Thu, Apr 25, 2024
[Archive]
Volume 19, Issue 2 (February 2021)
IJRM 2021, 19(2): 157-166 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Khodadadian A, Varghaiyan Y, Babakhanzadeh E, Alipourfard I, Haghi-Daredeh S, Ghobadi A, et al . Fertility preservation in women with ovarian cancer: Finding new pathways: A case-control study. IJRM 2021; 19 (2) :157-166
URL: http://ijrm.ir/article-1-1575-en.html
URL: http://ijrm.ir/article-1-1575-en.html
Ali Khodadadian * 

 1, Yasser Varghaiyan2
1, Yasser Varghaiyan2 

 , Emad Babakhanzadeh3
, Emad Babakhanzadeh3 

 , Iraj Alipourfard4
, Iraj Alipourfard4 

 , Saeed Haghi-Daredeh5
, Saeed Haghi-Daredeh5 

 , Amin Ghobadi6
, Amin Ghobadi6 

 , Mohsen Hemmati-Dinarvand7
, Mohsen Hemmati-Dinarvand7 

 , Mehrdad Talebi3
, Mehrdad Talebi3 

 , Nasrin Ghasemi8
, Nasrin Ghasemi8 




 1, Yasser Varghaiyan2
1, Yasser Varghaiyan2 

 , Emad Babakhanzadeh3
, Emad Babakhanzadeh3 

 , Iraj Alipourfard4
, Iraj Alipourfard4 

 , Saeed Haghi-Daredeh5
, Saeed Haghi-Daredeh5 

 , Amin Ghobadi6
, Amin Ghobadi6 

 , Mohsen Hemmati-Dinarvand7
, Mohsen Hemmati-Dinarvand7 

 , Mehrdad Talebi3
, Mehrdad Talebi3 

 , Nasrin Ghasemi8
, Nasrin Ghasemi8 


1- Department of Medical Genetics, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. , Ali.khodadadian@yahoo.com
2- Department of Immunology, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Department of Medical Genetics, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
4- Center of Pharmaceutical Sciences, Faculty of Life Sciences, University of Vienna, Vienna, Austria. School of Pharmacy, Faculty of Sciences, University of Rome Tor Vergata, Rome, Italy.
5- Department of Medical Nanotechnology, School of Medicine, Shahroud University of Medical Sciences, Shahroud, Iran.
6- Department of Clinical Biochemistry and Laboratory Medicine, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
7- Department of Clinical Biochemistry and Laboratory Medicine, Faculty of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
8- Abortion Research Centre, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
2- Department of Immunology, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Department of Medical Genetics, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
4- Center of Pharmaceutical Sciences, Faculty of Life Sciences, University of Vienna, Vienna, Austria. School of Pharmacy, Faculty of Sciences, University of Rome Tor Vergata, Rome, Italy.
5- Department of Medical Nanotechnology, School of Medicine, Shahroud University of Medical Sciences, Shahroud, Iran.
6- Department of Clinical Biochemistry and Laboratory Medicine, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
7- Department of Clinical Biochemistry and Laboratory Medicine, Faculty of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
8- Abortion Research Centre, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Full-Text [PDF 1729 kb]
(696 Downloads)
| Abstract (HTML) (1697 Views)
Full-Text: (320 Views)
- Introduction
Ovarian malignancies are the most common type of gynecological cancers and are often diagnosed at a later stage, when the cancer cells are migrating and invading other tissues and organs (1). Today, the standard treatments for cancer patients are chemotherapy and surgery, which in most cases lead to cure (>70% in cases of ovarian cancer); however, 90% of cases involving tumour recurrence remain incurable (2). Moreover, both methods have complications. One of the most pertinent challenges in ovarian cancer is fertility issues in women who undergo prolonged chemotherapy (especially when drug resistance is observed) or surgery (oophorectomy) (3). Oophorectomy can be bilateral or unilateral. When it is bilateral, a successful pregnancy will be impossible, and if it is unilateral, the reproductive ability will be reduced.
The effect of chemotherapy on male and female infertility has been investigated (4); that have shown a negative effect of chemotherapy drugs (including cisplatin) on fertility (5). Therefore, acquiring knowledge on all pathways involved in the formation, metastasis, growth, and drug resistance of cancer, such as ovarian cancer, can lead to the development of methods for early diagnosis and safer treatment of the disease- methods that will not require surgery and long-term prescription of chemotherapy drugs. These methods will not only reduce cancer symptoms but also minimize the complications in treated patients (e.g. infertility in ovarian cancer).
