Thu, Apr 25, 2024
[Archive]
Volume 19, Issue 12 (December 2021)
IJRM 2021, 19(12): 1045-1058 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Danafar A, Khoradmehr A, Hosseini Bondarabadi M, Mazaheri F, Tamadon A, Pourmasoumi S, et al . Impairment of sperm efficiency in mice following short-term nano-titanium dioxide exposure: An experimental study. IJRM 2021; 19 (12) :1045-1058
URL: http://ijrm.ir/article-1-1962-en.html
URL: http://ijrm.ir/article-1-1962-en.html
Amirhossein Danafar1 

 , Arezoo Khoradmehr2
, Arezoo Khoradmehr2 

 , Mahya Hosseini Bondarabadi3
, Mahya Hosseini Bondarabadi3 

 , Fahime Mazaheri4
, Fahime Mazaheri4 

 , Amin Tamadon5
, Amin Tamadon5 

 , Soheila Pourmasoumi6
, Soheila Pourmasoumi6 

 , Lida Gholizadeh7
, Lida Gholizadeh7 

 , Mojgan Moshrefi8
, Mojgan Moshrefi8 

 , Iman Halvaei9
, Iman Halvaei9 

 , Akram Hosseini10
, Akram Hosseini10 

 , Jalal Golzadeh11
, Jalal Golzadeh11 

 , Tahereh Rahiminia12
, Tahereh Rahiminia12 

 , Morteza Anvari *
, Morteza Anvari * 

 13
13


 , Arezoo Khoradmehr2
, Arezoo Khoradmehr2 

 , Mahya Hosseini Bondarabadi3
, Mahya Hosseini Bondarabadi3 

 , Fahime Mazaheri4
, Fahime Mazaheri4 

 , Amin Tamadon5
, Amin Tamadon5 

 , Soheila Pourmasoumi6
, Soheila Pourmasoumi6 

 , Lida Gholizadeh7
, Lida Gholizadeh7 

 , Mojgan Moshrefi8
, Mojgan Moshrefi8 

 , Iman Halvaei9
, Iman Halvaei9 

 , Akram Hosseini10
, Akram Hosseini10 

 , Jalal Golzadeh11
, Jalal Golzadeh11 

 , Tahereh Rahiminia12
, Tahereh Rahiminia12 

 , Morteza Anvari *
, Morteza Anvari * 

 13
13
1- Dr. Seyedhassani Medical Sciences Center, Yazd, Iran.
2- The Persian Gulf Marine Biotechnology Research Center, the Persian Gulf Biomedical Sciences Research Institute, Bushehr University of Medical Sciences, Bushehr, Iran. Research and Clinical Center of Infertility, Yazd Reproductive Science Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Université Clermont Auvergne, CNRS, LMGE, Clermont-Ferrand, France.
4- Research and Clinical Center of Infertility, Yazd Reproductive Science Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. Medical Nanotechnology and Tissue Engineering Research Centre, Yazd Reproductive Science Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
5- The Persian Gulf Marine Biotechnology Research Center, the Persian Gulf Biomedical Sciences Research Institute, Bushehr University of Medical Sciences, Bushehr, Iran.
6- Non-Communicable Diseases Research Center, Rafsanjan University of Medical Sciences, Rafsanjan, Iran. Clinical Research Development Unit (CRDU), Moradi Hospital, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
7- Research and Clinical Center of Infertility, Yazd Reproductive Science Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
8- Research and Clinical Center of Infertility, Yazd Reproductive Science Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. Medical Nanotechnology and Tissue Engineering Research Centre, Yazd Reproductive Science Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
9- Department of Anatomical Sciences, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran.
10- Department of Anatomical Sciences, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
11- Stem Cell Biology Research Center, Yazd Reproductive Science Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
12- Gametogenesis Research Center, Fertility and Infertility Center, Kashan University of Medical Sciences, Kashan, Iran.
13- Research and Clinical Center of Infertility, Yazd Reproductive Science Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. Department of Biology and Anatomical Sciences, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. , moanvari@gmail.com
2- The Persian Gulf Marine Biotechnology Research Center, the Persian Gulf Biomedical Sciences Research Institute, Bushehr University of Medical Sciences, Bushehr, Iran. Research and Clinical Center of Infertility, Yazd Reproductive Science Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Université Clermont Auvergne, CNRS, LMGE, Clermont-Ferrand, France.
4- Research and Clinical Center of Infertility, Yazd Reproductive Science Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. Medical Nanotechnology and Tissue Engineering Research Centre, Yazd Reproductive Science Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
5- The Persian Gulf Marine Biotechnology Research Center, the Persian Gulf Biomedical Sciences Research Institute, Bushehr University of Medical Sciences, Bushehr, Iran.
6- Non-Communicable Diseases Research Center, Rafsanjan University of Medical Sciences, Rafsanjan, Iran. Clinical Research Development Unit (CRDU), Moradi Hospital, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
7- Research and Clinical Center of Infertility, Yazd Reproductive Science Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
8- Research and Clinical Center of Infertility, Yazd Reproductive Science Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. Medical Nanotechnology and Tissue Engineering Research Centre, Yazd Reproductive Science Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
9- Department of Anatomical Sciences, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran.
10- Department of Anatomical Sciences, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
11- Stem Cell Biology Research Center, Yazd Reproductive Science Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
12- Gametogenesis Research Center, Fertility and Infertility Center, Kashan University of Medical Sciences, Kashan, Iran.
13- Research and Clinical Center of Infertility, Yazd Reproductive Science Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. Department of Biology and Anatomical Sciences, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. , moanvari@gmail.com
Full-Text [PDF 2821 kb]
(854 Downloads)
| Abstract (HTML) (1472 Views)
1. Introduction
Titanium dioxide nanoparticles (TiO2NPs) have recently become an area of concern due to their use in many compounds from food and medicine to industrial applications (1). Their hydrophilic and photo-catalytic properties make them useful for many purposes. In particular, these two properties are useful for water and air purification (2). Over the past decades, TiO2NPs have been used to keep food fresh and as a white additive color for foods and cosmetic products (3, 4). It was previously shown that TiO2NPs exhibit low toxicity to humans and animals compared to other nanoparticles (5). However, more recent evidence demonstrates that TiO2NPs display some cytotoxic features.
It has been reported that TiO2NPs can cross into the testes and that the reproductive system is very sensitive to nanoparticles (6), which may have negative effects on the germ line either by direct effects on germ cells or indirect effects on somatic nursing cells that could lead to infertility (7). It has been shown that TiO2NPs can enter mouse Leydig cells and male germ line cells and change gene expression (8). Also, they can cause testicular damage and impaired spermatogenesis in male mice (9).
The quality of sperm parameters is related to spermatogenesis, and oxidative stress, the cytoskeleton and the blood-testis barrier (BTB) have critical roles in the maturation and development of spermatozoa. The BTB can restrict exogenous components from entering into the seminiferous tubules from the blood vessels, and makes a stable environment for germ cells and spermatid growth (10) and cause lower spermatogenesis (9). TiO2NPs can downregulate BTB junction-related proteins by activating the mitogen-activated protein kinases (MAPK) signaling pathways, which causes male reproductive dysfunction (10).
The exact mechanism of TiO2NP impact on spermatozoa dysfunction is still unknown. However, it is proposed that nanozied particles such as TiO2NPs can increase reactive oxygen species (ROS) in cells (11). ROS play a critical role in the function of sperm and germ line cells, which can reduce male fertility (12). Normal levels of ROS in seminiferous tubules can have detrimental effects on the fertilization rate by affecting sperm functions including sperm maturation, hyperactivation, capacitation and the acrosome reaction. Generation of ROS causes oxidative damage in molecular structures. High levels of ROS can damage the plasma membrane due to lipid peroxidation, protein denaturation and DNA damage (13).
