Mon, May 6, 2024
[Archive]
Volume 19, Issue 9 (September 2021)
IJRM 2021, 19(9): 801-810 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hosseinalipour E, Karimipour M, Ahmadi A. Detrimental effects of cerium oxide nanoparticles on testis, sperm parameters quality, and in vitro fertilization in mice: An experimental study. IJRM 2021; 19 (9) :801-810
URL: http://ijrm.ir/article-1-1888-en.html
URL: http://ijrm.ir/article-1-1888-en.html
1- Department of Anatomy and Histology, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran.
2- Department of Anatomy and Histology, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran. Reproductive Health Research Center, Urmia University of Medical Sciences, Urmia, Iran. , mojtaba_karimipour@yahoo.com
3- Department of Basic Sciences, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran.
2- Department of Anatomy and Histology, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran. Reproductive Health Research Center, Urmia University of Medical Sciences, Urmia, Iran. , mojtaba_karimipour@yahoo.com
3- Department of Basic Sciences, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran.
Full-Text [PDF 555 kb]
(777 Downloads)
| Abstract (HTML) (1227 Views)
Full-Text: (235 Views)
- Introduction
Due to the growth of nanotechnology, studies related to the health risk of nanomaterials have been increasing rapidly (1). Nanoparticles (NPs) are material particles 1-100 nm in size and are commonly used in many industrial products (2). It has been reported that more than 1,814 nanomaterials are now used in different products (3). The size of these materials permits them to pass through biological membranes and barriers and penetrate the cells (4) and ultimately induce adverse effects such as oxidative stress, inflammation, gene mutations, and cell death (5, 6). NPs are widely used in medicine and their therapeutic application has been largely due to their oxidative stress-alleviating ability (7).
Nanoceria or cerium oxide nanoparticles (CeO2 NPs) are oxides of rare earth elements and are widely and extensively used in many industries such as the automotive industry, wood care application, and medicine (8, 9). These NPs are also used as diesel additives to reduce fuel consumption and decrease CO2 gas production (10). Due to the high emission of CeO2 NPs, the Organization for Economic Cooperation and Development has included these NPs in the list of NPs that need to be evaluated with high priority (11).
The existing publications related to the potential adverse effects of CeO2 NPs are largely controversial. Some reports have shown that CeO2 NPs are antioxidant agents that improve the total antioxidant capacity and prevent oxidative stress damage (12-14). CeO2 NPs have also been shown to possess anti-inflammatory properties (15). Conversely, several studies have reported the pro-oxidant effects and DNA damage in somatic cells and mouse oocytes following CeO2 NPs exposure (11, 16).
The publications on the effects of CeO2 NPs on the reproductive system are also controversial. In male mice, exposure to CeO2 NPs can lead to reproductive toxicity (17). In another study, it was shown that exposure of mice oocytes to CeO2 NPs caused oxidative stress and DNA damage (11). On the contrary, several publications have demonstrated the beneficial effects of CeO2 NPs on the reproductive system. For example, in a recent study, it was documented that CeO2 NPs improve oxidative stress-induced testicular toxicity due to cyclophosphamide administration (18). Or in another study, it was exhibited that exposure of ram sperm to CeO2 NPs had beneficial effects on motility and plasma membrane integrity (19). Furthermore, it was reported that pretreatment with CeO2 NPs relieved the adverse effects of radiofrequency radiation on testosterone synthesis through antioxidant capacity, enhanced the expression of testosterone synthase, and also elevated the resistance to oxidative damage in Leydig cells (20).
However, the available information regarding the adverse effects of CeO2 NP administration on the male reproductive system is controversial and incomplete. Thus, the objective of the present study was to determine the effects of in vivo CeO2 NPs exposure in male mice on testicular tissue, sperm parameters, sperm DNA fermentation, in vitro fertilization (IVF) rates, and in vitro embryo development. To the best of our knowledge, no publication has been reported on the effects of in vivo CeO2 NPs exposure in male mice on IVF and in vitro embryo growth.
Nanoceria or cerium oxide nanoparticles (CeO2 NPs) are oxides of rare earth elements and are widely and extensively used in many industries such as the automotive industry, wood care application, and medicine (8, 9). These NPs are also used as diesel additives to reduce fuel consumption and decrease CO2 gas production (10). Due to the high emission of CeO2 NPs, the Organization for Economic Cooperation and Development has included these NPs in the list of NPs that need to be evaluated with high priority (11).
The existing publications related to the potential adverse effects of CeO2 NPs are largely controversial. Some reports have shown that CeO2 NPs are antioxidant agents that improve the total antioxidant capacity and prevent oxidative stress damage (12-14). CeO2 NPs have also been shown to possess anti-inflammatory properties (15). Conversely, several studies have reported the pro-oxidant effects and DNA damage in somatic cells and mouse oocytes following CeO2 NPs exposure (11, 16).
The publications on the effects of CeO2 NPs on the reproductive system are also controversial. In male mice, exposure to CeO2 NPs can lead to reproductive toxicity (17). In another study, it was shown that exposure of mice oocytes to CeO2 NPs caused oxidative stress and DNA damage (11). On the contrary, several publications have demonstrated the beneficial effects of CeO2 NPs on the reproductive system. For example, in a recent study, it was documented that CeO2 NPs improve oxidative stress-induced testicular toxicity due to cyclophosphamide administration (18). Or in another study, it was exhibited that exposure of ram sperm to CeO2 NPs had beneficial effects on motility and plasma membrane integrity (19). Furthermore, it was reported that pretreatment with CeO2 NPs relieved the adverse effects of radiofrequency radiation on testosterone synthesis through antioxidant capacity, enhanced the expression of testosterone synthase, and also elevated the resistance to oxidative damage in Leydig cells (20).
However, the available information regarding the adverse effects of CeO2 NP administration on the male reproductive system is controversial and incomplete. Thus, the objective of the present study was to determine the effects of in vivo CeO2 NPs exposure in male mice on testicular tissue, sperm parameters, sperm DNA fermentation, in vitro fertilization (IVF) rates, and in vitro embryo development. To the best of our knowledge, no publication has been reported on the effects of in vivo CeO2 NPs exposure in male mice on IVF and in vitro embryo growth.
