Tue, Jul 8, 2025
[Archive]
Volume 20, Issue 3 (March 2022)
IJRM 2022, 20(3): 177-184 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shiravani Z, Atbaei S, Namavar Jahromi B, Hajisafari Tafti M, Moradi Alamdarloo S, Poordast T, et al . Comparing four different methods for the management of ectopic pregnancy: A cross-sectional study. IJRM 2022; 20 (3) :177-184
URL: http://ijrm.ir/article-1-2000-en.html
URL: http://ijrm.ir/article-1-2000-en.html
Zahra Shiravani1 

 , Sana Atbaei2
, Sana Atbaei2 

 , Bahia Namavar Jahromi3
, Bahia Namavar Jahromi3 

 , Mojgan Hajisafari Tafti4
, Mojgan Hajisafari Tafti4 

 , Shaghayegh Moradi Alamdarloo2
, Shaghayegh Moradi Alamdarloo2 

 , Tahereh Poordast2
, Tahereh Poordast2 

 , Adel Noori5
, Adel Noori5 

 , Sedighe Forouhari6
, Sedighe Forouhari6 

 , Soudabeh Sabetian *7
, Soudabeh Sabetian *7 




 , Sana Atbaei2
, Sana Atbaei2 

 , Bahia Namavar Jahromi3
, Bahia Namavar Jahromi3 

 , Mojgan Hajisafari Tafti4
, Mojgan Hajisafari Tafti4 

 , Shaghayegh Moradi Alamdarloo2
, Shaghayegh Moradi Alamdarloo2 

 , Tahereh Poordast2
, Tahereh Poordast2 

 , Adel Noori5
, Adel Noori5 

 , Sedighe Forouhari6
, Sedighe Forouhari6 

 , Soudabeh Sabetian *7
, Soudabeh Sabetian *7 


1- Gynecology Oncology Division, Department of Obstetrics and Gynecology, School of Medicine, Shiraz University of Medical Science, Shiraz, Iran. Maternal-Fetal Medicine Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
2- Department of Obstetrics and Gynecology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
3- Department of Obstetrics and Gynecology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran. Infertility Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
4- Department of Obstetrics and Gynecology, School of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
5- Shiraz University of Medical Sciences, Shiraz, Iran.
6- Infertility Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
7- Infertility Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. ,soudabehsabet@gmail.com
