Mon, Feb 2, 2026
[Archive]
Volume 18, Issue 7 (July 2020)
IJRM 2020, 18(7): 551-560 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Talebi A, Hayati Roodbari N, Sameni H R, Zarbakhsh S. Impact of coadministration of apigenin and bone marrow stromal cells on damaged ovaries due to chemotherapy in rat: An experimental study. IJRM 2020; 18 (7) :551-560
URL: http://ijrm.ir/article-1-1547-en.html
URL: http://ijrm.ir/article-1-1547-en.html
1- Department of Biology, Science and Research Branch, Islamic Azad University, Tehran, Iran.
2- Nervous System Stem Cells Research Center, Semnan University of Medical Sciences, Semnan, Iran.
3- Nervous System Stem Cells Research Center, Semnan University of Medical Sciences, Semnan, Iran. ,smzarbakhsh@gmail.com
2- Nervous System Stem Cells Research Center, Semnan University of Medical Sciences, Semnan, Iran.
3- Nervous System Stem Cells Research Center, Semnan University of Medical Sciences, Semnan, Iran. ,
Full-Text [PDF 1194 kb]
(1500 Downloads)
| Abstract (HTML) (3326 Views)
Although the useful impacts of apigenin and BMSCs on damaged ovaries have been studied individually, there is no report yet about the impact of coadministration of them on the improvement of damaged ovaries following chemotherapy. Thus, for the first time, we assessed the impact of coadministration of apigenin and BMSCs on ovarian function, structure, and apoptosis after creating a model of chemotherapy with cyclophosphamide in rat.
Materials and Methods

3. Discussion
In the current study, for the first time, we investigated the impact of coadministration of apigenin and BMSCs on damaged ovaries after creating a model of chemotherapy with cyclophosphamide in rats. Overall, the results showed that a coadministration of apigenin and BMSC significantly improved the function, structure, and apoptosis in the damaged ovaries.
We examined the ovaries in terms of function, structure, and apoptosis. To assess ovarian function, the ability of ovulation by superovulation and the level of AMH serum by ELISA kit were evaluated. AMH is a dimeric glycoprotein from transforming growth factor β family which is produced by granulosa cells of preantral follicles. Therefore, when apoptosis occurs in granulosa cells, the amount of AMH decreases. Indeed, AMH is an important indicator for the ovarian follicular content and can be used as a marker for ovarian dysfunction (25). To assess ovarian structure, the number of follicles in different stages were evaluated by H&E staining. Also to assess apoptosis in ovarian granulosa cells, the expression ratio of Bcl-2/Bax proteins was evaluated by western blot because the Bcl-2 and Bax genes are respectively characterized as antiapoptotic and proapoptotic agents (24, 26).
The results of the BMSC group were significantly more desirable than the control group that these results were consistent with other studies (7, 27). BMSCs as a type of MSCs are a suitable candidate for cell therapy in damaged ovaries. Liu and co-workers have reported that MSCs improve tissues chiefly via differentiation and paracrine effects (28). Various studies have demonstrated that BMSCs secrete some growth factors which can recover damaged ovaries and prevent cell apoptosis. Some of these growth factors are insulin-like growth factor 1, vascular endothelial growth factor, basic fibroblast growth factor, and hepatocyte growth factor (7, 27).
On the other hand, the results of apigenin group showed that apigenin as an antioxidant significantly improved the ovaries than the control group. These results consent with Tang’s study. Tang and co-workers showed that apigenin could protect ovaries by inhibiting the self-renewal capacity of human ovarian cancer (9). While the results of our study were contrary to Soyman’s study, Soyman and co-workers showed that a single dose of 15 mg/kg apigenin has no significant protective effect on ovarian injury following ischemia and reperfusion (29). The reason for this contradiction was probably due to the use of a single dose of apigenin in Soyman’s study against the administration of 10 mg/kg apigenin for 14 consecutive days in our study. Some articles have reported that apigenin has protective effects on various cell types. Anusha and co-workers showed that apigenin has protective effects on the rat model of Parkinson’s disease by suppressing neuro-inflammation factors and inhibiting apoptosis induced by oxidative stress (18). Zhang and co-workers showed that apigenin has neuroprotective effects on spinal cord injury in rats (30). Liu and co-workers showed that apigenin has a protective role in undifferentiated dental pulp cells (31). Apigenin plays an important role in fatty acid transport and lipid catabolism in mitochondria. Apigenin increases mitochondrial activity and produces ATP by increasing β-oxidation of fatty acids hence providing energy for cellular growth suppressing apoptosis (32). In addition, apigenin can prevent cell apoptosis by repressing ROS compounds (11). Accumulation of ROS in ovarian follicles leads to discharge in the ATP repository, which reduces follicle quality. Antioxidants as ROS scavengers and energy production facilitators may be responsible for beneficial effects on ovarian function and follicular survival (33).