Some studies suggest that TiO2NPs may disrupt endocrine functions and the regulation of the sex hormone profile (14, 15). It has been reported that TiO2NPs can induce accumulation of mutations and lead to genotoxicity in somatic cells. Intraperitoneal administration of TiO2NPs can significantly impact the reproductive system and increase sperm abnormalities (16).
Given the wide use of TiO2NPs, the purpose of this study was to examine the impact of TiO2NPs on spermatogenesis, as well as the histomorphometric cytotoxic effects of TiO2NPs in male mice. This study presents new data about sperm DNA integrity, sperm morphology and seminiferous tubule histopathology after daily oral TiO2NP administration.
2. Materials and Methods
2.1. Animal housing and treatment
In this experimental study, 8-12-wk-old NMRI male mice (35 ± 3 gr) were maintained in standard cages and animal house conditions (normal pellets and water ad libitum, humidity 60 ± 10%, room temperature 23 ± 2oC, and 12 hr light/dark cycle) in Yazd Reproductive Sciences Institute, Yazd, Iran. TiO2NPs (anatase/rutile, 99+%, 20 nm, Nanosony Co, Iran) were dissolved in water and the suspension was homogenized with a sonicator for 10 min. The TiO2NP suspension was prepared at the concentrations of 2.5, 5 and 10 mg/kg just before use in water (9). The mice were divided into four groups (n = 8/each group), including a control group and three treated groups (17). The control group received water intragastrically. The group I, group II and group II treated groups were fed orally with 2.5, 5 and 10 mg/kg/day TiO2NPs for 40 days, respectively (9).
After the 40 days (which is longer than needed for spermatogenesis in mice), the mice were sacrificed by cervical dislocation and the caudal epididymis was transferred into a petri dish. The testis was separated and fixed in Bouin’s solution for histological assessment. The suspension containing caudal epididymis and 1000 µl of Ham's F10 medium (Vitrolife, Gothenburg, Sweden) was incubated for 30 min at 37oC in a humidified atmosphere of 5% CO2 (17). After 30 min, sperm parameters were analyzed, and sperm DNA integrity and chromatin quality were assessed using standard procedures. All assays were summarized in figure 1.
2.2. Sperm parameters assessment
Sperm count was assessed by Makler’s counting chamber. To assess sperm motility, the percentages of sperm motility were classified into three classes of progressive, non-progressive and immotile sperm. Papanicolaou (Merck, Germany) and eosin-nigrosin staining (Merck, Germany) were used to examine sperm morphology and viability, respectively (18). In all groups, sperm viability was evaluated 30 min after sperm motility assessment. Sperm morphological abnormalities were classified as head (size, shape, double head, acrosomal section), neck (bent, cytoplasmic droplets) and tail (bent, coiled, double tail, cytoplasmic droplets). Eosin-nigrosin staining was used to assess sperm viability; white (unstained) and pink (or red) sperm heads were classified as live and dead sperm, respectively. To evaluate sperm motility, morphology and viability, 200 sperm were evaluated under a light microscope at x400 magnification (Motic, Spain).
2.3. Assessment of sperm DNA integrity and chromatin packaging quality
2.3.1. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
For detection of sperm DNA fragmentation, the TUNEL assay was applied. The slides were fixed with 4% paraformaldehyde, washed for 1 hr in phosphate-buffered saline, and then treated with 3% H2O2 and 0.1% Triton X-100 for 15 and 20 min, respectively. Afterward, the slides were incubated with enzyme (Roche, Germany): label solution (5:45 µl) for 1 hr. For positive and negative controls, the slides were incubated with DNase I grade I (3 U/ml in 50 mM Tris-HCl, pH 7.5, 1 mg/ml BSA) and 50 µl of label solution (without terminal transferase), respectively. Finally, for each slide, 200 sperm were evaluated under a fluorescence microscope at x400 magnification (Olympus BX51, Japan) (17) and were counted.
2.3.2. Chromomycin A3 staining
Protamine deficiency in sperm chromatin can be detected using chromomycin A3 assay. Methanol: glacial acetic acid (3:1) was used to fix the air-dried slides in a refrigerator. The fixed slides were treated with 100 µl of chromomycin A3 solution (Sigma, Germany) for 10 min and then, the slides were washed with McIlvain's buffer. For each slide, 200 sperm were evaluated under a fluorescent microscope at x400 magnification (Olympus BX51, Japan) with a 460-470 nm filter (17).
2.3.3. Toluidine blue (TB) staining
TB staining was used to determine chromatin condensation and DNA integrity via binding to phosphate groups of DNA strands. Ethanol: acetone (1:1) was used to fix the slides at 4oC for 30 min; these were then hydrolyzed in 0.1N HCl for 5 min. Then, the slides were rinsed three times in distilled water and finally, the samples were stained in 5% TB (Sigma, Germany) in 50% McIlvain's citrate phosphate buffer (pH: 3.5) (17). For each slide, 200 sperm were evaluated under a light microscope at x400 magnification (Olympus BX51, Japan).
2.3.4. Aniline blue (AB) staining
The acidic staining of AB reacts with lysine residues in histone proteins and leads to detection of sperm chromatin remodeling (19). 3% buffered glutaraldehyde in 0.2 M phosphate buffer (pH: 7.2) was used to fix the air-dried smears for 30 min. Then, the slides were stained with 5% AB (Sigma, Germany) in 4% acetic acid (pH: 3.5) for 5 min. Finally, the slides were washed in distilled water and 200 sperm were evaluated under a light microscope at x400 magnification (Olympus BX51, Japan).
2.4. Histomorphometric evaluation and cell numbers of seminiferous tubules
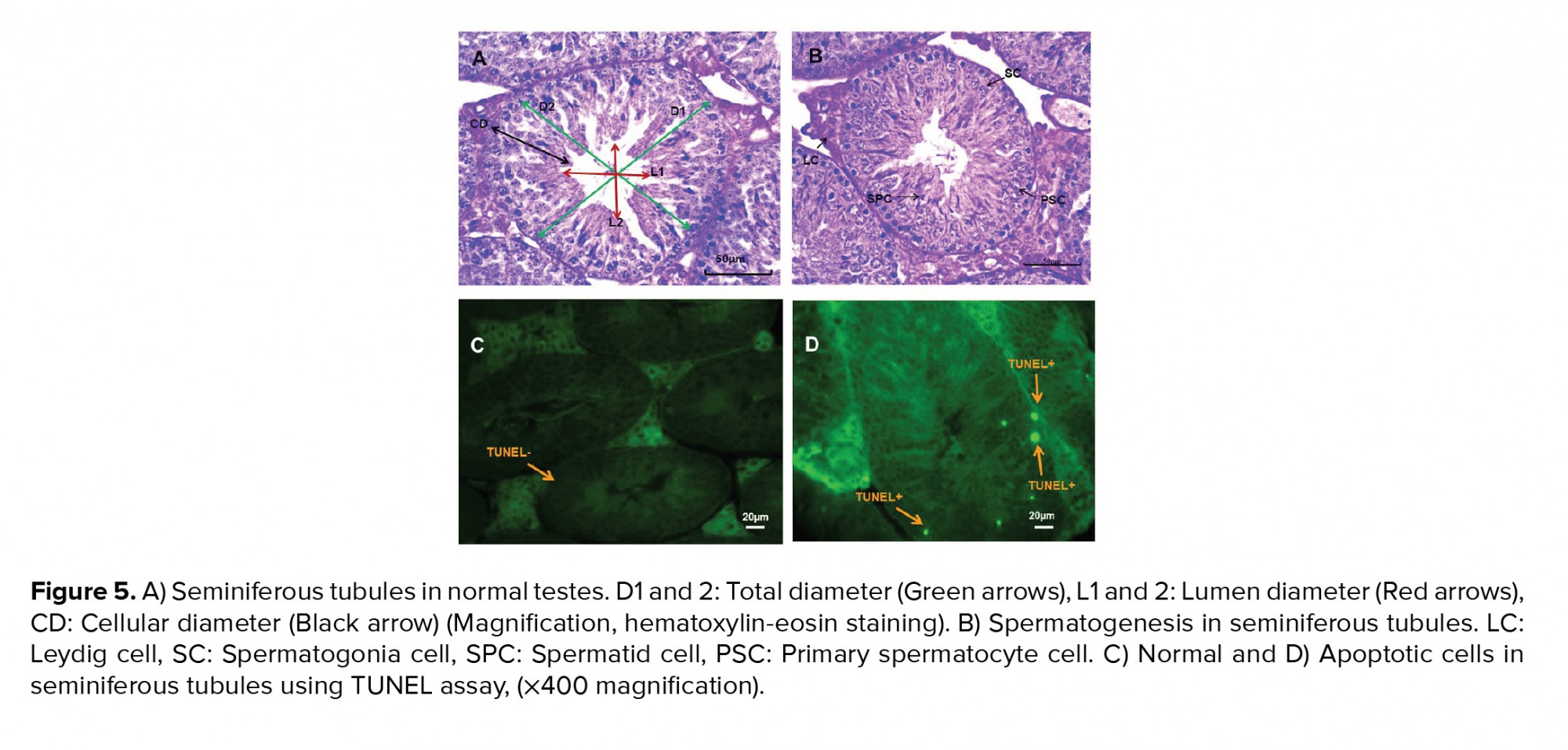
The five testis samples were fixed in Bouin’s solution for each group. Tissues were dehydrated in a graded alcohol series, cleared in xylene and embedded in paraffin wax. Samples were cut in 7 m (five slices from each testis) and then glass slides were stained with hematoxylin and eosin. A histopathologist unaware of the treatments evaluated the histological slides after hematoxylin-eosin staining. Total diameters, lumen diameter and cellular diameter (µm), luminal area, cellular area and cross-sectional area (×104 μm2) and numerical density (20) were measured using stereological methods under an optical microscope at x100 magnification (Olympus BX51, Japan). The mean seminiferous tubule diameter, and cross-sectional and numerical density area were calculated (21). For each slide, the mean of 10 random fields was calculated for measurements of Sertoli, Leydig, spermatogonia, primary spermatocyte and spermatid cell numbers (22) at x400 magnification. For the TUNEL assay in the testes for detection of cell apoptosis, sections were dewaxed in xylene and rehydrated through a graded series of ethanol, then washed for 1 hr in phosphate-buffered saline. The assay was performed according to the manufacturer’s instructions; sperm DNA fragmentation was detected under a fluorescence microscope (Olympus BX51, Japan) (23).
2.5. Plasma sex hormone levels
Serum samples were evaluated for their content of sex hormones (luteinizing hormone, follicle stimulating hormone, estradiol (E2) and testosterone), using the radioimmunoassay technique (DIA source Immuno-assays, S.A., Belgium), according to the manufacturer’s instructions (20).
2.6. Malondyaldehyde (MDA) assay
Seminal MDA levels were measured using a method that was based on a thiobarbituric acid (TBA) reaction and extraction with normal butanol (24), and 31.35 mg MDA (1 mM) and 57.66 mg TBA (4.0 mM) separately were dissolved in 100 mL glacial acetic acid. Semen was mixed with MDA and TBA solutions (1:1). After that, the seminal mixture was boiled in a water bath at 95°C for 60 min. The mixture was cooled at room temperature and spectrophotometric detection of absorbance was done at 532 nm wavelength and compared with a standard curve (25).