One of the most important and well-known pathways involved in the epithelial-to-mesenchymal transaction is the Wnt/β-catenin signaling pathway. The role of this pathway and its genes in many malignancies of ovarian cancer has been investigated (6). The leucine-rich repeat containing G protein-coupled receptor 5 (LGR5) is one of the most important genes in this pathway. The correlation between LGR5 and aggressiveness process has been studied in previous works and the results have proved the role of the gene in this process (7). The FOXO1 gene belongs to the forkhead transcription factors family. The role of this gene in a variety of malignancies has been examined in previous studies (8, 9). In addition, Choi and colleagues have studied the relationship between this gene and the LGR5 gene in gastric cancer cells (10). Moreover, the role of LGR5 and FOXO1 gene has been addressed in some cases of female infertility who suffered from gynecological malignancies (11-14).
Micro-RNAs are non-coding regulatory small RNAs, about 17-25 nucleotides in length (15), that act as important factor in regulating the expression of genes in many biological processes; for instance, cell proliferation, cell differentiation, and death of the cell (16, 17). Through bioinformatics analysis and based on a previous study, we conclude that the LGR5 and FOXO1 genes can be potential targets for the miR-340 (18). In addition, the role of mir-340, LGR5, and FOXO1 in drug resistance in several cases has also been studied (18, 19). However, the role of miR-340 and its potential association with the LGR5 and FOXO1 genes as well as their effects on the drug resistance in ovarian cancer have not been addressed till now. Therefore, the aim of this survey was to evaluate and compare the correlation between the expressions of miR-340, LGR5, and FOXO1 genes in both ovarian cancer samples and ovarian cancer cell lines (cisplatin-sensitive cell line [A2780S] and cisplatin-resistant cell line [A2780CP]) before and after the cisplatin treatment, so as to find new therapeutic procedures that do not have a negative effect on the normal fertility of ovarian cancer patients.
The effect of chemotherapy on male and female infertility has been investigated (4); that have shown a negative effect of chemotherapy drugs (including cisplatin) on fertility (5). Therefore, acquiring knowledge on all pathways involved in the formation, metastasis, growth, and drug resistance of cancer, such as ovarian cancer, can lead to the development of methods for early diagnosis and safer treatment of the disease- methods that will not require surgery and long-term prescription of chemotherapy drugs. These methods will not only reduce cancer symptoms but also minimize the complications in treated patients (e.g. infertility in ovarian cancer).
One of the most important and well-known pathways involved in the epithelial-to-mesenchymal transaction is the Wnt/β-catenin signaling pathway. The role of this pathway and its genes in many malignancies of ovarian cancer has been investigated (6). The leucine-rich repeat containing G protein-coupled receptor 5 (LGR5) is one of the most important genes in this pathway. The correlation between LGR5 and aggressiveness process has been studied in previous works and the results have proved the role of the gene in this process (7). The FOXO1 gene belongs to the forkhead transcription factors family. The role of this gene in a variety of malignancies has been examined in previous studies (8, 9). In addition, Choi and colleagues have studied the relationship between this gene and the LGR5 gene in gastric cancer cells (10). Moreover, the role of LGR5 and FOXO1 gene has been addressed in some cases of female infertility who suffered from gynecological malignancies (11-14).
Micro-RNAs are non-coding regulatory small RNAs, about 17-25 nucleotides in length (15), that act as important factor in regulating the expression of genes in many biological processes; for instance, cell proliferation, cell differentiation, and death of the cell (16, 17). Through bioinformatics analysis and based on a previous study, we conclude that the LGR5 and FOXO1 genes can be potential targets for the miR-340 (18). In addition, the role of mir-340, LGR5, and FOXO1 in drug resistance in several cases has also been studied (18, 19). However, the role of miR-340 and its potential association with the LGR5 and FOXO1 genes as well as their effects on the drug resistance in ovarian cancer have not been addressed till now. Therefore, the aim of this survey was to evaluate and compare the correlation between the expressions of miR-340, LGR5, and FOXO1 genes in both ovarian cancer samples and ovarian cancer cell lines (cisplatin-sensitive cell line [A2780S] and cisplatin-resistant cell line [A2780CP]) before and after the cisplatin treatment, so as to find new therapeutic procedures that do not have a negative effect on the normal fertility of ovarian cancer patients.