- Materials and Methods
- 1. Animal and treatment
In this experimental study, 24 adult male NMRI mice (8-10 wk old, 25-27 gr) were kept under standard animal house conditions (23 ± 2ºC; 12-hr light/dark cycles, and ad libitum access to food and water). The study was performed in the Anatomy and Histology Department of Urmia University of Medical Sciences, Urmia, Iran, between 2018 and 2019. After a one wk adaptation to the new environment, the animals were randomly divided into one control group and two experimental groups (n = 8/each) that received daily oral administration of CeO2 NPs at doses of 50 or 100 mg/kg body weight, respectively, for 35 consecutive days. The CeO2 NPs used in the current study (30 nm in diameter with 99.97% purity) were obtained from US Research Nanomaterials, Inc, USA. NPs were suspended in normal saline solution and vortexed before every administration. The mice in the control group received normal saline as the vehicle. The duration of the study was selected based on the timing (35 days) of the mouse spermatogenesis process reported in previous studies (21).
At the termination of the study, the mice were euthanized with an intraperitoneal injection of ketamine and testicular histomorphometry (seminiferous tubule diameter and epithelial height), spermiogenesis index (SPI), sperm parameters, and IVF assay were evaluated.
For evaluating the testicular histomorphometry and SPI, the left testes of each mouse was removed and fixed immediately in 10% formalin. Following the tissue processing, the 5-μm thick sections stained with hematoxylin and eosin were obtained. Then, 10 round-shaped tubules from each sample were randomly selected and the diameter of the seminiferous tubules (SNTs) and epithelial height were measured. In each SNT, the average of two diameters perpendicular to each other were determined. At two locations, the thickness from the basement membrane to the luminal surface was measured to obtain the average epithelial thickness of the same SNT (22, 23).
To determine the SPI in each sample, 20 SNTs were randomly selected and the percentage of SNTs with sperm in the lumen was calculated.
At the termination of the study, the mice were euthanized with an intraperitoneal injection of ketamine and testicular histomorphometry (seminiferous tubule diameter and epithelial height), spermiogenesis index (SPI), sperm parameters, and IVF assay were evaluated.
For evaluating the testicular histomorphometry and SPI, the left testes of each mouse was removed and fixed immediately in 10% formalin. Following the tissue processing, the 5-μm thick sections stained with hematoxylin and eosin were obtained. Then, 10 round-shaped tubules from each sample were randomly selected and the diameter of the seminiferous tubules (SNTs) and epithelial height were measured. In each SNT, the average of two diameters perpendicular to each other were determined. At two locations, the thickness from the basement membrane to the luminal surface was measured to obtain the average epithelial thickness of the same SNT (22, 23).
To determine the SPI in each sample, 20 SNTs were randomly selected and the percentage of SNTs with sperm in the lumen was calculated.
- 2. Collection of spermatozoa for the evaluation of sperm parameters
After 35 days, the animals were euthanized with an intraperitoneal injection of ketamine. To obtain sperm, both caudal part of the epididymides of each animal were cut into small pieces in pre-warm dishes containing 1-mL human tubal fluid medium (HTF; Sigma, USA) supplemented with 4 mg/mL bovine serum albumin (BSA; Sigma, USA) and incubated at 37ºC in 5% CO2 to release sperm from the epididymis into the medium.
After diluting the samples, sperms were counted using a Neubauer slide under a light microscope with 400× magnification. To assess the sperm motility, 10 μL of sperm sample was placed on a pre-warm Neubauer slide, and then, the percentage of motile sperms was calculated.
An aliquot (20 μL) of eosin solution was mixed with the same volume of sperm suspension to assess the sperm viability. Then 20 μL of nigrosine was added and a smear prepared. The slides was dyed at room temperature and the percentage of unstained colorless alive sperms and red-stained dead sperms was determined. At the end, the aniline blue staining method was implemented to evaluate the sperm morphology and the percentage of sperms with abnormal morphology was determined (23, 24).
After diluting the samples, sperms were counted using a Neubauer slide under a light microscope with 400× magnification. To assess the sperm motility, 10 μL of sperm sample was placed on a pre-warm Neubauer slide, and then, the percentage of motile sperms was calculated.
An aliquot (20 μL) of eosin solution was mixed with the same volume of sperm suspension to assess the sperm viability. Then 20 μL of nigrosine was added and a smear prepared. The slides was dyed at room temperature and the percentage of unstained colorless alive sperms and red-stained dead sperms was determined. At the end, the aniline blue staining method was implemented to evaluate the sperm morphology and the percentage of sperms with abnormal morphology was determined (23, 24).
- 3. Assessment of sperm DNA damage
Acridine orange is a fluorescence stain that can detect double- and single-stranded regions in sperm chromatin. Briefly, the sperm slides were placed in Carnoy's fixative (methanol/acetic acid 1:3) and after drying at room temperature, the slides were transferred to acridine orange solution. Eventually, using a fluorescent microscope, 200 sperms in each sample were evaluated and the percentage of normal sperms (green color) and abnormal ones (yellow to red color) was determined (23).
- 4. Assessment of sperm chromatin quality
Sperm nucleus maturity was analyzed using aniline blue staining. Briefly, the sperm smear slides were fixed in 3% glutaraldehyde and then stained with 5% aniline blue solution. In this test, abnormal immature sperms become dark and the normal sperms appear pale. At least 200 sperms were counted in each slide and the data were presented as a percentage (23).
- 5. IVF assays
Sixty adult female NMRI mice (8-10 wk old) were used to obtain enough oocytes for IVF assays. In order to induce superovulation and obtain mature oocytes from oviducts, female mice were intraperitoneally injected with 10 IU of pregnant mare's serum gonadotropin (PMSG; Folligon, Netherlands), followed by an injection of 10 IU of human chorionic gonadotropin (hCG; Folligon, Netherlands) 48 hr later. Next, 12-14 hr after the hCG injection, the mice were sacrificed and the fallopian tubes from each mouse were transferred to a petri dish containing HTF-BSA medium previously equilibrated in an incubator with 5% CO2 at 37 °C. After dissecting the oviducts, the oocytes were removed and washed, and then placed in the droplets of HTF-BSA medium which were under mineral oil. In order to evaluate IVF, 1 × 106 capacitated sperms from each sample were added to the oocytes. The fertilization process was evaluated under an inverted microscope after 3-5 hr by observing two pronuclei. Then these zygotes were cultured for 24 hr and the number of two-cell embryos was counted. At the end, the percentages of blastocysts formation after 120 hr and arrested embryos were determined (23, 25, 26).