Given the potential effects of apigenin on the differentiation of stem cells, coadministration of apigenin and BMSCs may offer a novel clinical approach to the recovery of damaged ovaries following chemotherapy. The results of the coadministration group were significantly more desirable than BMSC, apigenin, and control groups. The reasons were likely related to the compound of beneficial effects of MSCs and apigenin with various action mechanisms in the recovery of ovaries after chemotherapy. Additionally, apigenin might increase the differentiation of the transplanted BMSCs to replace damaged ovarian cells. Dawn and co-workers reported that the differentiation of adult bone marrow-derived cells into other cells of various organs has been repeatedly confirmed (8). Samet and coworkers have reported that apigenin has the potential synergistic effects on the differentiation of hematopoietic stem cells (13). Zhang and co-workers have shown that apigenin is effective on differentiating MSCs in vitro (12). They have reported that apigenin promotes the osteogenesis of hMSCs by increasing alkaline phosphatase activity and mineralization in hMSCs. Thereby using apigenin and MSCs in the ovary might cause osteogenic differentiation inside the ovaries. In this regard, Mao and co-workers have reported that the two main factors for osteogenic differentiation from MSCs are substrate stiffness and neighboring cells (34). Therefore, due to the lack of substrate stiffness and osteocyte in ovaries, the probability of BMSC differentiation into osteogenic cells was low. Zhang and co-workers in an in vitro study have shown that apigenin inhibits inflammatory factors and at doses < 40 µmol/L have no harmful effect on MSCs. However, at the dose of 80 µmol/L, apigenin significantly increased apoptosis in these cells (35). This issue shows that apigenin as an antioxidant may be harmful if consumed in too large quantities. In the present study, we observed that 10 mg/kg apigenin with BMSC transplantation has favorable effects on the rat ovarian restoration.
In the current study, BMSC transplantation was performed locally in the ovaries while apigenin injection was given intraperitoneally. According to related studies, our hypothesis was that the prescribed models would probably be the most effective mode. Liu and co-workers after comparing the local and systemic administration of MSCs reported that local administration of stem cells is the most efficient way for stem cell homing and differentiation (28). Also, the effectiveness of intraperitoneal injection of apigenin in other tissues has been repeatedly reported (18, 30, 31). While the local injection of apigenin in the ovary has not been done so far.
Limitation
This study had some limitations. The number of samples was small, so the larger sample size is required. Also, more research is necessary to clarify the molecular mechanisms underlying apigenin and BMSCs function in ovarian repair after chemotherapy. In addition, the effect of different doses of apigenin and the fate of transplanted BMSCs have not been investigated in this study.
4. Conclusion
The results suggest that the effect of co-administration of apigenin and BMSCs is maybe more effective than the effect of their administrations individually on the recovery of damaged ovaries following the chemotherapy with cyclophosphamide in rats.
Acknowledgments
This study was financially supported by the Science and Research Branch, Islamic Azad University, Tehran, Iran. We express our deep appreciation to Mrs. Parisa Hayat for her help in obtaining the results of flow cytometry and western blot and to Dr. Nasrin Khanmohammadi for her help in obtaining the results of superovulation.
Conflict of Interest
There is no conflict of interest in this article.
Full-Text: (562 Views)
- Introduction
Although the useful impacts of apigenin and BMSCs on damaged ovaries have been studied individually, there is no report yet about the impact of coadministration of them on the improvement of damaged ovaries following chemotherapy. Thus, for the first time, we assessed the impact of coadministration of apigenin and BMSCs on ovarian function, structure, and apoptosis after creating a model of chemotherapy with cyclophosphamide in rat.