2.7. Ethical considerations
Animal treatment methods were approved by the Ethics Committee of Yazd Reproduction Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran (Code: IR-SSU-RSI-REC-2169).
2.8. Statistical analysis
The Shapiro-Wilk test was applied to assess the normal distribution of the data. One-way ANOVA followed by Tukey post hoc test were used for comparison of the data between the different groups using the Statistical Package for the Social Sciences (SPSS), version 20 (SPSS Inc., USA). The data were presented as mean ± standard deviation. Analyses were considered as statistically significant at p < 0.05.
3. Results
3.1. Sperm parameter assessment
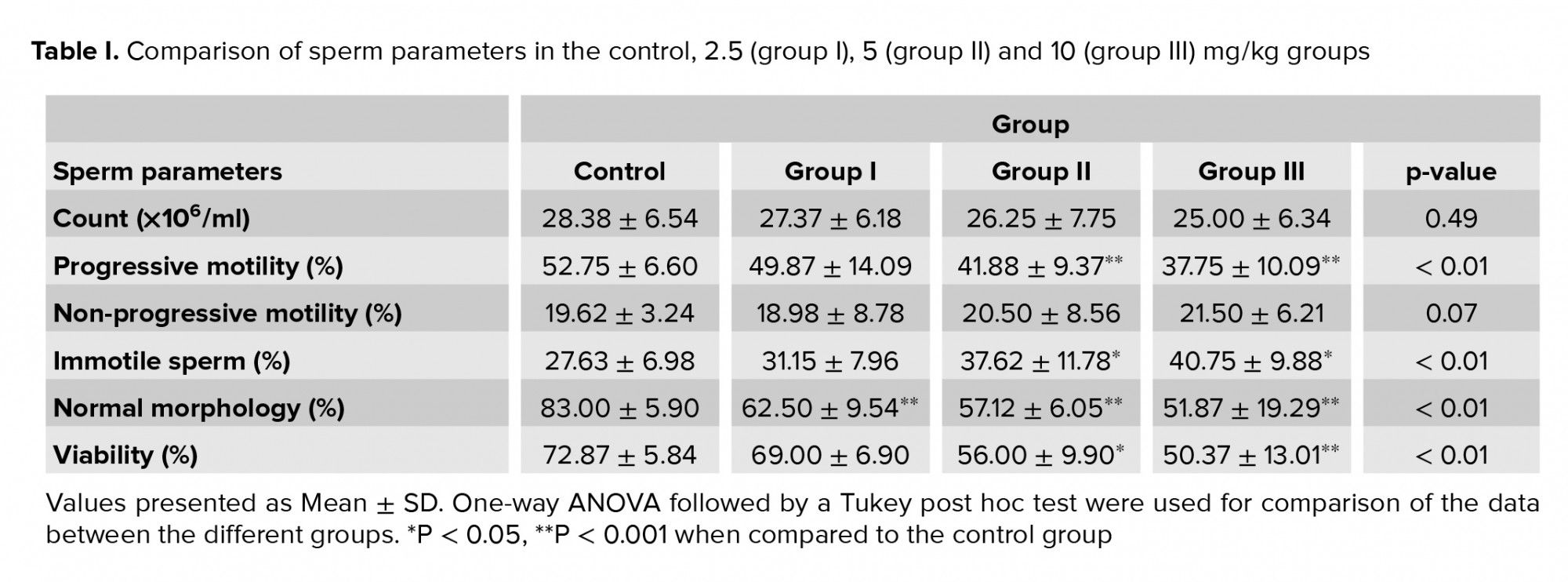
As shown in table I, the sperm count was lower in group III when compared to the control group, but this was not statistically significant (p = 0.49). It was found that the percentage of progressive motility was significantly higher in the control group than in groups II and III p ≤ 0.001 for both). The percentage of progressive motility was approximately 14% and 15% lower in the groups that received 5 and 10 mg/kg, respectively, compared to the control group. It was clear that there were no significant effects of different concentrations of TiO2NPs on the non-progressive sperm in the treated groups compared to the controls (p = 0.07). Moreover, the treatment of mice with different dosages of TiO2NPs was associated with a higher percentage of immotile sperm in groups II and III, compared to the control group (p ≤ 0.001). Normal morphology (Figure 2A) was significantly lower in all treated groups compared to the control group (Table I). In addition, sperm viability (Figure 2B) in groups II and III was approximately 16% and 22% less than in the control group (Table I).
3.2. Sperm DNA integrity and chromatin packaging quality
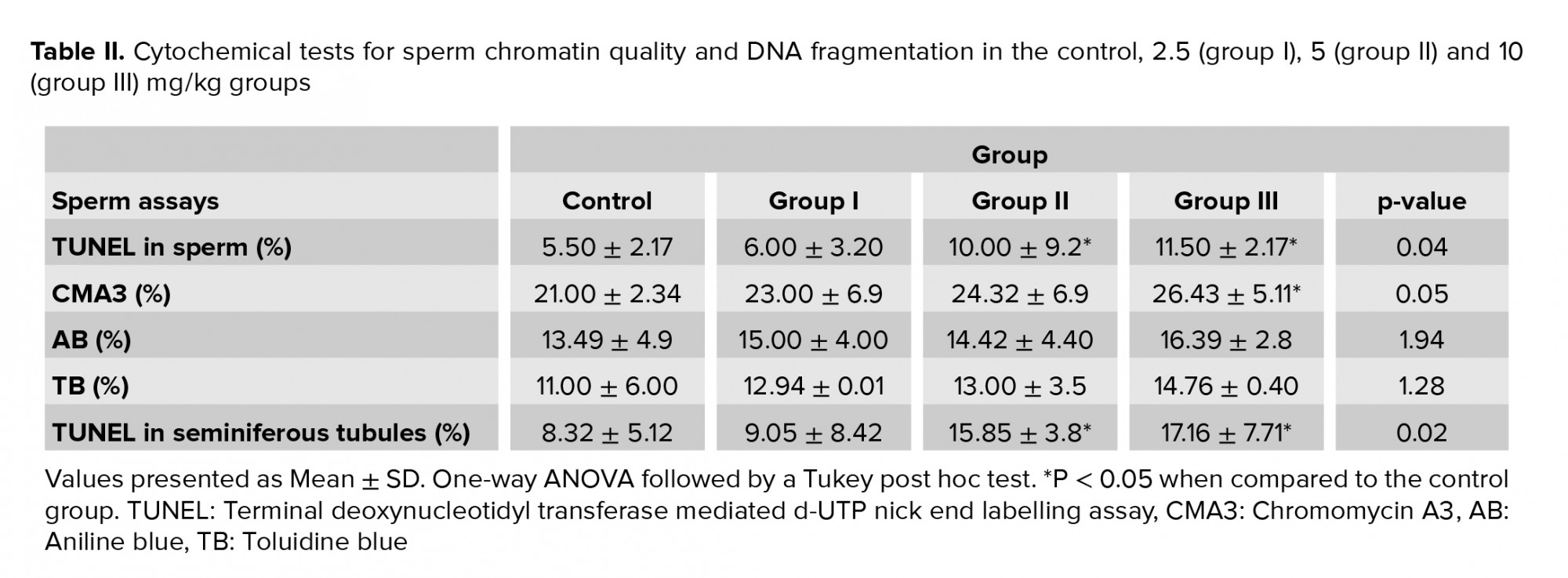
In the TUNEL assay, the apoptotic sperm cells exhibited intensive and brilliant fluorescent green, while the natural cells displayed pale and opaque green (Figure 2C). As shown in table II, a slight difference was observed in groups II and III compared to the control group in the TUNEL assay (p = 0.04) (Figure 2D). The results of CMA3 staining showed higher levels of abnormal sperm in group III in comparison with the control group (p = 0.05.