Mon, Feb 23, 2026
[Archive]
Volume 19, Issue 6 (June 2021)
IJRM 2021, 19(6): 493-504 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Asadi A, Ghahremani R, Abdolmaleki A, Rajaei F. Role of sperm apoptosis and oxidative stress in male infertility: A narrative review. IJRM 2021; 19 (6) :493-504
URL: http://ijrm.ir/article-1-1787-en.html
URL: http://ijrm.ir/article-1-1787-en.html
1- Department of Biology, Faculty of Science, University of Mohaghegh Ardabili, Ardabil, Iran.
2- Department of Engineering Sciences, Faculty of Advanced Technologies, University of Mohaghegh Ardabili, Namin, Iran. BioScience and Biotechnology Research Center (BBRC), Sabalan University of Advanced Technologies (SUAT), Namin, Iran. ,Abdolmalekiarash1364@gmail.com
3- Cellular and Molecular Research Center, Qazvin University of Medical Sciences, Qazvin, Iran.
2- Department of Engineering Sciences, Faculty of Advanced Technologies, University of Mohaghegh Ardabili, Namin, Iran. BioScience and Biotechnology Research Center (BBRC), Sabalan University of Advanced Technologies (SUAT), Namin, Iran. ,
3- Cellular and Molecular Research Center, Qazvin University of Medical Sciences, Qazvin, Iran.
Full-Text [PDF 1214 kb]
(2110 Downloads)
| Abstract (HTML) (3411 Views)
Full-Text: (776 Views)
- Introduction
Infertility is an important medical and behavioral issue that affects many people. Apoptosis is an important physiological mechanism which has been shown to play significant roles in various physiological processes. Apoptosis, which is characterized by chromatin disintegration during programmed cell death, is a distinct mechanism that leads to DNA fragmentation. This biological process is critical for proper germ cell formation and the maintenance of the germ cell-Sertoli ratio in the testis (1, 2). In the 1940s, reactive oxygen species (ROS) were reported as a potential contributor to male infertility (3). Oxidative stress (OS) leads to defect sperm operation. Recent studies have shown that ROS can be a contribute factor in 30-80% of infertile males (4). OS is caused by an imbalance in the physiology of body between antioxidants and ROS (5). The major causes of DNA damage are aberrant apoptosis and OS. DNA fragmentation is a common result of ROS-mediated damage, and it's most commonly seen in infertile men's spermatozoa. Direct or indirect ROS-mediated damage can result in single or double-stranded fragments and abnormal apoptosis (1). Semen analysis is an important first step in the laboratory assessment of an infertile male (6). It includes evaluation of the volume of ejaculates and sperm quantity, motility, and shape by the World Health Organization criteria (7). The effects of male elderly on quality of semen, DNA breakage, and chromosomal abnormalities have been studied since 1970 have been reported in infertile patients and fertile donors but the results are conflicting (8). Pollutants in nature cause harmful effects on sperm motility, vitality, membrane lipid composition, and acrosome status and is related to excessive ROS production (9).
This review aimed to assimilate and summarize recent findings of apoptosis as an important physiological process in male infertility.
This review aimed to assimilate and summarize recent findings of apoptosis as an important physiological process in male infertility.
- Materials and Methods
2.1. Search strategy
This study is a narrative review and the data were retrieved from Google Scholar, PubMed, Scopus and Science Direct. Publications were searched with no particular time restriction from 1943 to 2020 to get a holistic and comprehensive view of the research done on this topic so far with the following terms: "Apoptosis", "Fertility", "Male infertility", "Mitochondria in the apoptosis", "Spermatogenesis", "Apoptosis in a male germ cell", "Apoptosis pathways", "Hormones, and germ cell apoptosis", "Sperm morphology and ROS production"," Sperm apoptosis", "Sperm DNA integrity", and "DNA fragmentation Apoptosis, and semen quality".
2.2. Study selection
The study was done in three steps: first, the titles of the papers were searched according to the selected terms, and appropriate titles were selected for the next step. Second, abstracts were reviewed and eligible papers were selected. At the last step, full-texts of the eligible papers were evaluated. In total, 1,253 papers were evaluated, of which, 1,168 were excluded because of no consistency with the study goals or no new important data. Finally, 85 papers were included in this review. Also, 30 papers were omitted because of the language of the papers (such as Turkish or Arabic).
3. Results
3.1. Spermatogenesis
Spermatogenesis is one of the most active self-renewal processes in the body: The time taken to complete the cycle is unique and unalterable for any mammalian species. Since the process is supported by somatic Sertoli cells, cell-cell interaction between the germ and Sertoli cells is generally thought to control the duration of cell cycles and cell organization 10. Spermatogenesis is divided into three stages: (i): spermatogonia duplication and distinction; (ii) meiosis, and (iii) spermiogenesis, a complex mechanism which transforms round spermatids after meiosis into a complex structure called the spermatozoon. In humans, the spermatogenesis process begins at the time of puberty and continues throughout a men’s lifetime 13. During this process, an early wave of apoptosis that follows the first round of spermatogenesis in the testes will eliminate excess spermatogonia 14. Disorder in apoptosis results in a phenotype of male infertility due to an imbalance in germ- and Sertoli cell numbers 15. Later during the life, apoptosis plays a role in the elimination of germ cells that are damaged by exposure to environmental toxicants, chemotherapy drugs, or heat 16. Almost 75% of the spermatogonia die in the process of apoptosis before maturity 17. Therefore, apoptosis plays a significant role in controlling the spermatogenesis of different species of mammals, including humans 19. High apoptosis levels have been reported in infertile men testicular biopsies 20. Although spermatogenesis is affected by the hypothalamic-pituitary-testicular axis, exogenous factors such as infections, exposure to heavy metals, smoking of cigarettes, irradiation, chemicals/herbs, and medicines impair spermatogenesis and predispose sperm cells to harm.
3.2. Apoptosis
Apoptosis is a programmed cell death that involves the removal of genetically damaged cells (2). In the lack of special cell surface receptors, factors that can penetrate the cell straight and modulate the apoptotic cascade may cause apoptotic activation (21). Such factors include: heat shock, stressors, ROS, ultraviolet radiation, drug, synthetic peptides, and toxins (22). Nowadays, the attendance and activity of apoptotic signals in human sperm in response to different stimuli are widely accepted (23, 24). There are two distinct mechanisms for apoptosis initiation: extrinsic pathway or receptor apoptosis and apoptosis endogenous or mitochondrial (25). Specific mechanisms include the perforin-granzyme A and B pathway and P53 pathway that induces apoptosis (26, 27). The biochemical particularity of apoptosis consists of the transmission of phosphatidylserine to the plasma membrane external, caspase activation, and DNA fragmentation (28). Caspase activity is associated with sperm immaturity, low numbers, decreased motilities (29, 30), lower fertilization levels (31), and lack of plasma membrane integrity, as demonstrated by externalization of phosphatidylserine (32). Caspases are cysteine proteases which promote apoptosis in mammals (33). Apoptosis cycle is significant in the background of germ cells because both mitosis and meiosis occur in cells, and cell death may be necessary to remove cells with genetic defects during the process (34). The proportion of apoptotic sperm in infertile men's ejaculated semen samples is reported to be higher than in healthy men (35). Furthermore, during cryopreservation, infertile patients' sperm caspases become more active than healthy donors' (36). However, it is unclear whether the apoptotic markers detected in spermatozoa are remnants of an unsuccessful apoptotic cycle that occurred before to ejaculation, or if they are the product of apoptosis that occurred after ejaculation (37).
