Thu, Jul 3, 2025
[Archive]
Volume 18, Issue 5 (May 2020)
IJRM 2020, 18(5): 327-338 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sukhorum W, Umka Welbat J, Ktutsri S, Iamsaard S. Protective effect of melatonin against methotrexate-induced testicular damage in the rat model: An experimental study. IJRM 2020; 18 (5) :327-338
URL: http://ijrm.ir/article-1-1531-en.html
URL: http://ijrm.ir/article-1-1531-en.html
1- School of Medicine, Mae Fah Luang University, Chiang Rai, Thailand.
2- Department of Anatomy, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand.
3- Sitthichai Iamsaard; Department of Anatomy, Faculty of Medicine, Khon Kaen University. 123 Mitraparp Road, Ampoe Muang, Khon Kaen 40002, Thailand.
4- Research Institute for Human High Performance and Health Promotion (HHP & HP), Khon Kaen, Thailand. ,sittia@kku.ac.th
2- Department of Anatomy, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand.
3- Sitthichai Iamsaard; Department of Anatomy, Faculty of Medicine, Khon Kaen University. 123 Mitraparp Road, Ampoe Muang, Khon Kaen 40002, Thailand.
4- Research Institute for Human High Performance and Health Promotion (HHP & HP), Khon Kaen, Thailand. ,
Full-Text [PDF 907 kb]
(1115 Downloads)
| Abstract (HTML) (2866 Views)
Melatonin is an endocrine hormone produced by the pineal gland to regulate normal testicular function through the hypothalamic-adenohypophyseal axis (17, 18). Additionally, melatonin has been shown to have anti-inflammatory and proliferative effects (18, 19). Moreover, it has been demonstrated to reduce the expression of caspase-3 in ovaries and testes damaged by chemotherapeutic drugs (20-23). Based on the antioxidant, anti-inflammatory, and antiapoptotic activities of melatonin, the present study aimed to investigate its protective effect against testicular damage induced by MTX.
The separated proteins were transferred onto a nitrocellulose membrane (Bio-Red Laboratories, Inc., city, Germany) in a 10% methanol transfer buffer. Subsequently, the non-specific proteins were blocked with 5% skim milk. To examine the expression of tyrosine-phosphorylated protein, StAR protein, and caspase-3, the membrane was incubated with monoclonal anti-phosphotyrosine 4G10 antibody (1:1000; Millipore, city, state code, country), polyclonal anti-StAR antibody (1:2000; Santa Cruz Biotechnology), or monoclonal anti-caspase-3 antibody (1:100; Santa Cruz Biotechnology) at 4°C overnight. The membrane was also incubated with monoclonal anti-b-actin antibody as an internal control (1:1000; Santa Cruz Biotechnology).
Each of the non-binding antibodies was washed out of the membrane, and the membrane was further incubated with a non-binding antigen-antibody complex for each antibody. The complexes were composed of specific secondary antibodies conjugated to the primary antibodies with horseradish peroxidase at room temperature. After washing, each non-binding antigen-antibody complex was incubated with an enhanced chemiluminescence substrate kit reagent (GH Healthcare Life Science). The positive protein bands were detected using the ImageQuant LAS 500 imaging system (GH Healthcare Life Science) in the Vejawichakarn building of the Faculty of Medicine at Khon Kaen University in Thailand.
To quantify the level of target protein expression, the intensities of protein bands were measured using the Image J 1.52a program. In this study, BSA (Millipore), epidermal growth factor (Millipore), and StAR lysate (Santa Cruz Biotechnology) were used as specific negative and positive controls of tyrosine-phosphorylated protein and StAR protein, respectively (4).
