Med 2017; 32: 1167-1175. [DOI:10.1080/14767058.2017.1402876] [PMID]
16. Gasevic D, Frohlich J, Mancini GJ, Lear SA. Clinical usefulness of lipid ratios to identify men and women with metabolic syndrome: a cross-sectional study. Lipids Health Dis 2014; 13: 159-168. [DOI:10.1186/1476-511X-13-159] [PMID] [PMCID]
17. Pfalzgraf A, Frigg M, Steinhart H. Alpha.-tocopherol contents and lipid oxidation in pork muscle and adipose tissue during storage. J Agric Food Chem 1995; 43: 1339-1342. [DOI:10.1021/jf00053a039]
18. Siangproh W, Rattanarat P, Chailapakul O. Reverse-phase liquid chromatographic determination of α-lipoic acid in dietary supplements using a boron-doped diamond electrode. J Chromatogr A 2010; 1217: 7699-7705. [DOI:10.1016/j.chroma.2010.10.004] [PMID]
19. De Cicco S, Immediata V, Romualdi D, Policola C, Tropea A, Di Florio C, et al. Myoinositol combined with alpha-lipoic acid may improve the clinical and endocrine features of polycystic ovary syndrome through an insulin-independent action. Gynecol Endocrinol 2017; 33: 698-701. [DOI:10.1080/09513590.2017.1313972] [PMID]
20. Noori N, Tabibi H, Hosseinpanah F, Hedayati M, Nafar M. Effects of combined lipoic acid and pyridoxine on albuminuria, advanced glycation end-products, and blood pressure in diabetic nephropathy. Int J Vitam Nutr Res 2013; 83: 77-85. [DOI:10.1024/0300-9831/a000147] [PMID]
21. Li Y, Ma QG, Zhao LH, Wei H, Duan GX, Zhang JY, et al. Effects of lipoic acid on immune function, the antioxidant defense system, and inflammation-related genes expression of broiler chickens fed aflatoxin contaminated diets. Int J Mol Sci 2014; 15: 5649-5662. [DOI:10.3390/ijms15045649] [PMID] [PMCID]
22. Bao XH, Xu J, Chen Y, Yang CL, Ye ShD. Alleviation of podocyte injury: the possible pathway implicated in anti-inflammation of alpha-lipoic acid in type 2 diabetics. Aging Clin Exp Res 2014; 26: 483-489. [DOI:10.1007/s40520-014-0207-1] [PMID]
23. Chang JW, Lee EK, Kim TH, Min WK, Chun S, Lee KU, et al. Effects of α-lipoic acid on the plasma levels of asymmetric dimethylarginine in diabetic end-stage renal disease patients on hemodialysis: a pilot study. Am J Nephrol 2007; 27: 70-74. [DOI:10.1159/000099035] [PMID]
24. Porasuphatana S, Suddee S, Nartnampong A, Konsil J, Harnwong B, Santaweesuk A. Glycemic and oxidative status of patients with type 2 diabetes mellitus following oral administration of alphalipoic acid: A randomized double-blinded placebo-controlled study. Asia Pac J Clin Nutr 2012; 21: 12-21.
25. Di Tomo P, Di Silvestre S, Cordone VGP, Giardinelli A, Faricelli B, Pipino C, et al. Centella asiatica and lipoic acid, or a combination thereof, inhibit monocyte adhesion to endothelial cells from umbilical cords of gestational diabetic women. Nutr Metab Cardiovasc Dis 2015; 25: 659-666. [DOI:10.1016/j.numecd.2015.04.002] [PMID]
26. Jin JL, Sun D, Cao YX, Guo YL, Wu NQ, Zhu ChG, et al. Triglyceride glucose and haemoglobin glycation index for predicting outcomes in diabetes patients with new-onset, stable coronary artery disease: a nested case-control study. Ann Med 2018; 50: 576-586. [DOI:10.1080/07853890.2018.1523549] [PMID]
27. Wu TT, Gao Y, Zheng YY, Ma YT, Xie X. Atherogenic index of plasma (AIP): a novel predictive indicator for the coronary artery disease in postmenopausal women. Lipids Health Dis 2018; 17: 197-203. [DOI:10.1186/s12944-018-0828-z] [PMID] [PMCID]
28. Gasevic D, Frohlich J, Mancini GBJ, Lear SA. The association between triglyceride to high-density-lipoprotein cholesterol ratio and insulin resistance in a multiethnic primary prevention cohort. Metabolism 2012; 61: 583-589. [DOI:10.1016/j.metabol.2011.09.009] [PMID]
29. dos Santos-Weiss ICR, Réa RR, Fadel-Picheth CMT, Rego FGM, Pedrosa Fde O, Gillery P, et al. The plasma logarithm of the triglyceride/HDL-cholesterol ratio is a predictor of low risk gestational diabetes in early pregnancy. Clin Chim Acta 2013; 418: 1-4. [DOI:10.1016/j.cca.2012.12.004] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








