Fri, Apr 26, 2024
[Archive]
Volume 19, Issue 12 (December 2021)
IJRM 2021, 19(12): 1067-1074 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Efremova O, Ponomarenko I, Churnosov M. Maternal polymorphic loci of rs1979277 serine hydroxymethyl transferase and rs1805087 5-methylenetetrahydrofolate are correlated with the development of fetal growth restriction: A case-control study. IJRM 2021; 19 (12) :1067-1074
URL: http://ijrm.ir/article-1-1882-en.html
URL: http://ijrm.ir/article-1-1882-en.html
1- Department of Biomedical Disciplines, Belgorod State University, Belgorod, Russia. , efremova.bgu@gmail.com
2- Department of Biomedical Disciplines, Belgorod State University, Belgorod, Russia.
2- Department of Biomedical Disciplines, Belgorod State University, Belgorod, Russia.
Full-Text [PDF 278 kb]
(786 Downloads)
| Abstract (HTML) (1434 Views)
1. Introduction
Problems in folic acid metabolism can cause a range of consequences that complicate the course of pregnancy (1-3). Folic acid is known to be involved in the formation of the vascular bed. Changes in angiogenesis can cause placental dysfunction, which is associated with the pathogenesis of fetoplacental insufficiency and can lead to fetal growth restriction (FGR) (4, 5). FGR is a condition where the rate of fetal growth is lower than expected for the gender and race of the fetus (6, 7). Over the past few decades, the frequency of FGR and placental insufficiency has increased in many countries (8, 9).
Folic acid metabolism is carried out through a complex cascade process, accompanied by genetically determined enzymatic reactions, and occurs in most organs, including the placenta (2, 4). The serine hydroxymethyl transferase gene (SHMT1) encodes a pyridoxal phosphate-dependent enzyme that catalyzes the interconversion of serine and glycine, and enables the folate-dependent single-carbon metabolism necessary for the synthesis of purines and thymidylate, as well as for the conversion of homocysteine to methionine. Methionine is subsequently adenylated to S-adenosylmethionine, a cofactor that methylates deoxyribonuclease, ribonuclease, proteins, and many metabolites (10, 11).
Folate absorption occurs in specialized and multinucleated placental cells in the presence of the enzyme encoded by 5-methylenetetrahydrofolate (MTR). Methionine synthase is expressed in the villous syncytiotrophoblast, and 5,10-methylenetetrahydrofolate reductase is expressed in the extravillous trophoblast. It has been shown that the MTR-encoded enzyme in the villus trophoblast is involved in the metabolism of homocysteine using folate. The gene methionine synthase reductase (MTRR) encodes the cytoplasmic enzyme methionine synthase reductase, one of the functions of which is to reverse the conversion of homocysteine to methionine (10, 11).
Thus, metabolic enzymes such as 5,10-methylenetetrahydrofolate reductase and serine hydroxymethyl transferase in the mother's body play an important role in monocarbon folate metabolism and normal fetal development. Understanding the role of the SHMT1 gene polymorphisms in the process of intrauterine growth restriction is important for the development of effective methods for the diagnosis and prevention of this pregnancy complication.
This study aimed to evaluate the association between folate cycle gene polymorphisms in the maternal body with the development of FGR.
2. Materials and Methods
2.1. Design and participants
In this case-control study, we recruited 365 pregnant women in their third trimester, from whom anamnestic data were collected, and general clinical and biochemical parameters were studied. Given the available data about the allele frequencies of the studied folate cycle gene polymorphisms in the European population (data of the 1000 Genomes Project), we calculated that sample size of 365 should be sufficient to ensure the statistical power of 0.80 at α = 0.05 significance level. This research was conducted at the Regional Perinatal Center of the city of Belgorod in the Russian Federation, from June 2014 to December 2018. Participants included 122 pregnant women with FGR (defined as fetal weight of 10 or more percentiles below the standard) as the case group, and 243 pregnant women with normal birth weight as a control group. The diagnosis of FGR was based on clinical data, parameters of growth, weight after the birth, and ultrasound fetometry (TOSHIBA XARIO SSA-660A, manufacturer Toshiba (Canon), Japan) (4, 7). The sample for the genetic testing was taken only from the mother. The inclusion criteria were as follows: рatients in the third trimester of pregnancy; with spontaneous singleton pregnancy; and FGR. The control group consisted of pregnant women with a normally developed fetus. Exclusion criteria included: multiple pregnancies; treatment with insulin therapy for gestational diabetes mellitus; diagnosis in the mother of human immunodeficiency virus, viral hepatitis, or severe uncompensated extragenital diseases; diagnosis in the fetus of hemolytic disease, anomaly of fetal development, antiphospholipid syndrome, or congenital thrombophilia; or circulatory disorders in the mother-placenta-fetus interface.
