Wed, Apr 24, 2024
[Archive]
Volume 18, Issue 11 (November 2020)
IJRM 2020, 18(11): 921-934 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Dehghan Tezerjani M, Kalantar S M. Unraveling the dark matter, long non-coding RNAs, in male reproductive diseases: A narrative review. IJRM 2020; 18 (11) :921-934
URL: http://ijrm.ir/article-1-1729-en.html
URL: http://ijrm.ir/article-1-1729-en.html
1- Abortion Research Centre, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Science, Yazd, Iran. , masoud.det@gmail.com
2- Abortion Research Centre, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Science, Yazd, Iran. Department of Genetics, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
2- Abortion Research Centre, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Science, Yazd, Iran. Department of Genetics, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Keywords: Long noncoding RNA, Prostate cancer, Prostatic hyperplasia, Prostatitis, Varicocele, Sperm abnormalities.
Full-Text [PDF 9414 kb]
(774 Downloads)
| Abstract (HTML) (2041 Views)
Full-Text: (408 Views)
1. Introduction
The prevalence of infertility is 15% worldwide, and according to the global data, 20-70% of the total infertility cases is attributed to male infertility (1). Male infertility is characterized by heterogeneous and multifactorial conditions among which genetics factors encompass approximately 40% of cases with idiopathic infertility (2). The most common clinical abnormalities associated with male infertility are prostate cancer (PCa), prostatitis, benign prostatic hyperplasia (BPH), testicular cancer (TCa), varicocele, and inability to produce normal sperm (azoospermia, oligozoospermia, asthenozoospermia (AZS), and teratozoospermia). Although the main molecular mechanisms involved in the pathogenesis of these abnormalities are unknown, all of them have genetic background (3-6).
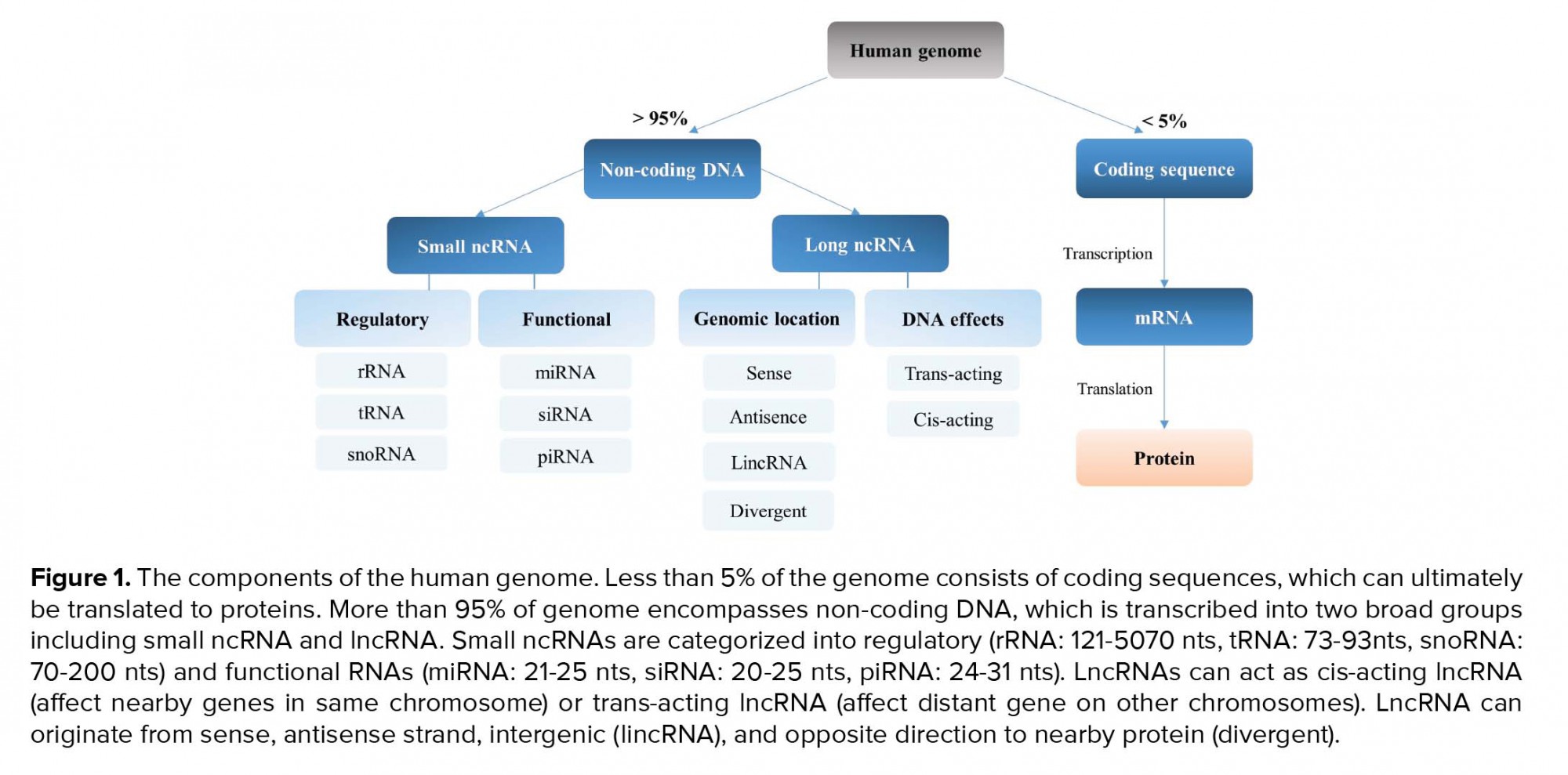
While more than 85% of the human genome is transcribed, only a low proportion of these transcribed RNAs encode proteins (7). Non-coding RNAs (ncRNAs) are categorized into two broad groups based on their size. Short ncRNAs are < 200 nucleotides (nts) in length and include microRNAs (miRNAs), piwi-interacting RNAs (piRNAs), and small nuclear RNAs (snoRNA) (8), while long ncRNAs (lncRNAs) are > 200 nts in length (Figure 1). lncRNAs are involved in several mechanisms such as chromatin remodeling, chromatin looping, recruitment of transcription factors. They can also be involved in RNA splicing and control of translation. Furthermore, they can also modulate RNA degradation as well as act as a miRNA sponge and sequester them to control gene expression (Figure 2) (9).
The gene regulatory process can occur at any stage such as RNA transcription, translation, and post-translational modification. This process includes DNA methylation, histone modification (methylation, phosphorylation, ubiquitylation, acetylation, and sumoylation) and tissue-specific transcription factors (TFs) (10, 11). In addition, ncRNAs have also been identified as prominent regulatory elements in gene expression (12). The dark matter of the genome, lncRNAs, was previously considered as transcriptional noise (13). However, advances in genomic technologies such as high-throughput sequencing technologies have led to the identification of thousands of lncRNA in different diseases including male reproductive ones. LncRNAs are involved in differentiation, proliferation, and self-renewal of spermatogonial stem cells (SSCs) (14). Furthermore, they modulate apoptosis, invasion, cell cycle, and cell signaling pathways in the male reproductive tract. Therefore, LncRNAs could be considered as a potential biomarker to detect any abnormalities in male reproductive tract. In this review, we will provide a short summary about the role of lncRNAs and their pathways in the pathogenesis of male reproductive-associated diseases such as PCa, prostatitis, BPH, TCa, varicocele, and sperm abnormalities (azoospermia, oligozoospermia, asthenozoospermia, and teratozoospermia).

2. Materials and Methods
The current narrative review study aimed to provide the latest information about the lncRNAs involved in the susceptibility to different male reproductive diseases and summarizing lncRNAs functions in the development of the related diseases. It was conducted through a comprehensive search in electronic databases, including PubMed, Scopus, and Google Scholar using Keywords such as lncRNA, prostate cancer, prostatic hyperplasia, prostatitis, testicular cancer, varicocele, sperm abnormalities. After evaluating 122 related articles including original, review, and meta-analysis, 93 articles were finally used in this study.
3. Diseases
3.1. Prostate cancer
PCa as a complex and noncutaneous malignancy is the second cause of cancer-associated death among men (15). Several studies have implicated the role of differentially expressed lncRNAs affecting pathological, treatment, and prognosis of PCa. In addition, lncRNAs can act as oncogene and tumor suppressor molecules in PCa tumorigenesis (16, 17). We will discuss some principal oncogenic lncRNAs later in the article. Table I mentions the further oncogenic lncRNAs with their summary of functions.