- Materials and Methods
- 1. Tissue preparation
In this case-control study, 30 ovarian tumor tissues samples with their peripheral tissue (as a control) were collected from Shahid Sadoughi Hospital, Iran, Yazd (November 2017 to January 2019). The exclusion criteria were: age range between 15-45 yr, addiction, long-term alcohol consumption, and family history of ovarian cancer or related malignancies. The samples were immediately put in RNAlater stabilization solution (Thermo Fisher Scientific, Waltham, MA). Next, following the histopathologic confirmation of the tumor tissues and marginal normal tissues; the collected samples were stored at a temperature of -80oC until the next initiation of steps.
- 2. Cell culture
Besides the tissue samples in this study, both the cisplatin-sensitive (A2780S) and cisplatin-resistant (A2780CP) ovarian cancer cell lines were provided by the Iranian Institute of Pasteur Cell Bank. These cell lines were cultured according to the relevant protocols. Briefly, the cells were purchased and thawed under the laminar hood cabinet and for the first time, were grown in the medium containing 80% RPMI1640 and 20% of FBS (fetal bovine serum). In order to improve the growth rate of cells, the initial culture medium was replaced with a medium containing 90% RPMI1640 and 10% FBS after two days.
Flasks containing cell lines were then incubated at 37ºC, 90% moisture, and 5% CO2 concentration. After the proliferation of the cells (covering the 80% of the surface of the flask), the cells were passaged and if needed, prepared for the next freeze. Next, the cells were transferred to 96-well cell culture plates for viability measurements. The IC50 was measured for cisplatin using MTT assay. Cultivate, passage, and treatment of cells was performed under conditions close to sterile and without contamination.
Flasks containing cell lines were then incubated at 37ºC, 90% moisture, and 5% CO2 concentration. After the proliferation of the cells (covering the 80% of the surface of the flask), the cells were passaged and if needed, prepared for the next freeze. Next, the cells were transferred to 96-well cell culture plates for viability measurements. The IC50 was measured for cisplatin using MTT assay. Cultivate, passage, and treatment of cells was performed under conditions close to sterile and without contamination.
- 3. MTT assay
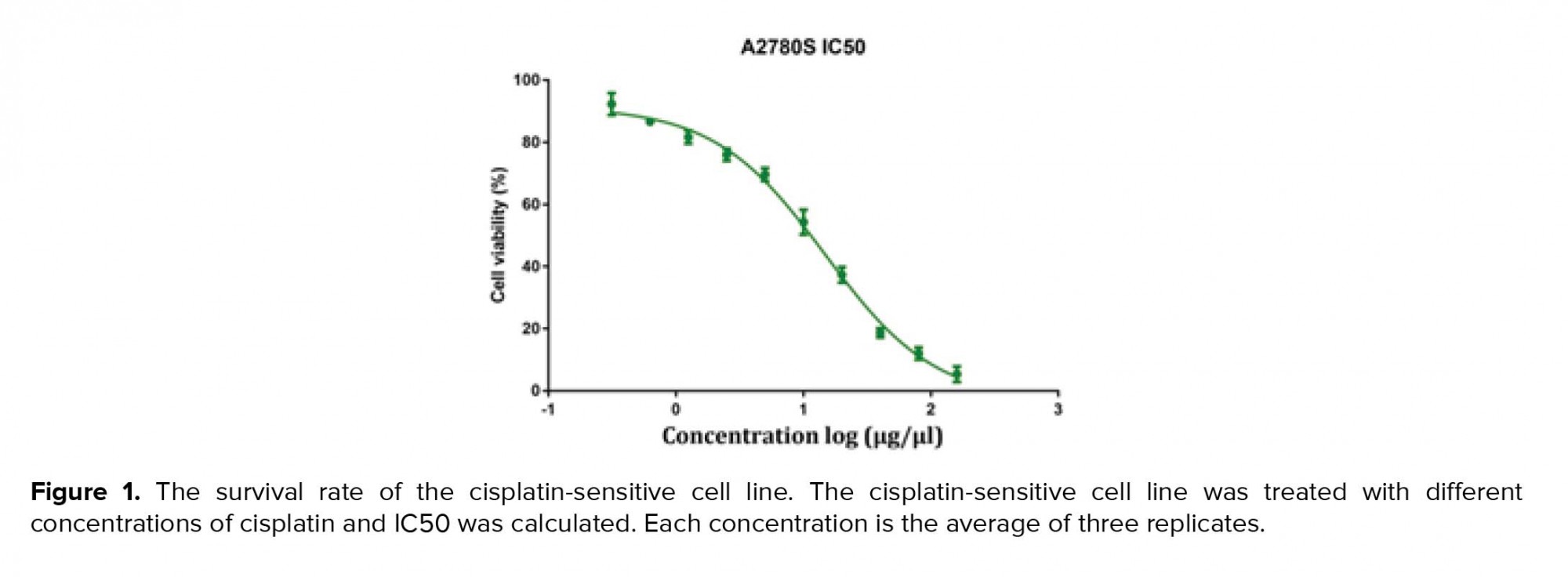
MTT assays were performed as previously defined. Shortly, cells were seeded in 96-well plates (6 × 106 cell/ well) and cultured in media containing 10% FBS for 1-3 days; then, the MTT solution (5 mg/mL, 20 μL) was added into each well. After incubation for 4 hr at 37ºC in a dark place, the media were taken away; 100 μL DMSO was added into each well and the plates were shaken for 30 min (in order to avoid direct contact with light and its effect on the solution color; the plate containing MTT and DMSO were wrapped in foil). The relative number of surviving cells was recorded in ELISA plate reader at 560 nm by means of evaluating the optical density (OD) of cell lysates. Finally, the cisplatin-sensitive cell line IC50 was calculated using Graph Pad Prism software (Figure 1).
- 4. RNA extraction, cDNA synthesis, and qRT-PCR
After obtaining IC50, the cells were cultivated for 24 hr in the presence of the cisplatin in six-well plates (approximately 1 million cells/ well) to obtain a sufficient amount of cell to extract RNA. RNA was extracted from cells using the total RNA purification kit (GeneAll® Hybrid-R, Cat.No. 305-101; Seoul, KOREA). The extracted RNA was then transferred and stored at a temperature of -70° C until the next stage was initiated. In the next step, the cDNA was synthesized for both miR-340 and the LGR5 gene according to the manufacturer’s protocols provided by the BONmiR High Sensitivity MicroRNA1st Strand cDNA Synthesis Kit (Bon Yakhteh, Cat.No 0011.17.1; Tehran, Iran) and the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Cat.No. 4368813, 4368814, 4374966, 4374967; Waltham, MA), respectively. The synthesized cDNAs were stored at -20° C until the remaining steps were completed. Subsequently, the qRT-PCR was performed for the miR-340 and the LGR5 gene. The SNORD and GADPH genes were used as an internal control for the qRT-PCR reaction for miR-340 and LGR5 gene, respectively. The forward and reverse primers of the LGR5, FOXO1, and GAPDH genes were designed using the Primer3 software (Table I). The forward and reverse primers of miR-340, coupled cDNA synthesis kit (Cat. No. BON209001), were purchased from the Bon Yakhteh Company.