2.2. Genetic measurements
DNA was extracted from the venous blood of the pregnant women using the phenol-chloroform method and was then checked for quality as described previously (12, 13). Five single nucleotide polymorphisms (SNPs) were selected for the analysis, based on having significant regulatory potential (14, 15): MTR (rs1805087), MTRR (rs1801394), SHMT1 (rs1979277) and TYMS (rs699517, rs2790). The study was carried out through polymerase chain reaction using appropriate oligonucleotide primers and probes. Then the polymorphisms were analyzed using the detection method of TaqMan probes (real-time polymerase chain reaction).
2.3. Ethical considerations
This study was approved by the Ethical Committee of the Medical Institute of Belgorod State University (reference number: 54). The study details were explained to the women before they participated in the study, and informed consent was obtained from all.
2.4. Statistical analysis
Statistical analysis of the biomedical and clinical characteristics of the studied groups was carried out using the STATISTICA 7,0 for Windows 10.0 software package. Differences in the studied traits between the compared independent groups (pregnant women with FGR and control) were evaluated using the Mann-Whitney test. Logistic regression was used to assess the associations between the clinical and clinical-anamnestic risk factors, and the development of FGR. The odds ratios (OR) and their 95% confidence intervals (95% CI) were calculated (16).
Logistic regression was also used to assess associations of the SNPs with FGR assuming additive, recessive, and dominant genetic models (17). Statistical calculations were performed using the gPLINK v2.050 software (http://zzz.bwh.harvard.edu/plink/). To correct for multiple comparisons, a permutation test was used (18).
3. Results
The case group (n = 122) and the control group (n = 243) did not differ by age or height of the pregnant women (Table I). The father's age also did not differ by groups: case group - 27.3 ± 7.7 yr; control group - 26.9 ± 6.2 yr (p = 0.69).
The findings showed that the women with FGR had a significantly lower weight before pregnancy than the control group (p = 0.01). The body mass index of the case group was also significantly lower than the control group (p < 0.01). The mean weight of the case group newborns was 2147.26 ± 621.15 g and in the control group was 3463.26 ± 438.26 g (p < 0.01). The growth of newborns in the case group was 40.27 ± 2.41 cm and in the control was 54.51 ± 2.26 cm (p < 0.01) (Table I).
For all of the studied SNPs, both in the case group and the control group, the frequencies of minor alleles were higher than 5%. For all the examined loci in both groups, the analysis of the observed distribution of genotypes did not reveal deviations from the expected distribution following the Hardy-Weinberg equilibrium (Table II).
An analysis of the association between folate cycle gene polymorphic loci alleles and the development of FGR (Table III) showed that the T rs1979277 allele of the SHMT1 gene was significantly associated with the development of FGR (OR = 1.67, 95% CI 1.20 - 2.33, p < 0.01, pperm < 0.01, Nperm = 6342).