3.1.1. Oncogenic lncRNAs in PCa
Prsenter and colleagues identified 121 unannotated ncRNA transcript in a cohort of 102 prostate tissues and cell lines using high-throughput RNA sequencing and named them as prostate cancer-associated ncRNA transcripts (PCATs) based on their fold-change in PCa samples compared to normal tissue. PCAT-1, ~ 1.9 kb lncRNA, is located upstream of the c-Myc gene at chromosome 8q24 (32). Its expression is significantly high and upregulated in most PCa cells especially metastatic samples; furthermore, it is mostly found in cytoplasm and in a small amount in the nucleus of PCa cells. It has also been reported that lncRNA PCAT-1 represses BRCA2 involved in homologous recombination (HR), resulting in unrepaired Double-stranded DNA breaks (DSBs) (33). Another study revealed that PCAT-1 with microRNAs (miR-3667-3p and miR-34a) upregulates the c-Myc protein level, which is essential for cell cycle progression, leading to proliferation of prostate tumor cell (34). PCAT-5 is another oncogenic lncRNA that is regulated by transcription factor ERG (ETS-related gene) and is significantly associated with cell proliferation and invasion of PCa cells. In addition, it is upregulated in castration-resistant prostate cancer (CRPC) tissue compared to normal prostate cells (35). The lncRNA Prostate Ovary Testis Expressed Family member Antisense 1 (POTEF-AS1) is the androgen-dependent regulator that is involved in apoptosis and toll-like receptor signaling pathways by interacting with TLR3 and TNFSF10. In addition, it has a fundamental role in the progression of docetaxel-treated cells by repressing apoptosis, resulting in chemoresistance (36).
HOXD antisense growth-associated lncRNA (HOXD-AS1) is upregulated in PCa. A study has reported that HOXD-AS1 regulated the expression of target genes and promoted cell proliferation by recruiting WDR5. This molecule is part of the MLL1/MLL complex and has a key role in histone H3 lysine 4 tri-methylation (H3K4me3) which is associated with transcriptional activation (37). Li and coworkers in 2017 reported that small nucleolar RNA host gene 1 (SNHG1) lncRNA promotes cell proliferation and is upregulated in PCa. By acting as ceRNA (competing endogenous RNA), this lncRNA suppresses the activity of miR-199a-3p that promotes the expression of its target, CDK7, resulting in increased cell proliferation and cell cycle progress in PCa (38). SOCS2-AS1 (Cytokine signaling 2-antisense transcript 1) is an androgen-induced lncRNA and regulates genes TNFSF10, FOXM1, and CENPF, which are involved in apoptosis and cellular proliferation in PCa. Moreover, by repressing apoptosis, it plays a fundamental role in the progression of CRPC (39).
CTBP1-AS, an androgen-responsive lncRNA, is located in the AS region of C-terminal-binding protein 1, which works as a corepressor for the androgen receptor. By recruiting the RNA-binding transcriptional repressor PSF and HDAC (Histon deacetylase), this lncRNA can repress the expression of the CTBP1 gene. Low expression of this gene is associated with overexpression of androgen-related genes and aberrant cell proliferation in prostate cells which causes PCa (40). PVT1 (Plasmacytoma Variant Translocation 1) lncRNA was identified to be associated with miR-146a expression. This lncRNA decreases the miR-146a expression by increasing methylation in CpG Island in the promoter of miR-146a. The silencing of miR-146a suppresses apoptosis and promotes cell viability in PCa (41). Another study by Yang and co-authors revealed that the level of PVT1 expression was notably high in PCa compared to normal prostate cells. In addition, they did knockdown this lncRNA in PCa cell lines and found the expression of cleaved caspase-3 and c-Myc was upregulated and downregulated, respectively. Therefore, this lncRNA increases cell growth in PCa in vitro and in vivo (42).
The gene encoding TTTY15 (Testis-specific transcript Y-linked 15) lncRNA is located at Yq11.2. Some studies revealed that the fusion of the TTTY15 gene with USP9Y (Ubiquitin Specific Peptidase 9 Y-linked) gene is a potential carcinogen in some cancers particularly in PCa (43, 44). Xia and colelagues found that this lncRNA is upregulated in patients with PCa compared to normal cases. They used the CRISPR-Cas9 technique to knock down this lncRNA and reached the conclusion that it suppressed the growth of PCa cells in vitro and in vivo. Moreover, TTTY15 increases CDK6 and FN1 expression by acting as a sponge for microRNA let-7 (45). A single study reported that the expression level of THBS4-003 (Thrombospondin 4) is significantly higher in PCa cells compared to non-tumor ones. It also revealed that knockdown of this lncRNA suppressed the invasive and migratory capability of PCa cell, the expression level of MMP-9 (matrix metalloproteinase-9) as well as p38 (3). Further, Jiang and colelagues found that lnc-MX1-1 (MX Dynamin like GTPase 1) is upregulated in PCa cells compared to adjacent normal prostate cells using array expression profiling. By using RNAi in LNCaP and 22Rv1 cell lines, they suppressed the expression of this lncRNA and identified proliferation and invasiveness of the cells were significantly reduced. In addition, their result indicated the significant association of this lncRNA with clinical features of patients with PCa such as PSA, metastasis, Gleason score, and recurrence-free survival (17).
3.1.2. Suppressive lncRNAs in PCa
Some of the identified lncRNAs are tumor-suppressive which show reduced expression in PCa cell and decrease the proliferation and migration of PCa cells through different molecular pathways. Some main suppressive lncRNAs will be discussed as follows. Table II presents the further suppressive lncRNAs with their summary of functions. BDNF-AS (brain-derived neurotrophic factor antisense), a naturally-occurring RNA antisense against BDNF, is downregulated in cancers such as retinoblastoma and lung cancer (46, 47). It was also downregulated in PSA-positive, PSA-negative PCa cell lines as well as PCa human tissue. Further investigation revealed that increasing the expression of this lncRNA using lentivirus-mediated BDNF-AS suppressed PCa cell development, invasion, and proliferation in two aforementioned PCa cell lines. This study also suggested the overexpression of this lncRNA could be considered as a potential method for therapeutic drug for PCa (48). GAS5 (Growth Arrest Specific 5) gene encodes a snoRNA from its intron and a lncRNA from its exonic sequence. The increased level of this lncRNA promotes apoptosis and inhibits the anti-apoptotic abilities of glucocorticoids by binding to the DNA-binding domain of glucocorticoids (49).
A study by Pickard and colleagues using GAS5-encoding plasmids or GAS5 siRNAs in PCa cell lines showed that the low expression of this lncRNA is significantly associated with increased apoptosis and decreased survival rate in PCa (50). In addition, a study found that this lncRNA targets mir-103 leading to inactivation of PI3KAKT-mTOR signaling pathway, low PCa cell growth, and proliferation (51). A single study reported that IGF2-AS (insulin growth factor 2 antisense) lncRNA was downregulated in PCa cell line and human PCa tissues. Lentivirus-induced IGF2AS overexpression decreased xenograft development in vivo, invasion, and proliferation of PCa cells in vitro. Through inverse regulation of IGF2, this lncRNA acted as an epigenetic tumor suppressor in PCa (16). LncRNA FENDRR (FOXF1 Adjacent Non-Coding Developmental Regulatory RNA) is located at 16q24.1. This lncRNA has a fundamental role in modifying chromatin by interacting with Trithorax group/MLL protein complexes (TrxG/MLL) and polycomb repressive complex 2 (PRC2) (52, 53). Zhang and colleagues identified that this lncRNA can also act as ceRNA for miR-18a-5p which upregulates the RUNX1 expression, resulting in decreased progression of PCa cell. Their study also indicated that there was a negative correlation between this lncRNA and the prognosis of PCa (54).
3.2. LncRNAs in the discrimination of BPH from PCa
BPH, another common prostate-associated disease, is non-cancerous enlargement of prostate characterized by over-proliferation of stromal and epithelial cells of the transition zone (61). It affects About 50% of males in the age range of 51-60 yr and reaches up to 70% among the age range of 61-70 (62). The measurement of prostate-specific antigen (PSA) has been used widely for screening PCa; however, its level can be increased by BPH as well (63). Therefore, using PSA is controversially debatable (64), and finding biomarkers that can exactly determine PCa from BPH would help clinicians to use proper therapeutic methods for these diseases. Several studies reported that because of tissue-specific expression pattern of lncRNAs, they could be considered as biomarkers to efficiently discriminate BPH from PCa. A study by Bayat and co-authors investigating four lncRNAs, Prcat17.3, Prcat38, Prcat47, and Cat2184.4, revealed low expression of lncRNA Cat2184.4 in PCa samples compared to BPH ones. In contrast, lncRNAs PRCAT17.3 and PRCAT38 showed significant upregulation in PCa samples compared to BPH ones. In addition, lncRNA PRCAT47 showed increased expression in PCa but not statistically significant compared to BPH samples. They concluded that this lncRNA can be considered as potential biomarkers to differentiate BPH from PCa (65).
Another study investigated the expression pattern of exosomal circulating lncRNAs in PCa, BPH and normal samples (66). It is worth mentioning that exosome is vesicle with 40-100 nm diameter, which has fundamental roles in the cell-to-cell communication and cell signaling by binding to their receptor on cells. Furthermore, they can carry a wide variety of molecules such as proteins, miRNAs, and lncRNAs. This structure keeps aforementioned molecules safe from degradation (67). This study revealed that exosomal lncRNA SAP30L-AS1 (SAP30L Antisense RNA 1 (Head To Head)) were significantly upregulated in BPH. However, their result showed that another exosomal lncRNA SChLAP1 (SWI/SNF Complex Antagonist Associated with Prostate Cancer 1) were upregulated in PCa samples compared to BPH and normal ones. The latter one is more useful as biomarker for discriminating BPH from PCa when PSA concentration is in a gray zone (66). lncRNA-p21 is another exosomal lncRNA which its expression pattern had been investigated in urine samples from patients with BPH and PCa. It is found in high level in urine samples of patients with PCa compared to samples from BPH patients (68).