- 6. Ethical considerations
The study was approved by the Ethics Committee of Urmia University of Medical Sciences and was performed according to the Guidelines for Care and Use of Laboratory Animals (Code: IR.umsu.rec.1395.228).
- 7. Statistical analysis
Statistical analysis for the data of testicular histomorphometry, SPI, and sperm parameters were done using SPSS 16. A one-way ANOVA with Tukey's post-hoc test was performed to quantify the results. The data of IVF assay were analyzed by two proportional test using Minitab software (version 15.1, Minitab Inc, USA). All results were expressed as means ± SD and P‐values < 0.05 were considered statistically significant.
- Results
- 1. Effects of CeO2 NP exposure on SPI and testicular histomorphometry
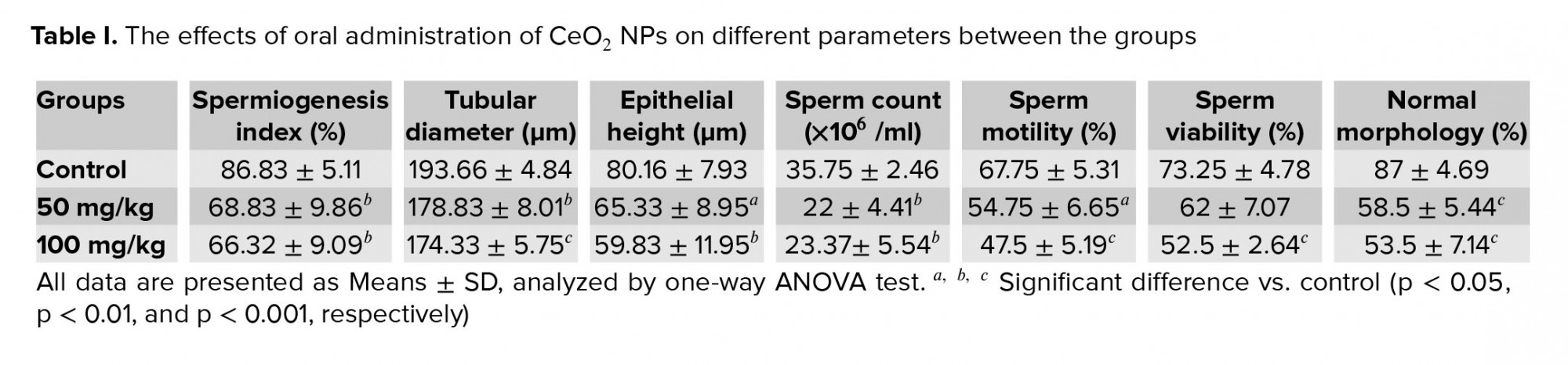
Microscopic analysis showed a significant reduction in the percentage of SNTs with positive SPI in the groups treated with 50 and 100 mg/kg CeO2 NPs compared with the control group (p = 0.005 and p = 0.002, respectively). Moreover, the mice in the CeO2 NPs receiving groups exhibited a significant decrease in the SNTs diameter (p = 0.003 and p < 0.001, respectively) and epithelial height (p = 0.047 and p = 0.007, respectively) in comparison with the control mice (Table I).
- 2. Effects of CeO2 NPs exposure on sperm parameters
Sperm parameters were evaluated 35 days after the NP administration. Sperm count in groups exposed to 50 and 100 mg/kg of CeO2 NPs was significantly reduced (p = 0.003 and p = 0.007, respectively) compared to the control group (Table I). Sperm motility in groups receiving 50 and 100 mg/kg was also significantly decreased compared to the control (p = 0.026 and p < 0.001, respectively). Moreover, it was found a significant decrease (p < 0.001) in the percentage of sperm viability with a dose of 100 mg/kg (Table I). As illustrated in Table I, the percentage of sperm with normal shape in mice exposed to CeO2 NPs was significantly decreased (p < 0.001) compared to the control group.
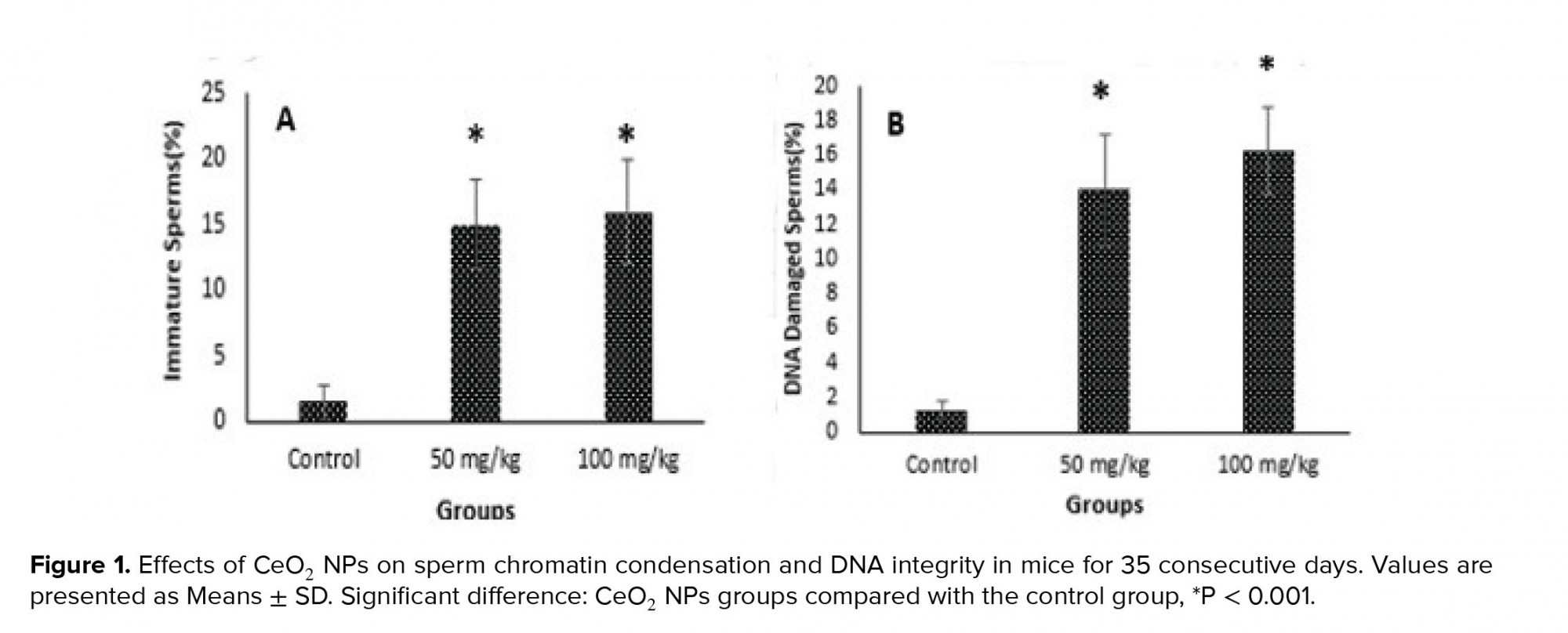
- 3. Effects of CeO2 NPs exposure on sperm chromatin condensation and DNA integrity
Figure 1 shows aniline blue and acridine orange staining tests and these results indicated that the percentages of immature sperms and DNA damage in sperms in groups exposed to CeO2 NPs were significantly higher (p < 0.001) than in the control group.