3.3. The role of mitochondria in the apoptosis process
Mitochondria are organelles that participate in the ATP synthesis, calcium signals, production of ROS, and regulation of apoptosis (21). Mitochondrial abnormalities trigger physiological disorders, including infertility (38). The mitochondria function at the heart of the apoptotic pathway by offering main factors, including those which trigger caspase activity and DNA fragmentation (39). Caspases are a family of proteases that are essential for the regulation of apoptosis (40). Cytochrome c, which is one of the main factors of apoptosis, mediates caspase 9 and caspase 3 activations, which leads to cell suicide (41). Bcl-2 is a family of regularizer proteins that play a role in the control of mitochondrial permeability and, therefore, in apoptosis regulation (2).
3.4. Apoptosis in male germ cell
3.4.1. Internal pathway of apoptosis
Primordial germ cells are sourced from the epiblast and finally migrate to the gonad. Surplus cells produced during this time are killed by apoptosis, which depends mainly on the harmony between Bcl-xL and Bax 42. The early wave of apoptosis removed in transgenic mice with overexpressing or Bclx results in the cumulation of spermatogonia and spermatocytes, further resulting in infertile animals 43. Bcl-x-knockout mice had severe defects in male germ cells during growth 44. Therefore, adult rats with two mutant Bcl-x alleles lack spermatogonia 42. Bcl-w mutant mice show almost perfect degeneration of the testicles 34. Although Bax, Bcl-w, and are the primary regulators of germ cell growth and maturity post-birth, Bcl-x is necessary for the durability of primordial germ cells in the embryonic gonad during early phases 45.
3.4.2. External pathway of apoptosis
Fas is a transmembrane molecule with 281 amino acids which are activated by FasL and intercede apoptosis. FasL and respective receptor Fas both interact, and the activated Fas induce apoptosis in the cell (46). It is widely accepted that Sertoli cells regulate the number of germ cells by one of the most common apoptotic pathways, the Fas/FasL paracrine signal transmission machine, in which the FasL expressed in Sertoli cells and Fas expressed in germ cells induce apoptosis when connecting with each other (47). Mice with a random loss of function mutation in the Fas gene (Faslpr) or FasL (Faslgld) develop apparent lymphoproliferative and autoimmune diseases. The consequence of the Fas/FasL-mediated cycle is hypospermatogenesis, such as maturity arrest and Sertoli cell syndrome. Germ cell maturity may be associated with Fas gene expression that is able to induce apoptosis to eliminate damaged germ cells (48). The expression of Fas/FasL in the human testis is regulated by gonadotropin. It is generally proven that the Fas/FasL system may have a role in the quality-control process of the manufactured gametes (34).
3.5. Hormones and germ cell apoptosis
In the mammalian testes, follicle-stimulating hormone (FSH), luteinizing hormone, gonadotropin, and testosterone regulate germ cells proliferation, differentiation, and viability 34. Luteinizing hormone helps with steroidogenesis by activating Leydig cells, but FSH activates the Sertoli cells to help with the spermatogenesis developmental stages 13. Getting exposed to excess hormones or hormone deficiency may result in cellular apoptosis in the testis 49. Sertoli cells have FSH and testosterone receptors, which are the major spermatogenesis hormonal regulators. Removal of the hormone causes apoptosis of germ cells 50. While testosterone and the synergistic activity of FSH with estradiol help germ cell survival during seminiferous tubular maturity, estradiol alone has an inhibitory effect and is an inducer of apoptosis (Figure 1) 34. Besides, excess testosterone can lead to increased expression of Fas/FasL in testis 51. Testosterone removal stimulates caspase activity and results in DNA fragmentation in Sertoli cells 52.
3.6. Sperm apoptosis
In the seminiferous epithelium, spermatogenesis is accompanied by germ cell apoptosis, a cycle that generally takes place during life. Apoptosis of germ cells is essential to retain the optimal germ cell ratio to Sertoli cells and to remove abnormal germ cells, especially during maturity. The exit of phosphatidylserine to the outer membrane of the sperm, caspases activation, and chromosome fragmentation are considered to mark apoptosis 53. Bcl-2, the apoptosis inhibitor gene, safeguards the cell by reducing the ROS generation. Although the FAS receptor frequently causes apoptosis, this fails to clean all the sperm intended for elimination, leading to a high population of defective sperm. The percentage of FAS-positive sperm can be > 50% in men with unusual sperm parameters (5).
3.7. Role of apoptosis in male infertility
In certain pathological circumstances, an enormous increase in germ cell apoptosis occurs, which involves idiopathic infertility in males 54. Apoptosis has been observed often in spermatocytes, less in spermatogonia, and rarely in spermatids 55. Fujisawa and co-workers documented the existence of apoptosis in the testes, especially in spermatocytes 56. A study by Martincic and colleagues determined the presence and abundance of germ cells apoptosis in infertile males whereas Sertoli cells do not undergo apoptosis 18. It has been shown that the Fas-system is involved in regulating the spontaneous apoptosis of germ cells. In the normal state, Sertoli cells express FasL which triggers the apoptosis in Fas-positive germ cells, and shows a paracrine interaction between germ and Sertoli cells 5758. Apoptosis increases with age in the testes, resulting in a decrease in germ cells. This may be linked with the reduction in androgen levels or a rise in OS 21. Measuring apoptosis rates can also be used as a sign forpursuing male infertility treatment 59.
3.8. The role of OS in apoptosis
OS is one of the major causes of male infertility. In recent years, the production of ROS and its influence on semen quality have been widely studied. OS occurs as a result of an imbalance between ROS production and antioxidants 160.
Mitochondrial exposure to ROS leads to the induction of apoptosis and thus causes the fragmentation of the DNA 1961. A direct association between increased sperm damage caused by ROS and high levels of cytochrome C, caspase 9, and 3 was demonstrated in the study by Soderquist and colleagues which showed positive apoptosis in infertile men. Studies in infertile men have shown that high levels of cytochrome C in plasma show remarkable mitochondrial harm by ROS 62. Increased age causes ROS to accumulate which induces lipid peroxidation. Excessive amounts of ROS and reduced antioxidant capacity during ageing can cause apoptosis or oxidative damage to DNA 63. The damaged paternal DNA, if not repaired, may through fertilization reach the couple’s offspring, causing a variety of diseases 64. In this regard, certain clinical research have found that giving antioxidants improves sperm DNA integrity. Vitamin C, vitamin E, and glutathione, when taken together for two months, dramatically reduced the levels of 8OHdG, a marker for OS-induced sperm DNA damage 65.