3.3. Prostatitis
Prostatitis is inflammation of the prostate gland that has important roles in the male reproductive system. Although it is amendable, its main pathophysiology and treatment in male infertility are still unknown. It also can affect the assisted reproduction; therefore, finding the main pathogenesis of prostatitis and its negative impact on sperm quantity and quality including apoptosis and DNA integrity is indispensable (69). A recent study by Xu and colleagues examined the association of lncRNA GAS5 (growth arrest-specific transcript 5), located at 1q25.1, with chronic non-bacterial prostatitis (CNP). Their study revealed that the expression of this lncRNA was decreased in prostatitis tissues. In addition, they found that this lncRNA prevented cell proliferation in prostatitis by downregulation of COX2 expression, an enzyme producing prostaglandins. Their study in the GAS5-overexpressed CNP rat model showed the overexpression of this lncRNA decreased prostate volume, inflammatory cells count, and locomotion score. Therefore, the overexpression of lncRNA can reduce the injury of CNP in vivo (4).
3.4. Testicular cancer
Although TCa is generally a rare form of cancer, with the annual incidence of approximately 1%, it is the most prevalent cancer among men with the age range of 14 to 44 years. Testicular germ cell tumors (TGCT) represent nearly 98% of TCa, while the remaining comprise stromal tumors including Sertoli cell tumors, Leydig cell tumors, and other more poorly defined histologic types (70, 71). Newly identified TC are responsive to treatment; however, therapeutic outcomes of theses tumors in high stages are not sufficient (72, 73). Therefore, finding molecular pathways and their regulatory elements involved in the progression and growth of TCa cells such as lncRNA would lead to the identification of the best treatment for TCa. A recent study used NCCIT cell lines (Human testicular embryonic carcinoma cells), a pluripotent extragonadal germ cell tumor cell line, to determine the effect of lncRNA Gm2044 on the growth and proliferation of TCa cells. It revealed that the overexpression of this lncRNA prohibited cell proliferation in vitro. In addition, its results showed this effect was mediated by the miR-202-Rbfox2 pathway (74). LncRNA OIP5-AS1 (Opa-interacting protein 5 antisense RNA 1), located at 15q15.1, is another lncRNA that was shown to be overexpressed and promote cell proliferation in many cancers (75). This lncRNA is highly overexpressed in TGCT compared to other many cancers such as thyroid, prostate, pheochromocytoma, and paraganglioma (76). Based on the study by Rezaie and co-authors lncRNA LINC-ROR (Long Intergenic Non-Protein Coding RNA, Regulator Of Reprogramming) was highly expressed in testicular tumor tissues compared to control cell lines (NT2) (77). This lncRNA is located at 18q21 and is involved in embryonic stem cells (ESC) maintenance (78). Moreover, this lncRNA interacts with heterogeneous nuclear ribonucleoprotein I and suppresses p53 activated by DNA damage (79).
3.5. Varicocele
Varicocele, a main abnormality contributing to male infertility, is an abnormal dilation of pampiniform venous plexus in the scrotum and is prevalent among 20% of adult and adolescent male (80). It causes increased oxidative stress, apoptosis, venous pressure, and temperature resulting in testicular and sperm damage (81). Although many genetics factors such as chromosome alteration and epigenetic changes have been identified to be associated with Varicocele, however, its main molecular mechanism is still unknown (82). lncRNAs as a functional modulator of biological processes may have important roles in the pathogenesis of the disease. The lncRNA gadd7 (growth arrested DNA-damage inducible gene 7), a 754‐nt polyadenylated lncRNA, is overexpressed after DNA damage and growth arrest signal. Its overexpression is associated with suppressed cell growth (83, 84). Furthermore, it is the modulator of endoplasmic reticulum stress and lipid-induced oxidative (85). A single study analyzed the expression level of this lncRNA in the ejaculated spermatozoa of patients with Varicocele and found that the expression level lncRNA gadd7 is negatively associated with sperm count. It also used mouse germ cell lines GC-1 and GC-2 transfected with either pcDNA3.1-gadd7 or negative control plasmid to investigate the effect of this lncRNA overexpression on cell features. Its result indicated the overexpression of this lncRNA suppressed cell proliferation and increased cell apoptosis. In addition, the study showed that gadd7 induced by stress can cause cell death through upregulation of Bax and downregulation of Bcl2, which are pro-apoptotic and anti-apoptotic regulators, respectively (86).
3.6. Sperm abnormalities
Male infertility can also be ascribed to uniform testicular maturation arrest (MA) and different types of sperm abnormalities such as oligozoospermia, non-obstructive azoospermia, AZS, and teratozoospermia (87). Spermatogenesis is a complex process regulated by different genes, proteins, and transcriptional network including ncRNAs. Based on the wide ability of lncRNA in proliferation, differentiation, and self-renewal of SSC, they have fundamental roles in spermatogenesis regulation (88). LncRNA HOTAIR (HOX Transcript Antisense Intergenic RNA), located at 12q13.13, is one of the well-studied lncRNA in many diseases and involved in chromatin regulation by binding to Polycomb repressive complex 2 (PRC2) (89). In addition, it plays a key role in epigenetic regulation by interacting with the lysine-specific demethylase 1 (LSD1), Enhancer of Zeste homolog 2 (EZH2) ,and methyltransferase specific to histone 3 lysine 27 (90). Zhang and colleagues investigated the expression of this lncRNA in the samples from patients with AZS and oligoasthenozoospermia, and found it had a low expression in AZS and oligoasthenozoospermia compared to normal samples. Furthermore, their results indicated that the low expression of HOTAIR is associated with low expression NRF2 (Nuclear factor erythroid 2-related factor 2) gene. HOTAIR is responsible for histone H4 acetylation in the NRF2 gene promoter, which leads to its activation (91). Since the expression level of NRF2 is associated with sperm quality and antioxidant gene expression (92), HOTAIR may protect spermatozoa against antioxidant activity (91). Another study analyzed the expression profile of lncRNA in AZS and normal sperm samples. The gene ontology and pathway analysis revealed that differentially expressed lncRNA in AZS and normal samples were related to sperm function and spermatogenesis. Moreover, among all identified differentially expressed lncRNAs, the expression level of three lncRNA including lnc32058, lnc09522, and lnc98487 were significantly correlated with sperm motility (93).
4. Conclusion
In conclusion, this review emphasizes the identified lncRNAs and their functions in male reproductive disorders including PCa, BPH, prostatitis, TCa, varicocele, and sperm abnormalities. With the advent of state-of-the-art molecular and genomics techniques such as high-throughput sequencing, thousands of lncRNA have been identified in susceptibility to different diseases. However, their main mechanisms and molecular pathways are still unknown and need more functional and in vitro studies. Identification of differentially expressed lncRNA in each disease can pave the way toward developing unique biomarker, approaches to treat diseases, as well as increase efficiency of assisted reproductive technologies. In addition, lncRNA can be considered as precious indicator of sperm quality.
Conflict of Interest
The authors declare that there is no conflict of interest.
The prevalence of infertility is 15% worldwide, and according to the global data, 20-70% of the total infertility cases is attributed to male infertility (1). Male infertility is characterized by heterogeneous and multifactorial conditions among which genetics factors encompass approximately 40% of cases with idiopathic infertility (2). The most common clinical abnormalities associated with male infertility are prostate cancer (PCa), prostatitis, benign prostatic hyperplasia (BPH), testicular cancer (TCa), varicocele, and inability to produce normal sperm (azoospermia, oligozoospermia, asthenozoospermia (AZS), and teratozoospermia). Although the main molecular mechanisms involved in the pathogenesis of these abnormalities are unknown, all of them have genetic background (3-6).
While more than 85% of the human genome is transcribed, only a low proportion of these transcribed RNAs encode proteins (7). Non-coding RNAs (ncRNAs) are categorized into two broad groups based on their size. Short ncRNAs are < 200 nucleotides (nts) in length and include microRNAs (miRNAs), piwi-interacting RNAs (piRNAs), and small nuclear RNAs (snoRNA) (8), while long ncRNAs (lncRNAs) are > 200 nts in length (Figure 1). lncRNAs are involved in several mechanisms such as chromatin remodeling, chromatin looping, recruitment of transcription factors. They can also be involved in RNA splicing and control of translation. Furthermore, they can also modulate RNA degradation as well as act as a miRNA sponge and sequester them to control gene expression (Figure 2) (9).
