Sun, Feb 1, 2026
[Archive]
Volume 18, Issue 8 (August 2020)
IJRM 2020, 18(8): 611-624 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ogunlade B, Adelakun S, Iteire K. Sulforaphane response on aluminum- induced oxidative stress, alterations in sperm characterization and testicular histomorphometry in Wistar rats. IJRM 2020; 18 (8) :611-624
URL: http://ijrm.ir/article-1-1633-en.html
URL: http://ijrm.ir/article-1-1633-en.html
1- Department of Human Anatomy, Federal University of Technology, Akure, Ondo State, Nigeria. , bogunlade@futa.edu.ng
2- Department of Human Anatomy, Federal University of Technology, Akure, Ondo State, Nigeria.
3- Department of Human Anatomy, University of Medical Sciences, Ondo city, Ondo State, Nigeria.
2- Department of Human Anatomy, Federal University of Technology, Akure, Ondo State, Nigeria.
3- Department of Human Anatomy, University of Medical Sciences, Ondo city, Ondo State, Nigeria.
Full-Text [PDF 2970 kb]
(1203 Downloads)
| Abstract (HTML) (2607 Views)
In addition, elevated concentrations of Aluminum in human sperm and seminal plasma were observed to decrease sperm viability and motility (8). Testicular Aluminum accumulations cause spermatocyte necrosis and trigger other reproductive toxicity through several mechanisms such as oxidative stress, which ultimately interferes with spermatogenesis and steroidogenesis, bloodtestis barrier, and endocrine disruption (9). The application of nutritional antioxidant supplements has increased over the years to tackle oxidative stress-induced tissue damage since they act as defense regulators and scavengers of reactive oxygen species. Sulforaphane (SFN) is the most active natural products found in crucifers such as broccoli sprout, cabbage, and kale with the potential of lowering the risk of cancer, oxidative stress-induced tissue injury, and age-related diseases (10). SFN possess antiproliferative activities and can effectively halt the initiation and progression of chemically induced tissue damage in animals (11). In addition, SFN has been suggested to have antidiabetic properties for normalizing changes in blood glucose and insulin sensitivity (12-14), and is used in cardiovascular and antihypertensive protection (15, 16). It has been reported that SFN can promote elimination and detoxification of aflatoxin (17), acetaldehyde (18), methylmercury (19), acrolein (20), benzene, crotonaldehyde (21), and free radicals (22) through the Nrf2-mediated mechanism. Furthermore, some clinical studies have demonstrated the effectiveness of SFN supplements in the prevention and/or improvement of skin erythema (23), autism (24), insulin resistance (13), Helicobacter pylori-infection (25), and liver abnormality (26). SNF also has the ability to cause programmed cell death(apoptosis) and cell cycle arrest linked to their ability to regulate several proteins such as Bcl-2 and Bax family proteins, caspases, p21, cyclins, and cyclin-dependent kinases (27).
This study was therefore designed to investigate the ameliorative response of SFN on the histomorphometric and enzymatic antioxidants on Aluminum chloride (AlCl3)-induced testicular toxicity of adult Wistar rats.
All animals were observed for any behavioral anomalies, illness, and physical anomalies. The experimental procedures were conducted in accordance with the provided recommendations in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences. The rats were fed with standard rat chow and drinking water was supplied ad libitum. The weight of the animals was recorded at procurement, during acclimatization, at commencement of the experiment, and weekly throughout the experimental period using a CAMRY electronic scale (EK5055, Indian).
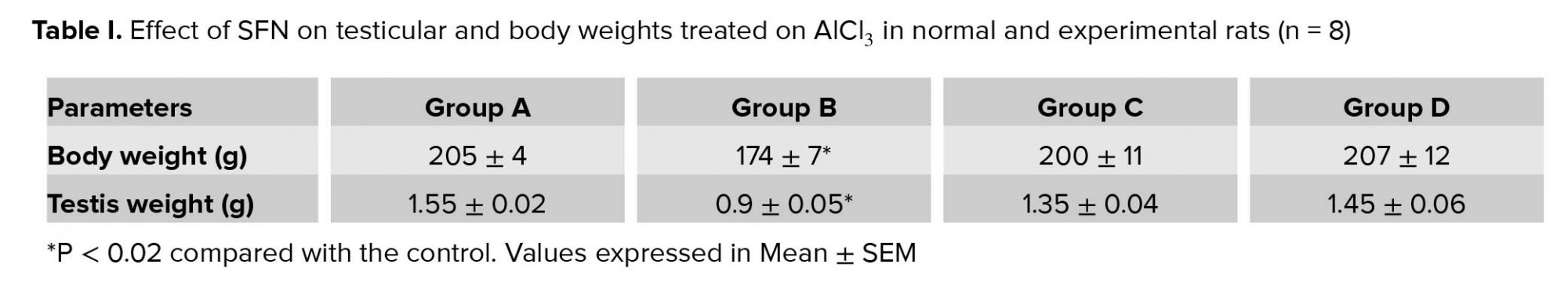
There was a significant decrease in the body weight in the rats administered with AlCl3 compared with the control (p = 0.02; Table I). However, there were no significant differences in body weight in the group administered with SFN only and combined administration of SFN and AlCl3 compared with the control.
In addition, there was a significant decrease in sperm motility in the group administered with AlCl3 only compared with the control (p = 0.02; Figure 1B). The group administered with a combination of AlCl3 and SFN showed improvement in the motility of the spermatozoa compared with the AlCl3 only group (p = 0.03; Figure 1B). However, there was no significant difference between the control and SFN only group.
Furthermore, the spermatozoa viability was significantly decreased after AlCl3 administration compared with the control (p = 0.02; Figure 1C). However, the viability of the spermatozoa in the SFN + AlCl3 group showed significant difference compared to the control and SFN only group (p = 0.03; Figure 1C).
The AlCl3 only group had significantly (p = 0.02) higher sperm head defects compared to the control (Figure 1D). However, there was no significant difference in the abnormal head defeat in the groups that received SFN only and a combination of AlCl3 and SFN compared with the control. Furthermore, the AlCl3 only group showed a significantly higher percentage of sperm abnormalities compared to the control (p = 0.02; Figure 1D). The percentage level of sperm abnormalities was drastically reduced in the combined administration of SFN and AlCl3, which was not statistically significant compared to the SFN only and control groups (Figure 1D).