3.9. Effect of sperm morphology on ROS production
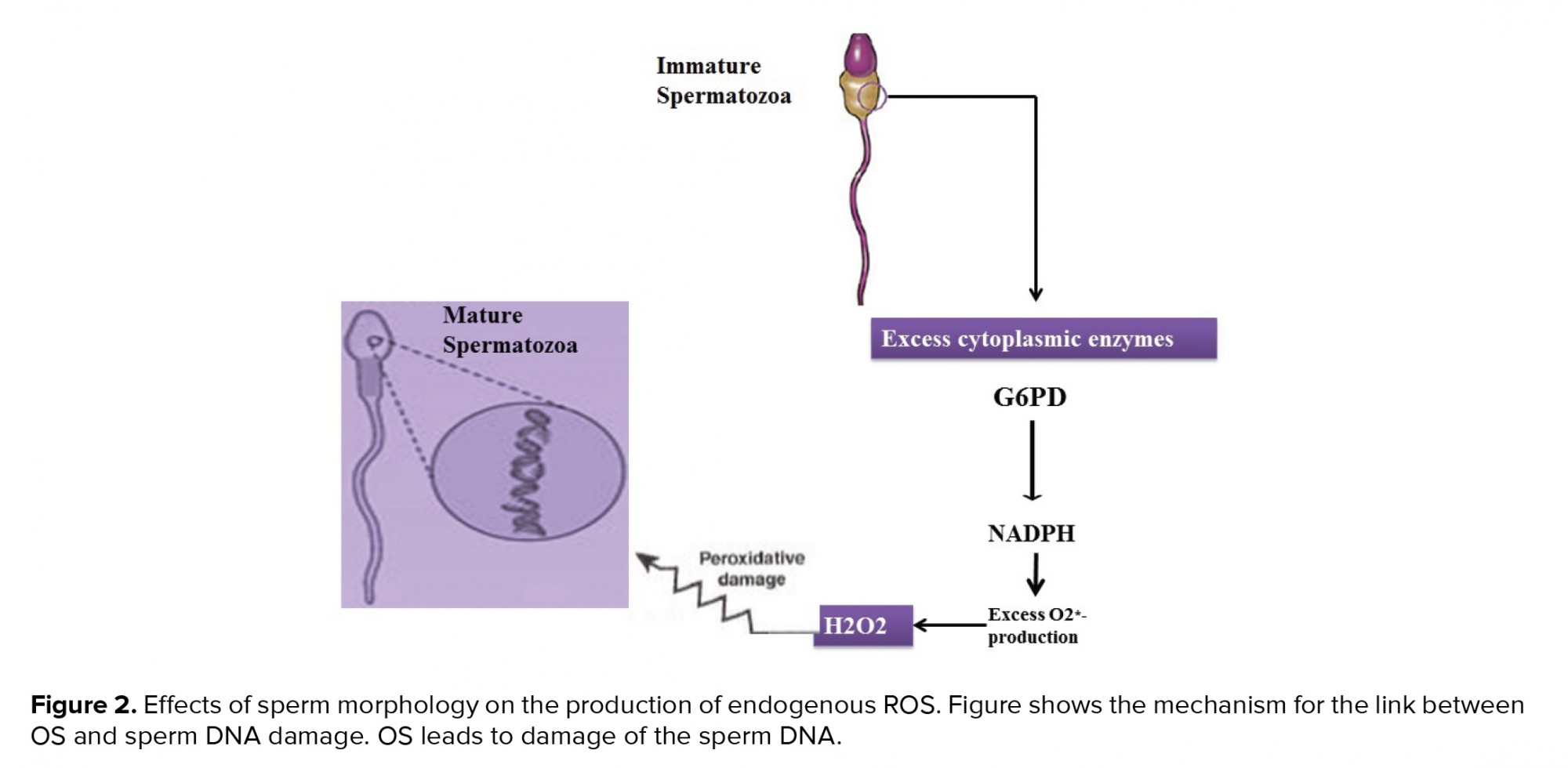
Teratozoospermia is caused by a defect in the spermatogenesis process and is determined by sperm with an excess cytoplasm 65. Remaining cytoplasm stimulates sperms to produce endogenous ROS through processes that can be mediated by the enzyme glucose-6-phosphate dehydrogenase. Teratozoospermia patients are at higher risk of pathogenic ROS, apoptosis, and DNA damage (Figure 2) 66. ROS generation is highest in immature sperm from males with abnormal semen values. Also, there is a direct relationship between the ROS generation and the spermatozoa deformation indicator, calculated by dividing the total count of abnormal sperms by the count of sperms evaluated 67.
3.10. The effect of ROS on sperm
Excessive ROS in human semen is severely correlated with male infertility 68. ROS are free radicals derived from oxygen that are required at a low level for capacitation, hyperactivation, motility, and acrosome reaction 69. Nonetheless, excessive ROS level can lead to OS and consequently cause DNA damage, lipid peroxidation, shorting of telomeres, epigenetic variations, Y chromosome microdeletions, and induction of apoptosis (Figure 3) 70. Aitken and Clarkson first observed ROS using the method of chemiluminescence in the human ejaculate 72. Also, high amounts of ROS negatively affect sperm concentration, motility, morphology, and male fertility 67. The sources of endogenous ROS are immature sperm and leukocytes, and there are different external causes 75. Genitourinary infections, varicocele, cigarette smoking, alcohol, recreational drug misuse, ionizing radiation, cell phone usage, stress, excessive exercise, spinal cord damage, parental age, and environmental pollution are all active variables in the OS production 76. Sperms are vulnerable to OS because it contains high values of polyunsaturated fatty acids that are sensitive to lipid peroxidation 70. The by-products of lipid oxidization include mutagenic molecules acrolein, malondialdehyde, and 4 hydroxy-nonenal (4-HNE), which indirectly lead to DNA damage 4. 4-HNE and acrolein lead to induction apoptosis and fragmentation of DNA 79. Malondialdehyde has been used in various biochemical studies to track the degree of sperm-related peroxidative damage 7980. More of the sperm genome (almost 85%) is linked to central nucleoproteins, which preserves it against free radical attacks 4. Therefore, the plasma membrane is the main purpose of ROS, which triggers a cascade of events that damage the genetic confirmation of sperm 69. In a study conducted by Venkatesh et al., the sperm count, sperm motility percentage, and normal sperm morphology percentage in infertile men reduced significantly in comparison with the control groups. Also, ROS levels in infertile men increased significantly in comparison with the control groups. No significant relation was observed between ROS levels and semen parameters 81.
3.11. Apoptosis as a marker of semen quality
Apoptosis of sperm was considered a potentially useful indicator of male fertility 21. Loss of germ cell, which happens via apoptosis, is a prevailing mechanism during spermatogenesis and is regulated by the expression levels of p53, p21, , FAS, and caspases 82. Several studies have reported increased apoptosis rates in samples of poor-quality semen 19. Apoptosis happens during spermatogenesis and has been proven too in ejaculated sperm 83. Studies showed the fragmentation of DNA in ejaculated sperm. OS causes damage to DNA and increased levels of oxidative damage in sperm DNA 85. The measurement of apoptosis may be an index of semen quality 21.


4. Conclusion
Apoptosis is one of the well-known mechanisms of quality control in the testis. Nevertheless, increased apoptosis may have adverse effects on sperm production, eventually compromising male fertility. Therefore, controlling the rate of apoptosis for fertility in men is of particular importance. Also, ROS and the consequent OS play a crucial role in apoptosis. ROS have been shown to cause abnormalities in semen analysis and sperm concentration. Therefore, ROS can cause infertility in men.
Acknowledgements
The authors would like to thank the research council of Mohaghegh Ardabili University for the financial support of this study.