The gene regulatory process can occur at any stage such as RNA transcription, translation, and post-translational modification. This process includes DNA methylation, histone modification (methylation, phosphorylation, ubiquitylation, acetylation, and sumoylation) and tissue-specific transcription factors (TFs) (10, 11). In addition, ncRNAs have also been identified as prominent regulatory elements in gene expression (12). The dark matter of the genome, lncRNAs, was previously considered as transcriptional noise (13). However, advances in genomic technologies such as high-throughput sequencing technologies have led to the identification of thousands of lncRNA in different diseases including male reproductive ones. LncRNAs are involved in differentiation, proliferation, and self-renewal of spermatogonial stem cells (SSCs) (14). Furthermore, they modulate apoptosis, invasion, cell cycle, and cell signaling pathways in the male reproductive tract. Therefore, LncRNAs could be considered as a potential biomarker to detect any abnormalities in male reproductive tract. In this review, we will provide a short summary about the role of lncRNAs and their pathways in the pathogenesis of male reproductive-associated diseases such as PCa, prostatitis, BPH, TCa, varicocele, and sperm abnormalities (azoospermia, oligozoospermia, asthenozoospermia, and teratozoospermia).


2. Materials and Methods
The current narrative review study aimed to provide the latest information about the lncRNAs involved in the susceptibility to different male reproductive diseases and summarizing lncRNAs functions in the development of the related diseases. It was conducted through a comprehensive search in electronic databases, including PubMed, Scopus, and Google Scholar using Keywords such as lncRNA, prostate cancer, prostatic hyperplasia, prostatitis, testicular cancer, varicocele, sperm abnormalities. After evaluating 122 related articles including original, review, and meta-analysis, 93 articles were finally used in this study.
3. Diseases
3.1. Prostate cancer
PCa as a complex and noncutaneous malignancy is the second cause of cancer-associated death among men (15). Several studies have implicated the role of differentially expressed lncRNAs affecting pathological, treatment, and prognosis of PCa. In addition, lncRNAs can act as oncogene and tumor suppressor molecules in PCa tumorigenesis (16, 17). We will discuss some principal oncogenic lncRNAs later in the article. Table I mentions the further oncogenic lncRNAs with their summary of functions.

3.1.1. Oncogenic lncRNAs in PCa
Prsenter and colleagues identified 121 unannotated ncRNA transcript in a cohort of 102 prostate tissues and cell lines using high-throughput RNA sequencing and named them as prostate cancer-associated ncRNA transcripts (PCATs) based on their fold-change in PCa samples compared to normal tissue. PCAT-1, ~ 1.9 kb lncRNA, is located upstream of the c-Myc gene at chromosome 8q24 (32). Its expression is significantly high and upregulated in most PCa cells especially metastatic samples; furthermore, it is mostly found in cytoplasm and in a small amount in the nucleus of PCa cells. It has also been reported that lncRNA PCAT-1 represses BRCA2 involved in homologous recombination (HR), resulting in unrepaired Double-stranded DNA breaks (DSBs) (33). Another study revealed that PCAT-1 with microRNAs (miR-3667-3p and miR-34a) upregulates the c-Myc protein level, which is essential for cell cycle progression, leading to proliferation of prostate tumor cell (34). PCAT-5 is another oncogenic lncRNA that is regulated by transcription factor ERG (ETS-related gene) and is significantly associated with cell proliferation and invasion of PCa cells. In addition, it is upregulated in castration-resistant prostate cancer (CRPC) tissue compared to normal prostate cells (35). The lncRNA Prostate Ovary Testis Expressed Family member Antisense 1 (POTEF-AS1) is the androgen-dependent regulator that is involved in apoptosis and toll-like receptor signaling pathways by interacting with TLR3 and TNFSF10. In addition, it has a fundamental role in the progression of docetaxel-treated cells by repressing apoptosis, resulting in chemoresistance (36).
HOXD antisense growth-associated lncRNA (HOXD-AS1) is upregulated in PCa. A study has reported that HOXD-AS1 regulated the expression of target genes and promoted cell proliferation by recruiting WDR5. This molecule is part of the MLL1/MLL complex and has a key role in histone H3 lysine 4 tri-methylation (H3K4me3) which is associated with transcriptional activation (37). Li and coworkers in 2017 reported that small nucleolar RNA host gene 1 (SNHG1) lncRNA promotes cell proliferation and is upregulated in PCa. By acting as ceRNA (competing endogenous RNA), this lncRNA suppresses the activity of miR-199a-3p that promotes the expression of its target, CDK7, resulting in increased cell proliferation and cell cycle progress in PCa (38). SOCS2-AS1 (Cytokine signaling 2-antisense transcript 1) is an androgen-induced lncRNA and regulates genes TNFSF10, FOXM1, and CENPF, which are involved in apoptosis and cellular proliferation in PCa. Moreover, by repressing apoptosis, it plays a fundamental role in the progression of CRPC (39).
CTBP1-AS, an androgen-responsive lncRNA, is located in the AS region of C-terminal-binding protein 1, which works as a corepressor for the androgen receptor. By recruiting the RNA-binding transcriptional repressor PSF and HDAC (Histon deacetylase), this lncRNA can repress the expression of the CTBP1 gene. Low expression of this gene is associated with overexpression of androgen-related genes and aberrant cell proliferation in prostate cells which causes PCa (40). PVT1 (Plasmacytoma Variant Translocation 1) lncRNA was identified to be associated with miR-146a expression. This lncRNA decreases the miR-146a expression by increasing methylation in CpG Island in the promoter of miR-146a. The silencing of miR-146a suppresses apoptosis and promotes cell viability in PCa (41). Another study by Yang and co-authors revealed that the level of PVT1 expression was notably high in PCa compared to normal prostate cells. In addition, they did knockdown this lncRNA in PCa cell lines and found the expression of cleaved caspase-3 and c-Myc was upregulated and downregulated, respectively. Therefore, this lncRNA increases cell growth in PCa in vitro and in vivo (42).
The gene encoding TTTY15 (Testis-specific transcript Y-linked 15) lncRNA is located at Yq11.2. Some studies revealed that the fusion of the TTTY15 gene with USP9Y (Ubiquitin Specific Peptidase 9 Y-linked) gene is a potential carcinogen in some cancers particularly in PCa (43, 44). Xia and colelagues found that this lncRNA is upregulated in patients with PCa compared to normal cases. They used the CRISPR-Cas9 technique to knock down this lncRNA and reached the conclusion that it suppressed the growth of PCa cells in vitro and in vivo. Moreover, TTTY15 increases CDK6 and FN1 expression by acting as a sponge for microRNA let-7 (45). A single study reported that the expression level of THBS4-003 (Thrombospondin 4) is significantly higher in PCa cells compared to non-tumor ones. It also revealed that knockdown of this lncRNA suppressed the invasive and migratory capability of PCa cell, the expression level of MMP-9 (matrix metalloproteinase-9) as well as p38 (3). Further, Jiang and colelagues found that lnc-MX1-1 (MX Dynamin like GTPase 1) is upregulated in PCa cells compared to adjacent normal prostate cells using array expression profiling. By using RNAi in LNCaP and 22Rv1 cell lines, they suppressed the expression of this lncRNA and identified proliferation and invasiveness of the cells were significantly reduced. In addition, their result indicated the significant association of this lncRNA with clinical features of patients with PCa such as PSA, metastasis, Gleason score, and recurrence-free survival (17).
3.1.2. Suppressive lncRNAs in PCa
Some of the identified lncRNAs are tumor-suppressive which show reduced expression in PCa cell and decrease the proliferation and migration of PCa cells through different molecular pathways. Some main suppressive lncRNAs will be discussed as follows. Table II presents the further suppressive lncRNAs with their summary of functions. BDNF-AS (brain-derived neurotrophic factor antisense), a naturally-occurring RNA antisense against BDNF, is downregulated in cancers such as retinoblastoma and lung cancer (46, 47). It was also downregulated in PSA-positive, PSA-negative PCa cell lines as well as PCa human tissue. Further investigation revealed that increasing the expression of this lncRNA using lentivirus-mediated BDNF-AS suppressed PCa cell development, invasion, and proliferation in two aforementioned PCa cell lines. This study also suggested the overexpression of this lncRNA could be considered as a potential method for therapeutic drug for PCa (48). GAS5 (Growth Arrest Specific 5) gene encodes a snoRNA from its intron and a lncRNA from its exonic sequence. The increased level of this lncRNA promotes apoptosis and inhibits the anti-apoptotic abilities of glucocorticoids by binding to the DNA-binding domain of glucocorticoids (49).
A study by Pickard and colleagues using GAS5-encoding plasmids or GAS5 siRNAs in PCa cell lines showed that the low expression of this lncRNA is significantly associated with increased apoptosis and decreased survival rate in PCa (50). In addition, a study found that this lncRNA targets mir-103 leading to inactivation of PI3KAKT-mTOR signaling pathway, low PCa cell growth, and proliferation (51). A single study reported that IGF2-AS (insulin growth factor 2 antisense) lncRNA was downregulated in PCa cell line and human PCa tissues. Lentivirus-induced IGF2AS overexpression decreased xenograft development in vivo, invasion, and proliferation of PCa cells in vitro. Through inverse regulation of IGF2, this lncRNA acted as an epigenetic tumor suppressor in PCa (16). LncRNA FENDRR (FOXF1 Adjacent Non-Coding Developmental Regulatory RNA) is located at 16q24.1. This lncRNA has a fundamental role in modifying chromatin by interacting with Trithorax group/MLL protein complexes (TrxG/MLL) and polycomb repressive complex 2 (PRC2) (52, 53). Zhang and colleagues identified that this lncRNA can also act as ceRNA for miR-18a-5p which upregulates the RUNX1 expression, resulting in decreased progression of PCa cell. Their study also indicated that there was a negative correlation between this lncRNA and the prognosis of PCa (54).