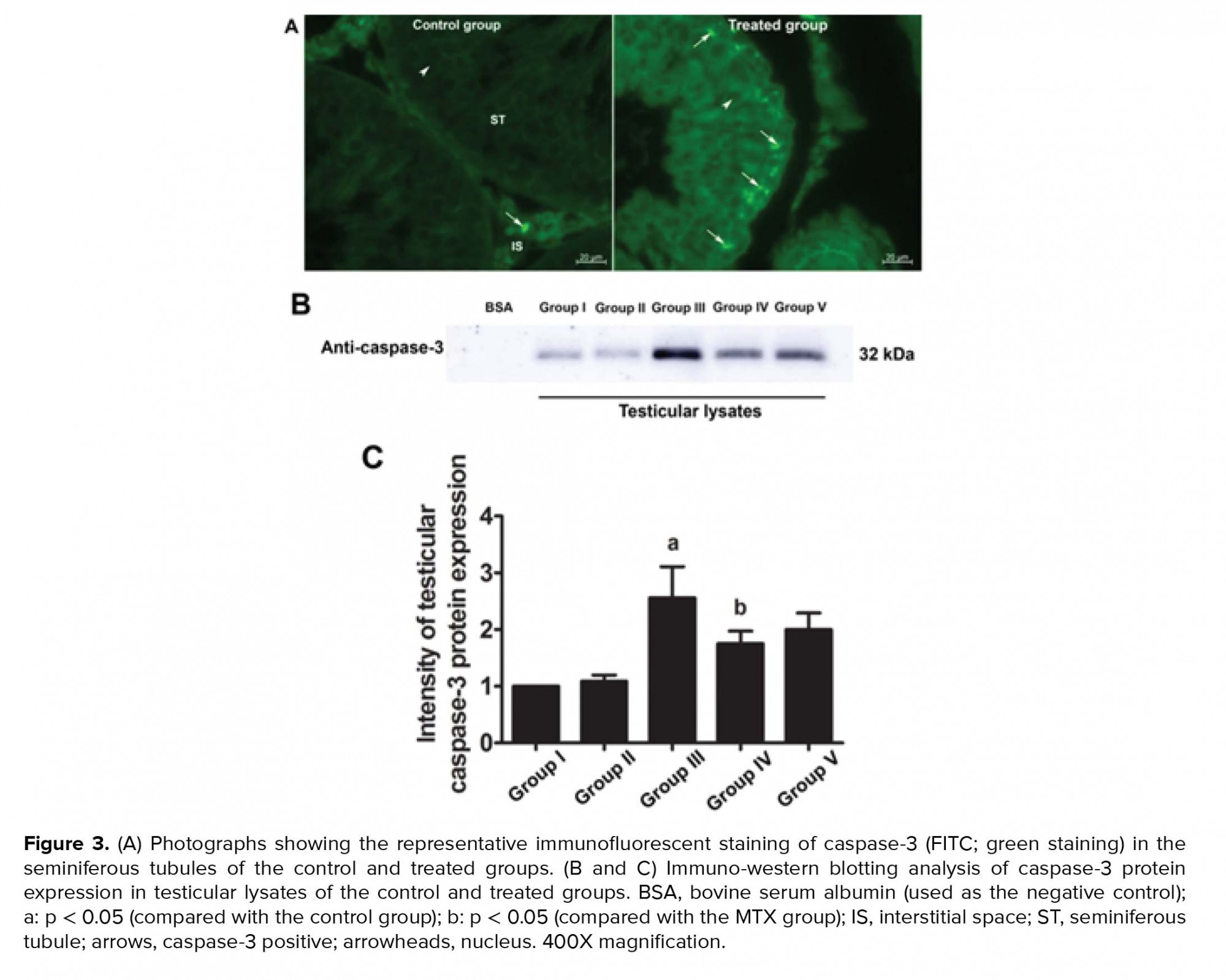
3.5. Effects of melatonin and MTX on testicular caspase-3
The immunofluorescent staining results for caspase-3 revealed that the protein was localized in both the seminiferous epithelium and interstitial tissues of all groups (Figure 3A). This finding was also confirmed by western blotting (Figure 3B). However, the intensity of caspase-3 expression was much greater in all the treated groups than in the control group (Figure 3A). Figures 3B and 5C further revealed that the expression of caspase-3 in the MTX group was increased compared with that in the other groups. The intensity of caspase-3 expression was also lower in the preventive (IV and V) groups than in the MTX group (Figure 3C).


4. Discussion
Chemotherapeutic agents have adverse effects on the male reproductive system, depending on the type and dosage of the drug used, and typically affect seminiferous epithelial cells (4). MTX is known as a low-risk gonadotoxic chemotherapeutic agent that affects the cell cycle via DNA and RNA synthesis inhibition, the inhibition of mitosis, and the deamination of proteins (26). In the present study, MTX was found to decrease the absolute testicular weight, the caudal epididymal sperm concentration, and the diameter of STs. These findings are supported by results from previous reports (14, 15, 27). In the present study, the significant decrease in testicular weight in the MTX group was correlated with the reduction of seminiferous epithelial height and diameter. This effect may have been caused by the increase in caspase-3 expression (Figure 3); our results thus indicate that MTX induces testicular apoptosis.