Materials and Methods
- 1. Experimental animals
- 2. Culture and characterization of BMSCs
- 3. Creating the chemotherapy model
- 4. Grouping and injection methods in the groups
- 5. Anti-Müllerian hormone evaluation
- 6. Evaluating the ability of ovulation
- 7. Histological evaluation
- 8. Western blot assays
- 9. Ethical consideration
- 10. Statistical analysis
- Results
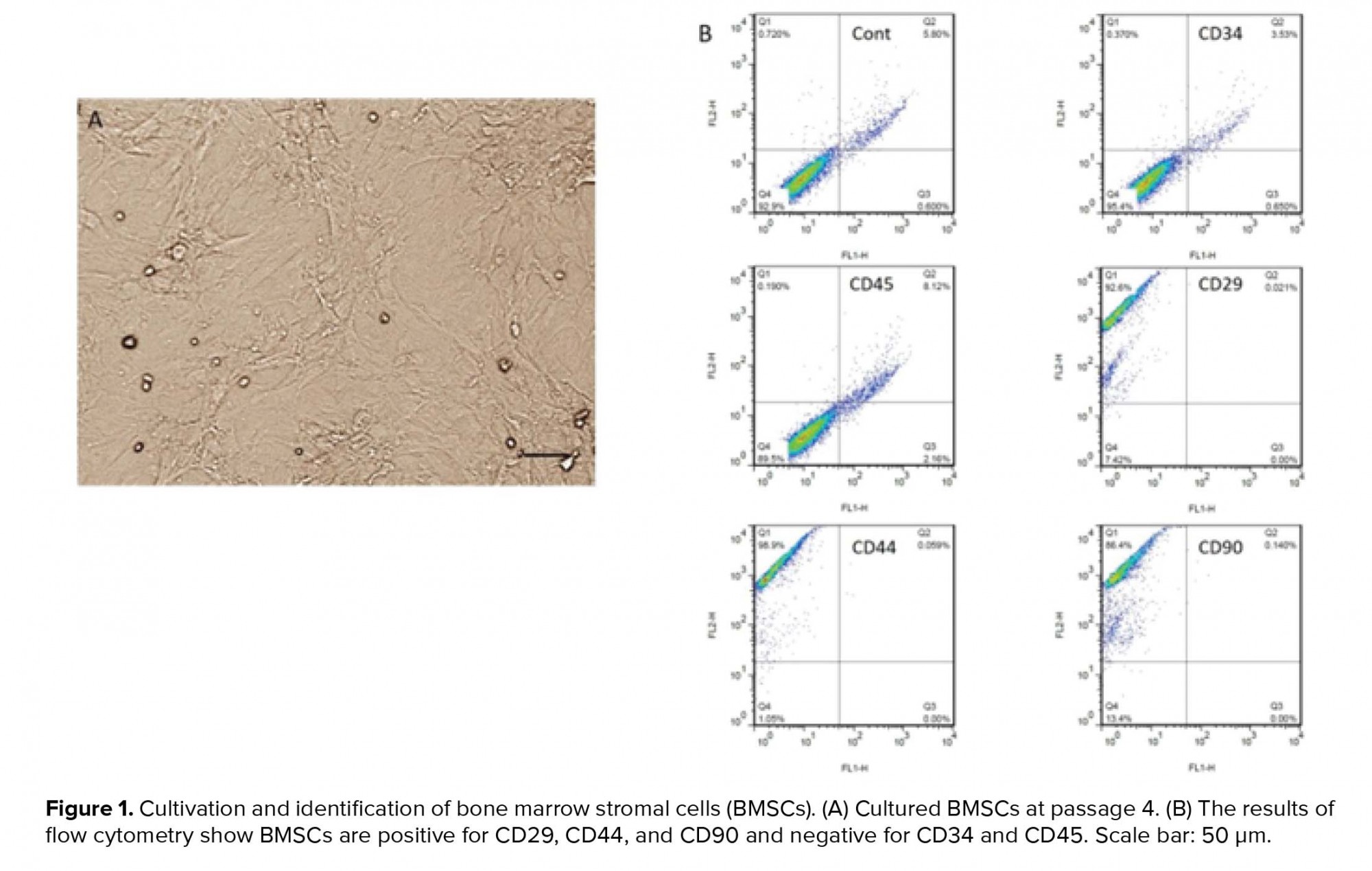
- 1. BMSC cultivation and characterization
- 2. Level of AMH serum
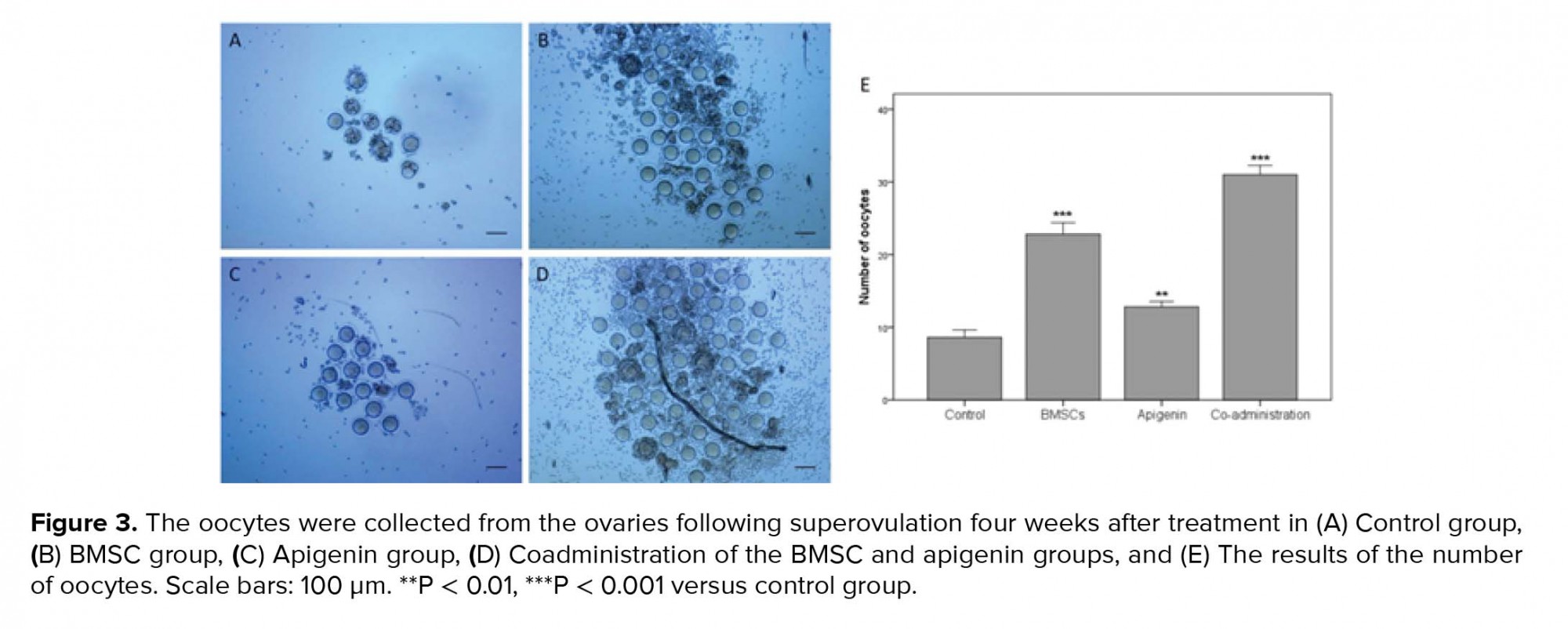
- 3. Evaluating the ability of ovulation
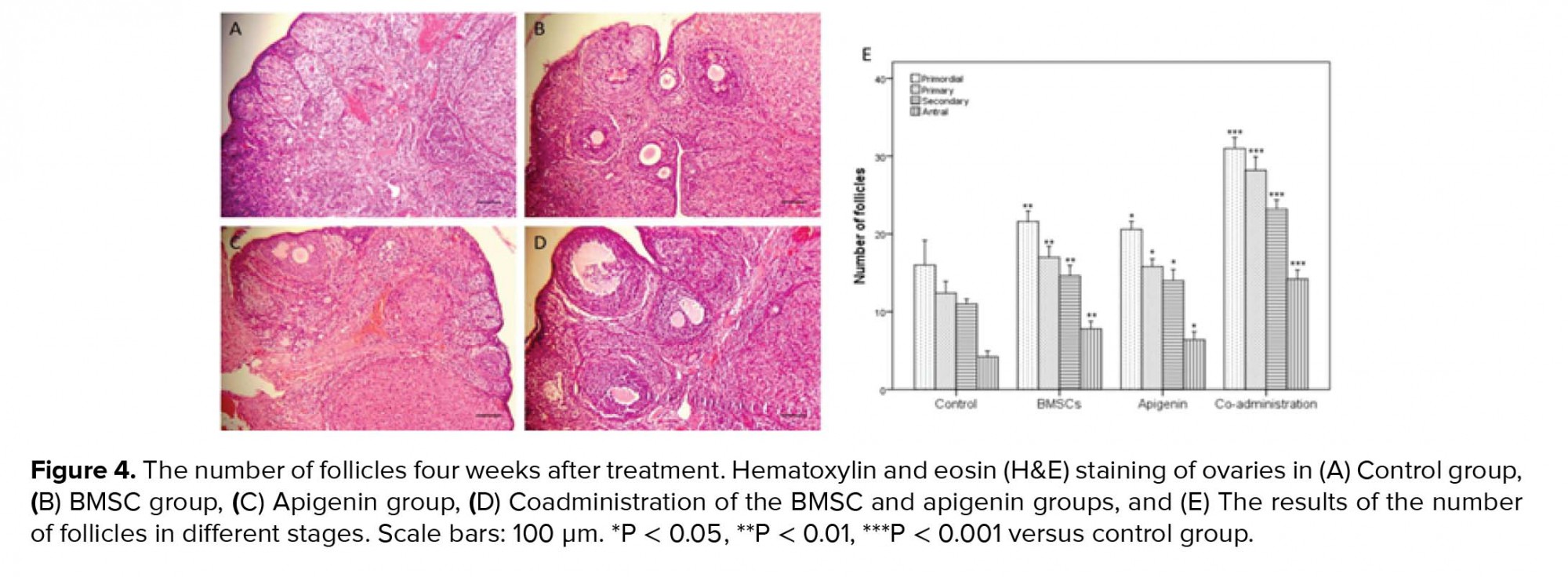
- 4. Histological evaluation of the ovaries
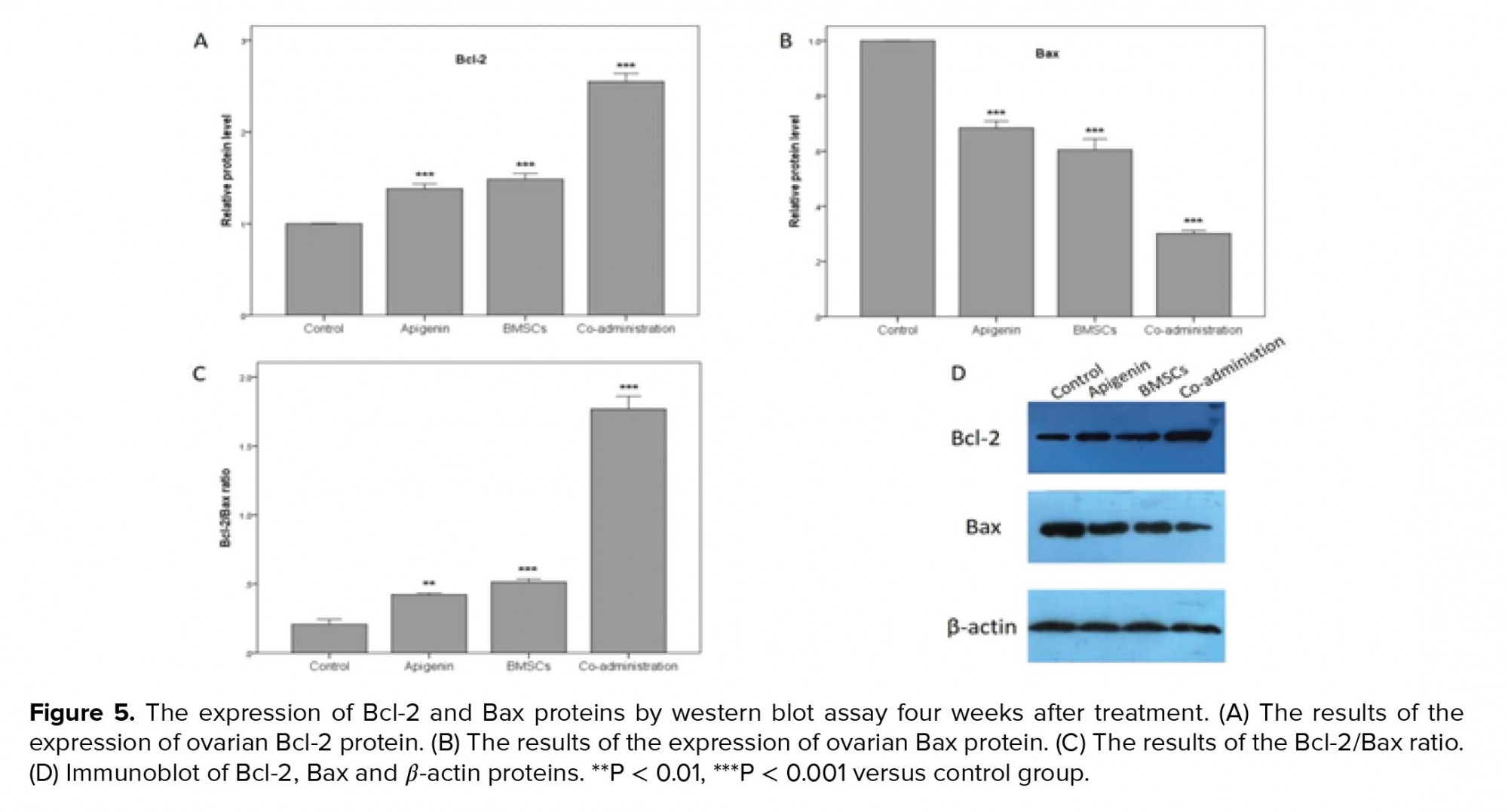
- 5. Analysis of the Bcl-2 and Bax expression in the ovaries





3. Discussion
In the current study, for the first time, we investigated the impact of coadministration of apigenin and BMSCs on damaged ovaries after creating a model of chemotherapy with cyclophosphamide in rats. Overall, the results showed that a coadministration of apigenin and BMSC significantly improved the function, structure, and apoptosis in the damaged ovaries.
We examined the ovaries in terms of function, structure, and apoptosis. To assess ovarian function, the ability of ovulation by superovulation and the level of AMH serum by ELISA kit were evaluated. AMH is a dimeric glycoprotein from transforming growth factor β family which is produced by granulosa cells of preantral follicles. Therefore, when apoptosis occurs in granulosa cells, the amount of AMH decreases. Indeed, AMH is an important indicator for the ovarian follicular content and can be used as a marker for ovarian dysfunction (25). To assess ovarian structure, the number of follicles in different stages were evaluated by H&E staining. Also to assess apoptosis in ovarian granulosa cells, the expression ratio of Bcl-2/Bax proteins was evaluated by western blot because the Bcl-2 and Bax genes are respectively characterized as antiapoptotic and proapoptotic agents (24, 26).