Conflict of interest
The authors declare that they have no competing interests.
This study is a narrative review and the data were retrieved from Google Scholar, PubMed, Scopus and Science Direct. Publications were searched with no particular time restriction from 1943 to 2020 to get a holistic and comprehensive view of the research done on this topic so far with the following terms: "Apoptosis", "Fertility", "Male infertility", "Mitochondria in the apoptosis", "Spermatogenesis", "Apoptosis in a male germ cell", "Apoptosis pathways", "Hormones, and germ cell apoptosis", "Sperm morphology and ROS production"," Sperm apoptosis", "Sperm DNA integrity", and "DNA fragmentation Apoptosis, and semen quality".
2.2. Study selection
The study was done in three steps: first, the titles of the papers were searched according to the selected terms, and appropriate titles were selected for the next step. Second, abstracts were reviewed and eligible papers were selected. At the last step, full-texts of the eligible papers were evaluated. In total, 1,253 papers were evaluated, of which, 1,168 were excluded because of no consistency with the study goals or no new important data. Finally, 85 papers were included in this review. Also, 30 papers were omitted because of the language of the papers (such as Turkish or Arabic).
3. Results
3.1. Spermatogenesis
Spermatogenesis is one of the most active self-renewal processes in the body: The time taken to complete the cycle is unique and unalterable for any mammalian species. Since the process is supported by somatic Sertoli cells, cell-cell interaction between the germ and Sertoli cells is generally thought to control the duration of cell cycles and cell organization 10. Spermatogenesis is divided into three stages: (i): spermatogonia duplication and distinction; (ii) meiosis, and (iii) spermiogenesis, a complex mechanism which transforms round spermatids after meiosis into a complex structure called the spermatozoon. In humans, the spermatogenesis process begins at the time of puberty and continues throughout a men’s lifetime 13. During this process, an early wave of apoptosis that follows the first round of spermatogenesis in the testes will eliminate excess spermatogonia 14. Disorder in apoptosis results in a phenotype of male infertility due to an imbalance in germ- and Sertoli cell numbers 15. Later during the life, apoptosis plays a role in the elimination of germ cells that are damaged by exposure to environmental toxicants, chemotherapy drugs, or heat 16. Almost 75% of the spermatogonia die in the process of apoptosis before maturity 17. Therefore, apoptosis plays a significant role in controlling the spermatogenesis of different species of mammals, including humans 19. High apoptosis levels have been reported in infertile men testicular biopsies 20. Although spermatogenesis is affected by the hypothalamic-pituitary-testicular axis, exogenous factors such as infections, exposure to heavy metals, smoking of cigarettes, irradiation, chemicals/herbs, and medicines impair spermatogenesis and predispose sperm cells to harm.
3.2. Apoptosis
Apoptosis is a programmed cell death that involves the removal of genetically damaged cells (2). In the lack of special cell surface receptors, factors that can penetrate the cell straight and modulate the apoptotic cascade may cause apoptotic activation (21). Such factors include: heat shock, stressors, ROS, ultraviolet radiation, drug, synthetic peptides, and toxins (22). Nowadays, the attendance and activity of apoptotic signals in human sperm in response to different stimuli are widely accepted (23, 24). There are two distinct mechanisms for apoptosis initiation: extrinsic pathway or receptor apoptosis and apoptosis endogenous or mitochondrial (25). Specific mechanisms include the perforin-granzyme A and B pathway and P53 pathway that induces apoptosis (26, 27). The biochemical particularity of apoptosis consists of the transmission of phosphatidylserine to the plasma membrane external, caspase activation, and DNA fragmentation (28). Caspase activity is associated with sperm immaturity, low numbers, decreased motilities (29, 30), lower fertilization levels (31), and lack of plasma membrane integrity, as demonstrated by externalization of phosphatidylserine (32). Caspases are cysteine proteases which promote apoptosis in mammals (33). Apoptosis cycle is significant in the background of germ cells because both mitosis and meiosis occur in cells, and cell death may be necessary to remove cells with genetic defects during the process (34). The proportion of apoptotic sperm in infertile men's ejaculated semen samples is reported to be higher than in healthy men (35). Furthermore, during cryopreservation, infertile patients' sperm caspases become more active than healthy donors' (36). However, it is unclear whether the apoptotic markers detected in spermatozoa are remnants of an unsuccessful apoptotic cycle that occurred before to ejaculation, or if they are the product of apoptosis that occurred after ejaculation (37).
3.3. The role of mitochondria in the apoptosis process
Mitochondria are organelles that participate in the ATP synthesis, calcium signals, production of ROS, and regulation of apoptosis (21). Mitochondrial abnormalities trigger physiological disorders, including infertility (38). The mitochondria function at the heart of the apoptotic pathway by offering main factors, including those which trigger caspase activity and DNA fragmentation (39). Caspases are a family of proteases that are essential for the regulation of apoptosis (40). Cytochrome c, which is one of the main factors of apoptosis, mediates caspase 9 and caspase 3 activations, which leads to cell suicide (41). Bcl-2 is a family of regularizer proteins that play a role in the control of mitochondrial permeability and, therefore, in apoptosis regulation (2).
3.4. Apoptosis in male germ cell
3.4.1. Internal pathway of apoptosis
Primordial germ cells are sourced from the epiblast and finally migrate to the gonad. Surplus cells produced during this time are killed by apoptosis, which depends mainly on the harmony between Bcl-xL and Bax 42. The early wave of apoptosis removed in transgenic mice with overexpressing or Bclx results in the cumulation of spermatogonia and spermatocytes, further resulting in infertile animals 43. Bcl-x-knockout mice had severe defects in male germ cells during growth 44. Therefore, adult rats with two mutant Bcl-x alleles lack spermatogonia 42. Bcl-w mutant mice show almost perfect degeneration of the testicles 34. Although Bax, Bcl-w, and are the primary regulators of germ cell growth and maturity post-birth, Bcl-x is necessary for the durability of primordial germ cells in the embryonic gonad during early phases 45.
3.4.2. External pathway of apoptosis
Fas is a transmembrane molecule with 281 amino acids which are activated by FasL and intercede apoptosis. FasL and respective receptor Fas both interact, and the activated Fas induce apoptosis in the cell (46). It is widely accepted that Sertoli cells regulate the number of germ cells by one of the most common apoptotic pathways, the Fas/FasL paracrine signal transmission machine, in which the FasL expressed in Sertoli cells and Fas expressed in germ cells induce apoptosis when connecting with each other (47). Mice with a random loss of function mutation in the Fas gene (Faslpr) or FasL (Faslgld) develop apparent lymphoproliferative and autoimmune diseases. The consequence of the Fas/FasL-mediated cycle is hypospermatogenesis, such as maturity arrest and Sertoli cell syndrome. Germ cell maturity may be associated with Fas gene expression that is able to induce apoptosis to eliminate damaged germ cells (48). The expression of Fas/FasL in the human testis is regulated by gonadotropin. It is generally proven that the Fas/FasL system may have a role in the quality-control process of the manufactured gametes (34).