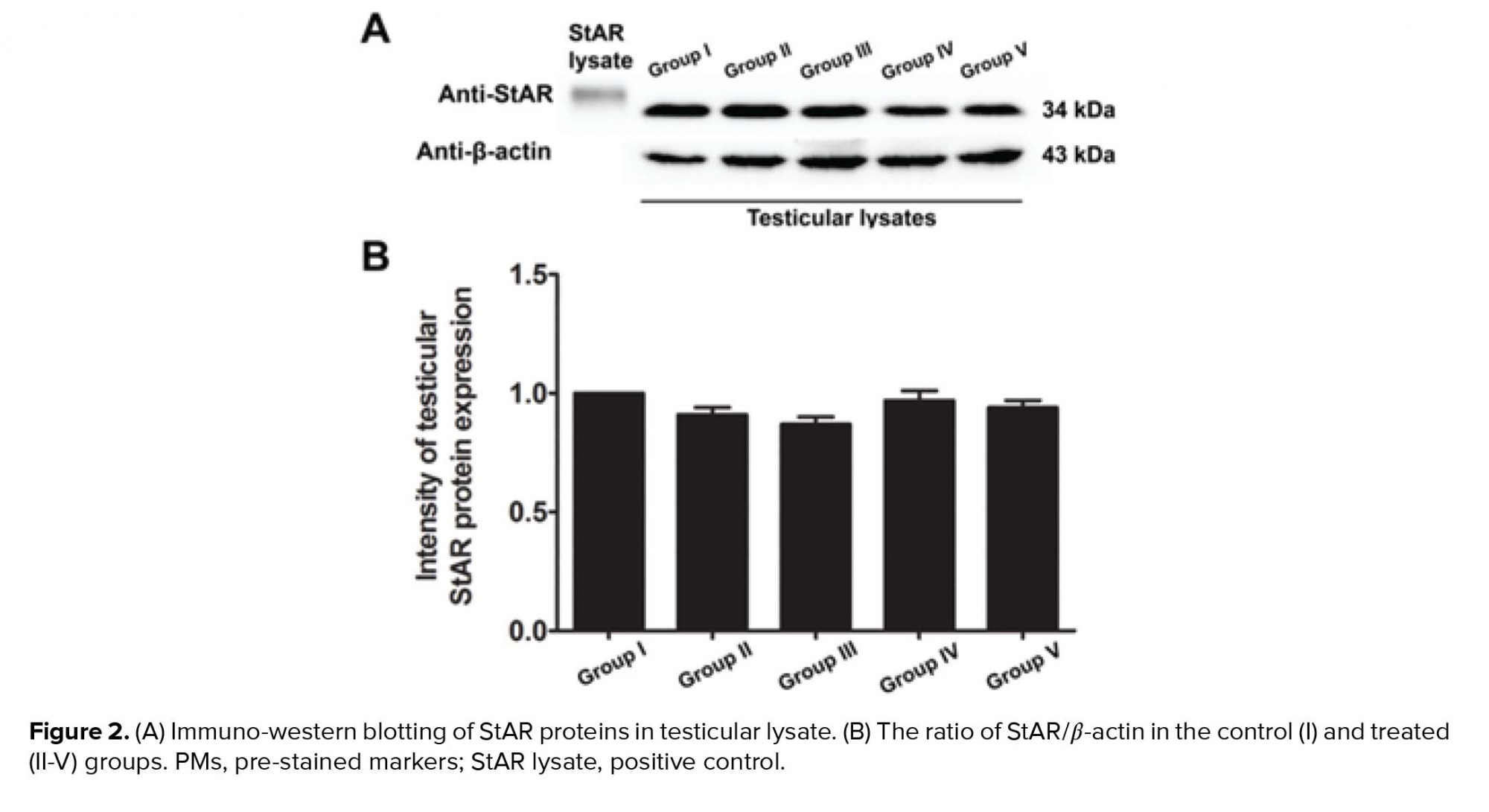
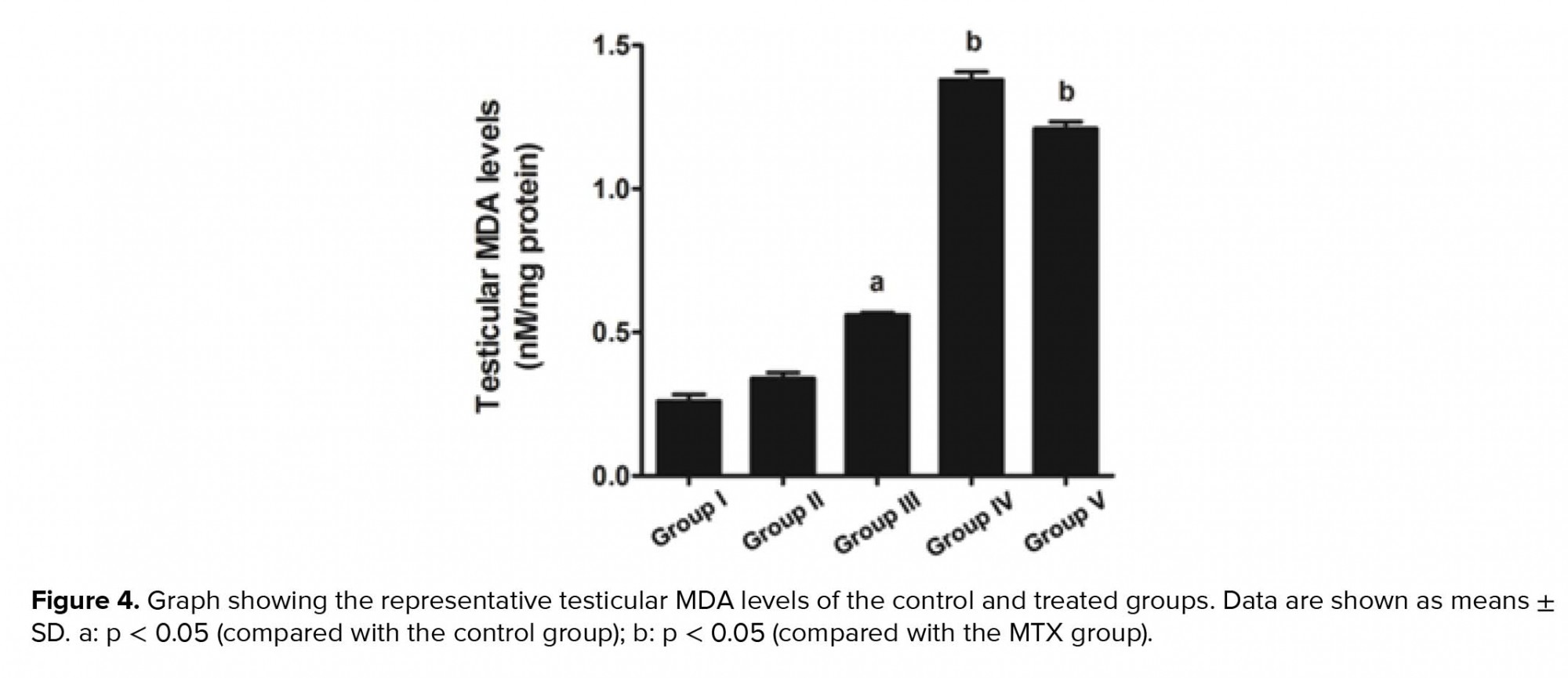
Our study further supports previous research that has shown the anti-inflammatory effect of melatonin via the nuclear factor erythroid 2-related factor 2 and nuclear factor kappa-light-chain-enhancer of activated B cell pathways in rat testes treated with MTX (28). Melatonin is further known to possess antioxidant capacities that can protect testicular injury in many animal models (29-31). In the present study, a significant decrease in sperm count was also associated with the histopathologies found in the testicular and caudal epididymis tissues. It should be obvious that MTX caused a reduction in seminiferous diameter and sperm mass within the epididymal lumen. These results might be due to the sensitivity of the mitotic rate of germinal epithelium to anticancer treatments (32). Although the level of testosterone in this study was not determined, the expression of the StAR protein revealed by western blotting showed no significant difference among the control and treated groups. Therefore, MTX may cause damage to the seminiferous epithelium via another mechanism, such as oxidative stress. In particular, the adverse effects of MTX may be caused by ROS, which create an imbalance between ROS production and antioxidant defense (33). The significantly increased testicular caspase-3 expression in the MTX group is consistent with an increase in mRNA expression reported by Sheikhbahaei and coworkers (34). In the present study, MTX significantly increased the testicular MDA level, which has also been reported previously (35, 36).
