Sat, May 4, 2024
[Archive]
Volume 18, Issue 9 (September 2020)
IJRM 2020, 18(9): 755-764 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mohammadzadeh M, Khalili M A, Ramezani V, Hamishehkar H, Dehghan Marvast L, Mangoli E, et al . Does resveratrol affect prepared sperm parameters and chromatin quality in normozoospermic and asthenozoospermic patients before and after freezing? A lab trial study. IJRM 2020; 18 (9) :755-764
URL: http://ijrm.ir/article-1-1620-en.html
URL: http://ijrm.ir/article-1-1620-en.html
Masoomeh Mohammadzadeh1 

 , Mohammad Ali Khalili1
, Mohammad Ali Khalili1 

 , Vahid Ramezani2
, Vahid Ramezani2 

 , Hamed Hamishehkar3
, Hamed Hamishehkar3 

 , Laleh Dehghan Marvast1
, Laleh Dehghan Marvast1 

 , Esmat Mangoli1
, Esmat Mangoli1 

 , Mahya Rajabi4
, Mahya Rajabi4 

 , Zhima Akhavan Sales5
, Zhima Akhavan Sales5 

 , Ali Reza Talebi *
, Ali Reza Talebi * 

 6
6


 , Mohammad Ali Khalili1
, Mohammad Ali Khalili1 

 , Vahid Ramezani2
, Vahid Ramezani2 

 , Hamed Hamishehkar3
, Hamed Hamishehkar3 

 , Laleh Dehghan Marvast1
, Laleh Dehghan Marvast1 

 , Esmat Mangoli1
, Esmat Mangoli1 

 , Mahya Rajabi4
, Mahya Rajabi4 

 , Zhima Akhavan Sales5
, Zhima Akhavan Sales5 

 , Ali Reza Talebi *
, Ali Reza Talebi * 

 6
6
1- Department of Reproductive Biology, Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
2- Department of Pharmaceutics, Faculty of Pharmacy, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
4- Abortion Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
5- Department of Immunology, International Campus, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
6- Department of Reproductive Biology, Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. , prof_talebi@hotmail.com
2- Department of Pharmaceutics, Faculty of Pharmacy, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
4- Abortion Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
5- Department of Immunology, International Campus, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
6- Department of Reproductive Biology, Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. , prof_talebi@hotmail.com
Full-Text [PDF 643 kb]
(623 Downloads)
| Abstract (HTML) (1807 Views)
The most important factors in ROS production in ART are sperm preparation and sperm freezing. Sperm freezing is done to maintain fertility, however, due to adverse changes during freezing and thawing processes, it damages the sperm viability and function. The production of osmotic pressure and the formation of ice crystals lead to the loss of water, making the conditions suitable for oxidative stress, instability, and decreased fluidity of the sperm membrane. Another consequence of freeze-thaw is the destruction of disulfide bands present in protamine, reducing the chromatin condensation and thus damaging the important components of sperm DNA. In fact, the main cause of oxidative stress is the imbalance between ROS produced during freezing and the antioxidants present in sperm (5, 6).
Sperm freezing removes the rich source of antioxidants from the semen. However, previous studies have shown that the application of antioxidants in culture media could protect the sperm from ROS's destructive effects (7, 8). One of these potent antioxidants, resveratrol (RSV), with a structure analogous to the estradiol, is associated with energy metabolism and production. In particular, it enhances adenosine monophosphate-activated protein kinase (AMPK) and reduces the rate of ROS and DNA apoptosis by preserving the mitochondrial membrane potential followed by semen freezing. Consequently, according to the effect of RSV on AMPK and the presence of AMPK at the whole flagellum and the post-equatorial region of the head, RSV has a main role in sperm motility (9, 10). Therefore, the freezing process has detrimental effects on the sperm parameters and chromatin quality (11). Because the IVF and ICSI processes are done by the prepared sperms lacking antioxidants during preparation, unlike semen, sperms are more affected by ROS factors due to centrifugation and incubation, ending in their impaired function. Many articles have suggested that the function of RSV is dose-dependent (12). However, no specific dose of this substance has been reported in the culture of human sperm preparation. Therefore, the aim of this study was to determine the specific dose of RSV and its effect on the sperms' parameters and chromatin before and after freezing in normozoospermic and asthenozoospermic individuals.
After 48 hr of sexual abstinence, the samples were obtained by masturbation. Semen analysis was performed according to the World Health Organization (WHO) guidelines (13). The samples were prepared using the swim-up method (14). The prepared spermatozoa were divided into control (without exposure) and experimental (exposure to 30 µmol/l of RSV) samples (15). First, each sample (control and experimental) of normozoospermic and asthenozoospermic was analyzed separately after preparation (0 hr), 1 hr of incubation in 37oC and after freezing. Next, the sperm parameters and the chromatin quality of each sample before and after freezing were compared to investigate the effect of RSV.