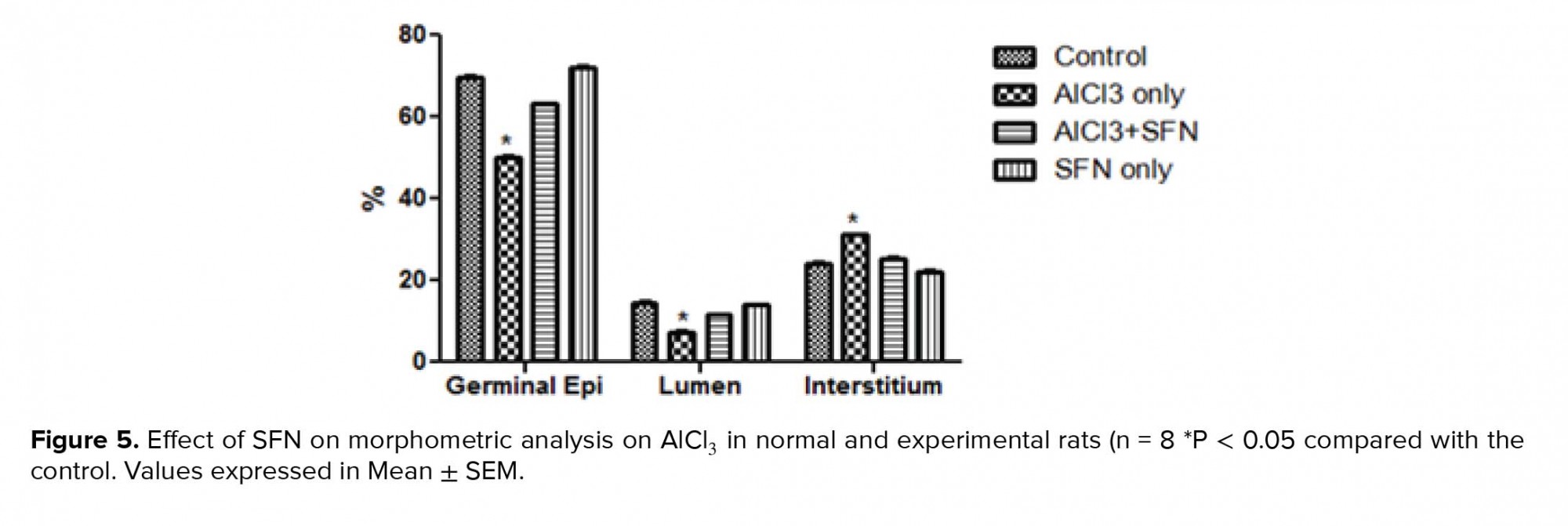
Concerning the EI, the AlCl3 only group showed a significant increase compared to the control (p = 0.02; Figure 5), while a corresponding decrease was observed in the combined SFN and AlCl3 group but it was not statistically significant compared to the SFN and control groups, respectively.
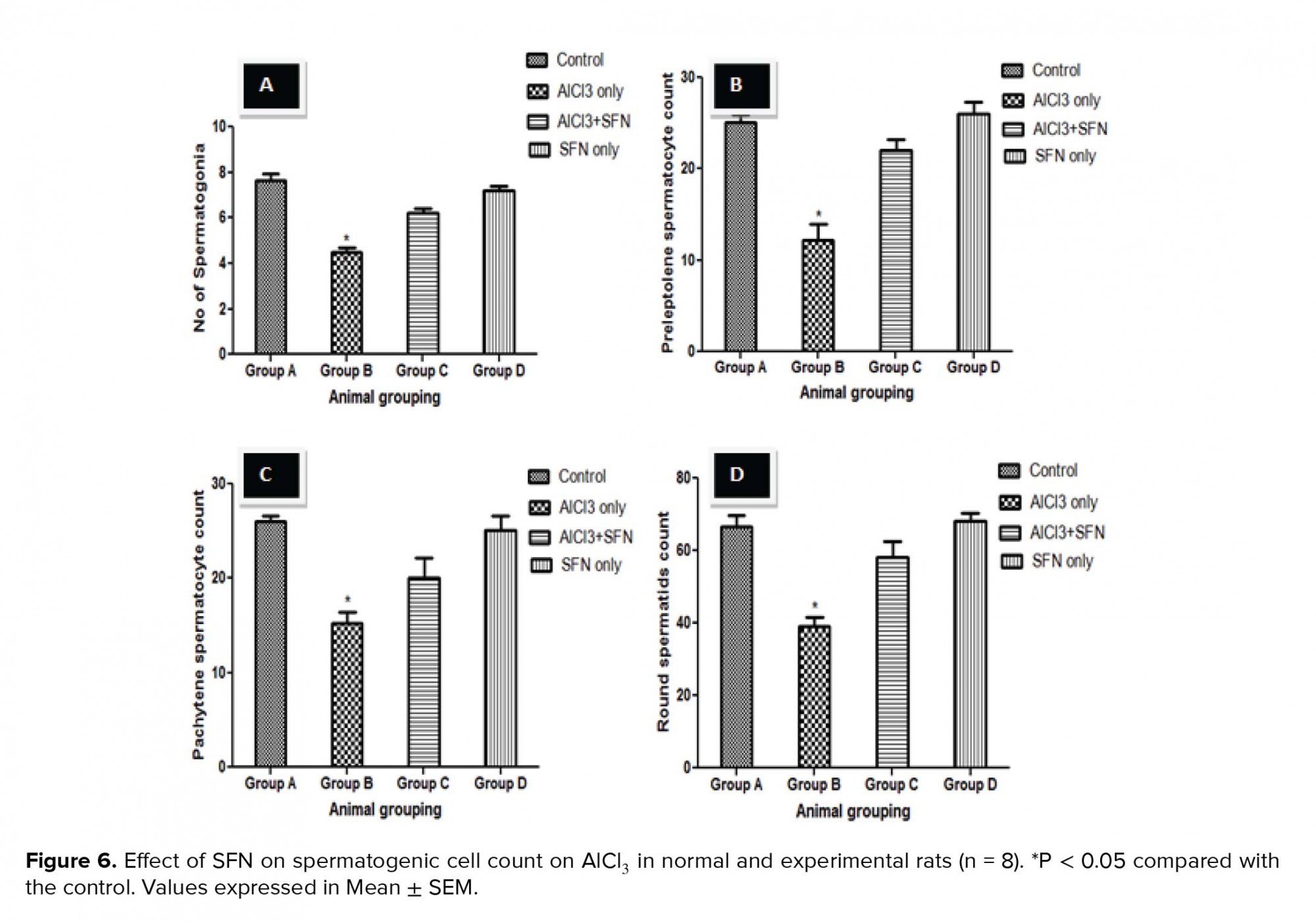
The testicular germ cell count such as spermatogonia, preleptotene and pachytene spermatocytes, and round spermatids count in the seminiferous tubules showed a significant decrease in the counts compared to the control (p = 0.02; Figure 6 A-D). Although, the germ cell count after the administration of SFN and AlCl3 was significantly improved compared to the AlCl3 only group, but it was not statistically significant compared to the SFN only and control groups, respectively.

4. Discussion
An emerging pandemic global public health issue after cancer and cardiovascular diseases is infertility due to increase in testicular cancer (35) and based on the analysis on semen parameters such as reduction in sperm counts and qualities in various countries (36, 37). The exposure of male individual to environmental toxicant is regarded as the channel that results in reduced sperm counts and infertility (1, 2). Aluminum is considered as the most common metallic element detectable in natural waters, animal, and plant tissues (3). Compounds of Al due to its reactivity with other elements such as Sulphur and chloride are widely used in many products such as storage utensils, household cookware, food additives, toothpaste ,and pharmaceuticals (antacids, vaccines, allergy injections, and anti-diarrhea) (3). The enormous rate of exposure to Al increases the chances of health-related issues to human due to increase metallic concentration in various organs thereby damaging various tissue of the body including testicular tissues of animals and humans (38). Testicular weight is crucial in the evaluation of male fertility test due to its important association in sperm production (39). In our study, the decrease in body and testicular weights observed after AlCl3 only administration could be correlated to the deleterious effect of the toxicant on body metabolism and testicular architecture, thereby resulting in spermatogenesis disruption. Previous research also concur that Al intoxication causes drastic decrease in testicular weight resulting in germinal EE disruption and inadequate TT production (40, 41). However, SFN attenuated the body and testicular weight loss in combined administration of SFN and AlCl3 group thereby restoring the testicular function.