The results of the BMSC group were significantly more desirable than the control group that these results were consistent with other studies (7, 27). BMSCs as a type of MSCs are a suitable candidate for cell therapy in damaged ovaries. Liu and co-workers have reported that MSCs improve tissues chiefly via differentiation and paracrine effects (28). Various studies have demonstrated that BMSCs secrete some growth factors which can recover damaged ovaries and prevent cell apoptosis. Some of these growth factors are insulin-like growth factor 1, vascular endothelial growth factor, basic fibroblast growth factor, and hepatocyte growth factor (7, 27).
On the other hand, the results of apigenin group showed that apigenin as an antioxidant significantly improved the ovaries than the control group. These results consent with Tang’s study. Tang and co-workers showed that apigenin could protect ovaries by inhibiting the self-renewal capacity of human ovarian cancer (9). While the results of our study were contrary to Soyman’s study, Soyman and co-workers showed that a single dose of 15 mg/kg apigenin has no significant protective effect on ovarian injury following ischemia and reperfusion (29). The reason for this contradiction was probably due to the use of a single dose of apigenin in Soyman’s study against the administration of 10 mg/kg apigenin for 14 consecutive days in our study. Some articles have reported that apigenin has protective effects on various cell types. Anusha and co-workers showed that apigenin has protective effects on the rat model of Parkinson’s disease by suppressing neuro-inflammation factors and inhibiting apoptosis induced by oxidative stress (18). Zhang and co-workers showed that apigenin has neuroprotective effects on spinal cord injury in rats (30). Liu and co-workers showed that apigenin has a protective role in undifferentiated dental pulp cells (31). Apigenin plays an important role in fatty acid transport and lipid catabolism in mitochondria. Apigenin increases mitochondrial activity and produces ATP by increasing β-oxidation of fatty acids hence providing energy for cellular growth suppressing apoptosis (32). In addition, apigenin can prevent cell apoptosis by repressing ROS compounds (11). Accumulation of ROS in ovarian follicles leads to discharge in the ATP repository, which reduces follicle quality. Antioxidants as ROS scavengers and energy production facilitators may be responsible for beneficial effects on ovarian function and follicular survival (33).
Given the potential effects of apigenin on the differentiation of stem cells, coadministration of apigenin and BMSCs may offer a novel clinical approach to the recovery of damaged ovaries following chemotherapy. The results of the coadministration group were significantly more desirable than BMSC, apigenin, and control groups. The reasons were likely related to the compound of beneficial effects of MSCs and apigenin with various action mechanisms in the recovery of ovaries after chemotherapy. Additionally, apigenin might increase the differentiation of the transplanted BMSCs to replace damaged ovarian cells. Dawn and co-workers reported that the differentiation of adult bone marrow-derived cells into other cells of various organs has been repeatedly confirmed (8). Samet and coworkers have reported that apigenin has the potential synergistic effects on the differentiation of hematopoietic stem cells (13). Zhang and co-workers have shown that apigenin is effective on differentiating MSCs in vitro (12). They have reported that apigenin promotes the osteogenesis of hMSCs by increasing alkaline phosphatase activity and mineralization in hMSCs. Thereby using apigenin and MSCs in the ovary might cause osteogenic differentiation inside the ovaries. In this regard, Mao and co-workers have reported that the two main factors for osteogenic differentiation from MSCs are substrate stiffness and neighboring cells (34). Therefore, due to the lack of substrate stiffness and osteocyte in ovaries, the probability of BMSC differentiation into osteogenic cells was low. Zhang and co-workers in an in vitro study have shown that apigenin inhibits inflammatory factors and at doses < 40 µmol/L have no harmful effect on MSCs. However, at the dose of 80 µmol/L, apigenin significantly increased apoptosis in these cells (35). This issue shows that apigenin as an antioxidant may be harmful if consumed in too large quantities. In the present study, we observed that 10 mg/kg apigenin with BMSC transplantation has favorable effects on the rat ovarian restoration.
In the current study, BMSC transplantation was performed locally in the ovaries while apigenin injection was given intraperitoneally. According to related studies, our hypothesis was that the prescribed models would probably be the most effective mode. Liu and co-workers after comparing the local and systemic administration of MSCs reported that local administration of stem cells is the most efficient way for stem cell homing and differentiation (28). Also, the effectiveness of intraperitoneal injection of apigenin in other tissues has been repeatedly reported (18, 30, 31). While the local injection of apigenin in the ovary has not been done so far.
Limitation
This study had some limitations. The number of samples was small, so the larger sample size is required. Also, more research is necessary to clarify the molecular mechanisms underlying apigenin and BMSCs function in ovarian repair after chemotherapy. In addition, the effect of different doses of apigenin and the fate of transplanted BMSCs have not been investigated in this study.