2.6. Ethical Consideration
All participants signed a written consent form prior to the study. This experimental study was approved by the Ethics Committee of Shahid Sadoughi University of Medical Sciences (IR.SSU.MEDICINE.REC.1395.285).
2.7. Statistical analysis
Considering the significant level of 5% and power test of 80% and the standard deviation of s = 24 for the RSV variable and the mean difference of 44, at least 10 people were needed. The data were analyzed by Graph pad prism. P < 0.05 was considered as the significant level. Moreover, the results of motility, morphology, and viability parameters were evaluated by ANOVA test, t test was used to evaluate the chromatin quality.
3. Results
The motility of the samples after preparation and 1 hr of incubation at 37ºC was significantly different from that of them after freezing in each sample of normozoospermic and asthenozoospermic groups (p < 0.0001). The mean motility of each sample confirmed that the result before freezing was better than after. However, the difference between the mobility of each sample was not significant after preparation, 1 hr of incubation at 37ºC, and after freezing in normozoospermic group (p = 0.99, p = 0.99, and p = 0.79, respectively) (Figure 4).
Interestingly, the results of viability were significantly different after preparation and 1 hr of incubation at 37ºC than after freezing in the control and the treated samples of normozoospermic group (p = 0.000). However, these results were similar for asthenozoospermic group.
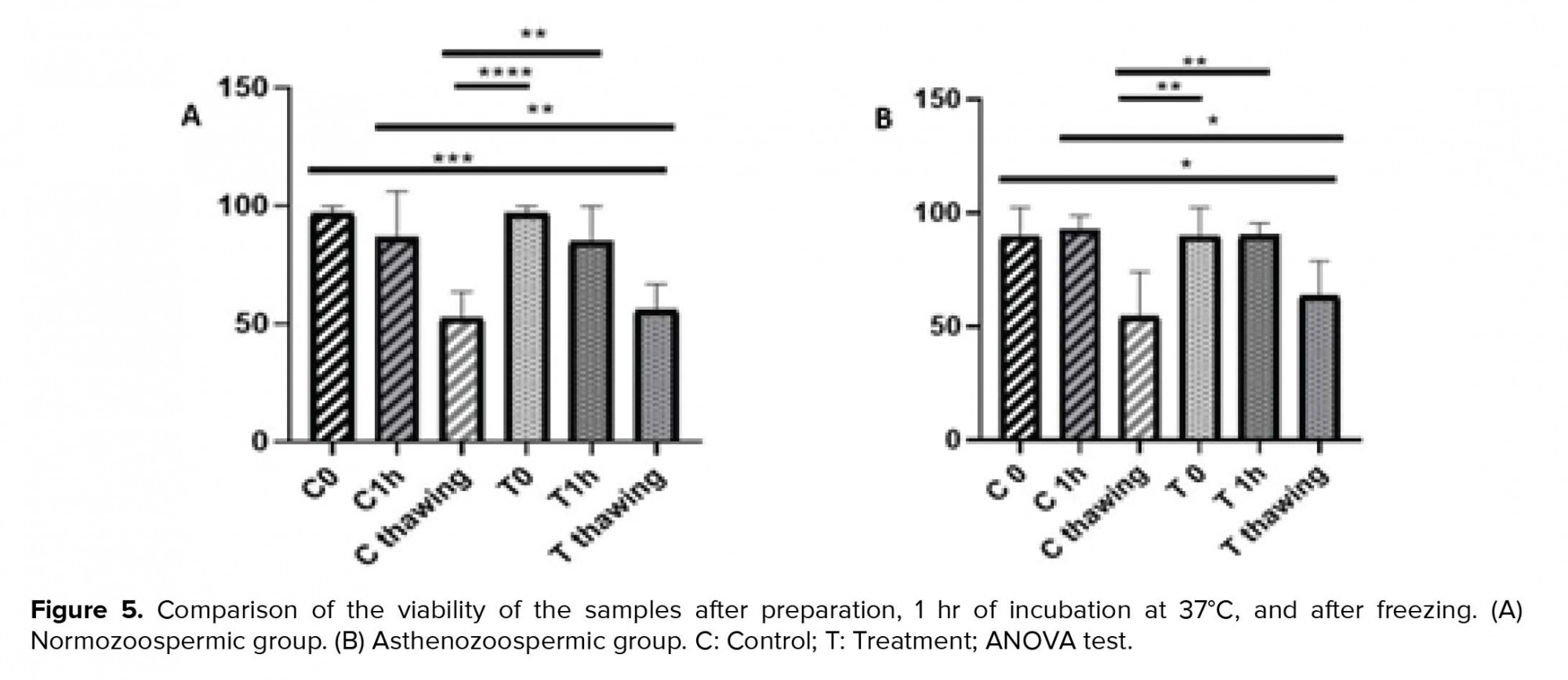