3.2. LncRNAs in the discrimination of BPH from PCa
BPH, another common prostate-associated disease, is non-cancerous enlargement of prostate characterized by over-proliferation of stromal and epithelial cells of the transition zone (61). It affects About 50% of males in the age range of 51-60 yr and reaches up to 70% among the age range of 61-70 (62). The measurement of prostate-specific antigen (PSA) has been used widely for screening PCa; however, its level can be increased by BPH as well (63). Therefore, using PSA is controversially debatable (64), and finding biomarkers that can exactly determine PCa from BPH would help clinicians to use proper therapeutic methods for these diseases. Several studies reported that because of tissue-specific expression pattern of lncRNAs, they could be considered as biomarkers to efficiently discriminate BPH from PCa. A study by Bayat and co-authors investigating four lncRNAs, Prcat17.3, Prcat38, Prcat47, and Cat2184.4, revealed low expression of lncRNA Cat2184.4 in PCa samples compared to BPH ones. In contrast, lncRNAs PRCAT17.3 and PRCAT38 showed significant upregulation in PCa samples compared to BPH ones. In addition, lncRNA PRCAT47 showed increased expression in PCa but not statistically significant compared to BPH samples. They concluded that this lncRNA can be considered as potential biomarkers to differentiate BPH from PCa (65).
Another study investigated the expression pattern of exosomal circulating lncRNAs in PCa, BPH and normal samples (66). It is worth mentioning that exosome is vesicle with 40-100 nm diameter, which has fundamental roles in the cell-to-cell communication and cell signaling by binding to their receptor on cells. Furthermore, they can carry a wide variety of molecules such as proteins, miRNAs, and lncRNAs. This structure keeps aforementioned molecules safe from degradation (67). This study revealed that exosomal lncRNA SAP30L-AS1 (SAP30L Antisense RNA 1 (Head To Head)) were significantly upregulated in BPH. However, their result showed that another exosomal lncRNA SChLAP1 (SWI/SNF Complex Antagonist Associated with Prostate Cancer 1) were upregulated in PCa samples compared to BPH and normal ones. The latter one is more useful as biomarker for discriminating BPH from PCa when PSA concentration is in a gray zone (66). lncRNA-p21 is another exosomal lncRNA which its expression pattern had been investigated in urine samples from patients with BPH and PCa. It is found in high level in urine samples of patients with PCa compared to samples from BPH patients (68).
3.3. Prostatitis
Prostatitis is inflammation of the prostate gland that has important roles in the male reproductive system. Although it is amendable, its main pathophysiology and treatment in male infertility are still unknown. It also can affect the assisted reproduction; therefore, finding the main pathogenesis of prostatitis and its negative impact on sperm quantity and quality including apoptosis and DNA integrity is indispensable (69). A recent study by Xu and colleagues examined the association of lncRNA GAS5 (growth arrest-specific transcript 5), located at 1q25.1, with chronic non-bacterial prostatitis (CNP). Their study revealed that the expression of this lncRNA was decreased in prostatitis tissues. In addition, they found that this lncRNA prevented cell proliferation in prostatitis by downregulation of COX2 expression, an enzyme producing prostaglandins. Their study in the GAS5-overexpressed CNP rat model showed the overexpression of this lncRNA decreased prostate volume, inflammatory cells count, and locomotion score. Therefore, the overexpression of lncRNA can reduce the injury of CNP in vivo (4).
3.4. Testicular cancer
Although TCa is generally a rare form of cancer, with the annual incidence of approximately 1%, it is the most prevalent cancer among men with the age range of 14 to 44 years. Testicular germ cell tumors (TGCT) represent nearly 98% of TCa, while the remaining comprise stromal tumors including Sertoli cell tumors, Leydig cell tumors, and other more poorly defined histologic types (70, 71). Newly identified TC are responsive to treatment; however, therapeutic outcomes of theses tumors in high stages are not sufficient (72, 73). Therefore, finding molecular pathways and their regulatory elements involved in the progression and growth of TCa cells such as lncRNA would lead to the identification of the best treatment for TCa. A recent study used NCCIT cell lines (Human testicular embryonic carcinoma cells), a pluripotent extragonadal germ cell tumor cell line, to determine the effect of lncRNA Gm2044 on the growth and proliferation of TCa cells. It revealed that the overexpression of this lncRNA prohibited cell proliferation in vitro. In addition, its results showed this effect was mediated by the miR-202-Rbfox2 pathway (74). LncRNA OIP5-AS1 (Opa-interacting protein 5 antisense RNA 1), located at 15q15.1, is another lncRNA that was shown to be overexpressed and promote cell proliferation in many cancers (75). This lncRNA is highly overexpressed in TGCT compared to other many cancers such as thyroid, prostate, pheochromocytoma, and paraganglioma (76). Based on the study by Rezaie and co-authors lncRNA LINC-ROR (Long Intergenic Non-Protein Coding RNA, Regulator Of Reprogramming) was highly expressed in testicular tumor tissues compared to control cell lines (NT2) (77). This lncRNA is located at 18q21 and is involved in embryonic stem cells (ESC) maintenance (78). Moreover, this lncRNA interacts with heterogeneous nuclear ribonucleoprotein I and suppresses p53 activated by DNA damage (79).
3.5. Varicocele
Varicocele, a main abnormality contributing to male infertility, is an abnormal dilation of pampiniform venous plexus in the scrotum and is prevalent among 20% of adult and adolescent male (80). It causes increased oxidative stress, apoptosis, venous pressure, and temperature resulting in testicular and sperm damage (81). Although many genetics factors such as chromosome alteration and epigenetic changes have been identified to be associated with Varicocele, however, its main molecular mechanism is still unknown (82). lncRNAs as a functional modulator of biological processes may have important roles in the pathogenesis of the disease. The lncRNA gadd7 (growth arrested DNA-damage inducible gene 7), a 754‐nt polyadenylated lncRNA, is overexpressed after DNA damage and growth arrest signal. Its overexpression is associated with suppressed cell growth (83, 84). Furthermore, it is the modulator of endoplasmic reticulum stress and lipid-induced oxidative (85). A single study analyzed the expression level of this lncRNA in the ejaculated spermatozoa of patients with Varicocele and found that the expression level lncRNA gadd7 is negatively associated with sperm count. It also used mouse germ cell lines GC-1 and GC-2 transfected with either pcDNA3.1-gadd7 or negative control plasmid to investigate the effect of this lncRNA overexpression on cell features. Its result indicated the overexpression of this lncRNA suppressed cell proliferation and increased cell apoptosis. In addition, the study showed that gadd7 induced by stress can cause cell death through upregulation of Bax and downregulation of Bcl2, which are pro-apoptotic and anti-apoptotic regulators, respectively (86).
3.6. Sperm abnormalities
Male infertility can also be ascribed to uniform testicular maturation arrest (MA) and different types of sperm abnormalities such as oligozoospermia, non-obstructive azoospermia, AZS, and teratozoospermia (87). Spermatogenesis is a complex process regulated by different genes, proteins, and transcriptional network including ncRNAs. Based on the wide ability of lncRNA in proliferation, differentiation, and self-renewal of SSC, they have fundamental roles in spermatogenesis regulation (88). LncRNA HOTAIR (HOX Transcript Antisense Intergenic RNA), located at 12q13.13, is one of the well-studied lncRNA in many diseases and involved in chromatin regulation by binding to Polycomb repressive complex 2 (PRC2) (89). In addition, it plays a key role in epigenetic regulation by interacting with the lysine-specific demethylase 1 (LSD1), Enhancer of Zeste homolog 2 (EZH2) ,and methyltransferase specific to histone 3 lysine 27 (90). Zhang and colleagues investigated the expression of this lncRNA in the samples from patients with AZS and oligoasthenozoospermia, and found it had a low expression in AZS and oligoasthenozoospermia compared to normal samples. Furthermore, their results indicated that the low expression of HOTAIR is associated with low expression NRF2 (Nuclear factor erythroid 2-related factor 2) gene. HOTAIR is responsible for histone H4 acetylation in the NRF2 gene promoter, which leads to its activation (91). Since the expression level of NRF2 is associated with sperm quality and antioxidant gene expression (92), HOTAIR may protect spermatozoa against antioxidant activity (91). Another study analyzed the expression profile of lncRNA in AZS and normal sperm samples. The gene ontology and pathway analysis revealed that differentially expressed lncRNA in AZS and normal samples were related to sperm function and spermatogenesis. Moreover, among all identified differentially expressed lncRNAs, the expression level of three lncRNA including lnc32058, lnc09522, and lnc98487 were significantly correlated with sperm motility (93).