The antioxidant system in the testes consists of a number of antioxidant enzymes, including SOD, CAT, and GPX, as well as non-enzyme factors, such as vitamin C, vitamin E, and melatonin. Melatonin can regulate normal testicular function (17, 18). Moreover, it has anti-inflammatory, proliferative, and ROS scavenging effects (18, 19). Herein, we revealed that melatonin could reduce testicular damage in the melatonin group, a finding similar to that made by Wang and co-workers (36). In the present study, it was demonstrated for the first time that melatonin may protect testicular apoptosis by decreasing caspase-3 expression. However, the MDA level was increased significantly in the preventive groups, which is not in concordance with the results of some previous reports (37). We assumed that the melatonin dose used in this study for the stimulation of neuronal progenitor cell survival had no effect on MDA reduction (24). Tyrosine phosphorylation in the testes is essential for spermatogenesis, and pattern changes are associated with increased sperm concentration (4, 14, 38, 39). The increased expression of the tyrosine-phosphorylated protein at 31 and 32 kDa in the MTX-treated testes is similar to that reported by Iamsaard et al. (14). In addition, the expression of tyrosine-phosphorylated protein at 47 kDa was increased significantly in the melatonin groups compared with the MTX group. It is possible that this improvement in tyrosine-phosphorylated protein expression caused by melatonin treatment plays an important role in normal spermatogenesis.