According to the viability test, the result after preparation and 1 hr of incubation at 37ºC was significantly different than that after freezing in the control sample (p = 0.004 and p = 0.002, respectively). Furthermore, the results in the treatment sample of asthenozoospermic group were significantly different (p = 0.01 and p = 0.009, respectively). The comparison of each sample were not significantly different after preparation, 1 hr of incubation at 37ºC and after freezing in normozoospermic and asthenozoospermic groups (p > 0.05) (Figure 5).
Further, the result of morphology showed no significant differences after preparation and 1 hr of incubation at 37ºC than after freezing in the control and the treatment sample of normozoospermic group (p > 0.05). The result of morphology showed no significant differences after 1 hr of incubation at 37ºC than after freezing, except in the case of preparation freezing in the control sample of asthenozoospermic (p = 0.029). However, no significant differences were observed after the preparation and 1 hr of incubation at 37ºC compared to after freezing in the treatment sample of asthenozoospermic group (Figure 6).
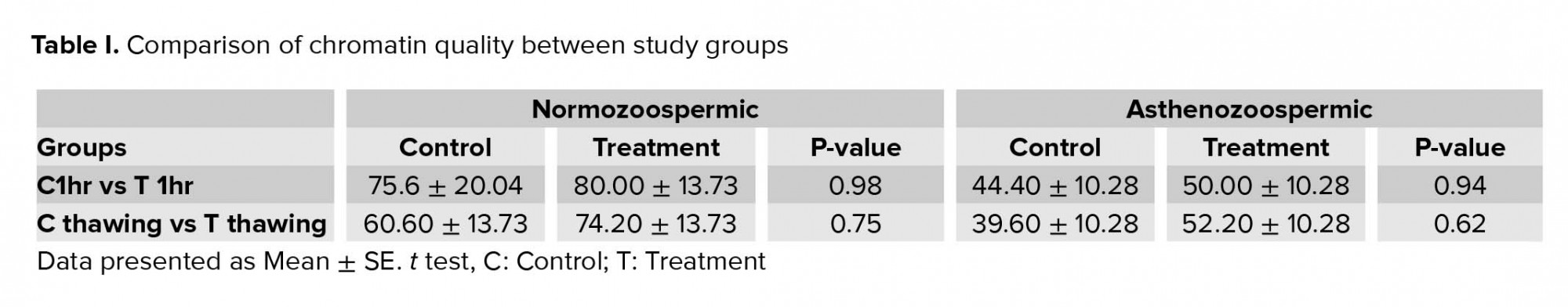
Likewise, for the chromatin quality, no significant difference was observed after preparation and 1 hr of incubation at 37ºC than freezing in each sample of normozoospermic; however, the mean of treatment samples in both groups showed that the result obtained before freezing was better than after freezing (Table I).