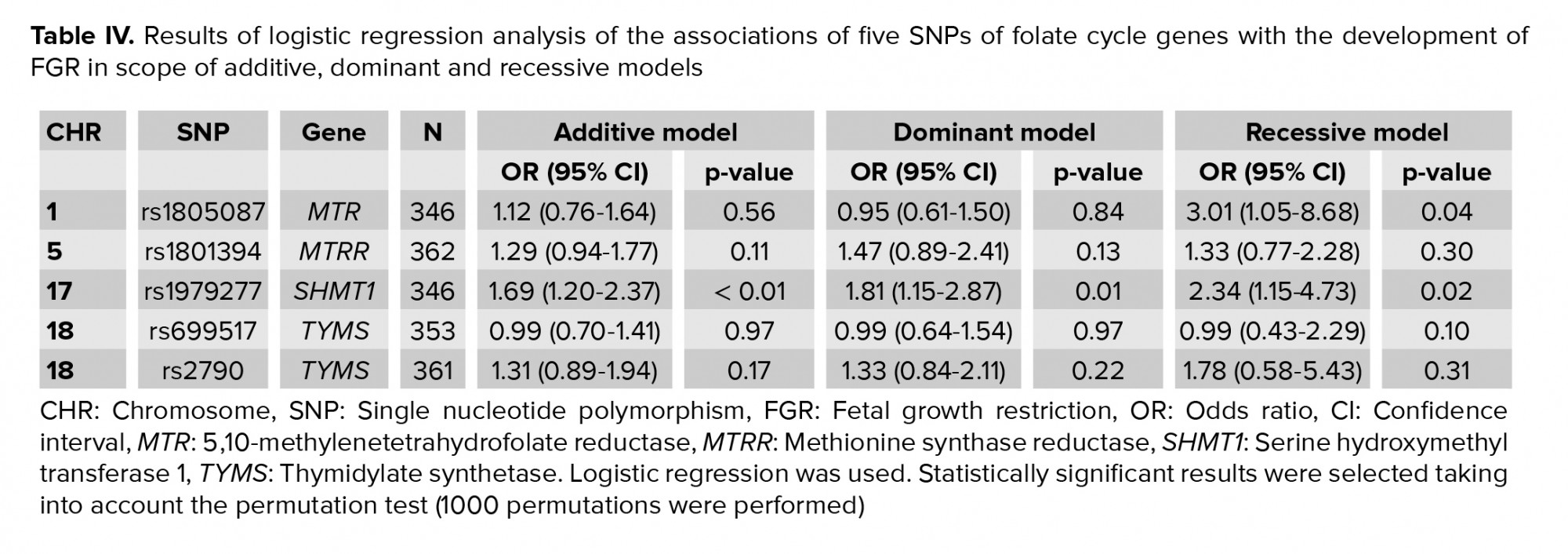
It was found that the T allele rs1979277 of the SHMT1 gene was associated with the development of FGR within the additive (OR = 1.69, 95% CI 1.20-2.37, p < 0.01, pperm < 0.01, Nperm = 8235), dominant (OR = 1.81, 95% CI 1.15-2.87, p = 0.01, pperm = 0.01, Nperm = 1706), and recessive (OR = 2.34, 95% CI 1.15-4.73, p = 0.02, pperm = 0.01, Nperm = 1352) models (Table IV). The association of the G rs1805087 allele of the MTR gene with the formation of FGR was also identified in accordance with the recessive model (OR = 3.01, 95% CI 1.05-8.68, p = 0.04, pperm = 0.04, Nperm = 506).



4. Discussion
In our study, it was found that polymorphic loci rs1979277 of the SHMT1 gene and rs1805087 of the MTR gene in the mother were associated with the development of FGR. It was identified that the alleles T rs1979277 of the SHMT1 gene and G rs1805087 were associated with an increased risk of FGR development (OR = 1.67-2.34 and OR = 3.01 respectively).
The results obtained are in accordance with the literature on the medico-biological effects of the studied genes. Epidemiological and experimental data consistently point to an association between folate deficiency in the first trimester of pregnancy, and poor fetal development and health of the offspring (19). Genetic disorders affecting folic acid metabolism have been found in patients with lung cancer (20). Some loci and genes that are associated with folic acid levels have been found using genome-wide associative studies, such as rs1801133 in MTR and rs1979277 in SHMT1 (21). The role of folic acid in reproductive activity has been shown. A number of authors have demonstrated that folic acid improves sperm quality and reduces the negative effect of high doses of drugs on sperm (22, 23). In many countries, to prevent the development of birth defects in the fetus, pregnant women are prescribed folic acid (4, 24). The role of the MTR A2756G polymorphism in the development of idiopathic male infertility has been demonstrated (25). MTR rs1805087 has been shown to have a statistically significant effect on the methylation levels of DNA methyltransferase 1, which is responsible for maintaining DNA methylation patterns during cell division (26).
A number of studies indicate that mitochondrial SHMT-derived monocarbon units are necessary for mediated folate of single-carbon metabolism in the cytoplasm (27). The relationship of SHMT1 gene polymorphisms with pregnancy and fetal development was demonstrated by Fekete et al. (28). Thus, in one case-control study, the relationship between SHMT1, dietary folic acid intake, preterm labor, and FGR was studied. It was shown that Caucasian carriers of SHMT1 T had an increased risk of spontaneous preterm delivery and development of FGR (2). Studies have also been carried out to examine the associations of SHMT1 with the development of acute lymphoblastic leukemia, tumors, neural tube defects, and sclerotic changes (29-32).