5. Conclusion
Our results indicate that melatonin can increase seminiferous morphometry and epididymal sperm concentration. However, the testicular MDA levels in co-treated groups were not improved. Melatonin also reduces testicular caspase-3 expression and increases the expression of tyrosine-phosphorylated proteins in rats treated with MTX.
Acknowledgments
This study was funded by the MFU Research Fund for Young Researchers 2018 (Project No. 611A08010) and the Research Institute for Human High Performance and Health Promotion (HHP&HP), Khon Kaen, Thailand.
Conflict of Interest
The authors declare no conflicts of interests with regard to the present study.
Full-Text: (605 Views)
- Introduction
Melatonin is an endocrine hormone produced by the pineal gland to regulate normal testicular function through the hypothalamic-adenohypophyseal axis (17, 18). Additionally, melatonin has been shown to have anti-inflammatory and proliferative effects (18, 19). Moreover, it has been demonstrated to reduce the expression of caspase-3 in ovaries and testes damaged by chemotherapeutic drugs (20-23). Based on the antioxidant, anti-inflammatory, and antiapoptotic activities of melatonin, the present study aimed to investigate its protective effect against testicular damage induced by MTX.
- Materials and Methods
- 1. Animals and experiment design
- Group I (control group) received an ethanol and normal saline solution similar to the treated groups.
- Group II (melatonin-treated group) received intraperitoneal injections of melatonin (Sigma-Aldrich, Inc., St. Louis, MO, USA) at a dose of 8 mg/kg for 15 consecutive days.
- Group III (MTX group) were intravenously injected with 0.5 ml/kg MTX (Pharmachemie B.V., Harsblem, the Netherlands) at a dose of 75 mg/kg on days 8 and 15 of the experiment. They were also intraperitoneally injected with leucovorin (Ben Venue Laboratories, Inc., Bedford, MA, USA) at a volume of 1 ml/kg (at a dose of 6 mg/kg) 18, 26, 42, and 50 hour after the MTX injections,
- Group IV were intraperitoneally injected with melatonin (8 mg/kg) for 15 consecutive days and MTX (75 mg/kg) on days 8 and 15 of the experimental period.
- Group V were intraperitoneally injected with melatonin (8 mg/kg) for 30 consecutive days and MTX (75 mg/kg) on days 8 and 15 of the experimental period.
- 2. Histological examination of male reproductive organs and sperm concentration
- 3. Activated caspase-3 immunofluorescence
- 4. Western blot analysis
The separated proteins were transferred onto a nitrocellulose membrane (Bio-Red Laboratories, Inc., city, Germany) in a 10% methanol transfer buffer. Subsequently, the non-specific proteins were blocked with 5% skim milk. To examine the expression of tyrosine-phosphorylated protein, StAR protein, and caspase-3, the membrane was incubated with monoclonal anti-phosphotyrosine 4G10 antibody (1:1000; Millipore, city, state code, country), polyclonal anti-StAR antibody (1:2000; Santa Cruz Biotechnology), or monoclonal anti-caspase-3 antibody (1:100; Santa Cruz Biotechnology) at 4°C overnight. The membrane was also incubated with monoclonal anti-b-actin antibody as an internal control (1:1000; Santa Cruz Biotechnology).
Each of the non-binding antibodies was washed out of the membrane, and the membrane was further incubated with a non-binding antigen-antibody complex for each antibody. The complexes were composed of specific secondary antibodies conjugated to the primary antibodies with horseradish peroxidase at room temperature. After washing, each non-binding antigen-antibody complex was incubated with an enhanced chemiluminescence substrate kit reagent (GH Healthcare Life Science). The positive protein bands were detected using the ImageQuant LAS 500 imaging system (GH Healthcare Life Science) in the Vejawichakarn building of the Faculty of Medicine at Khon Kaen University in Thailand.
To quantify the level of target protein expression, the intensities of protein bands were measured using the Image J 1.52a program. In this study, BSA (Millipore), epidermal growth factor (Millipore), and StAR lysate (Santa Cruz Biotechnology) were used as specific negative and positive controls of tyrosine-phosphorylated protein and StAR protein, respectively (4).