- 4. Effects of CeO2 NPs exposure on IVF rate and development of embryos
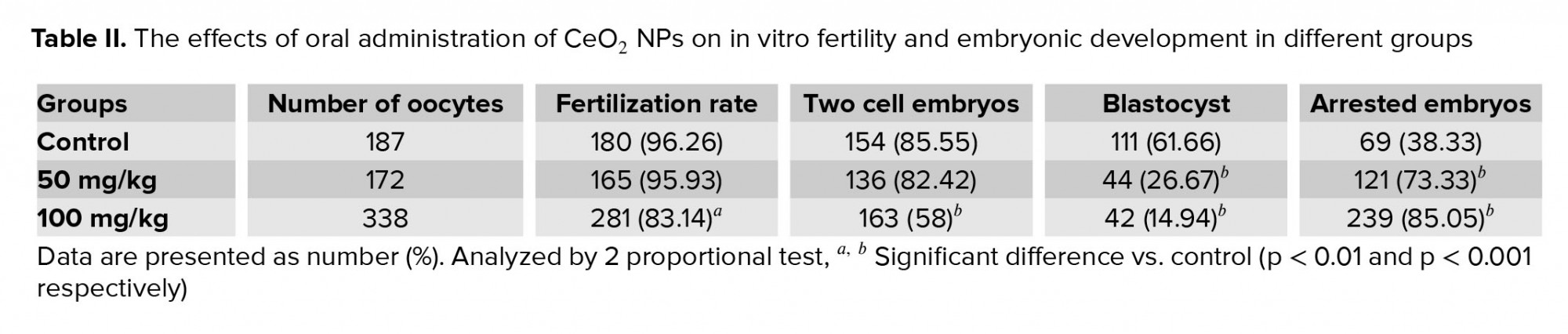
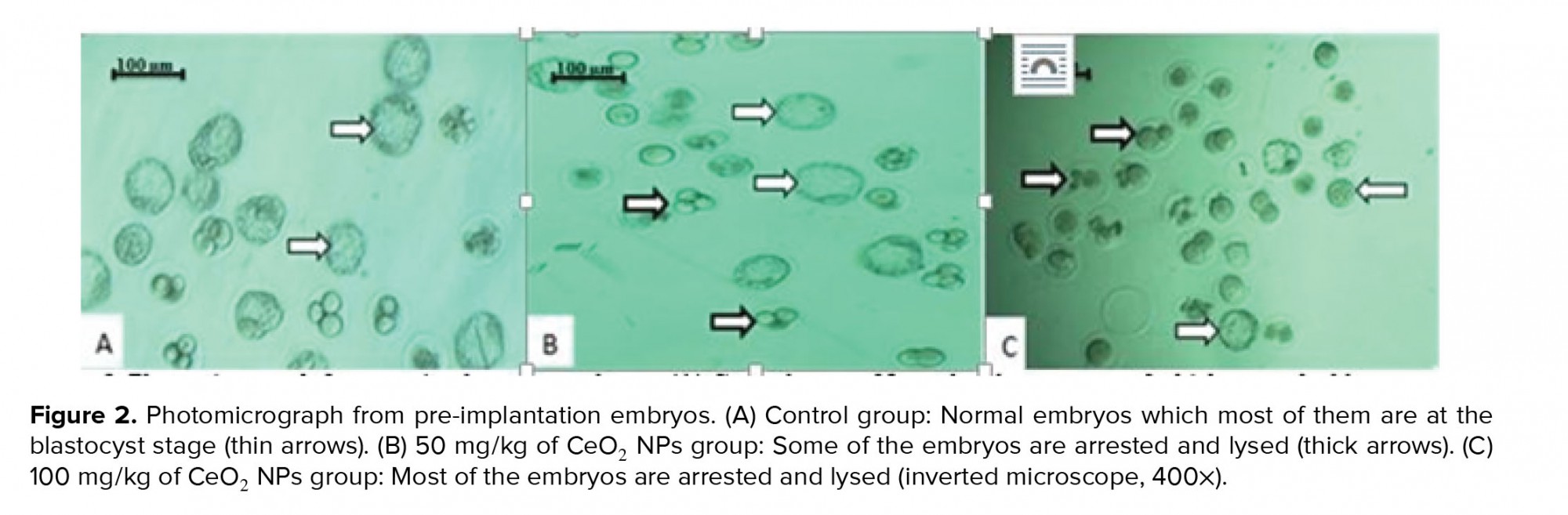
As shown in Table II, the percentages of fertilization and two-cell embryos formation in the group receiving 100 mg/kg CeO2 NPs were significantly lower (p < 0.01 and p < 0.001 respectively) than in the control, while no significant difference was observed between the group exposed to 50 mg/kg of CeO2 NPs and the control groups (p = 0.873 and p = 0.429, respectively). Additionally, the percentage of blastocyst formation was also reduced in both of the experimental groups compared to the control group (p < 0.001). Furthermore, the total percentage of arrested embryos was significantly higher (p < 0.001) in the groups receiving CeO2 NPs compared to the control group (Figure 2)
4. Discussion
The existing data from various experiments on potential toxic effects of cerium oxide on the male reproductive system are controversial. In the current study, we used sperm parameters and in vitro fertility potential evaluations to determine the toxic effects of CeO2 NPs on the male reproductive function. The findings of the current study revealed that all sperm parameters (count, motility, maturity, and morphology) had a significant decrease in mice following the CeO2 NPs exposure. Our findings also demonstrated that CeO2 NPs exposure induced adverse effects on IVF rate and embryonic development.