2- Department of Obstetrics and Gynecology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
3- Department of Obstetrics and Gynecology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran. Infertility Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
4- Department of Obstetrics and Gynecology, School of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
5- Shiraz University of Medical Sciences, Shiraz, Iran.
6- Infertility Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
7- Infertility Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. ,
Full-Text [PDF 417 kb]
(1223 Downloads)
| Abstract (HTML) (1639 Views)
Full-Text: (461 Views)
1. Introduction
Ectopic pregnancy (EP) is defined as implantation of a blastocyst outside the uterine cavity (1). It is a major reason for maternal morbidity and mortality during the first trimester (2). The incidence is about 2% of all pregnancies (3). Although the etiology of EP is not identified clearly, several risk factors such as tubal infection, previous tubal surgery and previous EP increase the probability of this occurrence (4). Usually, an EP is located in one of the fallopian tubes and requires emergent treatment; as it gets large, it can cause tubal rupture due to heavy internal bleeding and hypovolemic shock (5). Today, the options for the treatment of EP include surgery, medical treatment and expectant management (6). Surgery treatment includes salpingectomy, salpingotomy and salpingostomy, which can be performed by laparotomy or laparoscopy (7). Laparoscopy has significant advantages such as shorter hospital stays, quicker recovery and lower cost than laparotomy. Sometimes laparotomy is preferred because of easier access to tissue in hemodynamically unstable cases (8).