4. Conclusion
The results suggest that the effect of co-administration of apigenin and BMSCs is maybe more effective than the effect of their administrations individually on the recovery of damaged ovaries following the chemotherapy with cyclophosphamide in rats.
Acknowledgments
This study was financially supported by the Science and Research Branch, Islamic Azad University, Tehran, Iran. We express our deep appreciation to Mrs. Parisa Hayat for her help in obtaining the results of flow cytometry and western blot and to Dr. Nasrin Khanmohammadi for her help in obtaining the results of superovulation.
Conflict of Interest
There is no conflict of interest in this article.
Type of Study: Original Article |
Subject:
Stem Cell & Cloning
References
1. Khedr NF. Protective effect of mirtazapine and hesperidin on cyclophosphamide-induced oxidative damage and infertility in rat ovaries. Exp Biol Med (Maywood) 2015; 240: 1682-1689.
2. Dolmans MM, Demylle D, Martinez-Madrid B, Donnez J. Efficacy of in vitro fertilization after chemotherapy. Fertil Steril 2005; 83: 897-901.
3. Hamzeh M, Hosseinimehr SJ, Mohammadi HR, Yaghubi Beklar S, Dashti A, Talebpour Amiri F. Atorvastatin attenuates the ovarian damage induced by cyclophosphamide in rat: An experimental study. Int J Reprod Biomed 2018; 16: 323-334.
4. Liu T, Qin W, Huang Y, Zhao Y, Wang J. Induction of estrogen-sensitive epithelial cells derived from human-induced pluripotent stem cells to repair ovarian function in a chemotherapy-induced mouse model of premature ovarian failure. DNA Cell Biol 2013; 32: 685-698.
5. Nandy SB, Gangwani L, Nahleh Z, Subramani R, Arumugam A, de la Rosa JM, et al. Recurrence and metastasis of breast cancer is influenced by ovarian hormone's effect on breast cancer stem cells. Future Oncol 2015; 11: 983-995.
6. Varghese AC, du Plessis SS, Falcone T, Agarwal A. Cryopreservation/transplantation of ovarian tissue and in vitro maturation of follicles and oocytes: challenges for fertility preservation. Reprod Biol Endocrinol 2008; 6: 47-56.
7. Khanmohammadi N, Sameni HR, Mohammadi M, Pakdel A, Mirmohammadkhani M, Parsaie H, et al. Effect of transplantation of bone marrow stromal cell- conditioned medium on ovarian function, morphology and cell death in cyclophosphamide-treated rats. Cell J 2018; 20: 10-18.
8. Dawn B, Bolli R. Adult bone marrow-derived cells: regenerative potential, plasticity, and tissue commitment. Basic Res Cardiol 2005; 100: 494-503.
9. Tang AQ, Cao XC, Tian L, He L, Liu F. Apigenin inhibits the self-renewal capacity of human ovarian cancer SKOV3 derived sphere-forming cells. Mol Med Rep 2015; 11: 2221-2226.
10. Safari M, Parsaie H, Sameni HR, Aldaghi MR, Zarbakhsh S. Anti-oxidative and anti-apoptotic effects of apigenin on number of viable and apoptotic blastomeres, zona pellucida thickness and hatching rate of mouse embryos. Int J Fertil Steril 2018; 12: 257-262.
11. Sharma H, Kanwal R, Bhaskaran N, Gupta S. Plant flavone apigenin binds to nucleic acid bases and reduces oxidative DNA damage in prostate epithelial cells. PLoS One 2014; 9: e91588.
12. Zhang X, Zhou C, Zha X, Xu Z, Li L, Liu Y, et al. Apigenin promotes osteogenic differentiation of human mesenchymal stem cells through JNK and p38 MAPK pathways. Mol Cell Biochem 2015; 407: 41-50.
13. Samet I, Villareal MO, Motojima H, Han J, Sayadi S, Isoda H. Olive leaf components apigenin 7-glucoside and luteolin 7-glucoside direct human hematopoietic stem cell differentiation towards erythroid lineage. Differentiation 2015; 89: 146-155.
14. Safari M, Jafari B, Zarbakhsh S, Sameni H, Vafaei AA, Mohammadi NK, et al. G-CSF for mobilizing transplanted bone marrow stem cells in rat model of Parkinson's disease. Iran J Basic Med Sci 2016; 19: 1318-1324.
15. Haydari S, Safari M, Zarbakhsh S, Bandegi AR, Miladi-Gorji H. Effects of voluntary exercise on the viability, proliferation and BDNF levels of bone marrow stromal cells in rat pups born from morphine- dependent mothers during pregnancy. Neurosci Lett 2016; 634: 132-137.
16. Takehara Y, Yabuuchi A, Ezoe K, Kuroda T, Yamadera R, Sano C, et al. The restorative effects of adipose-derived mesenchymal stem cells on damaged ovarian function. Lab Invest 2013; 93: 181-193.