Sperm motility is known as a predictor of male fertility potential because it reflects the ability of sperm to fertilize the oocyte in the female genital tract (27). However, impairment of sperm motility leads to a decrease in the fertilization rate in both in vitro and in vivo.
Several experimental studies have previously reported conflicting findings regarding CeO2 NPs exposure (11, 14, 17, 18, 28-30). In the present study, sperm count and motility were significantly decreased following 35 consecutive days of CeO2 NPs administration. In the current study, the reduction in sperm count was also confirmed by evaluating SPI: it was observed that the percentage of SNTs without sperm in the lumen was significantly decreased in groups receiving CeO2 NPs. This is in agreement with a previous study that found that oral administration of CeO2 NPs led to a significant decrease in sperm motility and daily sperm production (17). A decline in sperm motility was also reported in mice whose mothers were exposed to a high dose of CeO2 NPs during pregnancy, and the testicular tissue examination in these mice showed that the number of spermatogonia, Sertoli, and Leydig cells was significantly decreased (28). In line with our study, Perrin and colleagues showed that in vitro exposure of human sperm cells to CeO2 NPs induced damage due to the aggregation of NPs in the membrane of sperm (29). Conversely, in vitro exposure with CeO2 NPs in rams did not affect sperm motility or the other sperm parameters which are known as indicators of fertility (30). In another study, it was also indicated that CeO2 NPs administration ameliorates male rats’ age-related infertility by elevating sex hormones production, and sperm concentration, as well as activating the spermatogenesis process through the antioxidant property (31).
The decrease in the quality of sperm parameters following CeO2 NPs administration may be induced due to translocation of CeO2 NPs from blood circulation to testicular tissue and damage to germ cells or directly induced damage to mature sperms. In a previous study, it was indicated that CeO2 NPs were found in the testes and epididymis of rats following inhalation (32).
Sperm morphology is another parameter that was evaluated in the current study and the results showed that both of the doses of CeO2 NPs (50 and 100 mg/kg) significantly increased the percentages of sperms with an abnormality in morphology. This parameter can also reveal the adverse effects of the chemical on the spermatogenesis process.
It has been indicated that spermatozoa with abnormal morphology is directly related to male infertility and can decline fertilization and pregnancy rates. In the literature, several mechanisms for the cause of abnormality in sperm morphology have been presented, including abnormal chromosome, point mutation in testicular DNA, and also impairment in the differentiation of spermatozoa during spermatogenesis following toxicant exposure (24, 33).
Regarding the sperm viability, our findings indicated that the oral administration of CeO2 NPs significantly decreased the percentages of alive sperms compared to the control group. This finding may have been caused by the direct effect of CeO2 NPs on the testis. However, further studies are needed to clarify this. Contrary to the results of our study, in a previous report, it was shown that exposure to CeO2 NPs for 14 days in rats decreases sperm and testicular damage induced by diabetes (34).
In the current study, we used aniline blue staining to evaluate the nucleus maturity and chromatin defects in sperm. The results showed that CeO2 NPs exposure via oral administration increased chromatin abnormality in sperm. Considering sperm maturation occurs in the epididymis, one possibility is that CeO2 NPs also induce their toxic effects in the epididymis. This result may be associated with oxidative stress damage induced by CeO2 NPs, although the potential of CeO2 NPs to induce oxidative stress is controversial.
The acridine orange staining test showed that CeO2 NPs caused DNA damage-DNA disintegration-in spermatozoa. This damage may transfer the abnormal genome into the oocyte which could reduce the fertilization and pregnancy rates. The IVF assay in the current study revealed that the percentages of fertilization and in vitro embryonic growth in mice exposed to CeO2 NPs were significantly decreased. There are conflicting reports about the genotoxic effects of CeO2 NPs. In a report by Preaubert and colleagues (35), it was shown that exposure to CeO2 NPs causes a significant increase in the DNA damage in mice sperm. Another study also confirmed the genotoxic effects of this NP in human spermatozoa (30), but conversely, no genotoxic effect was reported in ram spermatozoa exposed to CeO2 NPs (19). These aforementioned discrepancies indicate that the effects of CeO2 NPs on sperm may depend on several factors such as species sensitivity, surface chemistry, structure, size, and exposure time and dose.
Histomorphometric analyses in the testicular tissue showed significant decrease in epithelial height and diameter of SNTs in mice receiving CeO2 NPs comparing the control group. These alterations could be related to the decrease in the number of Sertoli cells because there is a direct correlation between the number of Sertoli cells and epithelial height and diameter of SNTs (36, 37). In the present study, the number of Sertoli cells was not evaluated. Further studies are needed to elucidate this relationship.
In addition to evaluating the testicular tissue and sperm parameters in the current study, we also evaluated the IVF assay. To the best of our knowledge, the current study is the first report describing the effects of in vivo exposure to CeO2 NPs on in vitro fertility and embryonic development. Findings from the present study indicated that oral administration of CeO2 NPs in male mice led to a significant decrease in the rate of fertilization and the percentages of two-cell embryos and blastocyst formation. The percentage of arrested embryos also increased. Sperm parameters quality is directly correlated to fertility and embryonic growth. The exact mechanisms by which CeO2 NPs caused damage to sperm, fertility, and embryonic growth were not obtained from the present study. Further investigation is required to support these findings and explore the involved mechanisms.
Our study had some limitations. For example, evaluations of oxidative stress and apoptosis in testicular tissue following CeO2 NPs exposure can provide additional information about mechanisms involved in CeO2 NPs toxicity.