4. Conclusion
In conclusion, this review emphasizes the identified lncRNAs and their functions in male reproductive disorders including PCa, BPH, prostatitis, TCa, varicocele, and sperm abnormalities. With the advent of state-of-the-art molecular and genomics techniques such as high-throughput sequencing, thousands of lncRNA have been identified in susceptibility to different diseases. However, their main mechanisms and molecular pathways are still unknown and need more functional and in vitro studies. Identification of differentially expressed lncRNA in each disease can pave the way toward developing unique biomarker, approaches to treat diseases, as well as increase efficiency of assisted reproductive technologies. In addition, lncRNA can be considered as precious indicator of sperm quality.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Review Article |
Subject:
Reproductive Genetics
References
1. Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol 2015; 13: 37. [DOI:10.1186/s12958-015-0032-1] [PMID] [PMCID]
2. Cooper TG, Noonan E, Von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010; 16: 231-245.
https://doi.org/10.1093/humupd/dmp048 [DOI:10.1093/humupd/dmq020] [PMID]
3. Liu J, Cheng G, Yang H, Deng X, Qin Ch, Hua L, et al. Reciprocal regulation of long noncoding RNAs THBS4-003 and THBS4 control migration and invasion in prostate cancer cell lines. Mol Med Rep 2016; 14: 1451-1458. [DOI:10.3892/mmr.2016.5443] [PMID] [PMCID]
4. Xu X, Hou J, Lv J, Huang Y, Pu J, Wang L. Overexpression of lncRNA GAS5 suppresses prostatic epithelial cell proliferation by regulating COX-2 in chronic non-bacterial prostatitis. Cell Cycle 2019; 18: 923-931. [DOI:10.1080/15384101.2019.1593644] [PMID] [PMCID]
5. Khademi Bami M, Dehghan Tezerjani M, Montazeri F, Ashrafzadeh Mehrjardi HR, Ghasemi-Esmailabad S, Sheikhha MH, et al. Tumor necrosis factor alpha-308 G/A single nucleotide polymorphism and risk of sperm abnormalities in Iranian males. Int J Fertil Steril 2017; 11: 112-116.
6. Ashrafzadeh HR, Nazari T, Dehghan Tezerjani M, Khademi Bami M, Ghasemi-Esmailabad S, Ghasemi N. Frequency of TNFR1 36 A/G gene polymorphism in azoospermic infertile men: A case-control study. Int J Reprod BioMed 2017; 15: 521-526. [DOI:10.29252/ijrm.15.8.521] [PMID] [PMCID]
7. Hangauer MJ, Vaughn IW, McManus MT. Pervasive transcription of the human genome produces thousands of previously unidentified long intergenic noncoding RNAs. Plos Genet 2013; 9: e1003569. 1-13. [DOI:10.1371/journal.pgen.1003569] [PMID] [PMCID]
8. Morceau F, Chateauvieux S, Gaigneaux A, Dicato M, Diederich M. Long and short non-coding RNAs as regulators of hematopoietic differentiation. Int J Mol Sci 2013; 14: 14744-14770. [DOI:10.3390/ijms140714744] [PMID] [PMCID]
9. Kadali VN, Chandran Sh, Murthy S. Long Non-coding RNAs and their "orchestration" in cancers. Journal of Applied Biology & Biotechnology 2018; 6: 57-60. [DOI:10.7324/JABB.2018.60509]
10. Tate PH, Bird AP. Effects of DNA methylation on DNA-binding proteins and gene expression. Curr Opin Genet Dev 1993; 3: 226-231. [DOI:10.1016/0959-437X(93)90027-M]
11. Nathan D, Sterner DE, Berger SL. Histone modifications: Now summoning sumoylation. Proc Natl Acad Sci USA 2003; 100: 13118-13120. [DOI:10.1073/pnas.2436173100] [PMID] [PMCID]
12. Engreitz JM, Haines JE, Perez EM, Munson G, Chen J, Kane M, et al. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature 2016; 539: 452-455. [DOI:10.1038/nature20149] [PMID] [PMCID]
13. Evans JR, Feng FY, Chinnaiyan AM. The bright side of dark matter: lncRNAs in cancer. J Clin Invest 2016; 126: 2775-2782. [DOI:10.1172/JCI84421] [PMID] [PMCID]
14. Mukherjee A, Koli S, Reddy K. Regulatory non‐coding transcripts in spermatogenesis: shedding light on 'dark matter'. Andrology 2014; 2: 360-369. [DOI:10.1111/j.2047-2927.2014.00183.x] [PMID]
15. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394-424. [DOI:10.3322/caac.21492] [PMID]
16. Chen Q, Sun T, Wang F, Gong B, Xie W, Ma M, et al. Long noncoding RNA IGF2AS is acting as an epigenetic tumor suppressor in human prostate cancer. Urology 2019; 124: 310-318. [DOI:10.1016/j.urology.2018.11.002] [PMID]
17. Jiang CY, Gao Y, Wang XJ, Ruan Y, Bei XY, Wang XH, et al. Long non-coding RNA lnc-MX1-1 is associated with poor clinical features and promotes cellular proliferation and invasiveness in prostate cancer. Biochem Biophys Res Commun 2016; 470: 721-727. [DOI:10.1016/j.bbrc.2016.01.056] [PMID]
18. Qin X, Zhu W, Lu A, Wang G, Ye X, Weng G. Long non-coding RNA SAP30L-AS1 promotes prostate cancer growth through repressing SAP30L. Gene 2019; 690: 120-128. [DOI:10.1016/j.gene.2018.12.047] [PMID]
19. Zhao R, Sun F, Bei X, Wang X, Zhu Y, Jiang C, et al. Upregulation of the long non‐coding RNA FALEC promotes proliferation and migration of prostate cancer cell lines and predicts prognosis of PCa patients. Prostate 2017; 77: 1107-1117. [DOI:10.1002/pros.23367] [PMID]
20. Wang J, Yang X, Li R, Wang L, Gu Y, Zhao Y, et al. Long non-coding RNA MYU promotes prostate cancer proliferation by mediating the miR-184/c-Myc axis. Oncol Rep 2018; 40: 2814-2825. [DOI:10.3892/or.2018.6661] [PMID]
21. Yang X, Wang L, Li R, Zhao Y, Gu Y, Liu S, et al. The long non-coding RNA PCSEAT exhibits an oncogenic property in prostate cancer and functions as a competing endogenous RNA that associates with EZH2. Biochem Biophys Res Commun 2018; 502: 262-268. [DOI:10.1016/j.bbrc.2018.05.157] [PMID]
22. Chen Y, Gu M, Liu Ch, Wan X, Shi Q, Chen Q, et al. Long noncoding RNA FOXC2-AS1 facilitates the proliferation and progression of prostate cancer via targeting miR-1253/EZH2. Gene 2019; 686: 37-42. [DOI:10.1016/j.gene.2018.10.085] [PMID]
23. Beaver LM, Kuintzle R, Buchanan A, Wiley MW, Glasser ST, Wong CP, et al. Long noncoding RNAs and sulforaphane: a target for chemoprevention and suppression of prostate cancer. J Nutr Biochem 2017; 42: 72-83. [DOI:10.1016/j.jnutbio.2017.01.001] [PMID] [PMCID]
24. Wang J, Cheng G, Li X, Pan Y, Qin Ch, Yang H, et al. Overexpression of long non-coding RNA LOC400891 promotes tumor progression and poor prognosis in prostate cancer. Tumor Biol 2016; 37: 9603-9613. [DOI:10.1007/s13277-016-4847-y] [PMID]
25. Liu T, Chi H, Chen J, Chen Ch, Huang Y, Xi H, et al. Curcumin suppresses proliferation and in vitro invasion of human prostate cancer stem cells by ceRNA effect of miR-145 and lncRNA-ROR. Gene 2017; 631: 29-38. [DOI:10.1016/j.gene.2017.08.008] [PMID]
26. Lemos AEG, Ferreira LB, Batoreu NM, de Freitas PP, Bonamino MH, Gimba ERP. PCA3 long noncoding RNA modulates the expression of key cancer-related genes in LNCaP prostate cancer cells. Tumour Biol 2016; 37: 11339-11348. [DOI:10.1007/s13277-016-5012-3] [PMID]
27. Lee B, Mazar J, Aftab MN, Qi F, Shelley J, Li JL, et al. Long noncoding RNAs as putative biomarkers for prostate cancer detection. J Mol Diagn 2014; 16: 615-626. [DOI:10.1016/j.jmoldx.2014.06.009] [PMID] [PMCID]