- 5. MDA examination
- 6. Ethical consideration
- 7. Statistical analysis
- Results
- 1. Effects of melatonin on the body weight and reproductive parameters of male rats treated with MTX
- 2. Effects of melatonin on the histology of the testes and cauda epididymis in the MTX group
- 3. Effects of melatonin and MTX on testicular tyrosine-phosphorylated protein
- 4. Effects of melatonin on testicular StAR protein expression in rats treated with MTX
3.5. Effects of melatonin and MTX on testicular caspase-3
The immunofluorescent staining results for caspase-3 revealed that the protein was localized in both the seminiferous epithelium and interstitial tissues of all groups (Figure 3A). This finding was also confirmed by western blotting (Figure 3B). However, the intensity of caspase-3 expression was much greater in all the treated groups than in the control group (Figure 3A). Figures 3B and 5C further revealed that the expression of caspase-3 in the MTX group was increased compared with that in the other groups. The intensity of caspase-3 expression was also lower in the preventive (IV and V) groups than in the MTX group (Figure 3C).
- 6. Effects of melatonin on testicular MDA levels in rats treated with MTX





4. Discussion
Chemotherapeutic agents have adverse effects on the male reproductive system, depending on the type and dosage of the drug used, and typically affect seminiferous epithelial cells (4). MTX is known as a low-risk gonadotoxic chemotherapeutic agent that affects the cell cycle via DNA and RNA synthesis inhibition, the inhibition of mitosis, and the deamination of proteins (26). In the present study, MTX was found to decrease the absolute testicular weight, the caudal epididymal sperm concentration, and the diameter of STs. These findings are supported by results from previous reports (14, 15, 27). In the present study, the significant decrease in testicular weight in the MTX group was correlated with the reduction of seminiferous epithelial height and diameter. This effect may have been caused by the increase in caspase-3 expression (Figure 3); our results thus indicate that MTX induces testicular apoptosis.
Our study further supports previous research that has shown the anti-inflammatory effect of melatonin via the nuclear factor erythroid 2-related factor 2 and nuclear factor kappa-light-chain-enhancer of activated B cell pathways in rat testes treated with MTX (28). Melatonin is further known to possess antioxidant capacities that can protect testicular injury in many animal models (29-31). In the present study, a significant decrease in sperm count was also associated with the histopathologies found in the testicular and caudal epididymis tissues. It should be obvious that MTX caused a reduction in seminiferous diameter and sperm mass within the epididymal lumen. These results might be due to the sensitivity of the mitotic rate of germinal epithelium to anticancer treatments (32). Although the level of testosterone in this study was not determined, the expression of the StAR protein revealed by western blotting showed no significant difference among the control and treated groups. Therefore, MTX may cause damage to the seminiferous epithelium via another mechanism, such as oxidative stress. In particular, the adverse effects of MTX may be caused by ROS, which create an imbalance between ROS production and antioxidant defense (33). The significantly increased testicular caspase-3 expression in the MTX group is consistent with an increase in mRNA expression reported by Sheikhbahaei and coworkers (34). In the present study, MTX significantly increased the testicular MDA level, which has also been reported previously (35, 36).
The antioxidant system in the testes consists of a number of antioxidant enzymes, including SOD, CAT, and GPX, as well as non-enzyme factors, such as vitamin C, vitamin E, and melatonin. Melatonin can regulate normal testicular function (17, 18). Moreover, it has anti-inflammatory, proliferative, and ROS scavenging effects (18, 19). Herein, we revealed that melatonin could reduce testicular damage in the melatonin group, a finding similar to that made by Wang and co-workers (36). In the present study, it was demonstrated for the first time that melatonin may protect testicular apoptosis by decreasing caspase-3 expression. However, the MDA level was increased significantly in the preventive groups, which is not in concordance with the results of some previous reports (37). We assumed that the melatonin dose used in this study for the stimulation of neuronal progenitor cell survival had no effect on MDA reduction (24). Tyrosine phosphorylation in the testes is essential for spermatogenesis, and pattern changes are associated with increased sperm concentration (4, 14, 38, 39). The increased expression of the tyrosine-phosphorylated protein at 31 and 32 kDa in the MTX-treated testes is similar to that reported by Iamsaard et al. (14). In addition, the expression of tyrosine-phosphorylated protein at 47 kDa was increased significantly in the melatonin groups compared with the MTX group. It is possible that this improvement in tyrosine-phosphorylated protein expression caused by melatonin treatment plays an important role in normal spermatogenesis.
5. Conclusion
Our results indicate that melatonin can increase seminiferous morphometry and epididymal sperm concentration. However, the testicular MDA levels in co-treated groups were not improved. Melatonin also reduces testicular caspase-3 expression and increases the expression of tyrosine-phosphorylated proteins in rats treated with MTX.
Acknowledgments
This study was funded by the MFU Research Fund for Young Researchers 2018 (Project No. 611A08010) and the Research Institute for Human High Performance and Health Promotion (HHP&HP), Khon Kaen, Thailand.
Conflict of Interest
The authors declare no conflicts of interests with regard to the present study.