It has been shown that a polymorphic variant of the SHMT1 gene affects the occurrence of intrauterine malformations. One study found that the rs1979277 A allele reduced the cytoplasmic activity of SHMT and had a higher frequency in the control vs. cases with non-syndromic cleft lip; the authors therefore suggested that a low enzyme activity may increase the cytoplasmic concentration of folates (33). SHMT1 provides the single-carbon units necessary for embryogenesis, and defects in the production of carbon alone lead to certain pathological conditions during pregnancy. Using intrauterine, maternal, and paternal groups and both triad and family approaches, it has been shown that the interaction between maternal and paternal SHMT1 C1420T predisposes the fetus to neural tube defects (34).
5. Conclusion
As a result of the study, a possible association of maternal polymorphic loci rs1979277 SHMT1 and rs1805087 MTR with FGR was established.
Acknowledgments
This work was financially supported by a grant from the President of the Russian Federation for leading scientific schools of the Russian Federation (NS2609.2020.7).
Conflict of Interest
The authors declare that they have no competing interest.
Full-Text: (379 Views)
1. Introduction
Problems in folic acid metabolism can cause a range of consequences that complicate the course of pregnancy (1-3). Folic acid is known to be involved in the formation of the vascular bed. Changes in angiogenesis can cause placental dysfunction, which is associated with the pathogenesis of fetoplacental insufficiency and can lead to fetal growth restriction (FGR) (4, 5). FGR is a condition where the rate of fetal growth is lower than expected for the gender and race of the fetus (6, 7). Over the past few decades, the frequency of FGR and placental insufficiency has increased in many countries (8, 9).
Folic acid metabolism is carried out through a complex cascade process, accompanied by genetically determined enzymatic reactions, and occurs in most organs, including the placenta (2, 4). The serine hydroxymethyl transferase gene (SHMT1) encodes a pyridoxal phosphate-dependent enzyme that catalyzes the interconversion of serine and glycine, and enables the folate-dependent single-carbon metabolism necessary for the synthesis of purines and thymidylate, as well as for the conversion of homocysteine to methionine. Methionine is subsequently adenylated to S-adenosylmethionine, a cofactor that methylates deoxyribonuclease, ribonuclease, proteins, and many metabolites (10, 11).
Folate absorption occurs in specialized and multinucleated placental cells in the presence of the enzyme encoded by 5-methylenetetrahydrofolate (MTR). Methionine synthase is expressed in the villous syncytiotrophoblast, and 5,10-methylenetetrahydrofolate reductase is expressed in the extravillous trophoblast. It has been shown that the MTR-encoded enzyme in the villus trophoblast is involved in the metabolism of homocysteine using folate. The gene methionine synthase reductase (MTRR) encodes the cytoplasmic enzyme methionine synthase reductase, one of the functions of which is to reverse the conversion of homocysteine to methionine (10, 11).
Thus, metabolic enzymes such as 5,10-methylenetetrahydrofolate reductase and serine hydroxymethyl transferase in the mother's body play an important role in monocarbon folate metabolism and normal fetal development. Understanding the role of the SHMT1 gene polymorphisms in the process of intrauterine growth restriction is important for the development of effective methods for the diagnosis and prevention of this pregnancy complication.
This study aimed to evaluate the association between folate cycle gene polymorphisms in the maternal body with the development of FGR.
2. Materials and Methods
2.1. Design and participants
In this case-control study, we recruited 365 pregnant women in their third trimester, from whom anamnestic data were collected, and general clinical and biochemical parameters were studied. Given the available data about the allele frequencies of the studied folate cycle gene polymorphisms in the European population (data of the 1000 Genomes Project), we calculated that sample size of 365 should be sufficient to ensure the statistical power of 0.80 at α = 0.05 significance level. This research was conducted at the Regional Perinatal Center of the city of Belgorod in the Russian Federation, from June 2014 to December 2018. Participants included 122 pregnant women with FGR (defined as fetal weight of 10 or more percentiles below the standard) as the case group, and 243 pregnant women with normal birth weight as a control group. The diagnosis of FGR was based on clinical data, parameters of growth, weight after the birth, and ultrasound fetometry (TOSHIBA XARIO SSA-660A, manufacturer Toshiba (Canon), Japan) (4, 7). The sample for the genetic testing was taken only from the mother. The inclusion criteria were as follows: рatients in the third trimester of pregnancy; with spontaneous singleton pregnancy; and FGR. The control group consisted of pregnant women with a normally developed fetus. Exclusion criteria included: multiple pregnancies; treatment with insulin therapy for gestational diabetes mellitus; diagnosis in the mother of human immunodeficiency virus, viral hepatitis, or severe uncompensated extragenital diseases; diagnosis in the fetus of hemolytic disease, anomaly of fetal development, antiphospholipid syndrome, or congenital thrombophilia; or circulatory disorders in the mother-placenta-fetus interface.