3.5. Hormones and germ cell apoptosis
In the mammalian testes, follicle-stimulating hormone (FSH), luteinizing hormone, gonadotropin, and testosterone regulate germ cells proliferation, differentiation, and viability 34. Luteinizing hormone helps with steroidogenesis by activating Leydig cells, but FSH activates the Sertoli cells to help with the spermatogenesis developmental stages 13. Getting exposed to excess hormones or hormone deficiency may result in cellular apoptosis in the testis 49. Sertoli cells have FSH and testosterone receptors, which are the major spermatogenesis hormonal regulators. Removal of the hormone causes apoptosis of germ cells 50. While testosterone and the synergistic activity of FSH with estradiol help germ cell survival during seminiferous tubular maturity, estradiol alone has an inhibitory effect and is an inducer of apoptosis (Figure 1) 34. Besides, excess testosterone can lead to increased expression of Fas/FasL in testis 51. Testosterone removal stimulates caspase activity and results in DNA fragmentation in Sertoli cells 52.
3.6. Sperm apoptosis
In the seminiferous epithelium, spermatogenesis is accompanied by germ cell apoptosis, a cycle that generally takes place during life. Apoptosis of germ cells is essential to retain the optimal germ cell ratio to Sertoli cells and to remove abnormal germ cells, especially during maturity. The exit of phosphatidylserine to the outer membrane of the sperm, caspases activation, and chromosome fragmentation are considered to mark apoptosis 53. Bcl-2, the apoptosis inhibitor gene, safeguards the cell by reducing the ROS generation. Although the FAS receptor frequently causes apoptosis, this fails to clean all the sperm intended for elimination, leading to a high population of defective sperm. The percentage of FAS-positive sperm can be > 50% in men with unusual sperm parameters (5).
3.7. Role of apoptosis in male infertility
In certain pathological circumstances, an enormous increase in germ cell apoptosis occurs, which involves idiopathic infertility in males 54. Apoptosis has been observed often in spermatocytes, less in spermatogonia, and rarely in spermatids 55. Fujisawa and co-workers documented the existence of apoptosis in the testes, especially in spermatocytes 56. A study by Martincic and colleagues determined the presence and abundance of germ cells apoptosis in infertile males whereas Sertoli cells do not undergo apoptosis 18. It has been shown that the Fas-system is involved in regulating the spontaneous apoptosis of germ cells. In the normal state, Sertoli cells express FasL which triggers the apoptosis in Fas-positive germ cells, and shows a paracrine interaction between germ and Sertoli cells 5758. Apoptosis increases with age in the testes, resulting in a decrease in germ cells. This may be linked with the reduction in androgen levels or a rise in OS 21. Measuring apoptosis rates can also be used as a sign forpursuing male infertility treatment 59.
3.8. The role of OS in apoptosis
OS is one of the major causes of male infertility. In recent years, the production of ROS and its influence on semen quality have been widely studied. OS occurs as a result of an imbalance between ROS production and antioxidants 160.
Mitochondrial exposure to ROS leads to the induction of apoptosis and thus causes the fragmentation of the DNA 1961. A direct association between increased sperm damage caused by ROS and high levels of cytochrome C, caspase 9, and 3 was demonstrated in the study by Soderquist and colleagues which showed positive apoptosis in infertile men. Studies in infertile men have shown that high levels of cytochrome C in plasma show remarkable mitochondrial harm by ROS 62. Increased age causes ROS to accumulate which induces lipid peroxidation. Excessive amounts of ROS and reduced antioxidant capacity during ageing can cause apoptosis or oxidative damage to DNA 63. The damaged paternal DNA, if not repaired, may through fertilization reach the couple’s offspring, causing a variety of diseases 64. In this regard, certain clinical research have found that giving antioxidants improves sperm DNA integrity. Vitamin C, vitamin E, and glutathione, when taken together for two months, dramatically reduced the levels of 8OHdG, a marker for OS-induced sperm DNA damage 65.
3.9. Effect of sperm morphology on ROS production
Teratozoospermia is caused by a defect in the spermatogenesis process and is determined by sperm with an excess cytoplasm 65. Remaining cytoplasm stimulates sperms to produce endogenous ROS through processes that can be mediated by the enzyme glucose-6-phosphate dehydrogenase. Teratozoospermia patients are at higher risk of pathogenic ROS, apoptosis, and DNA damage (Figure 2) 66. ROS generation is highest in immature sperm from males with abnormal semen values. Also, there is a direct relationship between the ROS generation and the spermatozoa deformation indicator, calculated by dividing the total count of abnormal sperms by the count of sperms evaluated 67.
3.10. The effect of ROS on sperm
Excessive ROS in human semen is severely correlated with male infertility 68. ROS are free radicals derived from oxygen that are required at a low level for capacitation, hyperactivation, motility, and acrosome reaction 69. Nonetheless, excessive ROS level can lead to OS and consequently cause DNA damage, lipid peroxidation, shorting of telomeres, epigenetic variations, Y chromosome microdeletions, and induction of apoptosis (Figure 3) 70. Aitken and Clarkson first observed ROS using the method of chemiluminescence in the human ejaculate 72. Also, high amounts of ROS negatively affect sperm concentration, motility, morphology, and male fertility 67. The sources of endogenous ROS are immature sperm and leukocytes, and there are different external causes 75. Genitourinary infections, varicocele, cigarette smoking, alcohol, recreational drug misuse, ionizing radiation, cell phone usage, stress, excessive exercise, spinal cord damage, parental age, and environmental pollution are all active variables in the OS production 76. Sperms are vulnerable to OS because it contains high values of polyunsaturated fatty acids that are sensitive to lipid peroxidation 70. The by-products of lipid oxidization include mutagenic molecules acrolein, malondialdehyde, and 4 hydroxy-nonenal (4-HNE), which indirectly lead to DNA damage 4. 4-HNE and acrolein lead to induction apoptosis and fragmentation of DNA 79. Malondialdehyde has been used in various biochemical studies to track the degree of sperm-related peroxidative damage 7980. More of the sperm genome (almost 85%) is linked to central nucleoproteins, which preserves it against free radical attacks 4. Therefore, the plasma membrane is the main purpose of ROS, which triggers a cascade of events that damage the genetic confirmation of sperm 69. In a study conducted by Venkatesh et al., the sperm count, sperm motility percentage, and normal sperm morphology percentage in infertile men reduced significantly in comparison with the control groups. Also, ROS levels in infertile men increased significantly in comparison with the control groups. No significant relation was observed between ROS levels and semen parameters 81.
3.11. Apoptosis as a marker of semen quality
Apoptosis of sperm was considered a potentially useful indicator of male fertility 21. Loss of germ cell, which happens via apoptosis, is a prevailing mechanism during spermatogenesis and is regulated by the expression levels of p53, p21, , FAS, and caspases 82. Several studies have reported increased apoptosis rates in samples of poor-quality semen 19. Apoptosis happens during spermatogenesis and has been proven too in ejaculated sperm 83. Studies showed the fragmentation of DNA in ejaculated sperm. OS causes damage to DNA and increased levels of oxidative damage in sperm DNA 85. The measurement of apoptosis may be an index of semen quality 21.



4. Conclusion
Apoptosis is one of the well-known mechanisms of quality control in the testis. Nevertheless, increased apoptosis may have adverse effects on sperm production, eventually compromising male fertility. Therefore, controlling the rate of apoptosis for fertility in men is of particular importance. Also, ROS and the consequent OS play a crucial role in apoptosis. ROS have been shown to cause abnormalities in semen analysis and sperm concentration. Therefore, ROS can cause infertility in men.