Type of Study: Original Article |
Subject:
Reproductive Biology
References
1. 1. Das UB, Mallick M, Debnath JM, Ghosh D. Protective effect of ascorbic acid on cyclophosphamide- induced testicular gametogenic and androgenic disorders in male rats. Asian J Androl 2002; 4: 201-207.
2. 2. Vassilakopoulou M, Boostandoost E, Papaxoinis G, de La Motte Rouge T, Khayat D, Psyrri A. Anticancer treatment and fertility: Effect of therapeutic modalities on reproductive system and functions. Crit Rev Oncol Hematol 2016; 97: 328-334.
3. 3. Iamsaard S, Sukhorum W, Arun S, Phunchago N, Uabundit N, Boonruangsri P, et al. Valproic acid induces histologic changes and decreases androgen receptor levels of testis and epididymis in rats. Int J Reprod Biomed 2017; 15: 217-224.
4. 4. Sukhorum W, Iamsaard S. Changes in testicular function proteins and sperm acrosome status in rats treated with valproic acid. Reprod Fertil Dev 2017; 29: 1585-1592.
5. 5. Cole PD, Zebala JA, Alcaraz MJ, Smith AK, Tan J, Kamen BA. Pharmacodynamic properties of methotrexate and aminotrexate during weekly therapy. Cancer Chemother Pharmacol 2006; 57: 826-834.
6. 6. Scalvenzi M, Patrì A, Costa C, Megna M, Napolitano M, Fabbrocini G, et al. Intralesional methotrexate for the treatment of keratoacanthoma: The neapolitan experience. Dermatol Ther 2019; 9: 369-372.
7. 7. Yuluğ E, Türedi S, Alver A, Türedi S, Kahraman C. Effects of resveratrol on methotrexate-induced testicular damage in rats. Scientific World Journal 2013; 2013: 489659.
8. 8. Sönmez MF, Çilenk KT, Karabulut D, Ünalmış S, Deligönül E, Öztürk İ, et al. Protective effects of propolis on methotrexate-induced testis injury in rat. Biomed Pharmacother 2016; 79: 44-51.
9. 9. Koc F, Erısgın Z, Tekelıoglu Y, Takır S. The effect of beta glucan on MTX induced testicular damage in rats. Biotech Histochem 2018; 93: 70-75.
10. 10. Johnson FE, Farr SA, Mawad M, Woo YC. Testicular cytotoxicity of intravenous methotrexate in rats. J Surg Oncol 1994; 55: 175-178.
11. 11. Chelab KG, Majeed SK. Histopathological effects of methotrexate on male and female reproductive organs in white mice. Bas J Vet Res 2009; 8: 166-175.
12. 12. Padmanabhan S, Tripathi DN, Vikram A, Ramarao P, Jena GB. Cytotoxic and genotoxic effects of methotrexate in germ cells of male Swiss mice. Mutat Res 2008; 655: 59-67.
13. 13. Güvenç M, Aksakal M. Ameliorating effect of kisspeptin-10 on methotrexate-induced sperm damages and testicular oxidative stress in rats. Andrologia 2018; 50: e13057.
14. 14. Iamsaard S, Welbat JU, Sukhorum W, Krutsri S, Arun S, Sawatpanich T. Methotrexate changes the testicular tyrosine phosphorylated protein expression and seminal vesicle epithelia of adult rats. Int J Morphol 2018; 36: 737-742.
15. 15. Vardi N, Parlakpinar H, Ates B, Cetin A, Otlu A. Antiapoptotic and antioxidant effects of 𝛽-carotene against methotrexate-induced testicular injury. Fertility and Sterility 2009; 92: 2028-2033.
16. 16. Armagan A, Uzar E, Uz E, Yilmaz HR, Kutluhan S, Koyuncuoglu HR, et al. Caffeic acid phenethyl ester modulates methotrexate-induced oxidative stress in testes of rat. Hum Exp Toxicol 2008; 27: 547-552.
17. 17. Awad H, Halawa F, Mostafa T, Atta H. Melatonin hormone profile in infertile males. Int J Androl 2006; 29: 409-413.
18. 18. Frungieri MB, Calandra RS, Rossi SP. Local actions of melatonin in somatic cells of the testis. Int J Mol Sci 2017; 18: 1170.
19. 19. Zhang HM, Zhang Y. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res 2014; 57: 131-146.
20. 20. Lombardi LA, Mattos LS, Simões RS, Florencio-Silva R, Sasso GRDS, Carbonel AAF, et al. Melatonin may prevent or reverse polycystic ovary syndrome in rats. Rev Assoc Med Bras 2019; 65: 1008-1014.