2.5. Ethical considerations
The study design was approved by the Institutional Ethics Committee of the Shahid Sadoughi University of Medical Sciences, Yazd (Code: IR.SSU.MEDICINE.REC.1396.220) and a written informed consent was obtained from each participant prior to the collection of tissue samples.
2.6. Statistical analysis
All experiments were repeated thrice. Results are shown as mean ± SEM. All data were analyzed using the GraphPad Prism, Version 6.0. The significant differences between groups were analyzed by Student’s t-test and two-way ANOVA. P-value < 0.05 was considered statistically significant.
3. Results
Compare to the paired adjacent normal tissues, a significant increase in the expression of the LGR5 gene and a significant decrease in expression of the miR-340 and FOXO1 genes were observed in the tissue samples of the patients with ovarian cancer (Figure 2). These primary results encouraged us to continue this study. In the next step, the expression level of these genes in both cisplatin-sensitive and cisplatin-resistance cell lines (A2780S and A2780CP) was measured after 12, 24, and 72 hr of cisplatin-treatment and the results were compared to untreated cisplatin-sensitive as control. The results at this stage, in addition to confirmation of the results obtained from the measurement of gene expression in tissue samples, indicated the role of these genes in drug resistance.
Moreover, after 24, 48, and, 72 hr of cisplatin treatment, a significant decrease (p < 0.001) was seen in the LGR5 expression compared to the untreated cisplatin-sensitive (control) but only in the A2780S cell line, while the A2780CP didn’t show any significant alterations in the LGR5 gene expression when compared with the untreated cisplatin-sensitive for the same periods of cisplatin treatment. Of course, the reduction of expression in the cisplatin-sensitive cell line after 48 and 72 hr of cisplatin treatment was more than that after 24 hr (Figure 3). In relation to FOXO1 and miR-340 genes, a significant increase in the expression after 24, 48, and, 72 hr of cisplatin treatment was seen in both genes. Although for miR-340, this increase was observed in all period of treatment in the A2780S cell line, the increase was very remarkable after 48 hr of cisplatin treatment (i.e., after 48 and 72 hr of cisplatin treatment), while for the FOXO1 gene, it is noteworthy that unlike all previous treatments, a significant increase (p = 0.012) was observed after 72 hr of cisplatin treatment in the A2780CP cell line (Figures 4, 5).