2.2. Genetic measurements
DNA was extracted from the venous blood of the pregnant women using the phenol-chloroform method and was then checked for quality as described previously (12, 13). Five single nucleotide polymorphisms (SNPs) were selected for the analysis, based on having significant regulatory potential (14, 15): MTR (rs1805087), MTRR (rs1801394), SHMT1 (rs1979277) and TYMS (rs699517, rs2790). The study was carried out through polymerase chain reaction using appropriate oligonucleotide primers and probes. Then the polymorphisms were analyzed using the detection method of TaqMan probes (real-time polymerase chain reaction).
2.3. Ethical considerations
This study was approved by the Ethical Committee of the Medical Institute of Belgorod State University (reference number: 54). The study details were explained to the women before they participated in the study, and informed consent was obtained from all.
2.4. Statistical analysis
Statistical analysis of the biomedical and clinical characteristics of the studied groups was carried out using the STATISTICA 7,0 for Windows 10.0 software package. Differences in the studied traits between the compared independent groups (pregnant women with FGR and control) were evaluated using the Mann-Whitney test. Logistic regression was used to assess the associations between the clinical and clinical-anamnestic risk factors, and the development of FGR. The odds ratios (OR) and their 95% confidence intervals (95% CI) were calculated (16).
Logistic regression was also used to assess associations of the SNPs with FGR assuming additive, recessive, and dominant genetic models (17). Statistical calculations were performed using the gPLINK v2.050 software (http://zzz.bwh.harvard.edu/plink/). To correct for multiple comparisons, a permutation test was used (18).
3. Results
The case group (n = 122) and the control group (n = 243) did not differ by age or height of the pregnant women (Table I). The father's age also did not differ by groups: case group - 27.3 ± 7.7 yr; control group - 26.9 ± 6.2 yr (p = 0.69).
The findings showed that the women with FGR had a significantly lower weight before pregnancy than the control group (p = 0.01). The body mass index of the case group was also significantly lower than the control group (p < 0.01). The mean weight of the case group newborns was 2147.26 ± 621.15 g and in the control group was 3463.26 ± 438.26 g (p < 0.01). The growth of newborns in the case group was 40.27 ± 2.41 cm and in the control was 54.51 ± 2.26 cm (p < 0.01) (Table I).
For all of the studied SNPs, both in the case group and the control group, the frequencies of minor alleles were higher than 5%. For all the examined loci in both groups, the analysis of the observed distribution of genotypes did not reveal deviations from the expected distribution following the Hardy-Weinberg equilibrium (Table II).
An analysis of the association between folate cycle gene polymorphic loci alleles and the development of FGR (Table III) showed that the T rs1979277 allele of the SHMT1 gene was significantly associated with the development of FGR (OR = 1.67, 95% CI 1.20 - 2.33, p < 0.01, pperm < 0.01, Nperm = 6342).
It was found that the T allele rs1979277 of the SHMT1 gene was associated with the development of FGR within the additive (OR = 1.69, 95% CI 1.20-2.37, p < 0.01, pperm < 0.01, Nperm = 8235), dominant (OR = 1.81, 95% CI 1.15-2.87, p = 0.01, pperm = 0.01, Nperm = 1706), and recessive (OR = 2.34, 95% CI 1.15-4.73, p = 0.02, pperm = 0.01, Nperm = 1352) models (Table IV). The association of the G rs1805087 allele of the MTR gene with the formation of FGR was also identified in accordance with the recessive model (OR = 3.01, 95% CI 1.05-8.68, p = 0.04, pperm = 0.04, Nperm = 506).




4. Discussion
In our study, it was found that polymorphic loci rs1979277 of the SHMT1 gene and rs1805087 of the MTR gene in the mother were associated with the development of FGR. It was identified that the alleles T rs1979277 of the SHMT1 gene and G rs1805087 were associated with an increased risk of FGR development (OR = 1.67-2.34 and OR = 3.01 respectively).