17. Song D, Zhong Y, Qian C, Zou Q, Ou J, Shi Y, et al. Human umbilical cord mesenchymal stem cells therapy in cyclophosphamide-induced premature ovarian failure rat model. Biomed Res Int 2016; 2016: 2517514.
18. Anusha C, Sumathi T, Joseph LD. Protective role of apigenin on rotenone induced rat model of Parkinson's disease: Suppression of neuroinflammation and oxidative stress mediated apoptosis. Chem Biol Interact 2017; 269: 67-79.
19. Ling L, Feng X, Wei T, Wang Y, Wang Y, Wang Z, et al. Human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation improves ovarian function in rats with premature ovarian insufficiency (POI) at least partly through a paracrine mechanism. Stem Cell Res Ther 2019; 10: 46-63.
20. Dayangan Sayan C, Tulmac OB, Karaca G, Ozkan ZS, Yalcin S, Devrim T, et al. Could erythropoietin reduce the ovarian damage of cisplatin in female rats? Gynecol Endocrinol 2018; 34: 309-313.
21. Sameni HR, Seiri M, Safari M, Tabrizi Amjad MH, Khanmohammadi N, Zarbakhsh S. Bone marrow stromal cells with the granulocyte colony-stimulating factor in the management of chemotherapy-induced ovarian failure in a rat model. Iran J Med Sci 2019; 44: 135-145.
22. Mesbah F, Bordbar H, Talaei Khozani T, Dehghani F, Mirkhani H. The non-preventive effects of human menopausal gonadotropins on ovarian tissues in Nandrolone decanoate-treated female rats: A histochemical and ultra-structural study. Int J Reprod Biomed 2018; 16: 159-174.
23. Kalhori Z, Soleimani Mehranjani M, Azadbakht M, Shariaatzadeh MA. Ovary stereological features and serum biochemical factors following induction of polycystic ovary syndrome with testosterone enanthate in mice: An experimental study. Int J Reprod Biomed 2018; 16: 267-274.
24. Bas D, Abramovich D, Hernandez F, Tesone M. Altered expression of Bcl-2 and Bax in follicles within dehydroepiandrosterone-induced polycystic ovaries in rats. Cell Biol Int 2011; 35: 423-429.
25. Peigné M, Decanter C. Serum AMH level as a marker of acute and long-term effects of chemotherapy on the ovarian follicular content: a systematic review. Reprod Biol Endocrinol 2014; 12: 26-35.
26. Zarbakhsh S, Safari R, Sameni HR, Yousefi B, Safari M, Khanmohammadi N, et al. Effects of co-administration of bone marrow stromal cells and L-carnitine on the recovery of damaged ovaries by performing chemotherapy model in rat. Int J Fertil Steril 2019; 13: 196-202.
27. Guo JQ, Gao X, Lin ZJ, Wu WZ, Huang LH, Dong HY, et al. BMSCs reduce rat granulosa cell apoptosis induced by cisplatin and perimenopause. BMC Cell Biol 2013; 14: 18-26.
28. Liu S, Zhou J, Zhang X, Liu Y, Chen J, Hu B, et al. Strategies to optimize adult stem cell therapy for tissue regeneration. Int J Mol Sci 2016; 17: 982-997.
29. Soyman Z, Kelekçi S, Sal V, Şevket O, Bayındır N, Uzun H. Effects of apigenin on experimental ischemia/reperfusion injury in the rat ovary. Balkan Med J 2017; 34: 444-449.
30. Zhang F, Li F, Chen G. Neuroprotective effect of apigenin in rats after contusive spinal cord injury. Neurol Sci 2014; 35: 583-588.
31. Liu L, Peng Z, Xu Z, Wei X. Effect of luteolin and apigenin on the expression of Oct-4, Sox2, and c-Myc in dental pulp cells with in vitro culture. Biomed Res Int 2015; 2015: 534952.
32. Jung UJ, Cho YY, Choi MS. Apigenin ameliorates dyslipidemia, hepatic steatosis and insulin resistance by modulating metabolic and transcriptional profiles in the liver of high-fat diet-induced obese mice. Nutrients 2016; 8: 305-320.
33. Wang S, He G, Chen M, Zuo T, Xu W, Liu X. The role of antioxidant enzymes in the ovaries. Oxid Med Cell Longev 2017; 2017: 4371714.
34. Mao AS, Shin JW, Mooney DJ. Effects of substrate stiffness and cell-cell contact on mesenchymal stem cell differentiation. Biomaterials 2016; 98: 184-191.
35. Zhang HT, Zha ZG, Cao JH, Liang ZJ, Wu H, He MT, et al. Apigenin accelerates lipopolysaccharide induced apoptosis in mesenchymal stem cells through suppressing vitamin D receptor expression. Chin Med J 2011; 124: 3537-3545.
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