4. Discussion
RSV is associated with energy metabolism by enhancing AMPK, which in turn preserves the mitochondrial membrane potential. As a result, it reduces the rates of ROS and DNA apoptosis followed by semen freezing. In this study, the concentration of 30 µmol/l RSV was selected as the most effective concentration. Despite the fact that RSV is known as a strong antioxidant in the literature, no significant difference was observed in the sperm parameters and chromatin quality between the control and treatment samples of each group. Various discussions can be run to explain this result. First, RSV might need other additional supplements for the semen contents to be effective. Therefore, RSV alone could not protect the prepared sperm against ROS. Furthermore, to enhance the RSV fusion to the sperm membrane, perhaps an appropriate carrier is required. As the last reason, RSV might have a long-term effect on the cell.
Another finding of our study was that the effect of RSV on sperm parameters and chromatin quality was more profound before freezing than after freezing in both normozoospermic and asthenozoospermic groups, attributable to the fact that the freezing process increased ROS (21). Consequently, this result can explain why RSV needs additional supplements to balance ROS. The rate of ROS was also lower in pre- than post-freezing; therefore, it could be concluded that freezing can cause devastating effects on sperm parameters and chromatin in exposure to large amounts of ROS.
Although many studies have investigated the effect of RSV, as an antioxidant, on the sperm function, the results of our research were inconsistent with other studies (15, 22-24). These inconsistencies can be due to the types of studied animal species, applied medium, and methods used in ART; as with regard to antioxidants, most studies were carried out on semen and not the prepared sperm. In line with our study, Shabani Nashteai and colleagues showed that 25 µm of RSV improved the cryopreserved sperm functions. However, they reached a different optimal concentration after freezing, which can be attributed to the fact that they studied semen including a wide variety of antioxidants and RSV.
Furthermore, the strong antioxidants contained in semen could better neutralize the effects of oxidative stress (10). However, this is contrary toour study; since our experiments were conducted on isolated sperms with no antioxidants from the semen. Meamar and colleagues demonstrated that the addition of 10 mM of RSV to cryopreservation media was not associated with significant differences in the sperm DNA fragmentation (25). This might be due to the high dose of RSV in medium or interactions between the antioxidants and the materials contained in cryopreservation media, probably reducing the effects of antioxidants or acting as toxic-like materials for the sperm.
Cui and coworker reported the effect of RSV on spermatozoa function in obese males. According to their results, 30 µmol/l of RSV had a significant effect on sperm function. The amount of dose selected in their study was similar to ours, however, the effect of RSV on sperm parameters was different in obese males after a 30-min exposure, probably due to the protective effects of RSV against hormonal abnormalities. In obese males, the considerably incremented level of estradiol could act as an exogenous estrogen, suppressing the destructive effects of intrinsic estrogen. Hence, it has a significant effect on the sperm function (15, 26).
Garcez and co-workers showed that RSV could avoid freezing damage (27). The different results achieved by them might be due to the type of applied cryopreservation media, which may produce less ROS compared to common methods. As mentioned earlier, when RSV was added to semen containing several different antioxidants, its functions were greatly affected. Contrary to our study, Garcez and co-authors showed that RSV minimized sperm DNA damage derived by cryopreservation only in infertile men, but not among the fertile men (27). This is because they used slow freezing method, which might have less risk factors on sperm compared to the rapid sperm freezing. However, the novelty of our study was the use of sperm preparation without antioxidants for exposure to RSV. We believe that several other studies are required to identify the functional mechanisms of RSV; for instance, future studies may deal with the status of chromatin quality and sperm parameters 4 and 24 hr after incubation to determine the long-term effects of RSV. On the other hand, due to time and budget constraints, we could not use RSV in other sperm factors including gene expression and fertility and the embryo development rate in these samples.