In women who are hemodynamically stable, without any sign of rupture of EP, systemic administration of intramuscular methotrexate (MTX) is acceptable by single-dose (SD), double-dose (DD) or multiple dose protocols (9). MTX is contraindicated with renal, hepatic, gastrointestinal or haemopoietic disorders (10). Weekly beta-human chorionic gonadotropin (β-hCG) monitoring follow-up is recommended until levels decline below 15 mIU/ml. If medical treatment is deemed unsuccessful, the patients will need surgical treatment (11).
It is known that some cases of tubal EP with no significant symptoms resolve spontaneously. Therefore, in selected women who are clinically stable with an undiagnosed EP location in ultrasound and a β-hCG less than 1000 mIU/ml, expectant management is a valuable option of treatment.
As there is no consensus on the best management for EP, we aimed to compare the different protocols for treatment of EP in terms of the gestational age, size of ectopic mass, duration of hospital stay, and history of pelvic surgery, pelvic infection, infertility, abortion and EP, as well as site of EP and β-hCG level. In order to discriminate properly between double-dose methotrexate (DD-MTX) and surgery management, we plotted receiver operating characteristics (ROC) curves to determine cut-off points for β-hCG levels and size of ectopic mass.
2. Materials and Methods
2.1. Study design
In this cross-sectional study, the data of 365 women aged between 15-44 yr with the diagnosis of EP were reviewed between March 2017 and March 2019 in hospitals affiliated to Shiraz University of Medical Sciences, Shiraz, Iran.
2.2. Inclusion and exclusion criteria
Women aged 15-44 yr who were diagnosed with EP were included. EP was diagnosed if the serum β-hCG level had a slower doubling time on serial measurement and the gestational sac was not seen in the uterine cavity by transvaginal sonography. The subjects who refused to complete their treatment in the current center or had incomplete historical data in the hospital records, were excluded.
2.3. Data collection