4. Discussion
As stated below previous studies have shown that the miR-340, LGR5, and FOXO1 genes have an effective role in the suppression or progression of various types of cancer as well as in the decrease or increase of drug sensitivity against chemotherapy.
In a study by Shi and colleagues, the function of miR-340 and LGR5 in breast cancer and their effect on drug resistance were discussed. As a result of this study, in addition to the introduction of the LGR5 gene as a downstream target for the mir-340, it has also been confirmed that the miR-340 and LGR5 genes have a suppressive and oncogenic role in breast cancer, respectively (18). The present study confirms the results of the previous studies.
Moreover, in 2017, in a study performed by Zhang and co-workers it was shown that in patients suffering from gastric cancer, the increase in Lgr5 markers caused by the disturbance in miR-132 expression resulted in more resistant to cisplatin during the treatment process (20). The finding of this work also confirms by the outcomes of our study.
Additionally, in studies by Shi and colleagues and Song et al it was shown that the increased expression of miR-340 in different pathways could be effective in increasing the sensitivity of cisplatin to hepatocellular carcinoma cell lines and osteosarcoma (21, 22). Furthermore, in recent studies, the role of FOXO1 gene as a tumor suppressor in the EMT pathway has been discussed (23). A study by Choi and colleagues showed that theinteraction between LGR5 and FOXO1 genes play an important role in the development of gastric cancer (10). Finally, in previous studies, it has been shown that the interesting genes in this study can affect the fertility process through various pathways. Among these, the following are worth mentioning.
According to the previous works, a significant increase in the LGR5 gene expression can play an important role in the infertility of women who suffer from endometriosis (11, 12). On the other hand, in some cases, disruption in the LGR5 gene expression can induce drug resistance in a number of cancers (24). Consequently, drug resistance leads to an increased need for chemotherapy drugs prescription, which can further lead to early menopause in women that has a negative effect on their fertility (25). In addition, in the drug resistance situation, it is possible not to respond to chemotherapy treatment, in which case the patients will have to remove their ovary or ovaries. Consequently, these patients may lose their opportunity to normal fertility. Studies have also shown the role of FOXO1 gene in infertility (26). Disturbance in the expression of the FOXO1 gene can also affect the drug resistance induction in a number of cancers, including ovarian cancer (27). Furthermore, miR-340 can also contribute to inducing drug resistance in cancer through the same pathways (28). As mentioned earlier, there is a significant relationship between the expressions of the LGR5 and FOXO1 genes (10). On the other hand, these genes are the potential downstream targets of miR-340. Consequently, the expression levels of the LGR5 and FOXO1 genes may also be affected by miR-340.
Inducing drug resistance can lead to late or lack of treatment in various cancers. In some cases, the patients may have to remove their ovaries. Therefore, finding new ways to reduce drug resistance can lead to easier treatment and maintaining reproductive ability in patients. The present study was designed to determine the pattern of the expressions of miR-340, LGR5, and FOXO1 genes in ovarian cancer tissue samples as well as their association with drug resistance in ovarian cancer cell lines. As a part of this study, we were looking for pathways involved in drug resistance. Therefore, we decided to use the cisplatin-sensitive IC50 for both cisplatin-sensitive and cisplatin-resistant cell lines; we have made an attempt to find answers to these questions: (a) if a cisplatin-resistant cell line does not show a good response under IC50 of the cisplatin-sensitive cell line, what potential problem can be the cause of this issue? (b) If the cisplatin-resistant cell line shows a good response, what alterations have occurred in the expression of genes that lead to a good response?
After treating cisplatin-sensitive cell line with cisplatin at different times (24, 48, and 72 hr), we observed a significant increase in the expressions of miR-340 and FOXO1 genes, as well as a significant decrease in the expression of the LGR5 gene. On the other hand, in the cisplatin-resistant cell line, significant differences in the expression of the FOXO1 gene was detected only after 72 hr of cisplatin treatment, this may be due to the prolonged treatment of cisplatin in cisplatin-resistant cell line (up to 72 hr) and loss or reduction of resistance properties in the cisplatin-resistant cell line, given the fact that cisplatin can be toxic in a culture medium up to six days (29). Although ovarian cancer often occurs in women over the age of 45 years, this malignancy may also be observed in younger people. In this study, the tissue samples were collected from individuals aged 15-45 years, as this period is considered as the best pregnancy age in women (30), people who develop cancer during this period may lose their reproductive ability during the treatment. However, if they are treated using safe procedures in a way that the functions of their ovaries (and other organs of the reproductive system) are not affected, they will have a chance to have a natural pregnancy.
Considering the aforementioned, our hypothesis was based on the notion that the LGR5, FOXO1, and miR-340 genes can contribute to the formation of cancer, induction of drug resistance, and infertility in women who have ovarian cancer or other gynecological malignancies. Ultimately, there is a hope that by conducting further studies, this goal can be achieved and that along with getting rid of cancer, the patients with ovarian cancer (and other related malignancies) will also have a chance to the experience natural fertility.
5. Conclusion
It can be concluded that the miR-340, LGR5, and FOXO1 genes can be targeted for safer treatment and preservation of reproductive ability in patients with ovarian cancer by prevention of oophorectomy and/or high prescription of chemotherapy drugs.
Acknowledgments
The authors would like to thank to Dr. Mohammadreza Dehghani and Dr. Mohammad Yahya Vahidi Mehrjardi for their valuable support. Part of this study has been funded by Shahid Sadoughi University of Medical Sciences of Yazd.
Conflict of Interest
The authors declare that they have no conflict of interest.