5. Conclusion
Despite all the protective effects of RSV on the motility and chromatin quality of the semen samples, it could not significantly affect the prepared sperms parameters and chromatin quality in normozoospermic and asthenozoospermic individuals. However, the chromatin quality of sperm was better in the treatment than the control groups.
Acknowledgments
The authors would like to thank the Yazd Reproductive Sciences Institute for their support in the current study.
Conflict of interest
The authors report no conflict of interest.
Full-Text: (360 Views)
- Introduction
The most important factors in ROS production in ART are sperm preparation and sperm freezing. Sperm freezing is done to maintain fertility, however, due to adverse changes during freezing and thawing processes, it damages the sperm viability and function. The production of osmotic pressure and the formation of ice crystals lead to the loss of water, making the conditions suitable for oxidative stress, instability, and decreased fluidity of the sperm membrane. Another consequence of freeze-thaw is the destruction of disulfide bands present in protamine, reducing the chromatin condensation and thus damaging the important components of sperm DNA. In fact, the main cause of oxidative stress is the imbalance between ROS produced during freezing and the antioxidants present in sperm (5, 6).
Sperm freezing removes the rich source of antioxidants from the semen. However, previous studies have shown that the application of antioxidants in culture media could protect the sperm from ROS's destructive effects (7, 8). One of these potent antioxidants, resveratrol (RSV), with a structure analogous to the estradiol, is associated with energy metabolism and production. In particular, it enhances adenosine monophosphate-activated protein kinase (AMPK) and reduces the rate of ROS and DNA apoptosis by preserving the mitochondrial membrane potential followed by semen freezing. Consequently, according to the effect of RSV on AMPK and the presence of AMPK at the whole flagellum and the post-equatorial region of the head, RSV has a main role in sperm motility (9, 10). Therefore, the freezing process has detrimental effects on the sperm parameters and chromatin quality (11). Because the IVF and ICSI processes are done by the prepared sperms lacking antioxidants during preparation, unlike semen, sperms are more affected by ROS factors due to centrifugation and incubation, ending in their impaired function. Many articles have suggested that the function of RSV is dose-dependent (12). However, no specific dose of this substance has been reported in the culture of human sperm preparation. Therefore, the aim of this study was to determine the specific dose of RSV and its effect on the sperms' parameters and chromatin before and after freezing in normozoospermic and asthenozoospermic individuals.
- Materials and Methods
After 48 hr of sexual abstinence, the samples were obtained by masturbation. Semen analysis was performed according to the World Health Organization (WHO) guidelines (13). The samples were prepared using the swim-up method (14). The prepared spermatozoa were divided into control (without exposure) and experimental (exposure to 30 µmol/l of RSV) samples (15). First, each sample (control and experimental) of normozoospermic and asthenozoospermic was analyzed separately after preparation (0 hr), 1 hr of incubation in 37oC and after freezing. Next, the sperm parameters and the chromatin quality of each sample before and after freezing were compared to investigate the effect of RSV.
- 1. Sperm motility assessment
- 2. Sperm morphology assessment
- 3. Sperm viability assessment
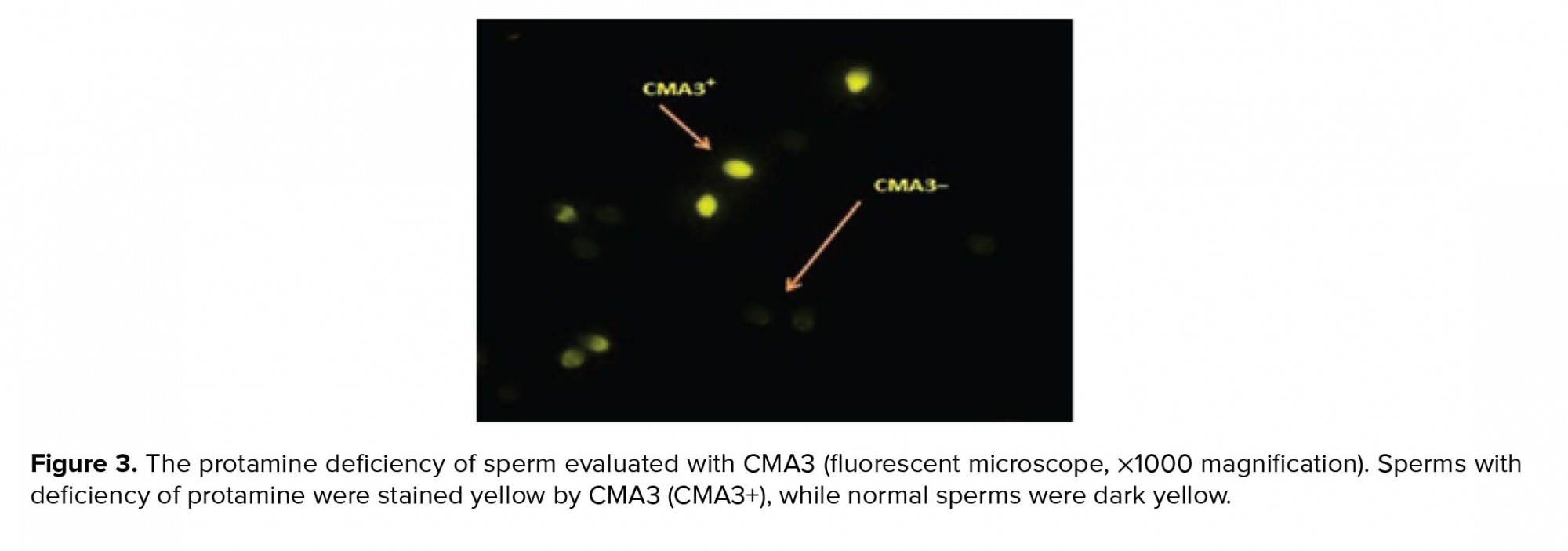
- 4. Evaluation of sperm chromatin quality by Chromomysin A3 (CMA3)
- 5. Cryopreservation and thawing of spermatozoa