The seminal fluid analysis (sperm count, sperm motility, sperm viability) were significantly declined in the AlCl3 only group thereby causing oligospermia due to increased oxidative stress-induced damage and decreased concentration of scavenging enzymes (42, 43). Previous studies also showed similar decrease in sperm count and sperm motility after exposure to various environmental toxicants in different experimental animal models (44-46). However, the combined administration of SFN and AlCl3 increases the motility, concentration, and viability of the spermatozoa thereby mitigating the effects of AlCl3 intoxication on testicular tissue.
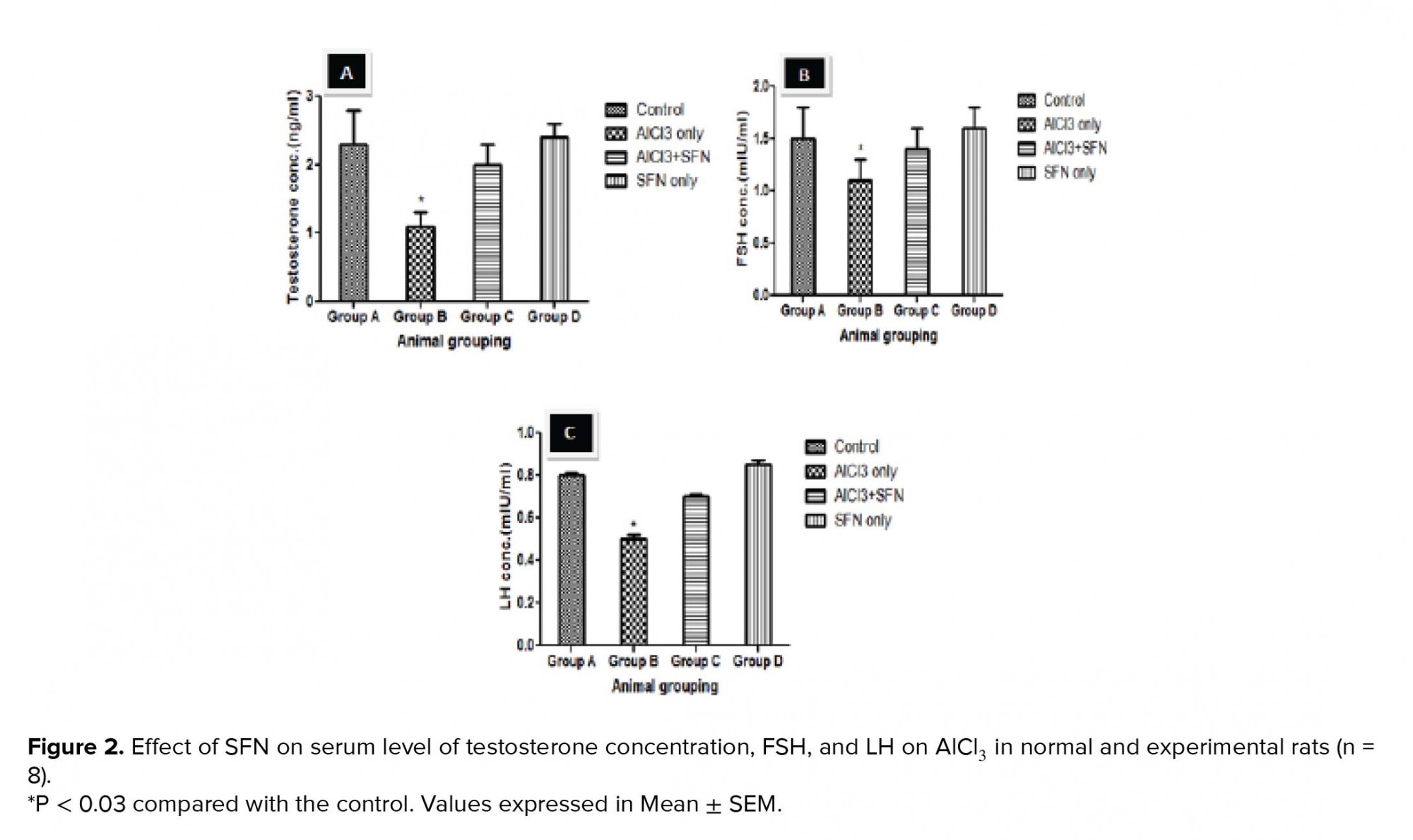
The process of spermatogenesis has been implicated to be under the regulation of reproductive hormones such as TT, FSH, and LH. In this present study, our results showed a decrease in this reproductive hormone after AlCl3 administration, thereby suggesting a decline in the role of anterior pituitary and Leydig cells. Previous studies have also observed that the decrease in the level of TT, FSH, and LH hormones in adult rats were due to several environmental agents (47-49). In addition, previous research deduced that the decrease in the level of TT synthesis could be due to the deleterious effects of testicular toxicant (such as NO, AlCl) on the Leydig cells and also the conversion of androsterone to TT due to decreased activity of 17-ketosteroid reductase enzyme (50, 51). However, the SFN and AlCl3 combined-treated group showed a significant improvement in serum FSH, LH, and TT levels that can be linked to the ameliorative potential of SFN on AlCl3 testicular toxicity in the release of gonadotrophin-releasing hormone (GnRH) secretion in the hypothalamus (52).
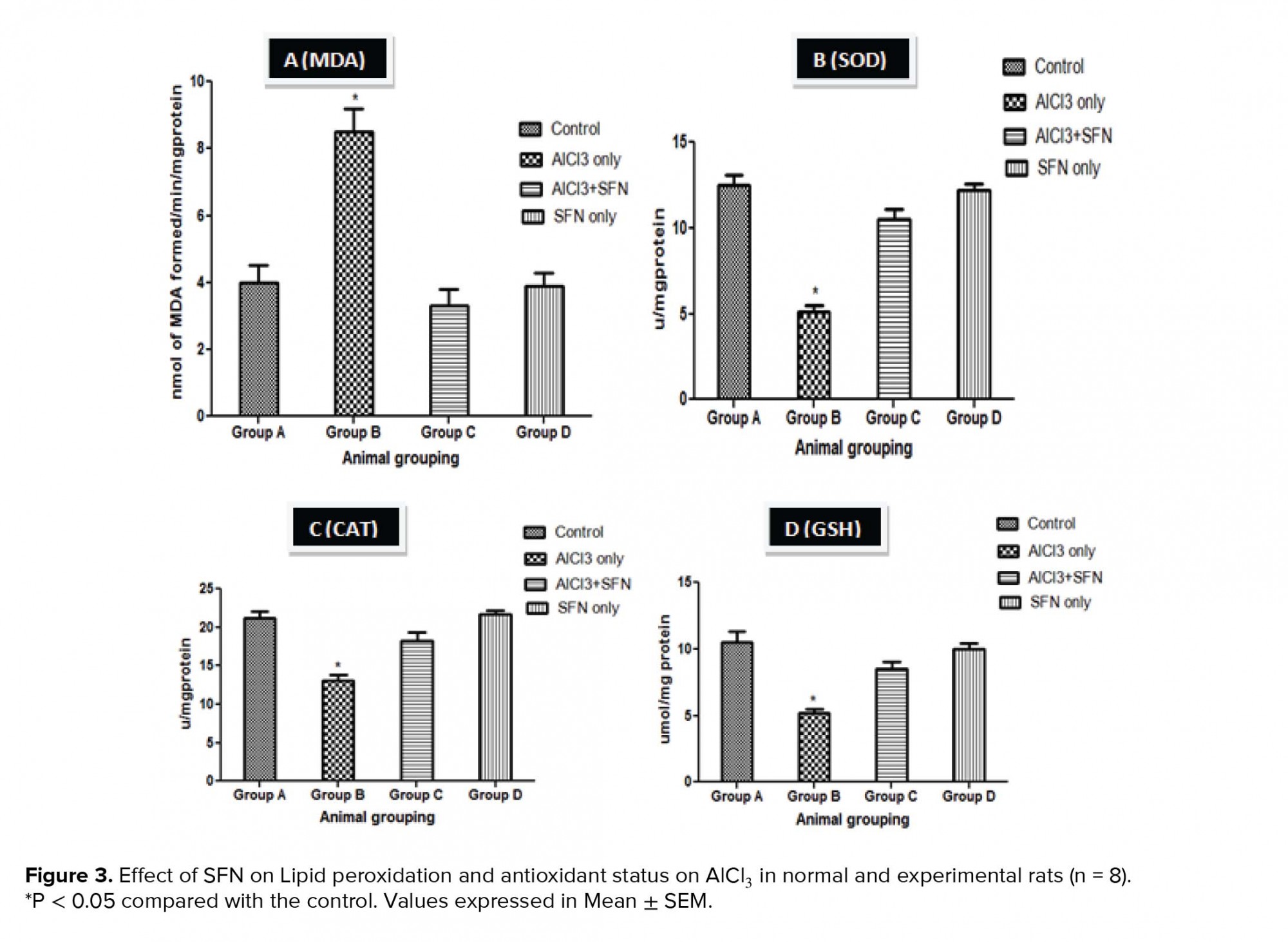
The antioxidant defense system prevents the cells of the body against the injurious effect of Reactive Oxygen Species (ROS) produced due to exposure to environmental toxicants, ultimately inducing toxicity to the reproductive system by perturbing the pro-oxidant and thereby leading to oxidative stress (53). Our study showed that exposure to AlCl3 decreased the antioxidant enzymes such as SOD, CAT, GSH and correspondingly increased the MDA level. The decline in the activities of the antioxidant enzymes observed in this study revealed that the antioxidant system was impaired, thereby inducing oxidative stress induced-testicular toxicity. Previous research have showed that the production of oxidative stress due to metallic exposure decrease enzyme defense mechanism, thereby causing spermatozoa cytotoxicity (54). In addition, the inhibition of sperm functions