Data on participants’ demographic, medical and clinical characteristics (age, gestational age, size and location of ectopic mass, duration of hospital stay, serial β-hCG level, type of surgery, and history of EP, infertility, pelvic infection, and pelvic surgery were retrieved from hospital records. The cases were classified according to treatment methods and based on the success of the treatment protocols (response vs. failure).
Surgery was the first plan for the cases with: 1) severe free fluid in the abdominal pelvic cavity and who were unstable hemodynamically; 2) severe abdominal pain; 3) based on participant’s preference; or 4) contraindication for medical treatment. The remaining cases were treated by SD- or DD-MTX (50 mg/m2, injected intramuscularly; Austria, Ebewe company) on day one, or days one and four, respectively. If the β-hCG level declined ≥ 15% between days four and seven, the MTX therapy was considered to be successful; otherwise, the management was assumed to have failed. Some cases with the criteria of clinical stability were treated by expectant management.
2.4. Ethical considerations
The local Medical Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran approved the study protocol (Code: IR.SUMS.REC.1395.S940). All of the enrolled participants signed a written informed consent form after complete explanation of the study design.
2.5. Statistical analysis
The Statistical Package for the Social Sciences (SPSS) version 22 was used to analyze the data. One-way analysis of variance (ANOVA) was used to statistically compare the means of the four treatment groups. Tukey’s test was used for a pairwise comparison of all means. The difference between 2 groups was analyzed by independent sample t test. Chi-square test was performed to determine whether there were any statistically significant differences between the descriptive data. Quantitative data were expressed as mean ± SD and the qualitative data were represented as frequencies or percentages. A p-value < 0.05 was considered as significant. ROC curves were generated to assess the optimal cut-off value for the size of ectopic mass and the initial β-hCG that discriminated between DD-MTX and surgery management. The cut-off points were denoted by the value that had the largest sum of sensitivity and specificity.