5. Conclusion
In the current study, long-term oral administration of CeO2 NPs at doses of 50 and 100 mg/kg impaired male reproductive functions by decreasing sperm parameters quality and inducing sperm DNA damage and testicular tissue alterations in mice. In addition, it was also observed that male mice receiving CeO2 NPs showed a decreased IVF rate and in vitro embryonic development. These findings provide useful information on the risk of CeO2 NPs application on the function of the male reproductive system. Finally, further investigations are needed to examine the potential influence of these particles on spermatogenesis in men.
Acknowledgements
This work was financially supported by the Reproductive Health Research Center, Urmia University of Medical Sciences, Urmia, Iran.
Conflict of interest
The authors report no conflicts of interest.




4. Discussion
The existing data from various experiments on potential toxic effects of cerium oxide on the male reproductive system are controversial. In the current study, we used sperm parameters and in vitro fertility potential evaluations to determine the toxic effects of CeO2 NPs on the male reproductive function. The findings of the current study revealed that all sperm parameters (count, motility, maturity, and morphology) had a significant decrease in mice following the CeO2 NPs exposure. Our findings also demonstrated that CeO2 NPs exposure induced adverse effects on IVF rate and embryonic development.
Sperm motility is known as a predictor of male fertility potential because it reflects the ability of sperm to fertilize the oocyte in the female genital tract (27). However, impairment of sperm motility leads to a decrease in the fertilization rate in both in vitro and in vivo.
Several experimental studies have previously reported conflicting findings regarding CeO2 NPs exposure (11, 14, 17, 18, 28-30). In the present study, sperm count and motility were significantly decreased following 35 consecutive days of CeO2 NPs administration. In the current study, the reduction in sperm count was also confirmed by evaluating SPI: it was observed that the percentage of SNTs without sperm in the lumen was significantly decreased in groups receiving CeO2 NPs. This is in agreement with a previous study that found that oral administration of CeO2 NPs led to a significant decrease in sperm motility and daily sperm production (17). A decline in sperm motility was also reported in mice whose mothers were exposed to a high dose of CeO2 NPs during pregnancy, and the testicular tissue examination in these mice showed that the number of spermatogonia, Sertoli, and Leydig cells was significantly decreased (28). In line with our study, Perrin and colleagues showed that in vitro exposure of human sperm cells to CeO2 NPs induced damage due to the aggregation of NPs in the membrane of sperm (29). Conversely, in vitro exposure with CeO2 NPs in rams did not affect sperm motility or the other sperm parameters which are known as indicators of fertility (30). In another study, it was also indicated that CeO2 NPs administration ameliorates male rats’ age-related infertility by elevating sex hormones production, and sperm concentration, as well as activating the spermatogenesis process through the antioxidant property (31).
The decrease in the quality of sperm parameters following CeO2 NPs administration may be induced due to translocation of CeO2 NPs from blood circulation to testicular tissue and damage to germ cells or directly induced damage to mature sperms. In a previous study, it was indicated that CeO2 NPs were found in the testes and epididymis of rats following inhalation (32).
Sperm morphology is another parameter that was evaluated in the current study and the results showed that both of the doses of CeO2 NPs (50 and 100 mg/kg) significantly increased the percentages of sperms with an abnormality in morphology. This parameter can also reveal the adverse effects of the chemical on the spermatogenesis process.
It has been indicated that spermatozoa with abnormal morphology is directly related to male infertility and can decline fertilization and pregnancy rates. In the literature, several mechanisms for the cause of abnormality in sperm morphology have been presented, including abnormal chromosome, point mutation in testicular DNA, and also impairment in the differentiation of spermatozoa during spermatogenesis following toxicant exposure (24, 33).
Regarding the sperm viability, our findings indicated that the oral administration of CeO2 NPs significantly decreased the percentages of alive sperms compared to the control group. This finding may have been caused by the direct effect of CeO2 NPs on the testis. However, further studies are needed to clarify this. Contrary to the results of our study, in a previous report, it was shown that exposure to CeO2 NPs for 14 days in rats decreases sperm and testicular damage induced by diabetes (34).
In the current study, we used aniline blue staining to evaluate the nucleus maturity and chromatin defects in sperm. The results showed that CeO2 NPs exposure via oral administration increased chromatin abnormality in sperm. Considering sperm maturation occurs in the epididymis, one possibility is that CeO2 NPs also induce their toxic effects in the epididymis. This result may be associated with oxidative stress damage induced by CeO2 NPs, although the potential of CeO2 NPs to induce oxidative stress is controversial.
The acridine orange staining test showed that CeO2 NPs caused DNA damage-DNA disintegration-in spermatozoa. This damage may transfer the abnormal genome into the oocyte which could reduce the fertilization and pregnancy rates. The IVF assay in the current study revealed that the percentages of fertilization and in vitro embryonic growth in mice exposed to CeO2 NPs were significantly decreased. There are conflicting reports about the genotoxic effects of CeO2 NPs. In a report by Preaubert and colleagues (35), it was shown that exposure to CeO2 NPs causes a significant increase in the DNA damage in mice sperm. Another study also confirmed the genotoxic effects of this NP in human spermatozoa (30), but conversely, no genotoxic effect was reported in ram spermatozoa exposed to CeO2 NPs (19). These aforementioned discrepancies indicate that the effects of CeO2 NPs on sperm may depend on several factors such as species sensitivity, surface chemistry, structure, size, and exposure time and dose.
Histomorphometric analyses in the testicular tissue showed significant decrease in epithelial height and diameter of SNTs in mice receiving CeO2 NPs comparing the control group. These alterations could be related to the decrease in the number of Sertoli cells because there is a direct correlation between the number of Sertoli cells and epithelial height and diameter of SNTs (36, 37). In the present study, the number of Sertoli cells was not evaluated. Further studies are needed to elucidate this relationship.
In addition to evaluating the testicular tissue and sperm parameters in the current study, we also evaluated the IVF assay. To the best of our knowledge, the current study is the first report describing the effects of in vivo exposure to CeO2 NPs on in vitro fertility and embryonic development. Findings from the present study indicated that oral administration of CeO2 NPs in male mice led to a significant decrease in the rate of fertilization and the percentages of two-cell embryos and blastocyst formation. The percentage of arrested embryos also increased. Sperm parameters quality is directly correlated to fertility and embryonic growth. The exact mechanisms by which CeO2 NPs caused damage to sperm, fertility, and embryonic growth were not obtained from the present study. Further investigation is required to support these findings and explore the involved mechanisms.