( In TB staining, sperm with normal chromatin were seen as colorless but sperm with chromatin abnormalities were observed as dark blue (Figure 2E). In AB staining, sperm with a dark blue and unstained nucleus were considered as having abnormal and normal chromatin, respectively (Figure 2F). The rate of abnormal chromatin in the AB and TB staining was higher in all treated groups compared with the control group, but this difference was not significant (p = 0.08 and p = 0.09, respectively) (Table II).
3.3. Stereological analysis
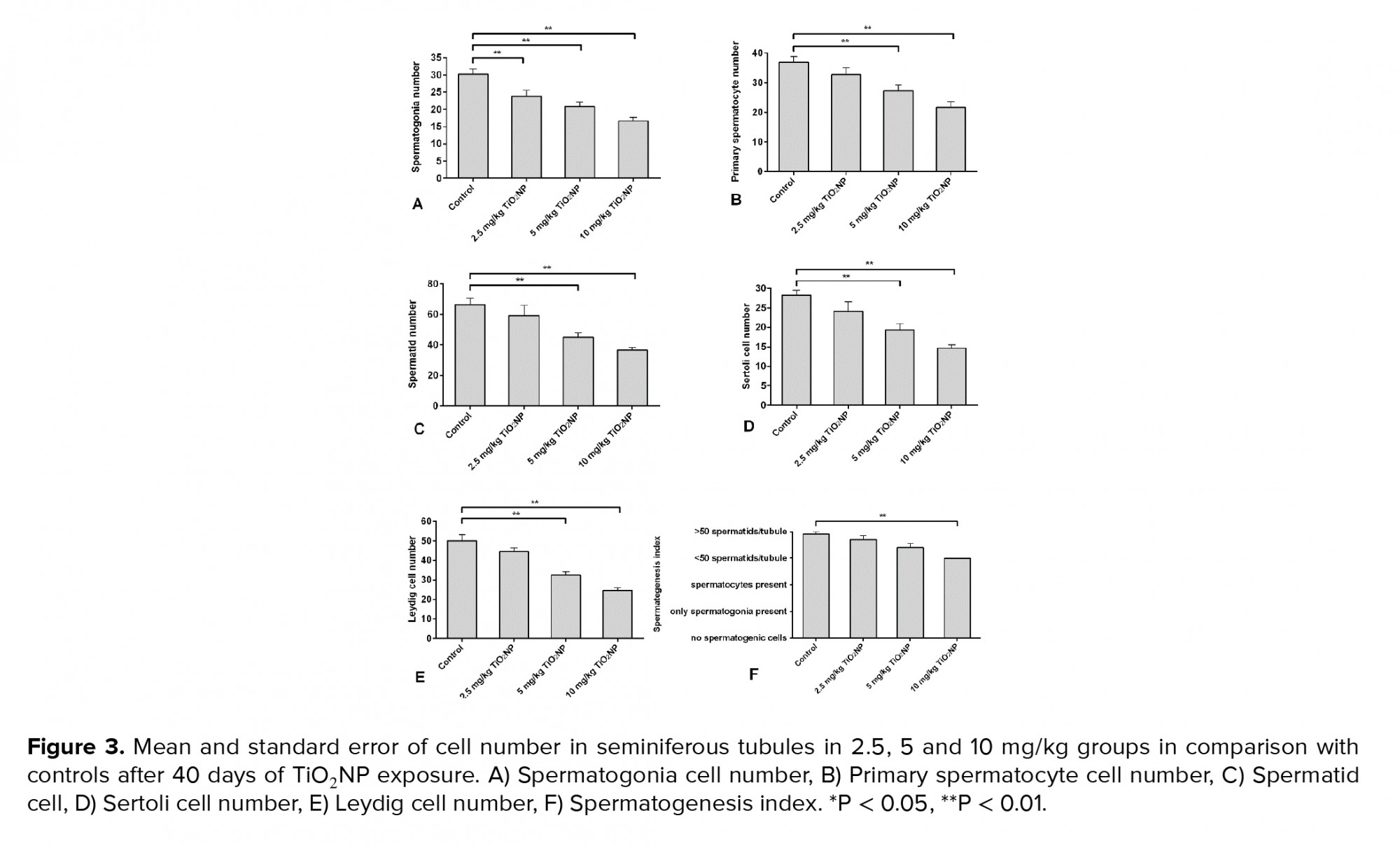
TiO2NP exposure was associated with differences in spermatogonia numbers compared to the control group (p < 0.01). Significantly fewer spermatogonia were found in groups I, II and III compared to the control group. Groups II and III displayed fewer primary spermatocytes and spermatid cells compared to controls 40 days after TiO2NP exposure (p = 0.02). There were significantly fewer Sertoli and Leydig cells in groups II and III (p ≤ 0.001). Overall, a significantly lower spermatogenesis index (p = 0.03) was observed in group III, but not in groups I and II, in comparison with the control group. The results of the histopathology assessments are shown in figure 3.
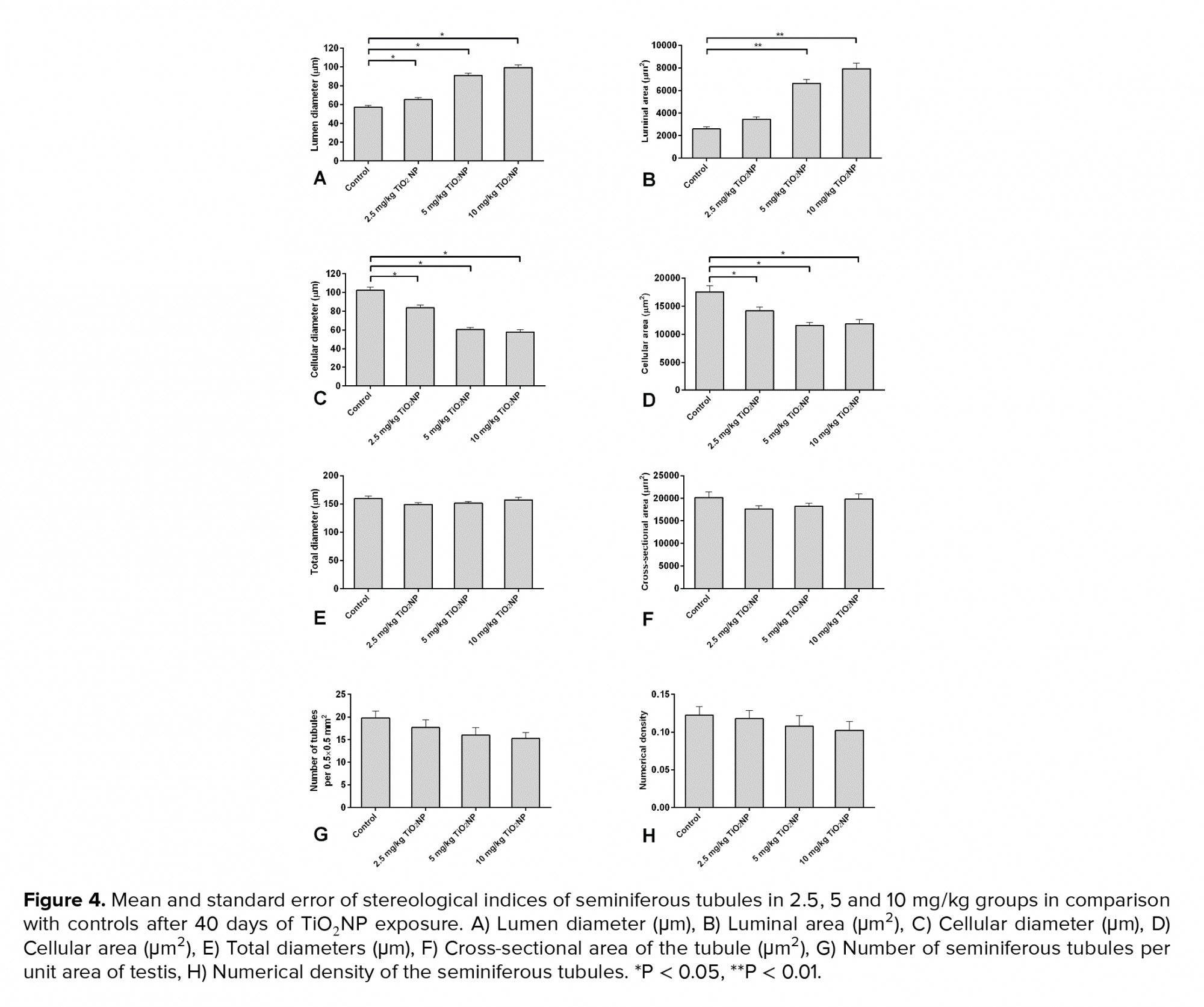
In groups I, II and III, the lumen diameter was significantly longer compared to in the control animals (p ≤ 0.001) (Figure 4A), and also there was a significantly larger lumen area in groups II and III (Figure 4B). The cellular diameters and area of the seminiferous tubules were smaller in groups I, II and III (Figures 4C and D). The total diameter and cross-sectional area were not varied across the groups (Figures 4E and F). However, there was a smaller number of tubules and density in all exposed groups compared to the control group, though this was not significant (Figures 4G and H). The results of the lumen studies are shown in figures 4 and 5. Also, there were significantly more apoptotic cells in the seminiferous tubules of groups II and III compared to in the control group (p = 0.02) (Table II).
3.4. Sex hormone levels and MDA
The effects of TiO2NPs on serum hormones of male mice are shown in table III. Following TiO2NP exposure, luteinizing hormone, follicle stimulating hormone and E2 levels were not different in the groups, whereas testosterone was markedly lower in group III compared with the control animals (p ≤ 0.001). The percentage of MDA in the group III seminal fluid was higher when compared with the control group (Table III).