2.5. Ethical considerations
The study design was approved by the Institutional Ethics Committee of the Shahid Sadoughi University of Medical Sciences, Yazd (Code: IR.SSU.MEDICINE.REC.1396.220) and a written informed consent was obtained from each participant prior to the collection of tissue samples.
2.6. Statistical analysis
All experiments were repeated thrice. Results are shown as mean ± SEM. All data were analyzed using the GraphPad Prism, Version 6.0. The significant differences between groups were analyzed by Student’s t-test and two-way ANOVA. P-value < 0.05 was considered statistically significant.
3. Results
Compare to the paired adjacent normal tissues, a significant increase in the expression of the LGR5 gene and a significant decrease in expression of the miR-340 and FOXO1 genes were observed in the tissue samples of the patients with ovarian cancer (Figure 2). These primary results encouraged us to continue this study. In the next step, the expression level of these genes in both cisplatin-sensitive and cisplatin-resistance cell lines (A2780S and A2780CP) was measured after 12, 24, and 72 hr of cisplatin-treatment and the results were compared to untreated cisplatin-sensitive as control. The results at this stage, in addition to confirmation of the results obtained from the measurement of gene expression in tissue samples, indicated the role of these genes in drug resistance.
Moreover, after 24, 48, and, 72 hr of cisplatin treatment, a significant decrease (p < 0.001) was seen in the LGR5 expression compared to the untreated cisplatin-sensitive (control) but only in the A2780S cell line, while the A2780CP didn’t show any significant alterations in the LGR5 gene expression when compared with the untreated cisplatin-sensitive for the same periods of cisplatin treatment. Of course, the reduction of expression in the cisplatin-sensitive cell line after 48 and 72 hr of cisplatin treatment was more than that after 24 hr (Figure 3). In relation to FOXO1 and miR-340 genes, a significant increase in the expression after 24, 48, and, 72 hr of cisplatin treatment was seen in both genes. Although for miR-340, this increase was observed in all period of treatment in the A2780S cell line, the increase was very remarkable after 48 hr of cisplatin treatment (i.e., after 48 and 72 hr of cisplatin treatment), while for the FOXO1 gene, it is noteworthy that unlike all previous treatments, a significant increase (p = 0.012) was observed after 72 hr of cisplatin treatment in the A2780CP cell line (Figures 4, 5).