and male infertility was also reported to occur through toxicity of lipid peroxides via generation of reactive oxygen species (54, 55). However, the co-administration of SFN and AlCl3 in this study showed ameliorative effects against oxidative injury by increasing the levels of antioxidant enzymes (SOD, CAT, GSH) with corresponding decrease in lipid peroxidation. It could be deduced that SFN decrease the free radicals levels via its free radical scavenging activity, especially oxygen radicals, and modulates several cytokines release and activities of testicular enzymes.
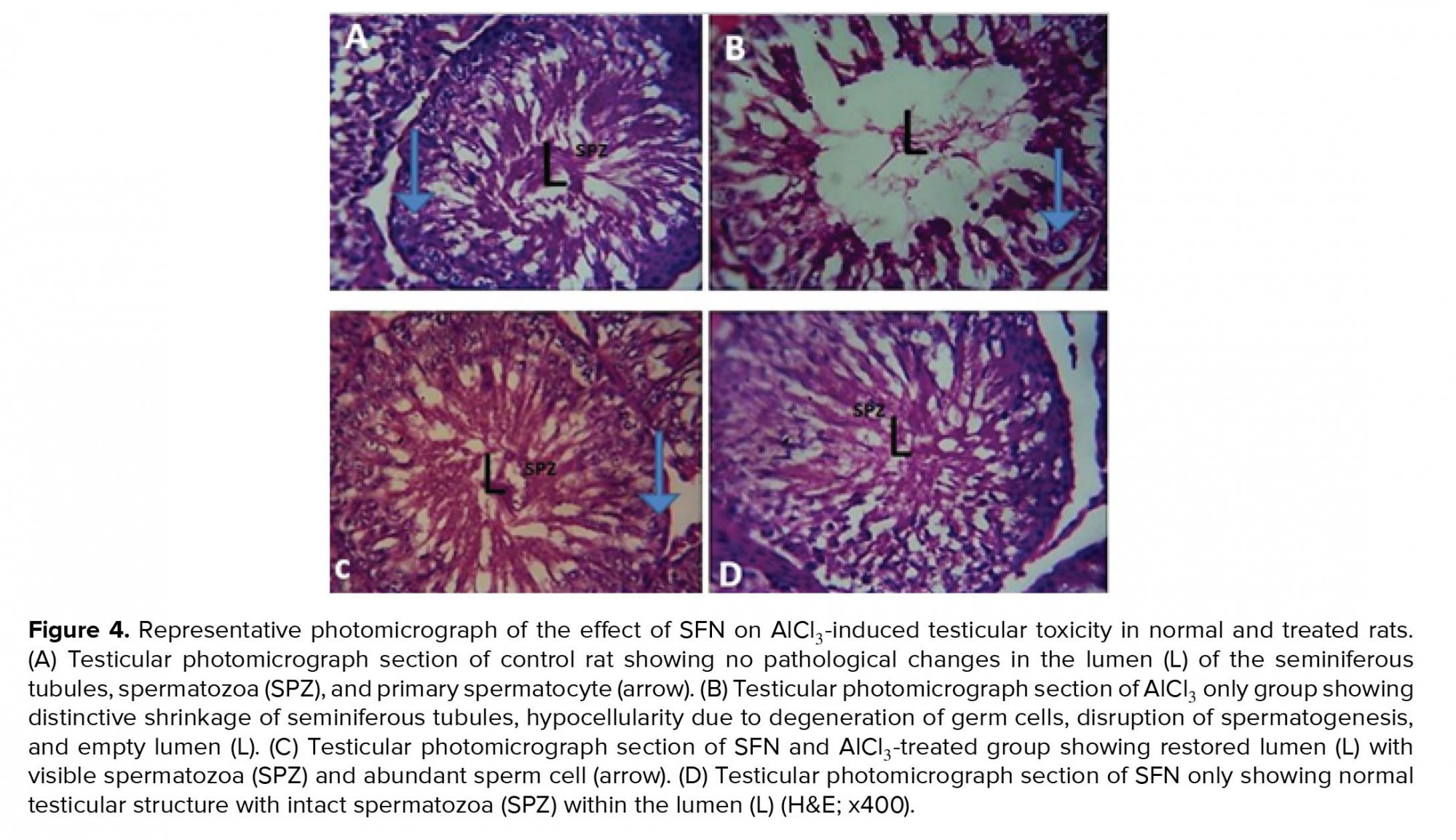
The histomorphological features of the testis are critical and usually refer to as the endpoint in the evaluation of male fertility assessment and reproductive toxicity (56). In our study, histological observation of animals that received AlCl3 only showed various distortions such as shrinked seminiferous tubules, degeneration of Leydig cells, thinner germinal EE, spermatogenesis disruption, and absence of spermatozoa in the EL. Previous studies have also reported similar changes in the histoarchitecture of the testis after exposure to different environmental toxicant (49, 57). The alteration in testicular histomorphology by metallic toxicant might be due to oxidative stress, thereby causing distortion of the steroidogenic activity of the Leydig cells by penetration through the blood-testis barrier. However, the administration of SFN in AlCl3-induced testicular damage showed its protective ability on spermatogenesis and tubular atrophy that was confirmed in our study by the histomorphological observation that showed distinctive increase in seminiferous tubules diameter and presence of spermatozoa in the EL. Previous studies have showed that normal spermatogenesis is achievable in oxidative stress-induced testicular toxicity caused by environmental toxicant by several antioxidant-rich agents, thereby increasing the endocrine activity of the Leydig and Sertoli cells (58-60). Furthermore, the histomorphological observation in our study corroborates with the decrease in the number of spermatogonia, preleptotene, pachytene spermatocytes, and round spermatids in AlCl3 only group suggesting declined spermatogenic activity. The increased oxidative stress and lipid peroxidation could increase apoptosis of the germ cells. Previous studies also showed that apoptosis of the spermatogonia and primary spermatocyte can occur via microtubule targeting and mitotic arrest after exposure of environmental toxicant (61) and decreased diameter of the seminiferous tubule could also be an indicator of defective spermatogenesis (62, 63).