3. Results
Among the 365 women with EP, 87 cases were treated by SD-MTX, 43 by DD-MTX, 154 by surgery, and 81 by expectant management.
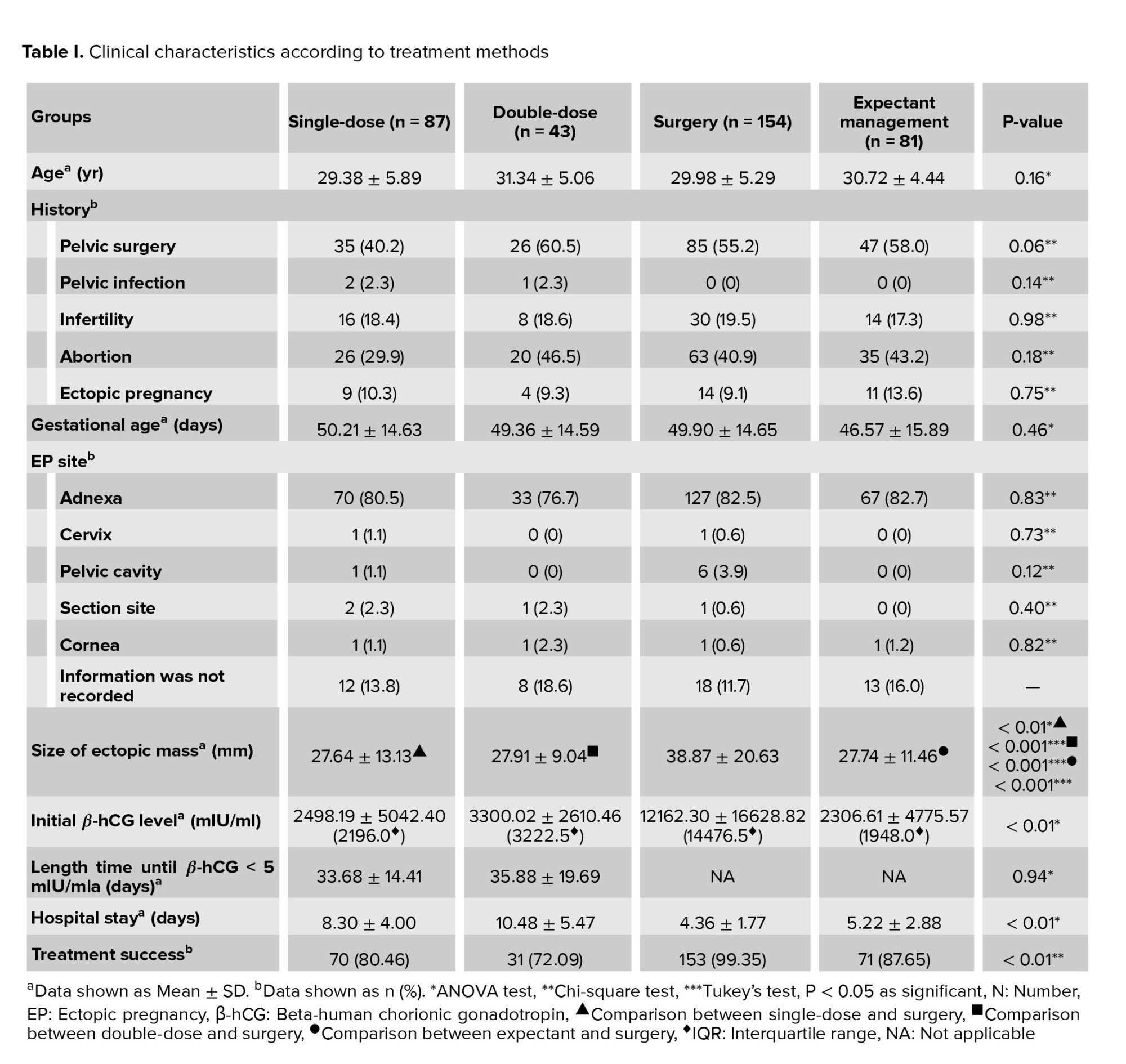
According to our results, the most common site of EP was right and left adnexa and there were no significant differences between different treatments. The women who underwent surgery had the largest ectopic mass, the highest β-hCG level, and the shortest hospital stay (p < 0.01) (Table I).
Surgery had the highest success rate (99.35%) and DD-MTX had the lowest (72.09%) (Table I). Among the surgery group, the treatment failed for only one case who had laparoscopy-salpingostomy.
The ROC analysis is presented in figure 1. The areas under the curve for the size of ectopic mass and β-hCG level were statistically significant (p < 0.01). The ROC curves suggested that a size of ectopic mass > 34.50 mm with 42% sensitivity and 67% specificity, and a β-hCG level > 6419 mIU/ml with 47% sensitivity and 90% specificity were suitable cut-off points for selecting surgery as the treatment plan.
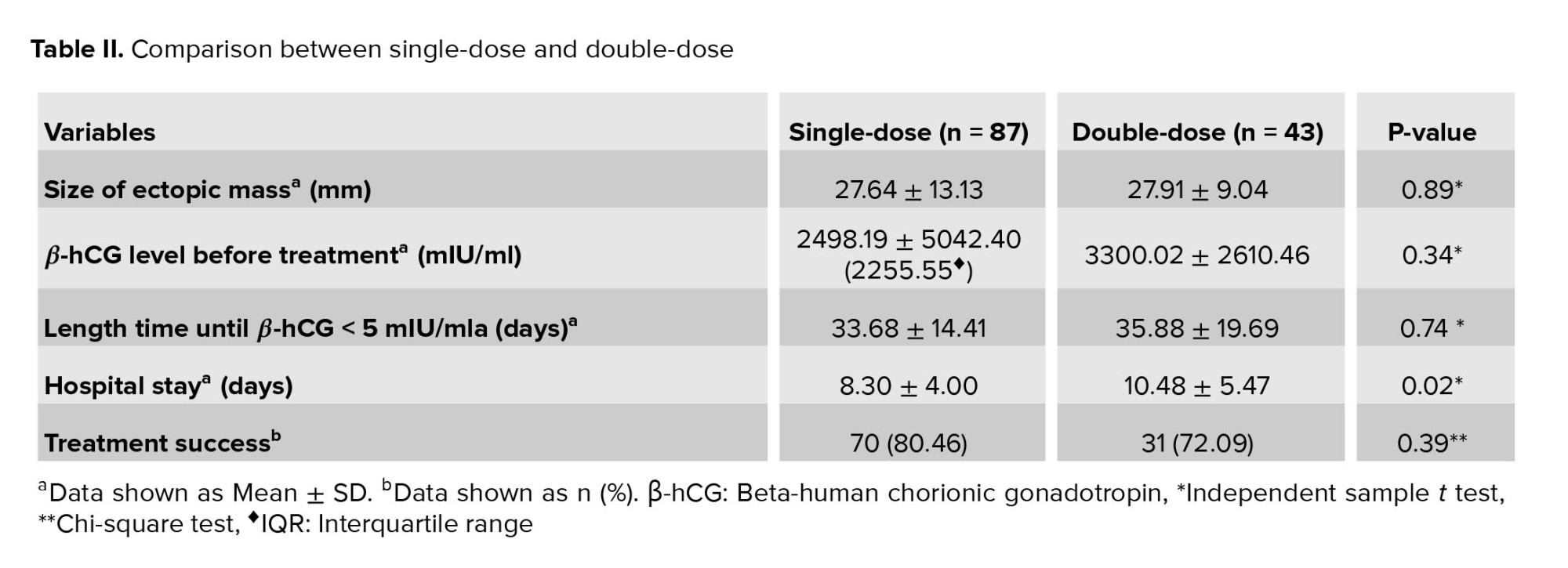
The results of comparison between the SD- and DD-MTX groups are summarized in table II. There were no significant differences in the size of ectopic mass, β-hCG level, duration to β-hCG < 5 mIU/ml or treatment success between the two groups. Hospital stays in the SD-MTX group were significantly shorter than in the DD-MTX group (p = 0.02) (Table II).
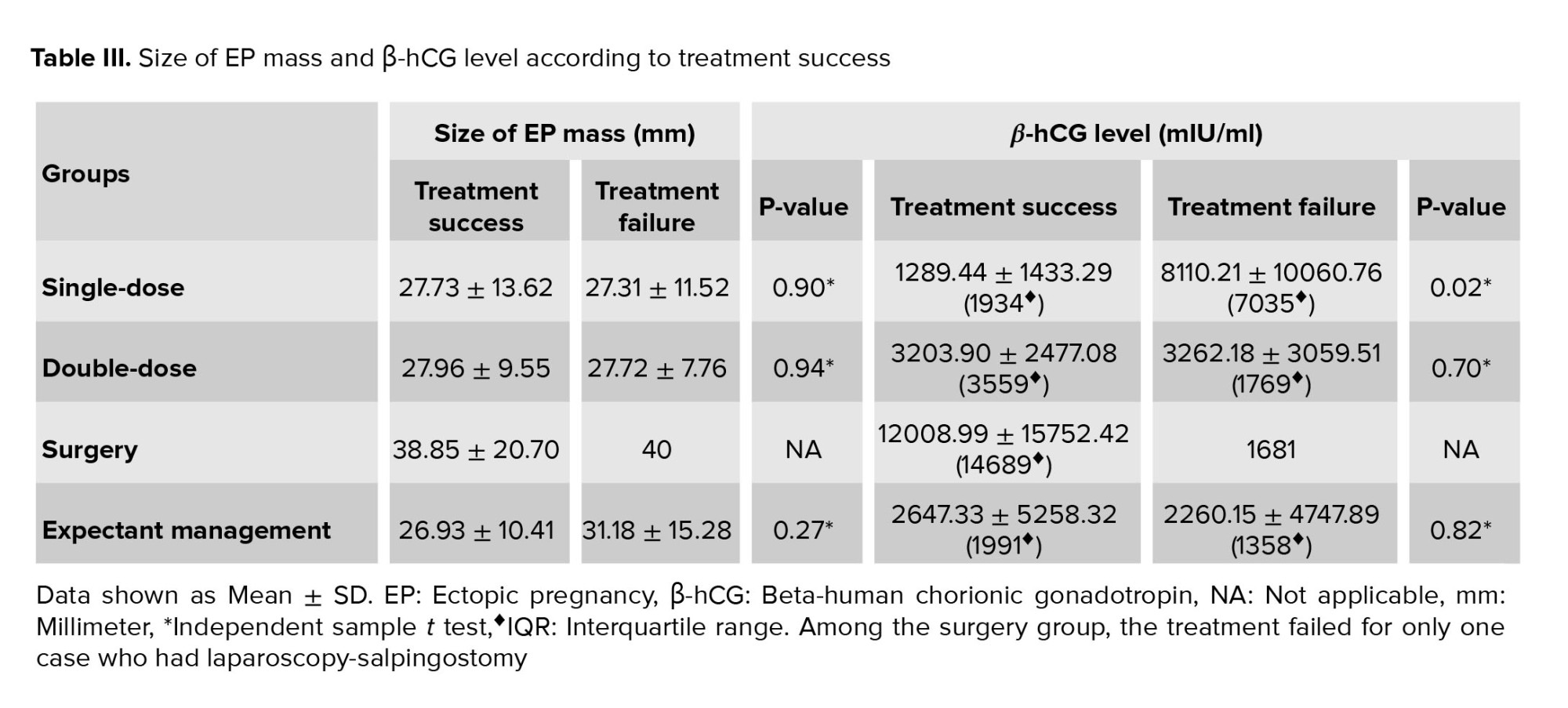
Within each group, the size of ectopic mass and β-hCG level of the women whose treatment was successful vs. in those whose treatment failed were compared (Table III). Only SD-MTX group represented a significant difference in β-hCG level between successful and failed treatment (p = 0.02).