2.6. Ethical Consideration
All participants signed a written consent form prior to the study. This experimental study was approved by the Ethics Committee of Shahid Sadoughi University of Medical Sciences (IR.SSU.MEDICINE.REC.1395.285).
2.7. Statistical analysis
Considering the significant level of 5% and power test of 80% and the standard deviation of s = 24 for the RSV variable and the mean difference of 44, at least 10 people were needed. The data were analyzed by Graph pad prism. P < 0.05 was considered as the significant level. Moreover, the results of motility, morphology, and viability parameters were evaluated by ANOVA test, t test was used to evaluate the chromatin quality.
3. Results
The motility of the samples after preparation and 1 hr of incubation at 37ºC was significantly different from that of them after freezing in each sample of normozoospermic and asthenozoospermic groups (p < 0.0001). The mean motility of each sample confirmed that the result before freezing was better than after. However, the difference between the mobility of each sample was not significant after preparation, 1 hr of incubation at 37ºC, and after freezing in normozoospermic group (p = 0.99, p = 0.99, and p = 0.79, respectively) (Figure 4).
Interestingly, the results of viability were significantly different after preparation and 1 hr of incubation at 37ºC than after freezing in the control and the treated samples of normozoospermic group (p = 0.000). However, these results were similar for asthenozoospermic group.
According to the viability test, the result after preparation and 1 hr of incubation at 37ºC was significantly different than that after freezing in the control sample (p = 0.004 and p = 0.002, respectively). Furthermore, the results in the treatment sample of asthenozoospermic group were significantly different (p = 0.01 and p = 0.009, respectively). The comparison of each sample were not significantly different after preparation, 1 hr of incubation at 37ºC and after freezing in normozoospermic and asthenozoospermic groups (p > 0.05) (Figure 5).
Further, the result of morphology showed no significant differences after preparation and 1 hr of incubation at 37ºC than after freezing in the control and the treatment sample of normozoospermic group (p > 0.05). The result of morphology showed no significant differences after 1 hr of incubation at 37ºC than after freezing, except in the case of preparation freezing in the control sample of asthenozoospermic (p = 0.029). However, no significant differences were observed after the preparation and 1 hr of incubation at 37ºC compared to after freezing in the treatment sample of asthenozoospermic group (Figure 6).
Likewise, for the chromatin quality, no significant difference was observed after preparation and 1 hr of incubation at 37ºC than freezing in each sample of normozoospermic; however, the mean of treatment samples in both groups showed that the result obtained before freezing was better than after freezing (Table I).