28. Zhang Ch, Liu Ch, Wu J, Zheng Y, Xu H, Cheng G, et al. Upregulation of long noncoding RNA LOC440040 promotes tumor progression and predicts poor prognosis in patients with prostate cancer. Onco Targets Ther 2017; 10: 4945-4954. [DOI:10.2147/OTT.S138354] [PMID] [PMCID]
29. Su W, Xu M, Chen X, Chen N, Gong J, Nie L, et al. Long noncoding RNA ZEB1-AS1 epigenetically regulates the expressions of ZEB1 and downstream molecules in prostate cancer. Molecular Cancer 2017; 16: 142. [DOI:10.1186/s12943-017-0711-y] [PMID] [PMCID]
30. Orfanelli U, Jachetti E, Chiacchiera F, Grioni M, Brambilla P, Briganti A, et al. Antisense transcription at the TRPM2 locus as a novel prognostic marker and therapeutic target in prostate cancer. Oncogene 2015; 34: 2094-2102. [DOI:10.1038/onc.2014.144] [PMID]
31. Lavorgna G, Chiacchiera F, Briganti A, Montorsi F, Pasini D, Salonia A. Expression-profiling of apoptosis induced by ablation of the long ncRNA TRPM2-AS in prostate cancer cell. Genom Data 2015; 3: 4-5. [DOI:10.1016/j.gdata.2014.10.020] [PMID] [PMCID]
32. Prensner JR, Iyer MK, Balbin OA, Dhanasekaran SM, Cao Q, Brenner JC, et al. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol 2011; 29: 742-749. [DOI:10.1038/nbt.1914] [PMID] [PMCID]
33. Prensner JR, Chen W, Iyer MK, Cao Q, Ma T, Han S, et al. PCAT-1, a long noncoding RNA, regulates BRCA2 and controls homologous recombination in cancer. Cancer Res 2014; 74: 1651-1660. [DOI:10.1158/0008-5472.CAN-13-3159] [PMID] [PMCID]
34. Prensner JR, Chen W, Han S, Iyer MK, Cao Q, Kothari V, et al. The long non-coding RNA PCAT-1 promotes prostate cancer cell proliferation through cMyc. Neoplasia 2014; 16: 900-908. [DOI:10.1016/j.neo.2014.09.001] [PMID] [PMCID]
35. Ylip A, Kivinummi K, Kohvakka A, Annala M, Latonen L, Scaravilli M, et al. Transcriptome sequencing reveals PCAT5 as a novel ERG-regulated long noncoding RNA in prostate cancer. Cancer Res 2015; 75: 4026-4031. [DOI:10.1158/0008-5472.CAN-15-0217] [PMID]
36. Misawa A, Takayama KI, Fujimura T, Homma Y, Suzuki Y, Inoue S. Androgen‐induced lncRNA POTEF‐AS1 regulates apoptosis‐related pathway to facilitate cell survival in prostate cancer cells. Cancer Sci 2017; 108: 373-379. [DOI:10.1111/cas.13151] [PMID] [PMCID]
37. Gu P, Chen X, Xie R, Han J, Xie W, Wang B, et al. lncRNA HOXD-AS1 regulates proliferation and chemo-resistance of castration-resistant prostate cancer via recruiting WDR5. Mol Ther 2017; 25: 1959-1973. [DOI:10.1016/j.ymthe.2017.04.016] [PMID] [PMCID]
38. Li J, Zhang Zh, Xiong L, Guo Ch, Jiang T, Zeng L, et al. SNHG1 lncRNA negatively regulates miR-199a-3p to enhance CDK7 expression and promote cell proliferation in prostate cancer. Biochem Biophys Res Commun 2017; 487: 146-152. [DOI:10.1016/j.bbrc.2017.03.169] [PMID]
39. Misawa A, Takayama KI, Urano T, Inoue S. Androgen-induced long noncoding RNA (lncRNA) SOCS2-AS1 promotes cell growth and inhibits apoptosis in prostate cancer cells. J Biol Chem 2016; 291: 17861-17880. [DOI:10.1074/jbc.M116.718536] [PMID] [PMCID]
40. Takayama KI, Horie‐Inoue K, Katayama Sh, Suzuki T, Tsutsumi Sh, Ikeda K, et al. Androgen‐responsive long noncoding RNA CTBP1‐AS promotes prostate cancer. EMBO J 2013; 32: 1665-1680. [DOI:10.1038/emboj.2013.99] [PMID] [PMCID]
41. Liu HT, Fang L, Cheng YX, Sun Q. LncRNA PVT1 regulates prostate cancer cell growth by inducing the methylation of miR‐146a. Cancer Med 2016; 5: 3512-3519. [DOI:10.1002/cam4.900] [PMID] [PMCID]
42. Yang J, Li C, Mudd A, Gu X. LncRNA PVT1 predicts prognosis and regulates tumor growth in prostate cancer. Biosci Biotechnol Biochem 2017; 81: 2301-2306. [DOI:10.1080/09168451.2017.1387048] [PMID]
43. Zhu Y, Ren Sh, Jing T, Cai X, Liu Y, Wang F, et al. Clinical utility of a novel urine-based gene fusion TTTY15-USP9Y in predicting prostate biopsy outcome. Urol Oncol 2015; 33: 384. e9-e20. [DOI:10.1016/j.urolonc.2015.01.019] [PMID]
44. Ren Sh, Peng Zh, Mao JH, Yu Y, Yin Ch, Gao X, et al. RNA-seq analysis of prostate cancer in the Chinese population identifies recurrent gene fusions, cancer-associated long noncoding RNAs and aberrant alternative splicings. Cell Res 2012; 22: 806-821. [DOI:10.1038/cr.2012.30] [PMID] [PMCID]
45. Yao J, Kong D, Ye C, Chen R, Li L, Zeng T, et al. The long noncoding RNA TTTY15, which is located on the Y chromosome, promotes prostate cancer progression by sponging let-7. Eur Urol 2019; 76: 315-326. [DOI:10.1016/j.eururo.2018.11.012] [PMID]
46. Shang W, Yang Y, Zhang J, Wu Q. Long noncoding RNA BDNF-AS is a potential biomarker and regulates cancer development in human retinoblastoma. Biochem Biophys Res Commun 2018; 497: 1142-1148. [DOI:10.1016/j.bbrc.2017.01.134] [PMID]
47. Shen M, Xu Zh, Jiang K, Xu W, Chen Y, Xu ZH. Long noncoding nature brain-derived neurotrophic factor antisense is associated with poor prognosis and functional regulation in non-small cell lung caner. Tumour Biol 2017; 39: 1010428317695948. 1-9. [DOI:10.1177/1010428317695948] [PMID]
48. Li W, Dou Zh, We Sh, Zhu Zh, Pan D, Jia Zh, et al. Long noncoding RNA BDNF-AS is associated with clinical outcomes and has functional role in human prostate cancer. Biomed Pharmacother 2018; 102: 1105-1110. [DOI:10.1016/j.biopha.2018.03.118] [PMID]
49. Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP. Noncoding RNA gas5 is a growth arrest-and starvation-associated repressor of the glucocorticoid receptor. Sci Signal 2010; 3: ra8. [DOI:10.1126/scisignal.2000568] [PMID] [PMCID]
50. Pickard MR, Mourtada-Maarabouni M, Williams GT. Long non-coding RNA GAS5 regulates apoptosis in prostate cancer cell lines. Biochim Biophys Acta 2013; 1832: 1613-1623. [DOI:10.1016/j.bbadis.2013.05.005] [PMID]
51. Xue D, Zhou C, Lu H, Xu R, Xu X, He X. LncRNA GAS5 inhibits proliferation and progression of prostate cancer by targeting miR-103 through AKT/mTOR signaling pathway. Tumour Biol 2016; 37: 16187-16197. [DOI:10.1007/s13277-016-5429-8] [PMID]
52. Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell 2007; 128: 735-745. [DOI:10.1016/j.cell.2007.02.009] [PMID]
53. Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Morales DR, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci USA 2009; 106: 11667-11672. [DOI:10.1073/pnas.0904715106] [PMID] [PMCID]
54. Zhang G, Han G, Zhang X, Yu Q, Li Z, Li Zh, et al. Long non-coding RNA FENDRR reduces prostate cancer malignancy by competitively binding miR-18a-5p with RUNX1. Biomarkers 2018; 23: 435-445. [DOI:10.1080/1354750X.2018.1443509] [PMID]
55. Sakurai K, Reon BJ, Anaya J, Dutta A. The lncRNA DRAIC/PCAT29 locus constitutes a tumor-suppressive nexus. Mol Cancer Res 2015; 13: 828-838. [DOI:10.1158/1541-7786.MCR-15-0016-T] [PMID] [PMCID]
56. Li JB, Liu F, Zhang BP, Bai WK, Cheng W, Zhang YH, et al. LncRNA625 modulates prostate cancer cells proliferation and apoptosis through regulating the Wnt/β-catenin pathway by targeting miR-432. Eur Rev Med Pharmacol Sci 2017; 21: 2586-2595.