The results obtained are in accordance with the literature on the medico-biological effects of the studied genes. Epidemiological and experimental data consistently point to an association between folate deficiency in the first trimester of pregnancy, and poor fetal development and health of the offspring (19). Genetic disorders affecting folic acid metabolism have been found in patients with lung cancer (20). Some loci and genes that are associated with folic acid levels have been found using genome-wide associative studies, such as rs1801133 in MTR and rs1979277 in SHMT1 (21). The role of folic acid in reproductive activity has been shown. A number of authors have demonstrated that folic acid improves sperm quality and reduces the negative effect of high doses of drugs on sperm (22, 23). In many countries, to prevent the development of birth defects in the fetus, pregnant women are prescribed folic acid (4, 24). The role of the MTR A2756G polymorphism in the development of idiopathic male infertility has been demonstrated (25). MTR rs1805087 has been shown to have a statistically significant effect on the methylation levels of DNA methyltransferase 1, which is responsible for maintaining DNA methylation patterns during cell division (26).
A number of studies indicate that mitochondrial SHMT-derived monocarbon units are necessary for mediated folate of single-carbon metabolism in the cytoplasm (27). The relationship of SHMT1 gene polymorphisms with pregnancy and fetal development was demonstrated by Fekete et al. (28). Thus, in one case-control study, the relationship between SHMT1, dietary folic acid intake, preterm labor, and FGR was studied. It was shown that Caucasian carriers of SHMT1 T had an increased risk of spontaneous preterm delivery and development of FGR (2). Studies have also been carried out to examine the associations of SHMT1 with the development of acute lymphoblastic leukemia, tumors, neural tube defects, and sclerotic changes (29-32).
It has been shown that a polymorphic variant of the SHMT1 gene affects the occurrence of intrauterine malformations. One study found that the rs1979277 A allele reduced the cytoplasmic activity of SHMT and had a higher frequency in the control vs. cases with non-syndromic cleft lip; the authors therefore suggested that a low enzyme activity may increase the cytoplasmic concentration of folates (33). SHMT1 provides the single-carbon units necessary for embryogenesis, and defects in the production of carbon alone lead to certain pathological conditions during pregnancy. Using intrauterine, maternal, and paternal groups and both triad and family approaches, it has been shown that the interaction between maternal and paternal SHMT1 C1420T predisposes the fetus to neural tube defects (34).
5. Conclusion
As a result of the study, a possible association of maternal polymorphic loci rs1979277 SHMT1 and rs1805087 MTR with FGR was established.
Acknowledgments
This work was financially supported by a grant from the President of the Russian Federation for leading scientific schools of the Russian Federation (NS2609.2020.7).
Conflict of Interest
The authors declare that they have no competing interest.
Type of Study: Original Article |
Subject:
Reproductive Genetics
References
1. Bailey RL, West KP, Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab 2015; 66 (Suppl.): 22-33. [DOI:10.1159/000371618] [PMID]
2. Williams PJ, Bulmer JN, Innes BA, Broughton Pipkin F. Possible roles for folic acid in the regulation of trophoblast invasion and placental development in normal early human pregnancy. Biol Reprod 2011; 84: 1148-1153. [DOI:10.1095/biolreprod.110.088351] [PMID]
3. Field MS, Kamynina E, Chon J, Stover PJ. Nuclear folate metabolism. Ann Rev Nutr 2018; 38: 219-243. [DOI:10.1146/annurev-nutr-071714-034441] [PMID]
4. Rosario FJ, Nathanielsz PW, Powell ThL, Jansson Th. Maternal folate deficiency causes inhibition of mTOR signaling, down-regulation of placental amino acid transporters and fetal growth restriction in mice. Sci Rep 2017; 7: 3982. [DOI:10.1038/s41598-017-03888-2] [PMID] [PMCID]
5. Lokeswara AW, Hiksas R, Irwinda R, Wibowo N. Preeclampsia: From cellular wellness to inappropriate cell death, and the roles of nutrition. Front Cell Dev Biol 2021; 9: 726513. [DOI:10.3389/fcell.2021.726513] [PMID] [PMCID]
6. Reshetnikov E, Zarudskaya O, Polonikov A, Bushueva O, Orlova V, Krikun E, et al. Genetic markers for inherited thrombophilia are associated with fetal growth retardation in the population of Central Russia. J Obstet Gynaecol Res 2017; 43: 1139-1144. [DOI:10.1111/jog.13329] [PMID]
7. Golovchenko O, Abramova M, Ponomarenko I, Reshetnikov E, Aristova I, Polonikov A, et al. Functionally significant polymorphisms of ESR1 and PGR and risk of intrauterine growth restriction in population of Central Russia. Eur J Obstet Gynecol Reprod Biol 2020; 253: 52-57. [DOI:10.1016/j.ejogrb.2020.07.045] [PMID]
8. Nardozza LMM, Caetano ACR, Zamarian ACP, Mozzalo JB, Silva CP, Marcal VMG, et al. Fetal growth restriction: Current knowledge. Arch Gynecol Obstet 2017; 295: 1061-1077. [DOI:10.1007/s00404-017-4341-9] [PMID]
9. Reshetnikov EA. [Study of associations of candidate genes differentially expressing in the placenta with the development of placental insufficiency with fetal growth restriction.] Res Result Biomed 2020; 6: 338-349. (in Russian)
10. Gordijn SJ, Beune IM, Ganzevoort W. Building consensus and standards in fetal growth restriction studies. Best Pract Res Clin Obstet Gynaecol 2018; 49: 117-126. [DOI:10.1016/j.bpobgyn.2018.02.002] [PMID]
11. Jones P, Beckett E, Yates Z, Veysey M, Lucock M. Converging evolutionary, environmental and clinical ideas on folate metabolism. Exp Res Hypothes Med 2016; 1: 34-41.