4. Discussion
RSV is associated with energy metabolism by enhancing AMPK, which in turn preserves the mitochondrial membrane potential. As a result, it reduces the rates of ROS and DNA apoptosis followed by semen freezing. In this study, the concentration of 30 µmol/l RSV was selected as the most effective concentration. Despite the fact that RSV is known as a strong antioxidant in the literature, no significant difference was observed in the sperm parameters and chromatin quality between the control and treatment samples of each group. Various discussions can be run to explain this result. First, RSV might need other additional supplements for the semen contents to be effective. Therefore, RSV alone could not protect the prepared sperm against ROS. Furthermore, to enhance the RSV fusion to the sperm membrane, perhaps an appropriate carrier is required. As the last reason, RSV might have a long-term effect on the cell.
Another finding of our study was that the effect of RSV on sperm parameters and chromatin quality was more profound before freezing than after freezing in both normozoospermic and asthenozoospermic groups, attributable to the fact that the freezing process increased ROS (21). Consequently, this result can explain why RSV needs additional supplements to balance ROS. The rate of ROS was also lower in pre- than post-freezing; therefore, it could be concluded that freezing can cause devastating effects on sperm parameters and chromatin in exposure to large amounts of ROS.
Although many studies have investigated the effect of RSV, as an antioxidant, on the sperm function, the results of our research were inconsistent with other studies (15, 22-24). These inconsistencies can be due to the types of studied animal species, applied medium, and methods used in ART; as with regard to antioxidants, most studies were carried out on semen and not the prepared sperm. In line with our study, Shabani Nashteai and colleagues showed that 25 µm of RSV improved the cryopreserved sperm functions. However, they reached a different optimal concentration after freezing, which can be attributed to the fact that they studied semen including a wide variety of antioxidants and RSV.
Furthermore, the strong antioxidants contained in semen could better neutralize the effects of oxidative stress (10). However, this is contrary toour study; since our experiments were conducted on isolated sperms with no antioxidants from the semen. Meamar and colleagues demonstrated that the addition of 10 mM of RSV to cryopreservation media was not associated with significant differences in the sperm DNA fragmentation (25). This might be due to the high dose of RSV in medium or interactions between the antioxidants and the materials contained in cryopreservation media, probably reducing the effects of antioxidants or acting as toxic-like materials for the sperm.
Cui and coworker reported the effect of RSV on spermatozoa function in obese males. According to their results, 30 µmol/l of RSV had a significant effect on sperm function. The amount of dose selected in their study was similar to ours, however, the effect of RSV on sperm parameters was different in obese males after a 30-min exposure, probably due to the protective effects of RSV against hormonal abnormalities. In obese males, the considerably incremented level of estradiol could act as an exogenous estrogen, suppressing the destructive effects of intrinsic estrogen. Hence, it has a significant effect on the sperm function (15, 26).
Garcez and co-workers showed that RSV could avoid freezing damage (27). The different results achieved by them might be due to the type of applied cryopreservation media, which may produce less ROS compared to common methods. As mentioned earlier, when RSV was added to semen containing several different antioxidants, its functions were greatly affected. Contrary to our study, Garcez and co-authors showed that RSV minimized sperm DNA damage derived by cryopreservation only in infertile men, but not among the fertile men (27). This is because they used slow freezing method, which might have less risk factors on sperm compared to the rapid sperm freezing. However, the novelty of our study was the use of sperm preparation without antioxidants for exposure to RSV. We believe that several other studies are required to identify the functional mechanisms of RSV; for instance, future studies may deal with the status of chromatin quality and sperm parameters 4 and 24 hr after incubation to determine the long-term effects of RSV. On the other hand, due to time and budget constraints, we could not use RSV in other sperm factors including gene expression and fertility and the embryo development rate in these samples.
5. Conclusion
Despite all the protective effects of RSV on the motility and chromatin quality of the semen samples, it could not significantly affect the prepared sperms parameters and chromatin quality in normozoospermic and asthenozoospermic individuals. However, the chromatin quality of sperm was better in the treatment than the control groups.
Acknowledgments
The authors would like to thank the Yazd Reproductive Sciences Institute for their support in the current study.
Conflict of interest
The authors report no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Andrology
References
1. Tremellen K. Oxidative stress and male infertility-a clinical perspective. Hum Reprod Update 2008; 14: 243-258. [DOI:10.1093/humupd/dmn004] [PMID]
2. Wagner H, Cheng JW, Ko EY. Role of reactive oxygen species in male infertility: an updated review of literature. Arab J Urol 2017; 16: 35-43. [DOI:10.1016/j.aju.2017.11.001] [PMID] [PMCID]