21. 21. Chen Z, Lei L, Wen D, Yang L. Melatonin attenuates palmitic acid-induced mouse granulosa cells apoptosis via endoplasmic reticulum stress. J Ovarian Res 2019; 12: 43.
22. 22. Gao Y, Wu X, Zhao S, Zhang Y, Ma H, Yang Z, et al. Melatonin receptor depletion suppressed hCG-induced testosterone expression in mouse Leydig cells. Cell Mol Biol Lett 2019; 24: 21.
23. 23. Riaz H, Yousuf MR, Liang A, Hua GH, Yang L. Effect of melatonin on regulation of apoptosis and steroidogenesis in cultured buffalo granulosa cells. Anim Sci J 2019; 90: 473-480.
24. 24. Sirichoat A, Krutsri S, Suwannakot K, Aranarochana A, Chaisawang P, Pannangrong W, et al. Melatonin protects against methotrexate-induced memory deficit and hippocampal neurogenesis impairment in a rat model. Biochem Pharmacol 2019; 163: 225-233.
25. 25. Iamsaard S, Prabsattroo T, Sukhorum W, Muchimapura S, Srisaard P, Uabundit N, et al. Anethum graveolens Linn. (dill) extract enhances the mounting frequency and level of testicular tyrosine protein phosphorylation in rats. J Zhejiang Univ Sci B 2013; 14: 247-252.
26. 26. Arnon J, Meirow D, Lewis-Roness H, Ornoy A. Genetic and teratogenic effects of cancer treatments on gametes and embryos. Hum Reprod Update 2001; 7: 394-403.
27. 27. Shrestha S, Dhungel S, Saxena AK, Bhattacharya S, Maskey D. Effect of methotrexate (mtx) administration on spermatogenesis: an experiment on animal model. Nepal Med Coll J 2007; 9: 230-233.
28. 28. Wang Y, Zhao TT, Zhao HY, Wang H. Melatonin protects methotrexate-induced testicular injury in rats. Eur Rev Med Pharmacol Sci 2018; 22: 7517-7525.
29. 29. Bahrami N, Goudarzi M, Hosseinzadeh A, Sabbagh S, Reiter RJ, Mehrzadi S. Evaluating the protective effects of melatonin on di(2-ethylhexyl) phthalate-induced testicular injury in adult mice. Biomed Pharmacother 2018; 108: 515-523.
30. 30. Muratoğlu S, Akarca Dizakar OS, Keskin Aktan A, Ömeroğlu S, Akbulut KG. The protective role of melatonin and curcumin in the testis of young and aged rats. Andrologia 2019; 51: e13203.
31. 31. El-Shafaei A, Abdelmaksoud R, Elshorbagy A, Zahran N, Elabd R. Protective effect of melatonin versus montelukast in cisplatin-induced seminiferous tubule damage in rats. Andrologia 2018; 50: e13077.
32. 32. Brydøy M, Fosså SD, Dahl O, Bjøro T. Gonadal dysfunction and fertility problems in cancer survivors. Acta Oncol 2007; 46: 480-489.
33. 33. Maneesh M, Jayalekshmi H. Role of reactive oxygen species and antioxidants on pathophysiology of male reproduction. Indian J Clin Biochem 2006; 21: 80-89.
34. 34. Sheikhbahaei F, Khazaei M, Rabzia A, Mansouri K, Ghanbari A. Protective effects of thymoquinone against methotrexate-induced germ cell apoptosis in male mice. Int J Fertil Steril 2016; 9: 541-547.
35. 35. Pınar N, Çakırca G, Özgür T, Kaplan M. The protective effects of alpha lipoic acid on methotrexate induced testis injury in rats. Biomed Pharmacother 2018; 97: 1486-1492.
36. 36. Wang Y, Zhao TT, Zhao HY, Wang H. Melatonin protects methotrexate-induced testicular injury in rats. Eur Rev Med Pharmacol Sci 2018; 22: 7517-7525.
37. 37. Kolli VK, Abraham P, Isaac B, Kasthuri N. Preclinical efficacy of melatonin to reduce methotrexate-induced oxidative stress and small intestinal damage in rats. Dig Dis Sci 2013; 58: 959-969.
38. 38. Iamsaard S, Arun S, Burawat J, Sukhorum W, Wattanathorn J, Nualkaew S, et al. Phenolic contents and antioxidant capacities of Thai- Makham Pom (Phyllanthus emblica L.) aqueous extracts. J Zhejiang Univ Sci B 2014; 15: 405-408.
39. 39. Arun S, Burawat J, Sukhorum W, Sampannang A, Uabundit N, Iamsaard S. Changes of testicular phosphorylated proteins in response to restraint stress in male rats. J Zhejiang Univ Sci B 2016; 17: 21-29.
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