12. Reshetnikov E, Ponomarenko I, Golovchenko O, Sorokina I, Batlutskaya I, Yakunchenko T, et al. The VNTR polymorphism of the endothelial nitric oxide synthase gene and blood pressure in women at the end of pregnancy. Taiwan J Obstet Gynecol 2019; 58: 390-395. [DOI:10.1016/j.tjog.2018.11.035] [PMID]
13. Ponomarenko I, Reshetnikov E, Polonikov A, Verzilina I, Sorokina I, Elgaeva EE, et al. Candidate genes for age at menarche are associated with endometriosis. Reprod Biomed Online 2020; 41: 943-956. [DOI:10.1016/j.rbmo.2020.04.016] [PMID]
14. Moskalenko M, Ponomarenko I, Reshetnikov E, Dvornyk V, Churnosov M. Polymorphisms of the matrix metalloproteinase genes are associated with essential hypertension in a Caucasian population of Central Russia. Sci Rep 2021; 11: 5224. [DOI:10.1038/s41598-021-84645-4] [PMID] [PMCID]
15. Dvornyk V, Ponomarenko I, Minyaylo O, Reshetnikov E, Churnosov M. Association of the functionally significant polymorphisms of the MMP9 gene with H. pylori-positive gastric ulcer in the Caucasian population of Central Russia. PLoS One 2021; 16: e0257060. [DOI:10.1371/journal.pone.0257060] [PMID] [PMCID]
16. Starikova D, Ponomarenko I, Reshetnikov E, Dvornyk V, Churnosov M. Novel data about association of the functionally significant polymorphisms of the MMP9 Gene with exfoliation glaucoma in the caucasian population of Central Russia. Ophthalmic Res 2021; 64: 458-464. [DOI:10.1159/000512507] [PMID] [PMCID]
17. Ponomarenko IV, Reshetnikov E, Altuchova O, Polonikov A, Sorokina I, Yermachenko A, et al. Association of genetic polymorphisms with age at menarche in Russian women. Gene 2019; 686: 228-236. [DOI:10.1016/j.gene.2018.11.042] [PMID]
18. Minyaylo O, Ponomarenko I, Reshetnikov E, Dvornyk V, Churnosov M. Functionally significant polymorphisms of the MMP-9 gene are associated with peptic ulcer disease in the Caucasian population of Central Russia. Sci Rep 2021; 11: 13515. [DOI:10.1038/s41598-021-92527-y] [PMID] [PMCID]
19. Liu HY, Liu SM, Zhang YZ. Maternal folic acid supplementation mediates offspring health via DNA methylation. Reprod Sci 2020; 27: 963-976. [DOI:10.1007/s43032-020-00161-2] [PMID]
20. Stanisławska-Sachadyn A, Borzyszkowska J, Krzemiński M, Janowicz A, Dziadziuszko R, Jassem J, et al. Folate/homocysteine metabolism and lung cancer risk among smokers. PLoS One 2019; 14: e0214462. [DOI:10.1371/journal.pone.0214462] [PMID] [PMCID]
21. Deng C, Tang Sh, Huang X, Gao J, Tian J, Zhou X, et al. Identification of three novel loci of ALDH2 gene for serum folate levels in a male Chinese population by genome-wide association study. Gene 2018; 674: 121-126. [DOI:10.1016/j.gene.2018.06.080] [PMID]
22. Salarkia E, Sepehri Gh, Torabzadeh P, Abshenas J, Saberi A. Effects of administration of co-trimoxazole and folic acid on sperm quality and histological changes of testes in male rats. Int J Reprod Biomed 2017; 15: 625-634. [DOI:10.29252/ijrm.15.10.5] [PMID] [PMCID]
23. Ahmadi S, Bashiri R, Ghadiri-Anari A, Nadjarzadeh A. Antioxidant supplements and semen parameters: An evidence based review. Int J Reprod Biomed 2016; 14: 729-736. [DOI:10.29252/ijrm.14.12.729] [PMID] [PMCID]
24. Ferreira FR, Akiba HRR, Araujo Júnior E, Figueiredo EN, Abrahão AR. Prevention of birth defects in the pre-conception period: Knowledge and practice of health care professionals (nurses and doctors) in a city of Southern Brazil. Iran J Reprod Med 2015; 13: 657-664.