Acknowledgements
The authors would like to thank the research council of Mohaghegh Ardabili University for the financial support of this study.
Conflict of interest
The authors declare that they have no competing interests.
Type of Study: Review Article |
Subject:
Reproductive Biology
References
1. Latchoumycandane C, Vaithinathan S, D'Cruz S, Mathur PP. Apoptosis and male infertility. In: Parekattil SJ, Esteves SC, Agarwal A. Male infertility. Switzeland: Springer; 2020. 479-486. [DOI:10.1007/978-3-030-32300-4_37]
2. Bejarano I, Rodríguez AB, Pariente JA. Apoptosis is a demanding selective tool during the development of fetal male germ cells. Front Cell Dev Biol 2018; 6: 65. 1-7. [DOI:10.3389/fcell.2018.00065] [PMID] [PMCID]
3. MacLeod J. The role of oxygen in the metabolism and motility of human spermatozoa. Am J Physiol-Leg Content 1943; 138: 512-518. [DOI:10.1152/ajplegacy.1943.138.3.512]
4. Bisht Sh, Faiq M, Tolahunase M, Dada R. Oxidative stress and male infertility. Nat Rev Urol 2017; 14: 470-485. [DOI:10.1038/nrurol.2017.69] [PMID]
5. Makker K, Agarwal A, Sharma R. Oxidative stress & male infertility. Indian J Med Res 2009; 129: 357-367.
6. Sharma R, Agarwal A, Rohra VK, Assidi M, Abu-Elmagd M, Turki RF. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod Biol Endocrinol 2015; 13: 35. 1-20. [DOI:10.1186/s12958-015-0028-x] [PMID] [PMCID]
7. World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. UK: Cambridge University press; 1999.
8. Brahem S, Mehdi M, Elghezal H, Saad A. The effects of male aging on semen quality, sperm DNA fragmentation and chromosomal abnormalities in an infertile population. J Assist Reprod Genet 2011; 28: 425-432. [DOI:10.1007/s10815-011-9537-5] [PMID] [PMCID]
9. Kutluyer F, Çakir Sahilli Y, Kocabaş M, Aksu Ö. Sperm quality and oxidative stress in chub Squalius orientalis and Padanian barbel Barbus plebejus (Teleostei: Cyprinidae) after in vitro exposure to low doses of bisphenol A. Drug Chem Toxicol 2020; 7: 1-6. [DOI:10.1080/01480545.2020.1726379] [PMID]
10. Roosen-Runge EC, Holstein AF. The human rete testis. Cell Tissue Res 1978; 189: 409-433. [DOI:10.1007/BF00209130] [PMID]
11. França LR, Ogawa T, Avarbock MR, Brinster RL, Russell LD. Germ cell genotype controls cell cycle during spermatogenesis in the rat. Biol Reprod 1998; 59: 1371-1377. [DOI:10.1095/biolreprod59.6.1371] [PMID]
12. Kuchakulla M, Narasimman M, Khodamoradi K, Khosravizadeh Z, Ramasamy R. How defective spermatogenesis affects sperm DNA integrity. Andrologia 2020; 53: 13615. 1-11. [DOI:10.1111/and.13615]
13. Sharma R, Agarwal A. Defective spermatogenesis and sperm DNA damage. In: Zini A, Agarwal A. A clinician's guide to sperm DNA and chromatin damage. Switzeland: Springer; 2018. 229-261. [DOI:10.1007/978-3-319-71815-6_14]
14. Aitken RJ, Findlay JK, Hutt KJ, Kerr JB. Apoptosis in the germ line. Reproduction 2011; 141: 139-150. [DOI:10.1530/REP-10-0232] [PMID]
15. Rodriguez I, Ody C, Araki K, Garcia I, Vassalli P. An early and massive wave of germinal cell apoptosis is required for the development of functional spermatogenesis. EMBO J 1997; 16: 2262-2270. [DOI:10.1093/emboj/16.9.2262] [PMID] [PMCID]
16. Wang Ch, Cui YG, Wang XH, Jia Y, Sinha Hikim A, Lue YH, et al. Transient scrotal hyperthermia and levonorgestrel enhance testosterone-induced spermatogenesis suppression in men through increased germ cell apoptosis. J Clin Endocrinol Metab 2007; 92: 3292-3304. [DOI:10.1210/jc.2007-0367] [PMID]
17. Print CG, Loveland KL. Germ cell suicide: New insights into apoptosis during spermatogenesis. Bioessays 2000; 22: 423-430.
https://doi.org/10.1002/(SICI)1521-1878(200005)22:5<423::AID-BIES4>3.0.CO;2-0 [DOI:10.1002/(SICI)1521-1878(200005)22:53.0.CO;2-0]
18. Aitken RJ, Koppers AJ. Apoptosis and DNA damage in human spermatozoa. Asian J Androl 2011; 13: 36-42. [DOI:10.1038/aja.2010.68] [PMID] [PMCID]
19. Abdolmaleki A, Ghayour MB, Behnam-Rassouli M. Protective effects of acetyl-l-carnitine against serum and glucose deprivation-induced apoptosis in rat adipose-derived mesenchymal stem cells. Cell Tissue Bank 2020; 21: 655-666. [DOI:10.1007/s10561-020-09844-1] [PMID]
20. Cavalcanti MCO, Steilmann C, Failing K, Bergmann M, Kliesch S, Weidner W, et al. Apoptotic gene expression in potentially fertile and subfertile men. Mol Hum Reprod 2011; 17: 415-420. [DOI:10.1093/molehr/gar011] [PMID]
21. Shukla KK, Mahdi AA, Rajender S. Apoptosis, spermatogenesis and male infertility. Front Biosci 2012; 4: 746-754. [DOI:10.2741/e415]
22. Fulda S, Gorman AM, Hori O, Samali A. Cellular stress responses: Cell survival and cell death. Int J Cell Biol 2010; 2010: 214074. 1-23. [DOI:10.1155/2010/214074] [PMID] [PMCID]
23. Espino J, Mediero M, Lozano GM, Bejarano I, Ortiz Á, García JF, et al. Reduced levels of intracellular calcium releasing in spermatozoa from asthenozoospermic patients. Reprod Biol Endocrinol 2009; 7: 1-11. [DOI:10.1186/1477-7827-7-11] [PMID] [PMCID]
24. Monllor F, Espino J, Marchena AM, Ortiz A, Lozano G, García JF, et al. Melatonin diminishes oxidative damage in sperm cells, improving assisted reproductive techniques. Turk J Biol 2017; 41: 881-889. [DOI:10.3906/biy-1704-45] [PMID] [PMCID]
25. Igney FH, Krammer PH. Death and anti-death: Tumour resistance to apoptosis. Nat Rev Cancer 2002; 2: 277-288. [DOI:10.1038/nrc776] [PMID]
26. Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature 2000; 408: 307-310. [DOI:10.1038/35042675] [PMID]
27. Martinvalet D, Zhu P, Lieberman J. Granzyme A induces caspase-independent mitochondrial damage, a required first step for apoptosis. Immunity 2005; 22: 355-370. [DOI:10.1016/j.immuni.2005.02.004] [PMID]
28. Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol 1992; 148: 2207-2216.