4. Discussion
It has been suggested that industrial NPs may increase the risk and incidence of reproductive disorders, and that occupational and environmental exposure to TiO2NPs may be one of the risk factors for increased infertility in humans (6). The present results showed that the most abnormal morphology was observed in sperm tails and the percentage of abnormal heads and necks was approximately six-fold higher in group III than in the control group. There were significantly more apoptotic sperm and the rate of abnormal chromatin in CMA3, AB and TB staining was significantly higher in the groups exposed to TiO2NPs, compared with the controls. A significantly lower spermatogenesis index, lumen parameters and testosterone level were observed and the percentage of MDA in seminal fluid was higher as well.
In 2020, a study reported that the administration of TiO2NPs led to negative effects on sperm parameters including count, motility and morphology (26). This was similar to the results of the present study. In another study, data showed that exposure to TiO2NPs and zinc oxide nanoparticles was associated with decreased sperm number and motility and with increased abnormal sperm in experimental mice (16). They concluded that TiO2NPs may pass through the BTB and create harmful damage to testes and sperm.
Small amounts of ROS are necessary for normal sperm functions such as capacitation and the acrosome reaction. High ROS levels can reduce normal sperm morphology and motility by peroxidation of spermatozoa mitochondria and flagellum structure. It has been shown that TiO2NPs produce ROS in animal models (13). It reported that high levels of ROS in seminal fluid have a positive correlation with abnormal sperm morphology (27). Our findings were similar to another study which demonstrated that sperm parameters (count, motility, viability and morphology) were reduced in the epididymis tail after frequent consumption and high dosage of TiO2NPs in male mice (28).
In the present study, MDA in seminal fluid and sperm viability were significantly lower in group III. We suggest that TiO2NPs may induce ROS generation in the testis, and that high levels of ROS are responsible for the increase in abnormal sperm morphology and reduced sperm viability. Another study showed that sperm flagellum abnormalities were significantly more common in the treated groups vs. the controls after 1, 2 or 5 days (29). To the best of our knowledge, this is the first study to analyze abnormal sperm morphology characteristics separately in three classifications (head, neck and tail defects) after TiO2NP exposure.
The BTB provides a unique environment for spermatogenesis and to control the transportation of components from vessels to the seminiferous tubules. It has been found that TiO2NPs activate the MAPK signal pathways, which decreases BTB junction-related proteins and finally destroys the normal BTB structure. Furthermore, MAPK signal pathways interfere with some cellular processes such as cell proliferation, cell differentiation, and apoptosis. It has been reported that activation of MAPK signal pathways results in related oxidative stress as well (28). It is known that TiO2NPs can induce apoptosis in testicular germ cells and epididymal sperm in laboratory animals (30, 31). This is the first study to examine the effects of TiO2NPs on mouse sperm DNA fragmentation by TUNEL assay and chromatin condensation tests including CMA3, AB, and TB. It has previously been shown that sperm chromatin defects are associated with apoptosis (32). Our results showed that the rate of AB positive sperm in all treated groups was higher than in the control group, but this was not significant. Consequently, it can be concluded that TiO2NPs probably do not substantially affect the histone profile of sperm chromatin when exposed to a concentration of 10 mg/kg for a short time, and a higher concentration may be needed. It can be hypothesized that the lower quality of sperm chromatin observed was probably caused by the toxic effects of TiO2NPs and hormonal profile defects.
It reported that exposure to TiO2NPs in different doses for 60 sequential days in male animals can cause many defects in testicular tissue, Sertoli cells and spermatogenic cell lines including elevation in apoptotic cells and variation in seminiferous tubule diameter (33). Apoptosis has an important role during spermiogenesis and proliferation of germ cells with the surrounding Sertoli cells (34). In one study, apoptotic cells in seminiferous tubules and interstitial spaces increased, and interstitial spaces revealed enlarged testicular histology after intraperitoneal injection of 2.5 and 5 mg/kg over 1, 2, 3, and 5 wk (29). In another, in the seminiferous tubules, the spermatogenic cell layers reduced in the groups with 50 and 250 mg/kg of body weight compared to the control group, but no histological changes were detected (35).
It has been reported that 30-80% of male infertility is related to oxidative stress (12). In mice, oxidative stress after TiO2NP exposure induces peroxidation of protein and DNA, leading to reduced Leydig cells and testosterone levels, which results in reduced spermatogenesis. This is because testosterone, released by the Leydig cells, is an essential factor for the spermatogenesis process (36). Testosterone and E2 are necessary for normal reproductive function. TiO2NPs alter feedback regulation of estrogen synthesis, which reduces testosterone and E2. It has been suggested that alteration in serum testosterone during TiO2NP exposure leads to reduced spermatogenesis and has a pivotal role in activating apoptosis in the spermatogonial cells (28).
5. Conclusion
Overexpression of the MAPK signal pathway after TiO2NP exposure disrupts the structure and function of the BTB, which may damage spermatogenesis. In this study, TiO2NP exposure likely increased oxidative stress, affecting testicular function by activating the MAPK signaling pathway and disrupting the BTB, leading to male reproductive dysfunction. Exact TiO2NPs mechanisms are not clear; however, they may relate to oxidative stress. According to our data, we can conclude that sperm function may be disrupted through cytotoxic and genotoxic effects of TiO2NP administration. Further investigation is required to reveal the underlying mechanisms. Considering the widespread use of TiO2NPs and the reported detrimental effects of them, this public health issue should be the focus of attention.
Acknowledgments
The authors thank staff from the Biotechnology group of Yazd Research and Clinical Center for Infertility. This study was funded by Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Conflict of Interest
The authors have no financial or nonfinancial conflict of interest.
Full-Text: (342 Views)
1. Introduction
Titanium dioxide nanoparticles (TiO2NPs) have recently become an area of concern due to their use in many compounds from food and medicine to industrial applications (1). Their hydrophilic and photo-catalytic properties make them useful for many purposes. In particular, these two properties are useful for water and air purification (2). Over the past decades, TiO2NPs have been used to keep food fresh and as a white additive color for foods and cosmetic products (3, 4). It was previously shown that TiO2NPs exhibit low toxicity to humans and animals compared to other nanoparticles (5). However, more recent evidence demonstrates that TiO2NPs display some cytotoxic features.
It has been reported that TiO2NPs can cross into the testes and that the reproductive system is very sensitive to nanoparticles (6), which may have negative effects on the germ line either by direct effects on germ cells or indirect effects on somatic nursing cells that could lead to infertility (7). It has been shown that TiO2NPs can enter mouse Leydig cells and male germ line cells and change gene expression (8). Also, they can cause testicular damage and impaired spermatogenesis in male mice (9).
The quality of sperm parameters is related to spermatogenesis, and oxidative stress, the cytoskeleton and the blood-testis barrier (BTB) have critical roles in the maturation and development of spermatozoa. The BTB can restrict exogenous components from entering into the seminiferous tubules from the blood vessels, and makes a stable environment for germ cells and spermatid growth (10) and cause lower spermatogenesis (9). TiO2NPs can downregulate BTB junction-related proteins by activating the mitogen-activated protein kinases (MAPK) signaling pathways, which causes male reproductive dysfunction (10).
The exact mechanism of TiO2NP impact on spermatozoa dysfunction is still unknown. However, it is proposed that nanozied particles such as TiO2NPs can increase reactive oxygen species (ROS) in cells (11). ROS play a critical role in the function of sperm and germ line cells, which can reduce male fertility (12). Normal levels of ROS in seminiferous tubules can have detrimental effects on the fertilization rate by affecting sperm functions including sperm maturation, hyperactivation, capacitation and the acrosome reaction. Generation of ROS causes oxidative damage in molecular structures. High levels of ROS can damage the plasma membrane due to lipid peroxidation, protein denaturation and DNA damage (13).
Some studies suggest that TiO2NPs may disrupt endocrine functions and the regulation of the sex hormone profile (14, 15). It has been reported that TiO2NPs can induce accumulation of mutations and lead to genotoxicity in somatic cells. Intraperitoneal administration of TiO2NPs can significantly impact the reproductive system and increase sperm abnormalities (16).
Given the wide use of TiO2NPs, the purpose of this study was to examine the impact of TiO2NPs on spermatogenesis, as well as the histomorphometric cytotoxic effects of TiO2NPs in male mice. This study presents new data about sperm DNA integrity, sperm morphology and seminiferous tubule histopathology after daily oral TiO2NP administration.
2. Materials and Methods
2.1. Animal housing and treatment
In this experimental study, 8-12-wk-old NMRI male mice (35 ± 3 gr) were maintained in standard cages and animal house conditions (normal pellets and water ad libitum, humidity 60 ± 10%, room temperature 23 ± 2oC, and 12 hr light/dark cycle) in Yazd Reproductive Sciences Institute, Yazd, Iran. TiO2NPs (anatase/rutile, 99+%, 20 nm, Nanosony Co, Iran) were dissolved in water and the suspension was homogenized with a sonicator for 10 min. The TiO2NP suspension was prepared at the concentrations of 2.5, 5 and 10 mg/kg just before use in water (9). The mice were divided into four groups (n = 8/each group), including a control group and three treated groups (17). The control group received water intragastrically. The group I, group II and group II treated groups were fed orally with 2.5, 5 and 10 mg/kg/day TiO2NPs for 40 days, respectively (9).
After the 40 days (which is longer than needed for spermatogenesis in mice), the mice were sacrificed by cervical dislocation and the caudal epididymis was transferred into a petri dish. The testis was separated and fixed in Bouin’s solution for histological assessment. The suspension containing caudal epididymis and 1000 µl of Ham's F10 medium (Vitrolife, Gothenburg, Sweden) was incubated for 30 min at 37oC in a humidified atmosphere of 5% CO2 (17). After 30 min, sperm parameters were analyzed, and sperm DNA integrity and chromatin quality were assessed using standard procedures. All assays were summarized in figure 1.
2.2. Sperm parameters assessment
Sperm count was assessed by Makler’s counting chamber. To assess sperm motility, the percentages of sperm motility were classified into three classes of progressive, non-progressive and immotile sperm. Papanicolaou (Merck, Germany) and eosin-nigrosin staining (Merck, Germany) were used to examine sperm morphology and viability, respectively (18). In all groups, sperm viability was evaluated 30 min after sperm motility assessment. Sperm morphological abnormalities were classified as head (size, shape, double head, acrosomal section), neck (bent, cytoplasmic droplets) and tail (bent, coiled, double tail, cytoplasmic droplets). Eosin-nigrosin staining was used to assess sperm viability; white (unstained) and pink (or red) sperm heads were classified as live and dead sperm, respectively. To evaluate sperm motility, morphology and viability, 200 sperm were evaluated under a light microscope at x400 magnification (Motic, Spain).
2.3. Assessment of sperm DNA integrity and chromatin packaging quality
2.3.1. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
For detection of sperm DNA fragmentation, the TUNEL assay was applied. The slides were fixed with 4% paraformaldehyde, washed for 1 hr in phosphate-buffered saline, and then treated with 3% H2O2 and 0.1% Triton X-100 for 15 and 20 min, respectively. Afterward, the slides were incubated with enzyme (Roche, Germany): label solution (5:45 µl) for 1 hr. For positive and negative controls, the slides were incubated with DNase I grade I (3 U/ml in 50 mM Tris-HCl, pH 7.5, 1 mg/ml BSA) and 50 µl of label solution (without terminal transferase), respectively. Finally, for each slide, 200 sperm were evaluated under a fluorescence microscope at x400 magnification (Olympus BX51, Japan) (17) and were counted.
2.3.2. Chromomycin A3 staining
Protamine deficiency in sperm chromatin can be detected using chromomycin A3 assay. Methanol: glacial acetic acid (3:1) was used to fix the air-dried slides in a refrigerator. The fixed slides were treated with 100 µl of chromomycin A3 solution (Sigma, Germany) for 10 min and then, the slides were washed with McIlvain's buffer. For each slide, 200 sperm were evaluated under a fluorescent microscope at x400 magnification (Olympus BX51, Japan) with a 460-470 nm filter (17).
2.3.3. Toluidine blue (TB) staining
TB staining was used to determine chromatin condensation and DNA integrity via binding to phosphate groups of DNA strands. Ethanol: acetone (1:1) was used to fix the slides at 4oC for 30 min; these were then hydrolyzed in 0.1N HCl for 5 min. Then, the slides were rinsed three times in distilled water and finally, the samples were stained in 5% TB (Sigma, Germany) in 50% McIlvain's citrate phosphate buffer (pH: 3.5) (17). For each slide, 200 sperm were evaluated under a light microscope at x400 magnification (Olympus BX51, Japan).
2.3.4. Aniline blue (AB) staining
The acidic staining of AB reacts with lysine residues in histone proteins and leads to detection of sperm chromatin remodeling (19). 3% buffered glutaraldehyde in 0.2 M phosphate buffer (pH: 7.2) was used to fix the air-dried smears for 30 min. Then, the slides were stained with 5% AB (Sigma, Germany) in 4% acetic acid (pH: 3.5) for 5 min. Finally, the slides were washed in distilled water and 200 sperm were evaluated under a light microscope at x400 magnification (Olympus BX51, Japan).
2.4. Histomorphometric evaluation and cell numbers of seminiferous tubules
The five testis samples were fixed in Bouin’s solution for each group. Tissues were dehydrated in a graded alcohol series, cleared in xylene and embedded in paraffin wax. Samples were cut in 7 m (five slices from each testis) and then glass slides were stained with hematoxylin and eosin. A histopathologist unaware of the treatments evaluated the histological slides after hematoxylin-eosin staining. Total diameters, lumen diameter and cellular diameter (µm), luminal area, cellular area and cross-sectional area (×104 μm2) and numerical density (20) were measured using stereological methods under an optical microscope at x100 magnification (Olympus BX51, Japan). The mean seminiferous tubule diameter, and cross-sectional and numerical density area were calculated (21). For each slide, the mean of 10 random fields was calculated for measurements of Sertoli, Leydig, spermatogonia, primary spermatocyte and spermatid cell numbers (22) at x400 magnification. For the TUNEL assay in the testes for detection of cell apoptosis, sections were dewaxed in xylene and rehydrated through a graded series of ethanol, then washed for 1 hr in phosphate-buffered saline. The assay was performed according to the manufacturer’s instructions; sperm DNA fragmentation was detected under a fluorescence microscope (Olympus BX51, Japan) (23).
2.5. Plasma sex hormone levels
Serum samples were evaluated for their content of sex hormones (luteinizing hormone, follicle stimulating hormone, estradiol (E2) and testosterone), using the radioimmunoassay technique (DIA source Immuno-assays, S.A., Belgium), according to the manufacturer’s instructions (20).
2.6. Malondyaldehyde (MDA) assay
Seminal MDA levels were measured using a method that was based on a thiobarbituric acid (TBA) reaction and extraction with normal butanol (24), and 31.35 mg MDA (1 mM) and 57.66 mg TBA (4.0 mM) separately were dissolved in 100 mL glacial acetic acid. Semen was mixed with MDA and TBA solutions (1:1). After that, the seminal mixture was boiled in a water bath at 95°C for 60 min. The mixture was cooled at room temperature and spectrophotometric detection of absorbance was done at 532 nm wavelength and compared with a standard curve (25).