5.Conclusion
The present study revealed the ameliorative response of SFN on AlCl3-induced testicular toxicity on blood LH, FSH, and TT through oxidative stress. The protective function of SFN may preserve the functional integrity of the testis against environmental toxicity.
Acknowledgements
The authors are grateful to Dr. Ijomone Neuro Laboratory, Department of Human Anatomy, Federal University of Technology Akure Nigeria for the photomicrograph capturing of slides. They are also thankful to Mr. Ige from Histology Laboratory, Department of Anatomy and Cell Biology, Obafemi Awolowo University, Ife, Nigeria for preparation of histology slide.
Conflict of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Full-Text: (553 Views)
- Introduction
In addition, elevated concentrations of Aluminum in human sperm and seminal plasma were observed to decrease sperm viability and motility (8). Testicular Aluminum accumulations cause spermatocyte necrosis and trigger other reproductive toxicity through several mechanisms such as oxidative stress, which ultimately interferes with spermatogenesis and steroidogenesis, bloodtestis barrier, and endocrine disruption (9). The application of nutritional antioxidant supplements has increased over the years to tackle oxidative stress-induced tissue damage since they act as defense regulators and scavengers of reactive oxygen species. Sulforaphane (SFN) is the most active natural products found in crucifers such as broccoli sprout, cabbage, and kale with the potential of lowering the risk of cancer, oxidative stress-induced tissue injury, and age-related diseases (10). SFN possess antiproliferative activities and can effectively halt the initiation and progression of chemically induced tissue damage in animals (11). In addition, SFN has been suggested to have antidiabetic properties for normalizing changes in blood glucose and insulin sensitivity (12-14), and is used in cardiovascular and antihypertensive protection (15, 16). It has been reported that SFN can promote elimination and detoxification of aflatoxin (17), acetaldehyde (18), methylmercury (19), acrolein (20), benzene, crotonaldehyde (21), and free radicals (22) through the Nrf2-mediated mechanism. Furthermore, some clinical studies have demonstrated the effectiveness of SFN supplements in the prevention and/or improvement of skin erythema (23), autism (24), insulin resistance (13), Helicobacter pylori-infection (25), and liver abnormality (26). SNF also has the ability to cause programmed cell death(apoptosis) and cell cycle arrest linked to their ability to regulate several proteins such as Bcl-2 and Bax family proteins, caspases, p21, cyclins, and cyclin-dependent kinases (27).
This study was therefore designed to investigate the ameliorative response of SFN on the histomorphometric and enzymatic antioxidants on Aluminum chloride (AlCl3)-induced testicular toxicity of adult Wistar rats.
- Materials and Methods
- 1. Chemicals
- 2. Animals
- 3. Experimental protocol
All animals were observed for any behavioral anomalies, illness, and physical anomalies. The experimental procedures were conducted in accordance with the provided recommendations in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences. The rats were fed with standard rat chow and drinking water was supplied ad libitum. The weight of the animals was recorded at procurement, during acclimatization, at commencement of the experiment, and weekly throughout the experimental period using a CAMRY electronic scale (EK5055, Indian).
- 4. Surgical procedure
- 5. Epididymis sperm count, viability, and motility
- 6. Biochemical estimations
- 7. Hormone determination
- 8. Testicular histology preparation
- 9. Morphometric studies
- 10. Quantitative evaluation of germ cells
- 11. Ethical consideration
- 12. Statistical analysis
- Results
- 1. Testes and body weight
There was a significant decrease in the body weight in the rats administered with AlCl3 compared with the control (p = 0.02; Table I). However, there were no significant differences in body weight in the group administered with SFN only and combined administration of SFN and AlCl3 compared with the control.
- 2. Effect of AlCl3 on sperm parameters
In addition, there was a significant decrease in sperm motility in the group administered with AlCl3 only compared with the control (p = 0.02; Figure 1B). The group administered with a combination of AlCl3 and SFN showed improvement in the motility of the spermatozoa compared with the AlCl3 only group (p = 0.03; Figure 1B). However, there was no significant difference between the control and SFN only group.
Furthermore, the spermatozoa viability was significantly decreased after AlCl3 administration compared with the control (p = 0.02; Figure 1C). However, the viability of the spermatozoa in the SFN + AlCl3 group showed significant difference compared to the control and SFN only group (p = 0.03; Figure 1C).
The AlCl3 only group had significantly (p = 0.02) higher sperm head defects compared to the control (Figure 1D). However, there was no significant difference in the abnormal head defeat in the groups that received SFN only and a combination of AlCl3 and SFN compared with the control. Furthermore, the AlCl3 only group showed a significantly higher percentage of sperm abnormalities compared to the control (p = 0.02; Figure 1D). The percentage level of sperm abnormalities was drastically reduced in the combined administration of SFN and AlCl3, which was not statistically significant compared to the SFN only and control groups (Figure 1D).
- 3. Serum TT, FSH, and LH
- 4. Lipid peroxidation and antioxidant status
- 5. Testicular histology
- 6. Stereological analysis
Concerning the EI, the AlCl3 only group showed a significant increase compared to the control (p = 0.02; Figure 5), while a corresponding decrease was observed in the combined SFN and AlCl3 group but it was not statistically significant compared to the SFN and control groups, respectively.
The testicular germ cell count such as spermatogonia, preleptotene and pachytene spermatocytes, and round spermatids count in the seminiferous tubules showed a significant decrease in the counts compared to the control (p = 0.02; Figure 6 A-D). Although, the germ cell count after the administration of SFN and AlCl3 was significantly improved compared to the AlCl3 only group, but it was not statistically significant compared to the SFN only and control groups, respectively.







4. Discussion
An emerging pandemic global public health issue after cancer and cardiovascular diseases is infertility due to increase in testicular cancer (35) and based on the analysis on semen parameters such as reduction in sperm counts and qualities in various countries (36, 37). The exposure of male individual to environmental toxicant is regarded as the channel that results in reduced sperm counts and infertility (1, 2). Aluminum is considered as the most common metallic element detectable in natural waters, animal, and plant tissues (3). Compounds of Al due to its reactivity with other elements such as Sulphur and chloride are widely used in many products such as storage utensils, household cookware, food additives, toothpaste ,and pharmaceuticals (antacids, vaccines, allergy injections, and anti-diarrhea) (3). The enormous rate of exposure to Al increases the chances of health-related issues to human due to increase metallic concentration in various organs thereby damaging various tissue of the body including testicular tissues of animals and humans (38). Testicular weight is crucial in the evaluation of male fertility test due to its important association in sperm production (39). In our study, the decrease in body and testicular weights observed after AlCl3 only administration could be correlated to the deleterious effect of the toxicant on body metabolism and testicular architecture, thereby resulting in spermatogenesis disruption. Previous research also concur that Al intoxication causes drastic decrease in testicular weight resulting in germinal EE disruption and inadequate TT production (40, 41). However, SFN attenuated the body and testicular weight loss in combined administration of SFN and AlCl3 group thereby restoring the testicular function.