29. Marchetti C, Jouy N, Leroy-Martin B, Defossez A, Formstecher P, Marchetti Ph. Comparison of four fluorochromes for the detection of the inner mitochondrial membrane potential in human spermatozoa and their correlation with sperm motility. Hum Reprod 2004; 19: 2267-2276. [DOI:10.1093/humrep/deh416] [PMID]
30. Lozano GM, Bejarano I, Espino J, Gonzalez D, Ortiz A, Garcia JF, et al. Relationship between caspase activity and apoptotic markers in human sperm in response to hydrogen peroxide and progesterone. J Reprod Dev 2009; 55: 615-621. [DOI:10.1262/jrd.20250] [PMID]
31. Grunewald S, Sharma R, Paasch U, Glander HJ, Agarwal A. Impact of caspase activation in human spermatozoa. Microsc Res Tech 2009; 72: 878-888. [DOI:10.1002/jemt.20732] [PMID]
32. Paasch U, Grunewald S, Agarwal A, Glandera HJ. Activation pattern of caspases in human spermatozoa. Fertil Steril 2004; 81 (Suppl.): 802-809. [DOI:10.1016/j.fertnstert.2003.09.030] [PMID]
33. Salvesen GS, Dixit VM. Caspases: Intracellular signaling by proteolysis. Cell 1997; 91: 443-446. [DOI:10.1016/S0092-8674(00)80430-4]
34. Shaha C, Tripathi R, Mishra DP. Male germ cell apoptosis: Regulation and biology. Philos Trans R Soc B Biol Sci 2010; 365: 1501-1515. [DOI:10.1098/rstb.2009.0124] [PMID] [PMCID]
35. Taylor SL, Weng SL, Fox P, Duran EH, Morshedi MS, Oehninger S, et al. Somatic cell apoptosis markers and pathways in human ejaculated sperm: Potential utility as indicators of sperm quality. Mol Hum Reprod 2004; 10: 825-834. [DOI:10.1093/molehr/gah099] [PMID]
36. Grunewald S, Paasch U, Wuendrich K, Glander HJ. Sperm caspases become more activated in infertility patients than in healthy donors during cryopreservation. Arch Androl 2005; 51: 449-460. [DOI:10.1080/014850190947813] [PMID]
37. Lachaud Ch, Tesarik J, Cañadas ML, Mendoza C. Apoptosis and necrosis in human ejaculated spermatozoa. Hum Reprod 2004; 19: 607-610. [DOI:10.1093/humrep/deh130] [PMID]
38. Rajender S, Rahul P, Mahdi AA. Mitochondria, spermatogenesis and male infertility. Mitochondrion 2010; 10: 419-428. [DOI:10.1016/j.mito.2010.05.015] [PMID]
39. Adams JM. Ways of dying: Multiple pathways to apoptosis. Genes Dev 2003; 17: 2481-2495. [DOI:10.1101/gad.1126903] [PMID]
40. Sakkas D, Moffatt O, Manicardi GC, Mariethoz E, Tarozzi N, Bizzaro D. Nature of DNA damage in ejaculated human spermatozoa and the possible involvement of apoptosis. Biol Reprod 2002; 66: 1061-1067. [DOI:10.1095/biolreprod66.4.1061] [PMID]
41. Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997; 91: 479-489. [DOI:10.1016/S0092-8674(00)80434-1]
42. Rucker EB, Dierisseau P, Wagner KU, Garrett L, Wynshaw-Boris A, Flaws JA, et al. Bcl-x and Bax regulate mouse primordial germ cell survival and apoptosis during embryogenesis. Mol Endocrinol 2000; 14: 1038-1052. [DOI:10.1210/mend.14.7.0465] [PMID]
43. Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 1995; 270: 96-99. [DOI:10.1126/science.270.5233.96] [PMID]
44. Kasai S, Chuma S, Motoyama N, Nakatsuji N. Haploinsufficiency of Bcl-x leads to male-specific defects in fetal germ cells: Differential regulation of germ cell apoptosis between the sexes. Dev Biol 2003; 264: 202-216. [DOI:10.1016/S0012-1606(03)00400-7]
45. Jeong SY, Seol DW. The role of mitochondria in apoptosis. BMB Rep 2008; 41: 11-22. [DOI:10.5483/BMBRep.2008.41.1.011] [PMID]
46. Janssen O, Qian J, Linkermann A, Kabelitz D. CD95 ligand-death factor and costimulatory molecule? Cell Death Differ 2003; 10: 1215-1225. [DOI:10.1038/sj.cdd.4401305] [PMID] [PMCID]
47. Porcelli F, Meggiolaro D, Carnevali A, Ferrandi B. Fas ligand in bull ejaculated spermatozoa: A quantitative immunocytochemical study. Acta Histochem 2006; 108: 287-292. [DOI:10.1016/j.acthis.2006.05.006] [PMID]
48. Francavilla S, D'Abrizio P, Cordeschi G, Pelliccione F, Necozione S, Ulisse S, et al. Fas expression correlates with human germ cell degeneration in meiotic and post-meiotic arrest of spermatogenesis. Mol Hum Reprod 2002; 8: 213-220. [DOI:10.1093/molehr/8.3.213] [PMID]
49. Shaha Ch. Germ cell apoptosis: Relevance to infertility and contraception. Immun Endoc Metab Agents Med Chem 2008; 8: 66-78. [DOI:10.2174/187152208783790714]
50. Sofikitis N, Giotitsas N, Tsounapi P, Baltogiannis D, Giannakis D, Pardalidis N. Hormonal regulation of spermatogenesis and spermiogenesis. J Steroid Biochem Mol Biol 2008; 109: 323-330. [DOI:10.1016/j.jsbmb.2008.03.004] [PMID]
51. Zhou XC, Wei P, Hu ZY, Gao F, Zou RJ, Liu YX. Role of Fas/FasL genes in azoospermia or oligozoospermia induced by testosterone undecanoate in rhesus monkey. Acta Pharmacol Sin 2001; 22: 1028-1033.