25. Tanoomand A, Hajibemani A, Abouhamzeh B. Investigation of the association of idiopathic male infertility with polymorphisms in the methionine synthase (MTR) gene. Clin Exp Reprod Med 2019; 46: 107-111. [DOI:10.5653/cerm.2018.00423] [PMID] [PMCID]
26. Coppedè F, Stoccoro A, Tannorella P, Migliore L. Plasma homocysteine and polymorphisms of genes involved in folate metabolism correlate with DNMT1 gene methylation levels. Metabolites 2019; 9: 298. [DOI:10.3390/metabo9120298] [PMID] [PMCID]
27. Fang Y, Zhang R, Zhi X, Zhao L, Cao L, Wang Y, et al. Association of main folate metabolic pathway gene polymorphisms with neural tube defects in Han population of Northern China. Childs Nerv Syst 2018; 34: 725-729.
https://doi.org/10.1007/s00381-018-3752-7 [DOI:10.1007/s00381-018-3730-0]
28. Fekete K, Berti C, Cetin I, Hermoso M, Koletzko BV, Decsi T. Perinatal folate supply: Relevance in health outcome parameters. Matern Child Nutr 2010; 6 (Suppl.): 23-38. [DOI:10.1111/j.1740-8709.2010.00261.x] [PMID] [PMCID]
29. Qu YY, Zhou ShX, Zhang X, Zhao R, Gu ChY, Chang K, et al. Functional variants of the 5-methyltetrahydrofolate-homocysteine methyltransferase gene significantly increase susceptibility to prostate cancer: Results from an ethnic Han Chinese population. Sci Rep 2016; 6: 36264. [DOI:10.1038/srep36264] [PMID] [PMCID]
30. Wang C, Lu D, Ling Q, Chen J, Liu Zh, Guo H, et al. Donor one-carbon metabolism gene single nucleotide polymorphisms predict the susceptibility of cancer recurrence after liver transplantation. Gene 2019; 689: 97-101. [DOI:10.1016/j.gene.2018.11.035] [PMID]
31. Bahari G, Hashemi M, Naderi M, Sadeghi-Bojd S, Taheri M. Association of SHMT1 gene polymorphisms with the risk of childhood acute lymphoblastic leukemia in a sample of Iranian population. Cell Mol Biol 2016; 62: 45-51. [DOI:10.3892/br.2017.1028] [PMID] [PMCID]
32. Nazari Mehrabani SZ, Shushizadeh MH, Abazari MF, Nouri Aleagha M, Ardalan A, Abdollahzadeh R, et al. Association of SHMT1, MAZ, ERG, and L3MBTL3 gene polymorphisms with susceptibility to multiple sclerosis. Biochem Genet 2019; 57: 355-370. [DOI:10.1007/s10528-018-9894-1] [PMID]
33. Salamanca C, González-Hormazábal P, Recabarren AS, Recabarren PA, Pantoja R, Leiva N, et al. A SHMT1 variant decreases the risk of nonsyndromic cleft lip with or without cleft palate in Chile. Oral Dis 2020; 26: 159-165. [DOI:10.1111/odi.13229] [PMID]
34. Rebekah KP, Tella S, Buragadda S, Tiruvatturu MK, Akka J. Interaction between maternal and paternal SHMT1 C1420T predisposes to neural tube defects in the fetus: Evidence from case-control and family‐based triad approaches. Birth Defects Res 2017; 109: 1020-1029. [DOI:10.1002/bdr2.23623] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