The seminal fluid analysis (sperm count, sperm motility, sperm viability) were significantly declined in the AlCl3 only group thereby causing oligospermia due to increased oxidative stress-induced damage and decreased concentration of scavenging enzymes (42, 43). Previous studies also showed similar decrease in sperm count and sperm motility after exposure to various environmental toxicants in different experimental animal models (44-46). However, the combined administration of SFN and AlCl3 increases the motility, concentration, and viability of the spermatozoa thereby mitigating the effects of AlCl3 intoxication on testicular tissue.
The process of spermatogenesis has been implicated to be under the regulation of reproductive hormones such as TT, FSH, and LH. In this present study, our results showed a decrease in this reproductive hormone after AlCl3 administration, thereby suggesting a decline in the role of anterior pituitary and Leydig cells. Previous studies have also observed that the decrease in the level of TT, FSH, and LH hormones in adult rats were due to several environmental agents (47-49). In addition, previous research deduced that the decrease in the level of TT synthesis could be due to the deleterious effects of testicular toxicant (such as NO, AlCl) on the Leydig cells and also the conversion of androsterone to TT due to decreased activity of 17-ketosteroid reductase enzyme (50, 51). However, the SFN and AlCl3 combined-treated group showed a significant improvement in serum FSH, LH, and TT levels that can be linked to the ameliorative potential of SFN on AlCl3 testicular toxicity in the release of gonadotrophin-releasing hormone (GnRH) secretion in the hypothalamus (52).
The antioxidant defense system prevents the cells of the body against the injurious effect of Reactive Oxygen Species (ROS) produced due to exposure to environmental toxicants, ultimately inducing toxicity to the reproductive system by perturbing the pro-oxidant and thereby leading to oxidative stress (53). Our study showed that exposure to AlCl3 decreased the antioxidant enzymes such as SOD, CAT, GSH and correspondingly increased the MDA level. The decline in the activities of the antioxidant enzymes observed in this study revealed that the antioxidant system was impaired, thereby inducing oxidative stress induced-testicular toxicity. Previous research have showed that the production of oxidative stress due to metallic exposure decrease enzyme defense mechanism, thereby causing spermatozoa cytotoxicity (54). In addition, the inhibition of sperm functions and male infertility was also reported to occur through toxicity of lipid peroxides via generation of reactive oxygen species (54, 55). However, the co-administration of SFN and AlCl3 in this study showed ameliorative effects against oxidative injury by increasing the levels of antioxidant enzymes (SOD, CAT, GSH) with corresponding decrease in lipid peroxidation. It could be deduced that SFN decrease the free radicals levels via its free radical scavenging activity, especially oxygen radicals, and modulates several cytokines release and activities of testicular enzymes.
The histomorphological features of the testis are critical and usually refer to as the endpoint in the evaluation of male fertility assessment and reproductive toxicity (56). In our study, histological observation of animals that received AlCl3 only showed various distortions such as shrinked seminiferous tubules, degeneration of Leydig cells, thinner germinal EE, spermatogenesis disruption, and absence of spermatozoa in the EL. Previous studies have also reported similar changes in the histoarchitecture of the testis after exposure to different environmental toxicant (49, 57). The alteration in testicular histomorphology by metallic toxicant might be due to oxidative stress, thereby causing distortion of the steroidogenic activity of the Leydig cells by penetration through the blood-testis barrier. However, the administration of SFN in AlCl3-induced testicular damage showed its protective ability on spermatogenesis and tubular atrophy that was confirmed in our study by the histomorphological observation that showed distinctive increase in seminiferous tubules diameter and presence of spermatozoa in the EL. Previous studies have showed that normal spermatogenesis is achievable in oxidative stress-induced testicular toxicity caused by environmental toxicant by several antioxidant-rich agents, thereby increasing the endocrine activity of the Leydig and Sertoli cells (58-60). Furthermore, the histomorphological observation in our study corroborates with the decrease in the number of spermatogonia, preleptotene, pachytene spermatocytes, and round spermatids in AlCl3 only group suggesting declined spermatogenic activity. The increased oxidative stress and lipid peroxidation could increase apoptosis of the germ cells. Previous studies also showed that apoptosis of the spermatogonia and primary spermatocyte can occur via microtubule targeting and mitotic arrest after exposure of environmental toxicant (61) and decreased diameter of the seminiferous tubule could also be an indicator of defective spermatogenesis (62, 63).
5.Conclusion
The present study revealed the ameliorative response of SFN on AlCl3-induced testicular toxicity on blood LH, FSH, and TT through oxidative stress. The protective function of SFN may preserve the functional integrity of the testis against environmental toxicity.
Acknowledgements
The authors are grateful to Dr. Ijomone Neuro Laboratory, Department of Human Anatomy, Federal University of Technology Akure Nigeria for the photomicrograph capturing of slides. They are also thankful to Mr. Ige from Histology Laboratory, Department of Anatomy and Cell Biology, Obafemi Awolowo University, Ife, Nigeria for preparation of histology slide.
Conflict of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Type of Study: Original Article |
Subject:
Reproductive Endocrinology
References
1. Nordkap L, Joensen UN, Blomberg Jensen M, Jørgensen N. Regional differences and temporal trends in male reproductive health disorders: semen quality may be a sensitive marker of environmental exposures. Mol Cell Endocrinol 2012; 355: 221-230. [DOI:10.1016/j.mce.2011.05.048] [PMID]
2. Mehrpour O, Karrari P, Zamani N, Tsatsakis AM, Abdollahi M. Occupational expxosure to pesticides and consequences on male semen and fertility: A review. Toxicol Lett 2014; 230: 146-156. [DOI:10.1016/j.toxlet.2014.01.029] [PMID]
3. Verstraeten SV, Aimo L, Oteiza PI. Aluminum and lead: Molecular mechanism of brain toxicity. Arch Toxicol 2008; 82: 789-802. [DOI:10.1007/s00204-008-0345-3] [PMID]
4. Venturini-Soriano M, Berthon G. Aluminum speciation studies in biological fluids. Part 7. A quantitative investigation of aluminum (III)-malate complexequilibria and their potential implications for aluminum metabolism and toxicity. J Inorg Biochem 2001; 85: 143-154. [DOI:10.1016/S0162-0134(01)00206-9]
5. Yousef MI, Salama AF. Propolis protection from reproductive toxicity caused by aluminium chloride in male rats. Food Chem Toxicol 2009; 47: 1168-1175. [DOI:10.1016/j.fct.2009.02.006] [PMID]
6. Choi EJ. Antioxidative effects of hesperetin against 7, 12- dimethylbenz (a) anthracene-induced oxidative stress in mice. Life Sci 2008; 82: 1059-1064. [DOI:10.1016/j.lfs.2008.03.002] [PMID]
7. Gurjar M, Baronia AK, Azim A, Sharma K. Managing aluminum phosphide poisonings. J Emerg Trauma Shock 2011; 4: 378-384. [DOI:10.4103/0974-2700.83868] [PMID] [PMCID]
8. Hirata A, Murakami Y, Shoji M, Kadoma Y, Fujisawa S. Kinetics of radical-Scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX-2 expression. Anticancer Res 2005; 25: 3367-3374.