Our study had some limitations. For example, evaluations of oxidative stress and apoptosis in testicular tissue following CeO2 NPs exposure can provide additional information about mechanisms involved in CeO2 NPs toxicity.
5. Conclusion
In the current study, long-term oral administration of CeO2 NPs at doses of 50 and 100 mg/kg impaired male reproductive functions by decreasing sperm parameters quality and inducing sperm DNA damage and testicular tissue alterations in mice. In addition, it was also observed that male mice receiving CeO2 NPs showed a decreased IVF rate and in vitro embryonic development. These findings provide useful information on the risk of CeO2 NPs application on the function of the male reproductive system. Finally, further investigations are needed to examine the potential influence of these particles on spermatogenesis in men.
Acknowledgements
This work was financially supported by the Reproductive Health Research Center, Urmia University of Medical Sciences, Urmia, Iran.
Conflict of interest
The authors report no conflicts of interest.
Type of Study: Original Article |
Subject:
Reproductive Biology
References
1. Park EJ, Cho WS, Jeong J, Yi Jh, Choi K, Kim Y, et al. Induction of inflammatory responses in mice treated with cerium oxide nanoparticles by intratracheal instillation. J Health Sci 2010; 56: 387-396. [DOI:10.1248/jhs.56.387]
2. Bottero JY, Rose J, Wiesner MR. Nanotechnologies: Tools for sustainability in a new wave of water treatment processes. Integr Environ Assess Manag 2006; 2: 391-395. [DOI:10.1002/ieam.5630020411] [PMID]
3. Vance ME, Kuiken T, Vejerano EP, McGinnis SP, Hochella Jr MF, Rejeski D, et al. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J Nanotechnol 2015; 6: 1769-1780. [DOI:10.3762/bjnano.6.181] [PMID] [PMCID]
4. Sarkar A, Fatima I, Mohammad Sajid Jamal Q, Sayeed U, Khan KA, Akhtar S, et al. Nanoparticles as a carrier system for drug delivery across blood brain barrier. Curr Drug Metab 2017; 18: 129-137. [DOI:10.2174/1389200218666170113125132] [PMID]
5. Das J, Choi YJ, Yasuda H, Han JW, Park C, Song H, et al. Efficient delivery of C/EBP beta gene into human mesenchymal stem cells via polyethylenimine-coated gold nanoparticles enhances adipogenic differentiation. Sci Rep 2016; 6: 33784. 1-17. [DOI:10.1038/srep37480] [PMID] [PMCID]
6. Soni D, Gandhi D, Tarale P, Bafana A, Pandey R, Sivanesan S. Oxidative stress and genotoxicity of zinc oxide nanoparticles to Pseudomonas species, human promyelocytic leukemic (HL-60), and blood cells. Biol Trace Elem Res 2017; 178: 218-227. [DOI:10.1007/s12011-016-0921-y] [PMID]
7. Nyoka M, Choonara YE, Kumar P, Kondiah PP, Pillay VJN. Synthesis of cerium oxide nanoparticles using various methods: Implications for biomedical applications. Nanomaterials (Basel) 2020; 10: 242. 1-21. [DOI:10.3390/nano10020242] [PMID] [PMCID]
8. Cassee FR, van Balen EC, Singh C, Green D, Muijser H, Weinstein J, et al. Exposure, health and ecological effects review of engineered nanoscale cerium and cerium oxide associated with its use as a fuel additive. Crit Rev Toxicol 2011; 41: 213-229. [DOI:10.3109/10408444.2010.529105] [PMID]
9. Blanchard V, Blanchet P. Color stability for wood products during use: Effects of inorganic nanoparticles. Bio Resourses 2011; 6: 1219-1229.
10. Park B, Donaldson K, Duffin R, Tran L, Kelly F, Mudway I, et al. Hazard and risk assessment of a nanoparticulate cerium oxide-based diesel fuel additive- a case study. Inhal Toxicol 2008; 20: 547-566. [DOI:10.1080/08958370801915309] [PMID]
11. Courbiere B, Auffan M, Rollais R, Tassistro V, Bonnefoy A, Botta A, et al. Ultrastructural interactions and genotoxicity assay of cerium dioxide nanoparticles on mouse oocytes. Int J Mol Sci 2013; 14: 21613-21628. [DOI:10.3390/ijms141121613] [PMID] [PMCID]
12. Celardo I, Pedersen JZ, Traversa E, Ghibelli L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale 2011; 3: 1411-1420. [DOI:10.1039/c0nr00875c] [PMID]
13. Hirst SM, Karakoti A, Singh S, Self W, Tyler R, Seal S, et al. Bio‐distribution and in vivo antioxidant effects of cerium oxide nanoparticles in mice. Environ Toxicol 2013; 28: 107-118. [DOI:10.1002/tox.20704] [PMID]
14. Vafaei-Pour Z, Shokrzadeh M, Jahani M, Shaki F. Embryo-protective effects of cerium oxide nanoparticles against gestational diabetes in mice. Iran J Pharm Res 2018; 17: 964-975.
15. Hirst SM, Karakoti AS, Tyler RD, Sriranganathan N, Seal S, Reilly CM. Anti‐inflammatory properties of cerium oxide nanoparticles. Small 2009; 5: 2848-2856. [DOI:10.1002/smll.200901048] [PMID]
16. Nemmar A, Al-Salam S, Beegam S, Yuvaraju P, Ali BH. The acute pulmonary and thrombotic effects of cerium oxide nanoparticles after intratracheal instillation in mice. Int J Nanomedicine 2017; 12: 2913-2922. [DOI:10.2147/IJN.S127180] [PMID] [PMCID]
17. Qin F, Shen T, Li J, Qian J, Zhang J, Zhou G, et al. SF-1 mediates reproductive toxicity induced by Cerium oxide nanoparticles in male mice. J Nanobiotechnology 2019; 17: 41. 1-13. [DOI:10.1186/s12951-019-0474-2] [PMID] [PMCID]
18. Hamzeh M, Hosseinimehr SJ, Karimpour A, Mohammadi HR, Khalatbary AR, Talebpour Amiri F. Cerium oxide nanoparticles protect cyclophosphamide-induced testicular toxicity in mice. Int J Prev Med 2019; 10: 5. [DOI:10.4103/ijpvm.IJPVM_184_18] [PMID] [PMCID]
19. Falchi L, Galleri G, Dore GM, Zedda MT, Pau S, Bogliolo L, et al. Effect of exposure to CeO2 nanoparticles on ram spermatozoa during storage at 4°C for 96 hours. Reprod Biol Endocrinol 2018; 16: 19. 1-10. [DOI:10.1186/s12958-018-0339-9] [PMID] [PMCID]
20. Qin F, Shen T, Cao H, Qian J, Zou D, Ye M, et al. CeO2 NPs relieve radiofrequency radiation, improve testosterone synthesis, and clock gene expression in Leydig cells by enhancing antioxidation. Int J Nanomedicine 2019; 14: 4601-4611. [DOI:10.2147/IJN.S206561] [PMID] [PMCID]
21. Hess RA, Chen P. Computer tracking of germ cells in the cycle of the seminiferous epithelium and prediction of changes in cycle duration in animals commonly used in reproductive biology and toxicology. J Androl 1992; 13: 185-190.