4. Discussion
As stated below previous studies have shown that the miR-340, LGR5, and FOXO1 genes have an effective role in the suppression or progression of various types of cancer as well as in the decrease or increase of drug sensitivity against chemotherapy.
In a study by Shi and colleagues, the function of miR-340 and LGR5 in breast cancer and their effect on drug resistance were discussed. As a result of this study, in addition to the introduction of the LGR5 gene as a downstream target for the mir-340, it has also been confirmed that the miR-340 and LGR5 genes have a suppressive and oncogenic role in breast cancer, respectively (18). The present study confirms the results of the previous studies.
Moreover, in 2017, in a study performed by Zhang and co-workers it was shown that in patients suffering from gastric cancer, the increase in Lgr5 markers caused by the disturbance in miR-132 expression resulted in more resistant to cisplatin during the treatment process (20). The finding of this work also confirms by the outcomes of our study.
Additionally, in studies by Shi and colleagues and Song et al it was shown that the increased expression of miR-340 in different pathways could be effective in increasing the sensitivity of cisplatin to hepatocellular carcinoma cell lines and osteosarcoma (21, 22). Furthermore, in recent studies, the role of FOXO1 gene as a tumor suppressor in the EMT pathway has been discussed (23). A study by Choi and colleagues showed that theinteraction between LGR5 and FOXO1 genes play an important role in the development of gastric cancer (10). Finally, in previous studies, it has been shown that the interesting genes in this study can affect the fertility process through various pathways. Among these, the following are worth mentioning.
According to the previous works, a significant increase in the LGR5 gene expression can play an important role in the infertility of women who suffer from endometriosis (11, 12). On the other hand, in some cases, disruption in the LGR5 gene expression can induce drug resistance in a number of cancers (24). Consequently, drug resistance leads to an increased need for chemotherapy drugs prescription, which can further lead to early menopause in women that has a negative effect on their fertility (25). In addition, in the drug resistance situation, it is possible not to respond to chemotherapy treatment, in which case the patients will have to remove their ovary or ovaries. Consequently, these patients may lose their opportunity to normal fertility. Studies have also shown the role of FOXO1 gene in infertility (26). Disturbance in the expression of the FOXO1 gene can also affect the drug resistance induction in a number of cancers, including ovarian cancer (27). Furthermore, miR-340 can also contribute to inducing drug resistance in cancer through the same pathways (28). As mentioned earlier, there is a significant relationship between the expressions of the LGR5 and FOXO1 genes (10). On the other hand, these genes are the potential downstream targets of miR-340. Consequently, the expression levels of the LGR5 and FOXO1 genes may also be affected by miR-340.
Inducing drug resistance can lead to late or lack of treatment in various cancers. In some cases, the patients may have to remove their ovaries. Therefore, finding new ways to reduce drug resistance can lead to easier treatment and maintaining reproductive ability in patients. The present study was designed to determine the pattern of the expressions of miR-340, LGR5, and FOXO1 genes in ovarian cancer tissue samples as well as their association with drug resistance in ovarian cancer cell lines. As a part of this study, we were looking for pathways involved in drug resistance. Therefore, we decided to use the cisplatin-sensitive IC50 for both cisplatin-sensitive and cisplatin-resistant cell lines; we have made an attempt to find answers to these questions: (a) if a cisplatin-resistant cell line does not show a good response under IC50 of the cisplatin-sensitive cell line, what potential problem can be the cause of this issue? (b) If the cisplatin-resistant cell line shows a good response, what alterations have occurred in the expression of genes that lead to a good response?
After treating cisplatin-sensitive cell line with cisplatin at different times (24, 48, and 72 hr), we observed a significant increase in the expressions of miR-340 and FOXO1 genes, as well as a significant decrease in the expression of the LGR5 gene. On the other hand, in the cisplatin-resistant cell line, significant differences in the expression of the FOXO1 gene was detected only after 72 hr of cisplatin treatment, this may be due to the prolonged treatment of cisplatin in cisplatin-resistant cell line (up to 72 hr) and loss or reduction of resistance properties in the cisplatin-resistant cell line, given the fact that cisplatin can be toxic in a culture medium up to six days (29). Although ovarian cancer often occurs in women over the age of 45 years, this malignancy may also be observed in younger people. In this study, the tissue samples were collected from individuals aged 15-45 years, as this period is considered as the best pregnancy age in women (30), people who develop cancer during this period may lose their reproductive ability during the treatment. However, if they are treated using safe procedures in a way that the functions of their ovaries (and other organs of the reproductive system) are not affected, they will have a chance to have a natural pregnancy.
Considering the aforementioned, our hypothesis was based on the notion that the LGR5, FOXO1, and miR-340 genes can contribute to the formation of cancer, induction of drug resistance, and infertility in women who have ovarian cancer or other gynecological malignancies. Ultimately, there is a hope that by conducting further studies, this goal can be achieved and that along with getting rid of cancer, the patients with ovarian cancer (and other related malignancies) will also have a chance to the experience natural fertility.
5. Conclusion
It can be concluded that the miR-340, LGR5, and FOXO1 genes can be targeted for safer treatment and preservation of reproductive ability in patients with ovarian cancer by prevention of oophorectomy and/or high prescription of chemotherapy drugs.
Acknowledgments
The authors would like to thank to Dr. Mohammadreza Dehghani and Dr. Mohammad Yahya Vahidi Mehrjardi for their valuable support. Part of this study has been funded by Shahid Sadoughi University of Medical Sciences of Yazd.
Conflict of Interest
The authors declare that they have no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Oncology
References
1. Wang Y, Sheng Q, Spillman MA, Behbakht K, Gu H. Gab2 regulates the migratory behaviors and E-cadherin expression via activation of the PI3K pathway in ovarian cancer cells. Oncogene 2012; 31: 2512-2520. [DOI:10.1038/onc.2011.435] [PMID] [PMCID]
2. Berry NB, Bapat SA. Ovarian cancer plasticity and epigenomics in the acquisition of a stem-like phenotype. J Ovarian Res 2008; 1: 8. [DOI:10.1186/1757-2215-1-8] [PMID] [PMCID]