9. Anand R, Kumari P, Kaushal A, Bal A, Wani WY, Sunkaria A, et al. Effect of acute aluminum phosphide exposure on rats- A biochemical and histological correlation. Toxicol Lett 2012; 215: 62-69. [DOI:10.1016/j.toxlet.2012.09.020] [PMID]
10. Zhao Z, Liao G, Zhao Q, Lv D, Holthfer H, Zou H. Sulforaphane attenuates contrast-induced nephropathy in rats via Nrf2/HO-1 pathway. Oxid Med Cell Longev 2016; 8: 25-32. [DOI:10.1155/2016/9825623] [PMID] [PMCID]
11. Lee JH, Moon MH, Jeong JK, Park YG, Lee YJ, Seol JW, et al. Sulforaphane induced adipolysis via hormone sensitive lipase activation, regulated by AMPK signaling pathway. Biochem Biophys Res Commun 2012; 426: 492-497. [DOI:10.1016/j.bbrc.2012.08.107] [PMID]
12. Choi KM, Lee YS, Kim W, Kim SJ, Shin KO, Yu JY, et al. Sulforaphane attenuates obesity by inhibiting adipogenesis and activating the AMPK pathway in obese mice. J Nutr Biochem 2014; 25: 201-207. [DOI:10.1016/j.jnutbio.2013.10.007] [PMID]
13. Bahadoran Z, Tobidi M, Nazeri P, Mehran M, Azizi F, Mirmiran P. Effect of broccoli sprouts on insulin resistance in type 2 diabetic patients: a randomized double-blind clinical trial. Int J Food Sci Nutr 2012; 63: 767-771. [DOI:10.3109/09637486.2012.665043] [PMID]
14. De Souza CG, Sattler JA, de Assis AM, Rech A, Santos Perry ML, Souza DO. Metabolic effects of sulforaphane oral treatment in streptozotocin-diabetic rats. J Med Food 2012; 15: 795-801. [DOI:10.1089/jmf.2012.0016] [PMID]
15. Bai Y, Cui W, Xin Y, Miao X, Barati MT, Zhang C, et al. Prevention by sulforaphane of diabetic cardiomyopathy is associated with upregulation of Nrf2 expression and transcription activation. J Mol Cel Cardiol 2013; 57: 82-95. [DOI:10.1016/j.yjmcc.2013.01.008] [PMID]
16. Shan Y, Zhao R, Geng W, Lin N, Wang X, Du X, et al. Protective effect of sulforaphane on human vascular endothelial cells against lipopolysaccharide-induced inflammatory damage. Cardiovascular Toxicology 2010; 10: 139-145. [DOI:10.1007/s12012-010-9072-0] [PMID]
17. Kensler TW, Chen JG, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin- DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, qidong, people's republic of China. Cancer Epidemiol Biomarkers Prev 2005; 14: 2605-2613. [DOI:10.1158/1055-9965.EPI-05-0368] [PMID]
18. Ushida Y, Talalay P. Sulforaphane accelerates acetaldehyde metabolism by inducing aldehyde dehydrogenases: Relevance to ethanol intolerance. Alcohol and Alcoholism 2013; 48: 526-534. [DOI:10.1093/alcalc/agt063] [PMID]
19. Toyama T, Shinkai Y, Yasutake A, Uchida K, Yamamoto M, Kumagai Y. Isothiocyanates reduce mercury accumulation via an Nrf2-dependent mechanism during exposure of mice to methylmercury. Environ Health Perspect 2011; 119: 1117-1122. [DOI:10.1289/ehp.1003123] [PMID] [PMCID]
20. Egner PA, Chen JG, Zarth AT, Ng DK, Wang JB, Kensler KH, et al. Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: Results of a randomized clinical trial in China. Cancer Prev Res 2014; 7: 813-823. [DOI:10.1158/1940-6207.CAPR-14-0103] [PMID] [PMCID]
21. Kensler TW, Egner PA, Agyeman AS, Visvanathan K, Groopman JD, Chen JG, et al. Keap1-nrf2 Signaling: A target for cancer prevention by sulforaphane. Top Curr Chem 2013; 329: 163-177. [DOI:10.1007/128_2012_339] [PMID] [PMCID]
22. Gaona-Gaona L, Molina-Jijón E, Tapia E, Zazueta C, Hernández-Pando R, Calderón-Oliver M, et al. Protective effect of sulforaphane pretreatment against cisplatin-induced liver and mitochondrial oxidant damage in rats. Toxicology 2011; 286: 20-27. [DOI:10.1016/j.tox.2011.04.014] [PMID]
23. Talalay P, Fahey JW, Healy ZR, Wehage SL, Benedict AL, Min C, et al. Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc Nati Acad Sci USA 2007; 104: 17500-17505. [DOI:10.1073/pnas.0708710104] [PMID] [PMCID]
24. Singh K, Connors SL, Macklin EA, Smith KD, Fahey JW, Talalay P, et al. Sulforaphane treatment of autism spectrum disorder (ASD). Proc Natl Acad Sci USA 2014; 111: 15550-15555. [DOI:10.1073/pnas.1416940111] [PMID] [PMCID]
25. Yanaka A, Fahey JW, Fukumoto A, Nakayama M, Inoue S, Zhang S, et al. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in helicobacter pylori-infected mice and humans. Cancer Prev Res 2009; 2: 353-360. [DOI:10.1158/1940-6207.CAPR-08-0192] [PMID]
26. Kikuchi M, Ushida Y, Shiozawa H, Umeda R, Tsuruya K, Aoki Y, et al. Sulforaphane-rich broccoli sprout extract improves hepatic abnormalities in male subjects. World J Gastroenterol 2015; 21: 12457-12467. [DOI:10.3748/wjg.v21.i43.12457] [PMID] [PMCID]
27. Zhang Y, Tang L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol Sin 2007; 28: 1343-1354. [DOI:10.1111/j.1745-7254.2007.00679.x] [PMID]
28. Avwioro OG. Histology and tissue pathology. Principles and techniques. 2nd ed. Ibadan: Claverianum Press; 2010.
29. Feng R, He W, Ochi H. A new murine oxidative stress model associated with senescence. Mech Ageing Dev 2001; 122: 547-559. [DOI:10.1016/S0047-6374(01)00232-9]
30. World Health Organization. WHO laboratory manual for the examination of human semen and semen-cervical mucus interaction. 4th ed. Cambridge: University Press; 1999.
31. Adelakun SA, Ukwenya VO, Ogunlade BS, Aniah JA, Ibiayo AG. Nitrite-induced testicular toxicity in rats: therapeutic potential of walnut oil. JBRA Assist Reprod 2019; 23: 15-23 [DOI:10.5935/1518-0557.20180062]
32. Akang EN, Oremosu AA, Osinubi AA, Dosumu OO, Kusemiju TO, Adelakun SA, et al. Histomorphometric studies of the effects of Telfairiaoccidentalis on alcohol-induced gonado-toxicity in male rats. Toxicol Rep 2015; 2: 968-975. [DOI:10.1016/j.toxrep.2015.06.009] [PMID] [PMCID]
33. Howard CV, Reed MG. Unbiased stereology: Three-dimensional measurement in microscopy. 2nd Ed. Abingdon: Garland Science/BIOS Scientific; 2005.