22. Narayana K, Prashanthi N, Nayanatara A, Bairy LK, D'Souza UJ. An organophosphate insecticide methyl parathion (o-o-dimethyl o-4-nitrophenyl phosphorothioate) induces cytotoxic damage and tubular atrophy in the testis despite elevated testosterone level in the rat. J Toxicol Sci 2006; 31: 177-189. [DOI:10.2131/jts.31.177] [PMID]
23. Karimipour M, Dibayi Z, Ahmadi A, Javanmard MZ, Hosseinalipour E. The protective effect of vitamin C on phenylhydrazine-induced hemolytic anemia on sperm quality and in-vitro embryo development in mice. Int J Reprod Biomed 2018; 16: 791-800. [DOI:10.18502/ijrm.v16i12.3685] [PMID] [PMCID]
24. Wyrobek AJ, Gordon LA, Burkhart JG, Francis MW, Kapp Jr RW, Letz G, et al. An evaluation of the mouse sperm morphology test and other sperm tests in nonhuman mammals: A report of the US environmental protection agency gene-tox program. Mutation Research 1983; 115: 1-72. [DOI:10.1016/0165-1110(83)90014-3]
25. Zahmatkesh E, Najafi G, Nejati V. Protective effect of royal jelly on in vitro fertilization (IVF) in male mice treated with oxymetholone. Cell J 2015; 17: 569-575.
26. Karimipour M, Javanmard MZ, Ahmadi A, Jafari A. Oral administration of titanium dioxide nanoparticle through ovarian tissue alterations impairs mice embryonic development. Int J Reprod Biomed 2018; 16: 397-404. [DOI:10.29252/ijrm.16.6.397] [PMID] [PMCID]
27. Robayo I, Montenegro V, Valdes C, Cox JF. CASA assessment of kinematic parameters of ram spermatozoa and their relationship to migration efficiency in ruminant cervical mucus. Reprod Domest Anim 2008; 43: 393-399. [DOI:10.1111/j.1439-0531.2007.00920.x] [PMID]
28. Nemati A, Farhadi A, Jalili C, Gholami M. The effect of cerium oxide during pregnancy on the development of the testicular tissue of newborn NMRI mice. Biol Trace Elem Res 2020; 195: 196-204. [DOI:10.1007/s12011-019-01836-x] [PMID]
29. Perrin J, Tassistro V, Auffan M, Liu W, Botta A, Sari-Minodier I, et al. Cerium dioxide nanoparticles induce DNA damage in human spermatozoa. Hum Reprod 2014; 29: 8-9.
30. Falchi L, Bogliolo L, Galleri G, Ariu F, Zedda MT, Pinna A, et al. Cerium dioxide nanoparticles did not alter the functional and morphologic characteristics of ram sperm during short-term exposure. Theriogenology 2016; 85: 1274-1281. e3. [DOI:10.1016/j.theriogenology.2015.12.011] [PMID]
31. Kobyliak NM, Falalyeyeva TM, Kuryk OG, Beregova TV, Bodnar PM, Zholobak NM, et al. Antioxidative effects of cerium dioxide nanoparticles ameliorate age-related male infertility: Optimistic results in rats and the review of clinical clues for integrative concept of men health and fertility. EPMA J 2015; 6: 12. 1-22. [DOI:10.1186/s13167-015-0034-2] [PMID] [PMCID]
32. Geraets L, Oomen AG, Schroeter JD, Coleman VA, Cassee FR. Tissue distribution of inhaled micro-and nano-sized cerium oxide particles in rats: results from a 28-day exposure study. Toxicol Sci 2012; 127: 463-473. [DOI:10.1093/toxsci/kfs113] [PMID]
33. Talebi AR, Sarcheshmeh AA, Khalili MA, Tabibnejad N. Effects of ethanol consumption on chromatin condensation and DNA integrity of epididymal spermatozoa in rat. Alcohol 2011; 45: 403-409. [DOI:10.1016/j.alcohol.2010.10.005] [PMID]
34. Artimani T, Amiri I, Soleimani Asl S, Saidijam M, Hasanvand D, Afshar S. Amelioration of diabetes‐induced testicular and sperm damage in rats by cerium oxide nanoparticle treatment. Andrologia 2018; 50: e13089. [DOI:10.1111/and.13089] [PMID]
35. Preaubert L, Courbiere B, Achard V, Tassistro V, Greco F, Orsiere T, et al. Cerium dioxide nanoparticles affect in vitro fertilization in mice. Nanotoxicology 2016; 10: 111-117. [DOI:10.3109/17435390.2015.1030792] [PMID]
36. Ramos AC, H Dos Santos A, Silveira KM, Kiss AC, Mesquita SFP, Gerardin DC, et al. Maternal treatment with fluoxetine promotes testicular alteration in male rat pups. Reprod Fertil Dev 2015; 28: 1206-1213. [DOI:10.1071/RD14199] [PMID]
37. Hooley RP, Paterson M, Brown P, Kerr K, Saunders PT. Intra-testicular injection of adenoviral constructs results in Sertoli cellspecific gene expression and disruption of the seminiferous epithelium. Reproduction 2009; 137: 361-370. [DOI:10.1530/REP-08-0247] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