3. AL-Hady FN. The level of reactive oxygen species (ROS) in fresh and aged Asthenospermic, Leukocytospermic and Normospermic semen specimens. Al-Qadisiyah Journal of Veterinary Medicine Sciences 2006; 5: 42-48.
4. Ko EY, Sabanegh Jr ES, Agarwal A. Male infertility testing: reactive oxygen species and antioxidant capacity. Fertil Steril 2014; 102: 1518-1527. [DOI:10.1016/j.fertnstert.2014.10.020] [PMID]
5. Abdillah DA, Setyawan EMN, Oh HJ, Ra K, Lee SH, Kim MJ, et al. Iodixanol supplementation during sperm cryopreservation improves protamine level and reduces reactive oxygen species of canine sperm. J Vet Sci 2019; 20: 79-86. [DOI:10.4142/jvs.2019.20.1.79] [PMID] [PMCID]
6. Hezavehei M, Mohseni Kouchesfahani H, Shahverdi AH, Sharafi M, Hosseini Salekdeh GH, Eftekhari-Yazdi P. Induction of sublethal oxidative stress on human sperm before cryopreservation: A time-dependent response in post-thawed sperm parameters. Cell J 2019; 20: 537-543.
7. Bansal AK, Bilaspuri GS. Impacts of oxidative stress and antioxidants on semen functions. Vet Med Int 2010; 2010: 1-8. [DOI:10.4061/2011/686137] [PMID] [PMCID]
8. Chi HJ, Kim JH, Ryu CS, Lee JY, Park JS, Chung DY, et al. Protective effect of antioxidant supplementation in sperm-preparation medium against oxidative stress in human spermatozoa. Hum Reprod 2008; 23: 1023-1028. [DOI:10.1093/humrep/den060] [PMID]
9. Juan ME, Gonzalez-Pons E, Munuera T, Ballester J, Rodriguez-Gil JE, Planas JM. trans-resveratrol, a natural antioxidant from grapes, increases sperm output in healthy rats. J Nutr 2005; 135: 757-760. [DOI:10.1093/jn/135.4.757] [PMID]
10. Shabani Nashtaei M, Amidi F, Sedighi Gilani MA, Aleyasin A, Bakhshalizadeh Sh, Naji M, et al. Protective features of resveratrol on human spermatozoa cryopreservation may be mediated through 5'AMP‐activated protein kinase activation. Andrology 2017; 5: 313-326. [DOI:10.1111/andr.12306] [PMID]
11. Mangoli E, Talebi AR, Anvari M, Taheri F, Vatanparast M, Rahiminia T, et al. Vitamin C attenuates negative effects of vitrification on sperm parameters, chromatin quality, apoptosis and acrosome reaction in neat and prepared normozoospermic samples. Taiwan J Obstet Gynecol 2018; 57: 200-204. [DOI:10.1016/j.tjog.2018.02.006] [PMID]
12. Matsumoto Y, Goto S, Hashimoto H, Kokeguchi S, Shiotani M, Okada H, et al. Andrology (Male Fertility, Spermatogenesis). Hum Reprod 2010; 25 (suppl.): i118-i152.
13. Organization WH. WHO laboratory manual for the examination and processing of human semen. Switzerland, World Health Organization Press; 2010.
14. Jayaraman V, Upadhya D, Narayan PK, Adiga SK. Sperm processing by swim-up and density gradient is effective in elimination of sperm with DNA damage. J Assist Reprod Genet 2012; 29: 557-563. [DOI:10.1007/s10815-012-9742-x] [PMID] [PMCID]
15. Cui X, Jing X, Wu X, Yan M. Protective effect of resveratrol on spermatozoa function in male infertility induced by excess weight and obesity. Mol Med Rep 2016; 14: 4659-4665. [DOI:10.3892/mmr.2016.5840] [PMID] [PMCID]
16. Kruger TF, Ackerman SB, Simmons KF, Swanson RJ, Brugo SS, Acosta AA. A quick, reliable staining technique for human sperm morphology. Arch Androl 1987; 18: 275-277. [DOI:10.3109/01485018708988493] [PMID]
17. Banihani S, Sharma R, Bayachou M, Sabanegh E, Agarwal A. Human sperm DNA oxidation, motility and viability in the presence of l‐carnitine during in vitro incubation and centrifugation. Andrologia 2012; 44 (Suppl.): 505-512. [DOI:10.1111/j.1439-0272.2011.01216.x] [PMID]
18. Talebi AR, Moein MR, Tabibnejad N, Ghasemzadeh J. Effect of varicocele on chromatin condensation and DNA integrity of ejaculated spermatozoa using cytochemical tests. Andrologia 2008; 40: 245-251. [DOI:10.1111/j.1439-0272.2008.00852.x] [PMID]
19. Mangoli E, Khalili MA, Talebi AR, Ghasemi-Esmailabad S, Hosseini A. Is there any correlation between sperm parameters and chromatin quality with embryo morphokinetics in patients with male infertility? Andrologia 2018; 50: e12997. [DOI:10.1111/and.12997] [PMID]
20. Di Santo M, Tarozzi N, Nadalini M, Borini A. Human sperm cryopreservation: update on techniques, effect on DNA integrity, and implications for ART. Adv Urol 2012; 2012: 85837: 1-12. [DOI:10.1155/2012/854837] [PMID] [PMCID]
21. Tatone C, Di Emidio G, Vento M, Ciriminna R, Artini PG. Cryopreservation and oxidative stress in reproductive cells. Gynecol Endocrinol 2010; 26: 563-567. [DOI:10.3109/09513591003686395] [PMID]
22. Branco CS, Garcez ME, Pasqualotto FF, Erdtman B, Salvador M. Resveratrol and ascorbic acid prevent DNA damage induced by cryopreservation in human semen. Cryobiology 2010; 60: 235-237. [DOI:10.1016/j.cryobiol.2009.10.012] [PMID]
23. Collodel G, Federico MG, Geminiani M, Martini S, Bonechi C, Rossi C, et al. Effect of trans-resveratrol on induced oxidative stress in human sperm and in rat germinal cells. Reprod Toxicol 2011; 31: 239-246. [DOI:10.1016/j.reprotox.2010.11.010] [PMID]
24. Tvrda E, Kovacik A, Tusimova E, Massanyi P, Lukac N. Resveratrol offers protection to oxidative stress induced by ferrous ascorbate in bovine spermatozoa. J Environ Sci Health A Tox Hazard Subst Environ Eng 2015; 50: 1440-1451. [DOI:10.1080/10934529.2015.1071153] [PMID]
25. Meamar M, Zribi N, Cambi M, Tamburrino L, Marchiani S, Filimberti E, et al. Sperm DNA fragmentation induced by cryopreservation: new insights and effect of a natural extract from Opuntia ficus-indica. Fertil Steril 2012; 98: 326-333. [DOI:10.1016/j.fertnstert.2012.05.001] [PMID]
26. Dobrzynska MM. Resveratrol as promising natural radioprotector. A review. Rocz Państw Zakł Hig 2013; 64: 255-262.
27. Garcez ME, dos Santos Branco C, Lara LV, Pasqualotto FF, Salvador M. Effects of resveratrol supplementation on cryopreservation medium of human semen. Fertil Steril 2010; 94: 2118-2121. [DOI:10.1016/j.fertnstert.2010.01.058] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