57. Das M, Renganathan A, Dighe SN, Bhaduri U, Shettar A, Mukherjee G, et al. DDX5/p68 associated lncRNA LOC284454 is differentially expressed in human cancers and modulates gene expression. RNA Biol 2018; 15: 214-230. [DOI:10.1080/15476286.2017.1397261] [PMID] [PMCID]
58. Lingadahalli Sh, Jadhao S, Sung YY, Chen M, Hu L, Chen X, et al. Novel lncRNA LINC00844 regulates prostate cancer cell migration and invasion through AR signaling. Mol Cancer Res 2018; 16: 1865-1878. [DOI:10.1158/1541-7786.MCR-18-0087] [PMID]
59. White NM, Zhao SG, Zhang J, Rozycki EB, Dang HX, McFadden SD, et al. Multi-institutional analysis shows that low PCAT-14 expression associates with poor outcomes in prostate cancer. Eur Urol 2017; 71: 257-266. [DOI:10.1016/j.eururo.2016.07.012] [PMID]
60. Luo G, Wang M, Wu X, Tao D, Xiao X, Wang L, et al. Long non-coding RNA MEG3 inhibits cell proliferation and induces apoptosis in prostate cancer. Cell Physiol Biochem 2015; 37: 2209-2220. [DOI:10.1159/000438577] [PMID]
61. Descazeaud A, Rubin MA, Hofer M, Setlur S, Nikolaief N, Vacherot F, et al. BPH gene expression profile associated to prostate gland volume. Diagn Mol Pathol 2008; 17: 207-213. [DOI:10.1097/PDM.0b013e31816f6352] [PMID] [PMCID]
62. McVary KT. BPH: epidemiology and comorbidities. Am J Manag Care 2006; 12: S122-S128.
63. Armitage TG, Cooper EH, Newling DW, Robinson MR, Appleyard I. The value of the measurement of serum prostate specific antigen in patients with benign prostatic hyperplasia and untreated prostate cancer. Br J Urol 1988; 62: 584-589. [DOI:10.1111/j.1464-410X.1988.tb04431.x] [PMID]
64. Bokhorst LP, Bangma ChH, van Leenders GJ, Lous JJ, Moss SM, Schröder FH, et al. Prostate-specific antigen-based prostate cancer screening: Reduction of prostate cancer mortality after correction for nonattendance and contamination in the rotterdam section of the European randomized study of screening for prostate cancer. Eur Urol 2014; 65: 329-336. [DOI:10.1016/j.eururo.2013.08.005] [PMID]
65. Bayat H, Narouie B, Ziaee SAM, Mowla SJ. Two long non‐coding RNAs, Prcat17. 3 and Prcat38, could efficiently discriminate benign prostate hyperplasia from prostate cancer. Prostate 2018; 78: 812-818. [DOI:10.1002/pros.23538] [PMID]
66. Wang YH, Ji J, Wang BC, Chen H, Yang ZhH, Wang K, et al. Tumor-derived exosomal long noncoding RNAs as promising diagnostic biomarkers for prostate cancer. Cell Physiol Biochem 2018; 46: 532-545. [DOI:10.1159/000488620] [PMID]
67. Lässer C. Identification and analysis of circulating exosomal microRNA in human body fluids. Methods Mol Biol 2013; 1024: 109-128. [DOI:10.1007/978-1-62703-453-1_9] [PMID]
68. Işin M, Uysaler E, Özgür E, Köseoğlu H, Şanlı Ö, Yücel ÖB, et al. Exosomal lncRNA-p21 levels may help to distinguish prostate cancer from benign disease. Front Genet 2015; 6: 168. [DOI:10.3389/fgene.2015.00168] [PMID] [PMCID]
69. Alshahrani S, McGill J, Agarwal A. Prostatitis and male infertility. J Reprod Immunol 2013; 100: 30-36. [DOI:10.1016/j.jri.2013.05.004] [PMID]
70. McGlynn KA, Cook MB. Etiologic factors in testicular germ-cell tumors. Future Oncol 2009; 5: 1389-1402. [DOI:10.2217/fon.09.116] [PMID] [PMCID]
71. De Toni L, Šabovic I, Cosci I, Ghezzi M, Foresta C, Garolla A. Testicular cancer: Genes, environment, hormones. Front Endocrinol 2019; 10: 408. [DOI:10.3389/fendo.2019.00408] [PMID] [PMCID]
72. van de Geijn GJM, Hersmus R, Looijenga LH. Recent developments in testicular germ cell tumor research. Birth Defects Res C 2009; 87: 96-113. [DOI:10.1002/bdrc.20140] [PMID]
73. Cost NG, Adibi M, Lubahn JD, Romman A, Raj GV, Sagalowsky AI, et al. Effect of testicular germ cell tumor therapy on renal function. Urology 2012; 80: 641-648. [DOI:10.1016/j.urology.2012.04.064] [PMID]
74. Liang M, Hu CK, He Ch, Zhou J, Liao Y. Upregulated lncRNA Gm2044 inhibits male germ cell development by acting as miR-202 host gene. Anim Cells Syst 2019; 23: 128-134. [DOI:10.1080/19768354.2019.1591506] [PMID] [PMCID]
75. Sun WL, Kang T, Wang YY, Sun JP, Li Ch, Liu HJ, et al. Long noncoding RNA OIP5-AS1 targets Wnt-7b to affect glioma progression via modulation of miR-410. Biosci Rep 2019; 39: BSR20180395. 1-11. [DOI:10.1042/BSR20180395] [PMID] [PMCID]
76. Arunkumar G, Anand Sh, Raksha P, Dhamodharan Sh, Rao HPS, Subbiah Sh, et al. LncRNA OIP5-AS1 is overexpressed in undifferentiated oral tumors and integrated analysis identifies AS a downstream effector of stemness-associated transcription factors. Sci Rep 2018; 8: 7018. 1-13. [DOI:10.1038/s41598-018-25451-3] [PMID] [PMCID]
77. Rezaei M, Emadi-Baygi M, Hoffmann MJ, Schulz WA, Nikpour P. Altered expression of LINC-ROR in cancer cell lines and tissues. Tumor Biol 2016; 37: 1763-1769. [DOI:10.1007/s13277-015-3933-x] [PMID]
78. Wang Y, Xu Zh, Jiang J, Xu Ch, Kang J, Xiao L, et al. Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev Cell 2013; 25: 69-80. [DOI:10.1016/j.devcel.2013.03.002] [PMID]
79. Zhang A, Zhou N, Huang J, Liu Q, Fukuda K, Ma D, et al. The human long non-coding RNA-RoR is a p53 repressor in response to DNA damage. Cell Res 2013; 23: 340-350. [DOI:10.1038/cr.2012.164] [PMID] [PMCID]
80. Weidner W, Pilatz A, Altinkilic B. Andrology: varicocele: an update. Urologe A 2010; 49 (Suppl.): 163-165. [DOI:10.1007/s00120-010-2374-9] [PMID]
81. Fretz PC, Sandlow JI. Varicocele: current concepts in pathophysiology, diagnosis, and treatment. Urol Clin North Am 2002; 29: 921-937. [DOI:10.1016/S0094-0143(02)00075-7]
82. Santana VP, Miranda-Furtado CL, de Oliveira-Gennaro FG, Dos Reis RM. Genetics and epigenetics of varicocele pathophysiology: An overview. J Assist Reprod Genet 2017; 34: 839-847. [DOI:10.1007/s10815-017-0931-5] [PMID] [PMCID]
83. Hollander MC, Alamo I, Fornace Jr AJ. A novel DNA damage-inducible transcript, gadd7, inhibits cell growth, but lacks a protein product. Nucleic Acids Res 1996; 24: 1589-1593. [DOI:10.1093/nar/24.9.1589] [PMID] [PMCID]
84. Fornace AJ, Alamo I, Hollander MC. DNA damage-inducible transcripts in mammalian cells. Proc Natl Acad Sci USA 1988; 85: 8800-8804. [DOI:10.1073/pnas.85.23.8800] [PMID] [PMCID]
85. Brookheart RT, Michel CI, Listenberger LL, Ory DS, Schaffer JE. The non-coding RNA gadd7 is a regulator of lipid-induced oxidative and endoplasmic reticulum stress. J Biol Chem 2009; 284: 7446-7454. [DOI:10.1074/jbc.M806209200] [PMID] [PMCID]
86. Zhao J, Li H, Deng H, Zhu L, Zhou B, Yang M, et al. LncRNA gadd7, increased in varicocele patients, suppresses cell proliferation and promotes cell apoptosis. Oncotarget 2018; 9: 5105-5110. [DOI:10.18632/oncotarget.23696] [PMID] [PMCID]
87. Hung AJ, King P, Schlegel PN. Uniform testicular maturation arrest: a unique subset of men with nonobstructive azoospermia. J Urol 2007; 178: 608-612. [DOI:10.1016/j.juro.2007.03.125] [PMID]
88. Zhang X, Gao F, Fu J, Zhang P, Wang Y, Zeng X. Systematic identification and characterization of long non-coding RNAs in mouse mature sperm. PLoS One 2017; 12: e0173402. [DOI:10.1371/journal.pone.0173402] [PMID] [PMCID]
89. Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010; 464: 1071-1076. [DOI:10.1038/nature08975] [PMID] [PMCID]
90. Tsai MCh, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science 2010; 329: 689-693. [DOI:10.1126/science.1192002] [PMID] [PMCID]
91. Zhang L, Liu Zh, Li X, Zhang P, Wang J, Zhu D, et al. Low long non-coding RNA HOTAIR expression is associated with down-regulation of Nrf2 in the spermatozoa of patients with asthenozoospermia or oligoasthenozoospermia. Int J Clin Exp Pathol 2015; 8: 14198-14205.
92. Chen K, Mai Z, Zhou Y, Gao X, Yu B. Low NRF2 mRNA expression in spermatozoa from men with low sperm motility. Tohoku J Exp Med 2012; 228: 259-266.
https://doi.org/10.1620/tjem.226.259 [DOI:10.1620/tjem.228.259]
93. Zhang X, Zhang P, Song D, Xiong S, Zhang H, Fu J, et al. Expression profiles and characteristics of human lncRNA in normal and asthenozoospermia sperm. Biol Reprod 2018; 100: 982-993. [DOI:10.1093/biolre/ioy253] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