3. Hancke K, Isachenko V, Isachenko E, Weiss JM. Prevention of ovarian damage and infertility in young female cancer patients awaiting chemotherapy--clinical approach and unsolved issues. Support Care Cancer 2011; 19: 1909-1919. [DOI:10.1007/s00520-011-1261-2] [PMID]
4. Gaffan J, Holden L, Newlands ES, Short D, Fuller S, Begent RH, et al. Infertility rates following POMB/ACE chemotherapy for male and female germ cell tumours - a retrospective long-term follow-up study. Br J Cancer 2003; 89: 1849-1854. [DOI:10.1038/sj.bjc.6601383] [PMID] [PMCID]
5. Kerr JB, Hutt KJ, Cook M, Speed TP, Strasser A, Findlay JK, et al. Cisplatin-induced primordial follicle oocyte killing and loss of fertility are not prevented by imatinib. Nat Med 2012; 18: 1170-1172. [DOI:10.1038/nm.2889] [PMID] [PMCID]
6. Hseu Y-C, Lin Y-C, Rajendran P, Thigarajan V, Mathew DC, Lin K-Y, et al. Antrodia salmonea suppresses invasion and metastasis in triple-negative breast cancer cells by reversing EMT through the NF-κB and Wnt/β-catenin signaling pathway. Food Chem Toxicol 2018; 124: 219-230. [DOI:10.1016/j.fct.2018.12.009] [PMID]
7. Kobayashi S, Yamada‐Okabe H, Suzuki M, Natori O, Kato A, Matsubara K, et al. LGR5‐positive colon cancer stem cells interconvert with drug‐resistant LGR5‐negative cells and are capable of tumor reconstitution. Stem Cells 2012; 30: 2631-2644. [DOI:10.1002/stem.1257] [PMID]
8. Wachtel M, Schafer BW. PAX3-FOXO1: Zooming in on an "undruggable" target. Semin Cancer Biol 2018; 50: 115-123. [DOI:10.1016/j.semcancer.2017.11.006] [PMID]
9. Zhao Y, Tindall DJ, Huang H. Modulation of androgen receptor by FOXA1 and FOXO1 factors in prostate cancer. Int J Biol Sci 2014; 10: 614-619. [DOI:10.7150/ijbs.8389] [PMID] [PMCID]
10. Choi Y, Park J, San Ko Y, Kim Y, Pyo J-S, Jang BG, et al. FOXO1 reduces tumorsphere formation capacity and has crosstalk with LGR5 signaling in gastric cancer cells. Biochem Biophys Res Commun 2017; 493: 1349-1355. [DOI:10.1016/j.bbrc.2017.09.163] [PMID]
11. Vallvé-Juanico J, Barón C, Suárez-Salvador E, Castellví J, Ballesteros A, Gil-Moreno A, et al. Lgr5 does not vary throughout the menstrual cycle in endometriotic human eutopic endometrium. Int J Mol Sci 2019; 20: 22. [DOI:10.3390/ijms20010022] [PMID] [PMCID]
12. Vallvé-Juanico J, Suárez-Salvador E, Castellví J, Ballesteros A, Taylor HS, Gil-Moreno A, et al. Aberrant expression of epithelial leucine-rich repeat containing G protein-coupled receptor 5-positive cells in the eutopic endometrium in endometriosis and implications in deep-infiltrating endometriosis. Fertil Steril 2017; 108: 858-867. [DOI:10.1016/j.fertnstert.2017.08.018] [PMID]
13. Vasquez YM, Wang X, Wetendorf M, Franco HL, Mo Q, Wang T, et al. FOXO1 regulates uterine epithelial integrity and progesterone receptor expression critical for embryo implantation. PLoS Genet 2018; 14: e1007787. [DOI:10.1371/journal.pgen.1007787] [PMID] [PMCID]
14. Ting AY, Zelinski MB. Characterization of FOXO1, 3 and 4 transcription factors in ovaries of fetal, prepubertal and adult rhesus macaques. Biol Reprod 2017; 96: 1052-1059. [DOI:10.1093/biolre/iox034] [PMID] [PMCID]
15. Karami N, Mirabutalebi SH, Montazeri F, Kalantar SM, Sheikhha MH, Eftekhar M. Aberrant expression of microRNAs 16 and 21 and gene targets in women with unexplained recurrent miscarriage: A case-control study. Int J Reprod BioMed 2018; 16: 617-622. [DOI:10.29252/ijrm.16.10.617] [PMID] [PMCID]
16. Khodadadian A, Hemmati-Dinarvand M, Kalantary-Charvadeh A, Ghobadi A, Mazaheri M. Candidate biomarkers for Parkinson's disease. Biomed Pharmacother 2018; 104: 699-704. [DOI:10.1016/j.biopha.2018.05.026] [PMID]
17. Mirabutalebi SH, Karami N, Montazeri F, Fesahat F, Sheikhha MH, Hajimaqsoodi E, et al. The relationship between the expression levels of miR-135a and HOXA10 gene in the eutopic and ectopic endometrium. Int J Reprod BioMed 2018; 16: 501-506. [DOI:10.29252/ijrm.16.8.501] [PMID] [PMCID]
18. Shi S, Chen X, Liu H, Yu K, Bao Y, Chai J, et al. LGR5 acts as a target of miR-340-5p in the suppression of cell progression and drug resistance in breast cancer via Wnt/beta-catenin pathway. Gene 2019; 683: 47-53. [DOI:10.1016/j.gene.2018.10.014] [PMID]
19. Park J, Choi Y, San Ko Y, Kim Y, Pyo J-S, Jang BG, et al. FOXO1 suppression is a determinant of acquired lapatinib-resistance in HER2-positive gastric cancer cells through MET upregulation. Cancer Res Treat 2018; 50: 239-254. [DOI:10.4143/crt.2016.580] [PMID] [PMCID]
20. Zhang L, Guo X, Zhang D, Fan Y, Qin L, Dong S, et al. Upregulated miR-132 in Lgr5(+) gastric cancer stem cell-like cells contributes to cisplatin-resistance via SIRT1/CREB/ABCG2 signaling pathway. Mol Carcinog 2017; 56: 2022-2034. [DOI:10.1002/mc.22656] [PMID]
21. Shi L, Chen ZG, Wu LL, Zheng JJ, Yang JR, Chen XF, et al. miR-340 reverses cisplatin resistance of hepatocellular carcinoma cell lines by targeting Nrf2-dependent antioxidant pathway. Asian Pac J Cancer Prev 2014; 15: 10439-10444. [DOI:10.7314/APJCP.2014.15.23.10439] [PMID]
22. Song L, Duan P, Gan Y, Li P, Zhao C, Xu J, et al. MicroRNA-340-5p modulates cisplatin resistance by targeting LPAATβ in osteosarcoma. Braz J Med Biol Res 2017; 50: e6359. [DOI:10.1590/1414-431x20176359] [PMID] [PMCID]
23. Gao Zh, Liu R, Ye N, Liu Ch, Li X, Guo X, et al. FOXO1 inhibits tumor cell migration via regulating cell surface morphology in non-small cell lung cancer cells. Cell Physiol Biochem 2018; 48: 138-148. [DOI:10.1159/000491670] [PMID]
24. Shi S, Chen X, Liu H, Yu K, Bao Y, Chai J, et al. LGR5 acts as a target of miR-340-5p in the suppression of cell progression and drug resistance in breast cancer via Wnt/β-catenin pathway. Gene 2019; 683: 47-53. [DOI:10.1016/j.gene.2018.10.014] [PMID]
25. Cao HZ, Liu XF, Yang WT, Chen Q, Zheng PS. LGR5 promotes cancer stem cell traits and chemoresistance in cervical cancer. Cell Death Dis 2017; 8: e3039. [DOI:10.1038/cddis.2017.393] [PMID] [PMCID]
26. Vasquez YM, Wang X, Wetendorf M, Franco HL, Mo Q, Wang T, et al. FOXO1 regulates uterine epithelial integrity and progesterone receptor expression critical for embryo implantation. PLos Genet 2018; 14: e1007787. [DOI:10.1371/journal.pgen.1007787] [PMID] [PMCID]
27. Park J, Choi Y, San Ko Y, Kim Y, Pyo J-S, Jang BG, et al. FOXO1 suppression is a determinant of acquired lapatinib-resistance in HER2-positive gastric cancer cells through MET upregulation. Cancer Res Treat 2018; 50: 239-254. [DOI:10.4143/crt.2016.580] [PMID] [PMCID]
28. Rezaei Z, Sebzari A, Kordi-Tamandani DM, Dastjerdi K. Involvement of the dysregulation of Mir-23b-3p, Mir-195-5p, Mir-656-5p, and Mir-340-5p in trastuzumab resistance of HER2-positive breast cancer cells and system biology approach to predict their targets involved in resistance. DNA Cell Biol 2019; 38: 184-192. [DOI:10.1089/dna.2018.4427] [PMID]
29. Schuldes H, Bade S, Knobloch J, Jonas D. Loss of in vitro cytotoxicity of cisplatin after storage as stock solution in cell culture medium at various temperatures. Cancer 1997; 79: 1723-1728.
https://doi.org/10.1002/(SICI)1097-0142(19970501)79:9<1723::AID-CNCR13>3.0.CO;2-# [DOI:10.1002/(SICI)1097-0142(19970501)79:93.0.CO;2-#]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