34. Baines H, Nwagwu MO, Hastie GR, Wiles RA, Mayhew TM, Ebling FJ. Effects of estradiol and FSH on maturation of the testis in the hypogonadal (hpg) mouse. Reprod Biol Endocrinol 2008; 6: 4-13. [DOI:10.1186/1477-7827-6-4] [PMID] [PMCID]
35. Jørgensen N, Vierula M, Jacobsen R, Pukkala E, Perheentupa A, Virtanen HE, et al. Recent adverse trends in semen quality and testis cancer incidence among Finnish men. Int J Androl 2011; 34: e37-e48. [DOI:10.1111/j.1365-2605.2010.01133.x] [PMID] [PMCID]
36. Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decresing quality of semen during past 50 years. BMJ 1992; 305: 609-613. [DOI:10.1136/bmj.305.6854.609] [PMID] [PMCID]
37. Geoffroy-Siraudin C, Loundou Ad, Romain F, Achard V, Courbiere B, Perrard MH, et al. Decline of semen quality among 10932 males consulting for couple infertility over a 20-year period in Marseille, France. Asian J Androl 2012; 14: 584-590. [DOI:10.1038/aja.2011.173] [PMID] [PMCID]
38. Cheraghi E, Golkar A, Roshanaei K, Alani B. Aluminium-induced oxidative stress, apoptosis and alterations in testicular tissue and sperm quality in wistar rats: Ameliorative effects of curcumin. Int J Fertil Steril 2017; 11: 166-175.
39. Franca LR, Godinho CL. Testis Morphometry, Seminiferous Epithelium Cycle Length, and Daily Sperm Production in Domestic Cats (Felis catus). Biol Reprod 2003; 68: 1554-1561. [DOI:10.1095/biolreprod.102.010652] [PMID]
40. Pandey G, Jain GC. Aluminium chloride-induced testicular effects in rats: A histomorphometrical study. Asian Journal of Applied Science and Technology 2017; 1: 46-52.
41. Trif A, Muselin F, Argherie D, Dumitrescu E, Măcinic I. The consequences of chronic exposure to aluminium on some morphological biomarkers of reproductive function (body, genital organs, sexual accessory glands weight, seminiferous tubules diameter) in male rats. Lucrări Stiinłifice Medicină Veterinară 2007; 40: 652-658.
42. Saleh RA, Agarwal A. Oxidative stress and male infertility: from research bench to clinical practice. J Androl 2002; 23: 737-752.
43. Bansal AK, Bilaspuri GS. Impacts of oxidative stress and antioxidants on semen functions. Vet Med Int 2010; 2011: 137-143. [DOI:10.4061/2011/686137] [PMID] [PMCID]
44. Akinloye O, Arowojolu AO, Shittu OB, Anetor JI. Cadmium toxicity: A possible cause of male infertility in Nigeria. Reprod Biol 2006; 6: 17-30.
45. Isaac JA, Bolanle AM, Oluyemi A. Modulatory effects of kolaviron (Garcina kola extract) on spermogram and reproductive system of adult male wistar rats in lead acetate induced toxicity. J Toxicol Environ Health Sci 2013; 5: 121-130. [DOI:10.5897/JTEHS2013.0262]
46. Al-Attar AM. Antioxidant effect of vitamin E treatment on some heavymetals-induced renal and testicular injuries inmale mice. Saudi J Biol Sci 2011; 18: 63-72. [DOI:10.1016/j.sjbs.2010.10.004] [PMID] [PMCID]
47. Guo CH, Lin CY, Yeh MS, Wang Hsu GS. Aluminum-induced suppression of testosterone through nitric oxide production in male mice. Environ Toxicol Pharmacol 2005; 19: 33-40. [DOI:10.1016/j.etap.2004.02.009] [PMID]
48. Yakubu OE, Nwodo OFC, Imo C, Ogwoni HA. Spermatogenic and haematological effects of aqueous and ethanolic extracts of hymenocardiaacida stem bark on aluminium-induced toxicity in male wistar rats. Insights in Biomedicine 2017; 2: 1-5.
49. Nuhair RS. Effects of aluminum chloride on some hormones levele and reproductive organs of male rats (Rattusnorvegicus). University of Thi-Qar Journal of Science 2015; 5: 3-9.
50. Dobashi M, Fujisawa M, Yamazaki T, Okuda Y, Kanzaki M, Tatsumi N, et al. Inhibition of steroidogenesis in Leydig cells by exogenous nitric oxide occurs independently of steroidogenic acute regulatory protein(star) mRNA. Arch Androl 2001; 47: 203-209. [DOI:10.1080/014850101753145915] [PMID]
51. Dobashi M, Fujisawa M, Yamazaki T, Okuda Y, Kanzaki M, Tatsumi N, et al. Inhibition of steroidogenesis in Leydig cells by exogenous nitric oxide occurs independently of steroidogenic acute regulatory protein (star) mRNA. Arch Androl 2001; 47: 203-209. [DOI:10.1080/014850101753145915] [PMID]
52. Shahraki MR, Zahedi Asl S, Sarkaki AR. The effect of aluminum injection in lateral ventricle on sex hormones in male rat. Shiraz E-Medical Journal 2004; 5: 1-10.
53. Sikka SC, Rajasekaran M, Hellstorm WJ. Role of oxidative stress and antioxidants in male infertility. J Androl 1995; 16: 464-468.
54. Rao MV, Gangadharan B. Antioxidative potential of melatonin against mercury induced intoxication in spermatozoa in vitro. Toxicol In Vitro 2008; 22: 935-942. [DOI:10.1016/j.tiv.2008.01.014] [PMID]
55. Anane R, Creppy EE. Lipid peroxidation as pathway of aluminium cytotoxicity in human skin fibroblast cultures: prevention by superoxide dismutase catalase and vitamins E and C. Hum Exp Toxicol 2001; 20: 477-481. [DOI:10.1191/096032701682693053] [PMID]
56. Turner RMO. Pathogenesis, diagnosis, and management of testicular degeneration in stallions. Clinical Techniques in Equine Practice 2007; 6: 278-284. [DOI:10.1053/j.ctep.2007.09.006]
57. Khattab FKI. Histological and ultrastructural studies on the testis of rat after treatment with aluminium chloride. Aust J Basic and Appl Sci 2007; 1: 63-72.
58. Kang IS, Kim C. Taurine chloramine administered in vivo increases NRF2-regulated antioxidant enzyme expression in murine peritoneal macrophages. Adv Exp Med Biol 2013; 775: 259-267. [DOI:10.1007/978-1-4614-6130-2_22] [PMID]
59. Kim C, Cha YN. Taurine chloramine produced from taurine under inflammation provides anti-inflammatory and cytoprotective effects. Amino Acids 2014; 46: 89-100. [DOI:10.1007/s00726-013-1545-6] [PMID]
60. Kim W, Kim HU, Lee HN, Kim SH, Kim C, Cha YN, et al. Taurine chloramine stimulates efferocytosis through upregulation of Nrf2-Mediated heme oxygenase-1 expression in murine macrophages: Possible involvement of carbon monoxide. Antioxid Redox Signal 2015; 23: 163-177. [DOI:10.1089/ars.2013.5825] [PMID] [PMCID]
61. Ahmed YF, Mahmoud GHMK, Farghaly AA, Abo-Zeid MA, Ismail EM. Some studies on the toxic effects of prolonged lead exposure in male rabbits: chromosomal and testicular alterations. Global Veterinaria 2012; 8: 360-366.
62. Aruldhas MM, Subramanian S, Sekar P, Vengatesh G, Chandrahasan G, Govindarajulu P, et al. Chronic chromium exposure-induced changes in testicular histoarchitecture are associated with oxidative stress: study in a non-human primate (Macaca radiate Geoffroy). Hum Reprod 2005; 20: 2801-2813. [DOI:10.1093/humrep/dei148] [PMID]
63. Pires VC, Gollucke APB, Ribeiro DA, Lungato L, Almeida VD, Aguiar Jr O. Grape juice concentrate protects reproductive parameters of male rats against cadmium-induced damage: A chronic assay. Br J Nutr 2013; 110: 2020-2029. [DOI:10.1017/S0007114513001360] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