52. Tesarik J, Martinez F, Rienzi L, Iacobelli M, Ubaldi F, Mendoza C, et al. In-vitro effects of FSH and testosterone withdrawal on caspase activation and DNA fragmentation in different cell types of human seminiferous epithelium. Hum Reprod 2002; 17: 1811-1819. [DOI:10.1093/humrep/17.7.1811] [PMID]
53. Evenson DP. Loss of livestock breeding efficiency due to uncompensable sperm nuclear defects. Reprod Fertil Dev 1999; 11: 1-16. [DOI:10.1071/RD98023] [PMID]
54. Pareek TK, Joshi AR, Sanyal A, Dighe RR. Insights into male germ cell apoptosis due to depletion of gonadotropins caused by GnRH antagonists. Apoptosis 2007; 12: 1085-1100. [DOI:10.1007/s10495-006-0039-3] [PMID]
55. Sasagawa I, Matsuki S, Suzuki Y, Iuchi Y, Tohya K, Kimura M, et al. Possible involvement of the membrane‐bound form of peroxiredoxin 4 in acrosome formation during spermiogenesis of rats. Eur J Biochem 2001; 268: 3053-3061. [DOI:10.1046/j.1432-1327.2001.02200.x] [PMID]
56. Fujisawa M, Hiramine C, Tanaka H, Okada H, Arakawa S, Kamidono S. Decrease in apoptosis of germ cells in the testes of infertile men with varicocele. World J Urol 1999; 17: 296-300. [DOI:10.1007/s003450050149] [PMID]
57. Lee J, Richburg JH, Younkin SC, Boekelheide K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology 1997; 138: 2081-2088. [DOI:10.1210/endo.138.5.5110] [PMID]
58. Pentikäinen V, Erkkilä K, Dunkel L. Fas regulates germ cell apoptosis in the human testis in vitro. Am J Physiol 1999; 276: 310-316. [DOI:10.1152/ajpendo.1999.276.2.E310] [PMID]
59. Aitken RJ, Baker MA. Causes and consequences of apoptosis in spermatozoa; contributions to infertility and impacts on development. Int J Dev Biol 2013; 57: 265-272. [DOI:10.1387/ijdb.130146ja] [PMID]
60. Agarwal A, Makker K, Sharma R. Clinical relevance of oxidative stress in male factor infertility: An update. Am J Reprod Immunol 2008; 59: 2-11. [DOI:10.1111/j.1600-0897.2007.00559.x] [PMID]
61. Vashisht A, Gahlay GK. Using miRNAs as diagnostic biomarkers for male infertility: Opportunities and challenges. Mol Hum Reprod 2020; 26: 199-214. [DOI:10.1093/molehr/gaaa016] [PMID]
62. Söderquist L, Rodriguez‐Martinez H, Janson L. Post‐thaw motility, ATP content and cytochrome C oxidase activity of AI bull spermatozoa in relation to fertility. Zentralbl Veterinarmed A 1991; 38: 165-174. [DOI:10.1111/j.1439-0442.1991.tb00998.x] [PMID]
63. Petersen CG, Mauri AL, Vagnini LD, Renzi A, Petersen B, Mattila M, et al. The effects of male age on sperm DNA damage: An evaluation of 2,178 semen samples. JBRA Assist Reprod 2018; 22: 323-330.
64. Gunes S, Hekim GNT, Arslan MA, Asci R. Effects of aging on the male reproductive system. J Assist Reprod Genet 2016; 33: 441-454. [DOI:10.1007/s10815-016-0663-y] [PMID] [PMCID]
65. Agarwal A, Said TM. Oxidative stress, DNA damage and apoptosis in male infertility: A clinical approach. BJU Int 2005; 95: 503-507. [DOI:10.1111/j.1464-410X.2005.05328.x] [PMID]
66. Aitken RJ. The amoroso lecture. The human spermatozoon-a cell in crisis? J Reprod Fertil 1999; 115: 1-7. [DOI:10.1530/jrf.0.1150001] [PMID]
67. Aziz N, Saleh RA, Sharma RK, Lewis-Jones I, Esfandiari N, Thomas Jr AJ, et al. Novel association between sperm reactive oxygen species production, sperm morphological defects, and the sperm deformity index. Fertil Steril 2004; 81: 349-354. [DOI:10.1016/j.fertnstert.2003.06.026] [PMID]
68. Agarwal A, Sharma RK, Nallella KP, Thomas Jr AJ, Alvarez JG, Sikka SC. Reactive oxygen species as an independent marker of male factor infertility. Fertil Steril 2006; 86: 878-885. [DOI:10.1016/j.fertnstert.2006.02.111] [PMID]
69. Bui AD, Sharma R, Henkel R, Agarwal A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia 2018; 50: e13012. 1-10. [DOI:10.1111/and.13012] [PMID]
70. Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril 2003; 79: 829-843. [DOI:10.1016/S0015-0282(02)04948-8]
71. Sawyer DE, Mercer BG, Wiklendt AM, Aitken RJ. Quantitative analysis of gene-specific DNA damage in human spermatozoa. Mutat Res 2003; 529: 21-34. [DOI:10.1016/S0027-5107(03)00101-5]
72. Aitken RJ, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil 1987; 81: 459-469. [DOI:10.1530/jrf.0.0810459] [PMID]
73. Yumura Y, Iwasaki A, Saito K, Ogawa T, Hirokawa M. Effect of reactive oxygen species in semen on the pregnancy of infertile couples. Int J Urol 2009; 16: 202-207. [DOI:10.1111/j.1442-2042.2008.02213.x] [PMID]
74. Agarwal A, Mulgund A, Sharma R, Sabanegh E. Mechanisms of oligozoospermia: an oxidative stress perspective. Syst Biol Reprod Med 2014; 60: 206-216. [DOI:10.3109/19396368.2014.918675] [PMID]
75. Takeshima T, Kuroda S, Yumura Y. Reactive oxygen species and sperm cells. In: Cristiana F. Reactive oxygen species (ROS) in living cells. UK: IntechOpen; 2018. [DOI:10.5772/intechopen.73037]
76. Harlev A, Agarwal A, Gunes SO, Shetty A, du Plessis SS. Smoking and male infertility: An evidence-based review. World J Mens Health 2015; 33: 143-160. [DOI:10.5534/wjmh.2015.33.3.143] [PMID] [PMCID]
77. Sharma R, Harlev A, Agarwal A, Esteves SC. Cigarette smoking and semen quality: A new meta-analysis examining the effect of the 2010 World Health Organization laboratory methods for the examination of human semen. Eur Urol 2016; 70: 635-645. [DOI:10.1016/j.eururo.2016.04.010] [PMID]
78. Bisht S, Dada R. Oxidative stress: Major executioner in disease pathology, role in sperm DNA damage and preventive strategies. Front Biosci (Schol Ed) 2017; 9: 420-447. [DOI:10.2741/s495] [PMID]
79. Aitken RJ, Whiting S, De Iuliis GN, McClymont S, Mitchell LA, Baker MA. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J Biol Chem 2012; 287: 33048-33060. [DOI:10.1074/jbc.M112.366690] [PMID] [PMCID]
80. Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 1991; 11: 81-128. [DOI:10.1016/0891-5849(91)90192-6]
81. Venkatesh S, Riyaz AM, Shamsi MB, Kumar R, Gupta NP, Mittal S, et al. Clinical significance of reactive oxygen species in semen of infertile Indian men. Andrologia 2009; 41: 251-256. [DOI:10.1111/j.1439-0272.2009.00943.x] [PMID]
82. Tesarik J, Greco E, Cohen-Bacrie P, Mendoza C. Germ cell apoptosis in men with complete and incomplete spermiogenesis failure. Mol Hum Reprod 1998; 4: 757-762. [DOI:10.1093/molehr/4.8.757] [PMID]
83. Shen HM, Dai J, Chia SE, Lim A, Ong CN. Detection of apoptotic alterations in sperm in subfertile patients and their correlations with sperm quality. Hum Reprod 2002; 17: 1266-1273. [DOI:10.1093/humrep/17.5.1266] [PMID]
84. Saleh RA, Agarwal A. Oxidative stress and male infertility: From research bench to clinical practice. J Androl 2002; 23: 737-752.
85. Xu Q, Lin HY, Yeh SD, Yu IC, Wang RS, Chen YT, et al. Infertility with defective spermatogenesis and steroidogenesis in male mice lacking androgen receptor in Leydig cells. Endocrine 2007; 32: 96-106. [DOI:10.1007/s12020-007-9015-0] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