2.7. Ethical considerations
Animal treatment methods were approved by the Ethics Committee of Yazd Reproduction Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran (Code: IR-SSU-RSI-REC-2169).
2.8. Statistical analysis
The Shapiro-Wilk test was applied to assess the normal distribution of the data. One-way ANOVA followed by Tukey post hoc test were used for comparison of the data between the different groups using the Statistical Package for the Social Sciences (SPSS), version 20 (SPSS Inc., USA). The data were presented as mean ± standard deviation. Analyses were considered as statistically significant at p < 0.05.
3. Results
3.1. Sperm parameter assessment
As shown in table I, the sperm count was lower in group III when compared to the control group, but this was not statistically significant (p = 0.49). It was found that the percentage of progressive motility was significantly higher in the control group than in groups II and III p ≤ 0.001 for both). The percentage of progressive motility was approximately 14% and 15% lower in the groups that received 5 and 10 mg/kg, respectively, compared to the control group. It was clear that there were no significant effects of different concentrations of TiO2NPs on the non-progressive sperm in the treated groups compared to the controls (p = 0.07). Moreover, the treatment of mice with different dosages of TiO2NPs was associated with a higher percentage of immotile sperm in groups II and III, compared to the control group (p ≤ 0.001). Normal morphology (Figure 2A) was significantly lower in all treated groups compared to the control group (Table I). In addition, sperm viability (Figure 2B) in groups II and III was approximately 16% and 22% less than in the control group (Table I).
3.2. Sperm DNA integrity and chromatin packaging quality
In the TUNEL assay, the apoptotic sperm cells exhibited intensive and brilliant fluorescent green, while the natural cells displayed pale and opaque green (Figure 2C). As shown in table II, a slight difference was observed in groups II and III compared to the control group in the TUNEL assay (p = 0.04) (Figure 2D). The results of CMA3 staining showed higher levels of abnormal sperm in group III in comparison with the control group (p = 0.05.( In TB staining, sperm with normal chromatin were seen as colorless but sperm with chromatin abnormalities were observed as dark blue (Figure 2E). In AB staining, sperm with a dark blue and unstained nucleus were considered as having abnormal and normal chromatin, respectively (Figure 2F). The rate of abnormal chromatin in the AB and TB staining was higher in all treated groups compared with the control group, but this difference was not significant (p = 0.08 and p = 0.09, respectively) (Table II).
3.3. Stereological analysis
TiO2NP exposure was associated with differences in spermatogonia numbers compared to the control group (p < 0.01). Significantly fewer spermatogonia were found in groups I, II and III compared to the control group. Groups II and III displayed fewer primary spermatocytes and spermatid cells compared to controls 40 days after TiO2NP exposure (p = 0.02). There were significantly fewer Sertoli and Leydig cells in groups II and III (p ≤ 0.001). Overall, a significantly lower spermatogenesis index (p = 0.03) was observed in group III, but not in groups I and II, in comparison with the control group. The results of the histopathology assessments are shown in figure 3.
In groups I, II and III, the lumen diameter was significantly longer compared to in the control animals (p ≤ 0.001) (Figure 4A), and also there was a significantly larger lumen area in groups II and III (Figure 4B). The cellular diameters and area of the seminiferous tubules were smaller in groups I, II and III (Figures 4C and D). The total diameter and cross-sectional area were not varied across the groups (Figures 4E and F). However, there was a smaller number of tubules and density in all exposed groups compared to the control group, though this was not significant (Figures 4G and H). The results of the lumen studies are shown in figures 4 and 5. Also, there were significantly more apoptotic cells in the seminiferous tubules of groups II and III compared to in the control group (p = 0.02) (Table II).
3.4. Sex hormone levels and MDA
The effects of TiO2NPs on serum hormones of male mice are shown in table III. Following TiO2NP exposure, luteinizing hormone, follicle stimulating hormone and E2 levels were not different in the groups, whereas testosterone was markedly lower in group III compared with the control animals (p ≤ 0.001). The percentage of MDA in the group III seminal fluid was higher when compared with the control group (Table III).







4. Discussion
It has been suggested that industrial NPs may increase the risk and incidence of reproductive disorders, and that occupational and environmental exposure to TiO2NPs may be one of the risk factors for increased infertility in humans (6). The present results showed that the most abnormal morphology was observed in sperm tails and the percentage of abnormal heads and necks was approximately six-fold higher in group III than in the control group. There were significantly more apoptotic sperm and the rate of abnormal chromatin in CMA3, AB and TB staining was significantly higher in the groups exposed to TiO2NPs, compared with the controls. A significantly lower spermatogenesis index, lumen parameters and testosterone level were observed and the percentage of MDA in seminal fluid was higher as well.
In 2020, a study reported that the administration of TiO2NPs led to negative effects on sperm parameters including count, motility and morphology (26). This was similar to the results of the present study. In another study, data showed that exposure to TiO2NPs and zinc oxide nanoparticles was associated with decreased sperm number and motility and with increased abnormal sperm in experimental mice (16). They concluded that TiO2NPs may pass through the BTB and create harmful damage to testes and sperm.
Small amounts of ROS are necessary for normal sperm functions such as capacitation and the acrosome reaction. High ROS levels can reduce normal sperm morphology and motility by peroxidation of spermatozoa mitochondria and flagellum structure. It has been shown that TiO2NPs produce ROS in animal models (13). It reported that high levels of ROS in seminal fluid have a positive correlation with abnormal sperm morphology (27). Our findings were similar to another study which demonstrated that sperm parameters (count, motility, viability and morphology) were reduced in the epididymis tail after frequent consumption and high dosage of TiO2NPs in male mice (28).
In the present study, MDA in seminal fluid and sperm viability were significantly lower in group III. We suggest that TiO2NPs may induce ROS generation in the testis, and that high levels of ROS are responsible for the increase in abnormal sperm morphology and reduced sperm viability. Another study showed that sperm flagellum abnormalities were significantly more common in the treated groups vs. the controls after 1, 2 or 5 days (29). To the best of our knowledge, this is the first study to analyze abnormal sperm morphology characteristics separately in three classifications (head, neck and tail defects) after TiO2NP exposure.
The BTB provides a unique environment for spermatogenesis and to control the transportation of components from vessels to the seminiferous tubules. It has been found that TiO2NPs activate the MAPK signal pathways, which decreases BTB junction-related proteins and finally destroys the normal BTB structure. Furthermore, MAPK signal pathways interfere with some cellular processes such as cell proliferation, cell differentiation, and apoptosis. It has been reported that activation of MAPK signal pathways results in related oxidative stress as well (28). It is known that TiO2NPs can induce apoptosis in testicular germ cells and epididymal sperm in laboratory animals (30, 31). This is the first study to examine the effects of TiO2NPs on mouse sperm DNA fragmentation by TUNEL assay and chromatin condensation tests including CMA3, AB, and TB. It has previously been shown that sperm chromatin defects are associated with apoptosis (32). Our results showed that the rate of AB positive sperm in all treated groups was higher than in the control group, but this was not significant. Consequently, it can be concluded that TiO2NPs probably do not substantially affect the histone profile of sperm chromatin when exposed to a concentration of 10 mg/kg for a short time, and a higher concentration may be needed. It can be hypothesized that the lower quality of sperm chromatin observed was probably caused by the toxic effects of TiO2NPs and hormonal profile defects.
It reported that exposure to TiO2NPs in different doses for 60 sequential days in male animals can cause many defects in testicular tissue, Sertoli cells and spermatogenic cell lines including elevation in apoptotic cells and variation in seminiferous tubule diameter (33). Apoptosis has an important role during spermiogenesis and proliferation of germ cells with the surrounding Sertoli cells (34). In one study, apoptotic cells in seminiferous tubules and interstitial spaces increased, and interstitial spaces revealed enlarged testicular histology after intraperitoneal injection of 2.5 and 5 mg/kg over 1, 2, 3, and 5 wk (29). In another, in the seminiferous tubules, the spermatogenic cell layers reduced in the groups with 50 and 250 mg/kg of body weight compared to the control group, but no histological changes were detected (35).
It has been reported that 30-80% of male infertility is related to oxidative stress (12). In mice, oxidative stress after TiO2NP exposure induces peroxidation of protein and DNA, leading to reduced Leydig cells and testosterone levels, which results in reduced spermatogenesis. This is because testosterone, released by the Leydig cells, is an essential factor for the spermatogenesis process (36). Testosterone and E2 are necessary for normal reproductive function. TiO2NPs alter feedback regulation of estrogen synthesis, which reduces testosterone and E2. It has been suggested that alteration in serum testosterone during TiO2NP exposure leads to reduced spermatogenesis and has a pivotal role in activating apoptosis in the spermatogonial cells (28).
5. Conclusion
Overexpression of the MAPK signal pathway after TiO2NP exposure disrupts the structure and function of the BTB, which may damage spermatogenesis. In this study, TiO2NP exposure likely increased oxidative stress, affecting testicular function by activating the MAPK signaling pathway and disrupting the BTB, leading to male reproductive dysfunction. Exact TiO2NPs mechanisms are not clear; however, they may relate to oxidative stress. According to our data, we can conclude that sperm function may be disrupted through cytotoxic and genotoxic effects of TiO2NP administration. Further investigation is required to reveal the underlying mechanisms. Considering the widespread use of TiO2NPs and the reported detrimental effects of them, this public health issue should be the focus of attention.
Acknowledgments
The authors thank staff from the Biotechnology group of Yazd Research and Clinical Center for Infertility. This study was funded by Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Conflict of Interest
The authors have no financial or nonfinancial conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Andrology
References
1. Akasaka H, Mukumoto N, Nakayama M, Wang T, Yada R, Shimizu Y, et al. Investigation of the potential of using TiO 2 nanoparticles as a contrast agent in computed tomography and magnetic resonance imaging. Appl Nanosci 2020; 10: 3143-3148. [DOI:10.1007/s13204-019-01098-y]
2. Powell JJ, Faria N, Thomas-McKay E, Pele LC. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J Autoimmun 2010; 34: J226-J233. [DOI:10.1016/j.jaut.2009.11.006] [PMID]
3. Chaudhry Q, Scotter M, Blackburn J, Ross B, Boxall A, Castle L, et al. Applications and implications of nanotechnologies for the food sector. Food Addit Contam 2008; 25: 241-258. [DOI:10.1080/02652030701744538] [PMID]
4. Peters RJB, van Bemmel G, Herrera-Rivera Z, Helsper HPFG, Marvin HJP, Weigel S, et al. Characterization of titanium dioxide nanoparticles in food products: Analytical methods to define nanoparticles. J Agric Food Chem 2014; 62: 6285-6293. [DOI:10.1021/jf5011885] [PMID]
5. Zhao L, Zhu Y, Chen Z, Xu H, Zhou J, Tang S, et al. Cardiopulmonary effects induced by occupational exposure to titanium dioxide nanoparticles. Nanotoxicology 2018; 12: 169-184. [DOI:10.1080/17435390.2018.1425502] [PMID]
6. Wang R, Song B, Wu J, Zhang Y, Chen A, Shao L. Potential adverse effects of nanoparticles on the reproductive system. Int J Nanomedicine 2018; 13: 8487-8506. [DOI:10.2147/IJN.S170723] [PMID] [PMCID]
7. Braydich-Stolle L, Hussain S, Schlager JJ, Hofmann MC. In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicol Sci 2005; 88: 412-419. [DOI:10.1093/toxsci/kfi256] [PMID] [PMCID]
8. Asare N, Instanes Ch, Sandberg WJ, Refsnes M, Schwarze P, Kruszewski M, et al. Cytotoxic and genotoxic effects of silver nanoparticles in testicular cells. Toxicology 2012; 291: 65-72. [DOI:10.1016/j.tox.2011.10.022] [PMID]
9. Gao G, Ze Y, Zhao X, Sang X, Zheng L, Ze X, et al. Titanium dioxide nanoparticle-induced testicular damage, spermatogenesis suppression, and gene expression alterations in male mice. J Hazard Mater 2013; 258: 133-143. [DOI:10.1016/j.jhazmat.2013.04.046] [PMID]
10. Lu Y, Luo B, Li J, Dai J. Perfluorooctanoic acid disrupts the blood-testis barrier and activates the TNFα/p38 MAPK signaling pathway in vivo and in vitro. Arch Toxicol 2016; 90: 971-983. [DOI:10.1007/s00204-015-1492-y] [PMID]
11. Zhu Z, Kawai T, Umehara T, Hoque SM, Zeng W, Shimada M. Negative effects of ROS generated during linear sperm motility on gene expression and ATP generation in boar sperm mitochondria. Free Radic Biol Med 2019; 141: 159-171. [DOI:10.1016/j.freeradbiomed.2019.06.018] [PMID]
12. Layali E, Tahmasbpour E, Jorsaraei SGhA. Effects of silver nanoparticles on lipid peroxidation and quality of sperm parameters in male rats. J Babol Univ Med Sci 2016; 18: 48-55.
13. Aitken RJ, Smith TB, Jobling MS, Baker MA, De Iuliis GN. Oxidative stress and male reproductive health. Asian J Androl 2014; 16: 31-38. [DOI:10.4103/1008-682X.122203] [PMID] [PMCID]
14. Tassinari R, Cubadda F, Moracci G, Aureli F, D'Amato M, Valeri M, et al. Oral, short-term exposure to titanium dioxide nanoparticles in Sprague-Dawley rat: Focus on reproductive and endocrine systems and spleen. Nanotoxicology 2014; 8: 654-662. [DOI:10.3109/17435390.2013.822114] [PMID]
15. Mohammadi Fartkhooni F, Noori A, Momayez M, Sadeghi L, Shirani K, Yousefi Babadi V. The effects of nano titanium dioxide (TiO2) in spermatogenesis in wistar rat. Euro J Exp Bio 2013; 3: 145-149.
16. Ogunsuyi OM, Ogunsuyi OI, Akanni O, Alabi OA, Alimba CG, Adaramoye OA, et al. Alteration of sperm parameters and reproductive hormones in Swiss mice via oxidative stress after co‐exposure to titanium dioxide and zinc oxide nanoparticles. Andrologia 2020; 52: e13758. [DOI:10.1111/and.13758] [PMID]
17. Sabour M, Khoradmehr A, Kalantar SM, Danafar AH, Omidi M, Halvaei I, et al. Administration of high dose of methamphetamine has detrimental effects on sperm parameters and DNA integrity in mice. Int J Reprod Biomed 2017; 15: 161-168. [DOI:10.29252/ijrm.15.3.161] [PMID] [PMCID]
18. Seed J, Chapin RE, Clegg ED, Dostal LA, Foote RH, Hurtt ME, et al. Methods for assessing sperm motility, morphology, and counts in the rat, rabbit, and dog: A consensus report. Reprod Toxicol 1996; 10: 237-244. [DOI:10.1016/0890-6238(96)00028-7]
19. Nazar M, Talebi AR, Sharifabad MH, Abbasi A, Khoradmehr A, Danafar AH. Acute and chronic effects of gold nanoparticles on sperm parameters and chromatin structure in Mice. Int J Reprod Biomed 2016; 14: 637-642. [DOI:10.29252/ijrm.14.10.637] [PMID] [PMCID]
20. Bahaodini A, Owjfard M, Tamadon A, Jafari SM. Low frequency electromagnetic fields long-term exposure effects on testicular histology, sperm quality and testosterone levels of male rats. Asian Pac J Reprod 2015; 4: 195-200. [DOI:10.1016/j.apjr.2015.06.001]
21. Panahi M, Keshavarz S, Rahmanifar F, Tamadon A, Mehrabani D, Karimaghai N, et al. Busulfan induced azoospermia: Stereological evaluation of testes in rat. Vet Res Forum 2015; 6: 273-278.
22. Panahi M, Karimaghai N, Rahmanifar F, Tamadon A, Vahdati A, Mehrabani D, et al. Stereological evaluation of testes in busulfan-induced infertility of hamster. Comparat Clin Pathol 2015; 24: 1051-1056. [DOI:10.1007/s00580-014-2029-0]
23. Khoradmehr A, Danafar AH, Halvaei I, Golzadeh J, Hosseini M, Mirjalili T, et al. Effect of prenatal methamphetamine administration during gestational days on mice. Iran J Reprod Med 2015; 13: 41-48.
24. Bandegi L, Anvari M, Vakili M, Khoradmehr A, Mirjalili A, Talebi AR. Effects of antidepressants on parameters, melondiadehyde, and diphenyl-2-picryl-hydrazyl levels in mice spermatozoa. Int J Reprod Biomed 2018; 16: 365-372. [DOI:10.29252/ijrm.16.6.365] [PMID] [PMCID]
25. Zeb A, Ullah F. A simple spectrophotometric method for the determination of thiobarbituric acid reactive substances in fried fast foods. J Anal Method Chem 2016; 2016: 9412767. [DOI:10.1155/2016/9412767] [PMID] [PMCID]
26. Keramati Khiarak B, Karimipour M, Ahmadi A, Farjah GhH. Effects of oral administration of titanium dioxide particles on sperm parameters and in vitro fertilization potential in mice: A comparison between nano-and fine-sized particles. Vet Res Forum 2020; 11: 401-408.
27. Sabeti P, Pourmasumi S, Rahiminia T, Akyash F, Talebi AR. Etiologies of sperm oxidative stress. Int J Reprod Biomed 2016; 14: 231-240. [DOI:10.29252/ijrm.14.4.231] [PMID] [PMCID]
28. Lu T, Ling Ch, Hu M, Meng X, Deng Y, An H, et al. Effect of nano-titanium dioxide on blood-testis barrier and MAPK signaling pathway in male mice. Biol Trace Elem Res 2021; 199: 2961-2971. [DOI:10.1007/s12011-020-02404-4] [PMID]
29. Smith MA, Michael R, Aravindan RG, Dash S, Shah SI, Galileo DS, et al. Anatase titanium dioxide nanoparticles in mice: Evidence for induced structural and functional sperm defects after short-, but not long-, term exposure. Asian J Androl 2015; 17: 261-268. [DOI:10.4103/1008-682X.143247] [PMID] [PMCID]
30. Morgan AM, Abd El-Hamid MI, Noshy PA. Reproductive toxicity investigation of titanium dioxide nanoparticles in male albino rats. World J Pharm Pharmaceut Sci 2015; 4: 34-49.
31. Hong F, Si W, Zhao X, Wang L, Zhou Y, Chen M, et al. TiO2 nanoparticle exposure decreases spermatogenesis via biochemical dysfunctions in the testis of male mice. J Agric Food Chem 2015; 63: 7084-7092. [DOI:10.1021/acs.jafc.5b02652] [PMID]
32. Ribas‐Maynou J, García‐Peiró A, Fernández‐Encinas A, Abad C, Amengual M, Prada E, et al. Comprehensive analysis of sperm DNA fragmentation by five different assays: TUNEL assay, SCSA, SCD test and alkaline and neutral Comet assay. Andrology 2013; 1: 715-722. [DOI:10.1111/j.2047-2927.2013.00111.x] [PMID]
33. Han Zh, Yan Q, Ge W, Liu ZG, Gurunathan S, De Felici M, et al. Cytotoxic effects of ZnO nanoparticles on mouse testicular cells. Int J Nanomedicine 2016; 11: 5187-5203. [DOI:10.2147/IJN.S111447] [PMID] [PMCID]
34. Liu B, Wu SD, Shen LJ, Zhao TX, Wei Y, Tang XL, et al. Spermatogenesis dysfunction induced by PM2. 5 from automobile exhaust via the ROS-mediated MAPK signaling pathway. Ecotoxicol Environ Saf 2019; 167: 161-168. [DOI:10.1016/j.ecoenv.2018.09.118] [PMID]
35. Jia F, Sun Z, Yan X, Zhou B, Wang J. Effect of pubertal nano-TiO2 exposure on testosterone synthesis and spermatogenesis in mice. Arch Toxicol 2014; 88: 781-788. [DOI:10.1007/s00204-013-1167-5]
36. Boekelheide K, Fleming SL, Johnson KJ, Patel SR, Schoenfeld HA. Role of Sertoli cells in injury‐associated testicular germ cell apoptosis. Proc Soc Exp Biol Med 2000; 225: 105-115.
https://doi.org/10.1046/j.1525-1373.2000.22513.x [DOI:10.1111/j.1525-1373.2000.22513.x] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