4. Discussion
The current study was performed to compare different EP treatments including surgery, medical treatment and expectant management. EP treatment depends on the hemodynamic stability of patients, β-hCG levels, pregnancy planning, the size of the EP and the presence of fetal cardiac activity (12). Specialists have agreed that medical therapy is not recommended if the initial serum β-hCG values are < 1500 mIU/ml in women with unruptured tubal EP who may be treated by expectant management (13). MTX is a folic-acid antagonist that is accepted as a non-invasive effective treatment for cases with particular criteria (e.g. hemodynamic stability, fertility need) (14).
The SD-MTX method is the most commonly used protocol for cases with EP. The success rate of this protocol has been reported in previous studies to range between 52% and 94% (12, 15). In our study, the success of SD-MTX was 80.46%. Although there are no specific criteria used to recommend SD-MTX, the treatment success may be affected by β-hCG levels, positive fetal cardiac cavity, presence of a yolk sac, and the size of EP (16). It has been observed that the treatment success of the SD protocol decreases when the initial serum β-hCG values are > 2000 mIU/ml (17). Our results are in accordance with those reported in the literature. We demonstrated a significant difference in initial serum β-hCG values between the women who had a successful vs. failed SD treatment (1289.44 ± 1433.29 vs. 8110.21 ± 10060.76 (IQR = 7035) with p = 0.02). In this study, we did not find any significant difference in treatment success between SD and DD medical therapy which is in agreement with the findings reported in previous research. Therefore, we suggest that the SD-MTX protocol should be preferred since the use of the DD-MTX protocol may amplify the side effects, lengthen the hospital stay, cause more anxiety in patients, and also increase medical costs (18).
Surgery is approved as the first option for women who present with signs of tubal rupture and probable intra-abdominal bleeding (19). We found that the women who were selected for surgery had significantly larger ectopic masses and shorter hospital stays. According to the results of this study, we recommend surgery for cases with an ectopic mass size > 34.5 mm or a β-hCG level > 6419 mIU/ml.
Our findings are compatible with previous studies which reported that a high failure rate of medical therapy occurred if the size of the EP mass was > 35 mm (20, 21). Previously it was reported that a serum β-hCG level < 5000 mIU/ml could be considered for successful medical treatment (1, 22). In the current study, the predictive cut-off value for successful DD-MTX was ≤ 6419 mIU/ml), which was higher than prior studies.
In this study, among the management groups, surgery had a 99.35% success rate and so was the most effective method, and DD-MTX, with a 72.09% success rate, was the least. Previous studies have found a success rate from 72-87% for SD- and DD-MTX treatment (23, 24). Some studies have reported that the success rates of DD and multiple dose MTX regimens are higher than that of SD (24); and Song et al., in a prospective study, showed that the SD-MTX method, with the option of second dose administration for the cases with treatment failure, could be an appropriate medical treatment of EP for eligible women (23). In our study, women who were given SD-MTX and DD-MTX treatment did not significantly differ in their size of ectopic mass, β-hCG level or the treatment success; furthermore, the hospital stays in the SD-MTX group were significantly shorter than in the DD-MTX group. We conclude that SD-MTX is preferable to DD-MTX for eligible cases.
The limitation of this study was that characteristics like former EP in the same location, child-bearing characteristics and others that may influence the specialist’s opinion on treatment method were not examined. It is recommended to categorize the patients according to different criteria in further studies.
5. Conclusion
Based on our results, surgery is suggested as the best option for cases with a size of ectopic mass > 34.50 mm in diameter or a β-hCG level > 6419 mIU/ml. SD-MTX had a higher success rate and shorter duration of hospitalization compared to the DD-MTX protocol, and so was found to be an efficient and safe alternative. However, further randomized clinical trials with larger sample sizes are required to confirm the current results.
Acknowledgments
This article was partially extracted from the M.D. thesis done by Dr. Sana Atbaei with the grant number of 95-01-50-12789 and financially supported by the Vice Chancellor for Research of Shiraz University of Medical Sciences, Shiraz, Iran.
Conflict of Interest
The authors declare that there is no conflict of interest.
Ectopic pregnancy (EP) is defined as implantation of a blastocyst outside the uterine cavity (1). It is a major reason for maternal morbidity and mortality during the first trimester (2). The incidence is about 2% of all pregnancies (3). Although the etiology of EP is not identified clearly, several risk factors such as tubal infection, previous tubal surgery and previous EP increase the probability of this occurrence (4). Usually, an EP is located in one of the fallopian tubes and requires emergent treatment; as it gets large, it can cause tubal rupture due to heavy internal bleeding and hypovolemic shock (5). Today, the options for the treatment of EP include surgery, medical treatment and expectant management (6). Surgery treatment includes salpingectomy, salpingotomy and salpingostomy, which can be performed by laparotomy or laparoscopy (7). Laparoscopy has significant advantages such as shorter hospital stays, quicker recovery and lower cost than laparotomy. Sometimes laparotomy is preferred because of easier access to tissue in hemodynamically unstable cases (8).
In women who are hemodynamically stable, without any sign of rupture of EP, systemic administration of intramuscular methotrexate (MTX) is acceptable by single-dose (SD), double-dose (DD) or multiple dose protocols (9). MTX is contraindicated with renal, hepatic, gastrointestinal or haemopoietic disorders (10). Weekly beta-human chorionic gonadotropin (β-hCG) monitoring follow-up is recommended until levels decline below 15 mIU/ml. If medical treatment is deemed unsuccessful, the patients will need surgical treatment (11).
It is known that some cases of tubal EP with no significant symptoms resolve spontaneously. Therefore, in selected women who are clinically stable with an undiagnosed EP location in ultrasound and a β-hCG less than 1000 mIU/ml, expectant management is a valuable option of treatment.
As there is no consensus on the best management for EP, we aimed to compare the different protocols for treatment of EP in terms of the gestational age, size of ectopic mass, duration of hospital stay, and history of pelvic surgery, pelvic infection, infertility, abortion and EP, as well as site of EP and β-hCG level. In order to discriminate properly between double-dose methotrexate (DD-MTX) and surgery management, we plotted receiver operating characteristics (ROC) curves to determine cut-off points for β-hCG levels and size of ectopic mass.
2. Materials and Methods
2.1. Study design
In this cross-sectional study, the data of 365 women aged between 15-44 yr with the diagnosis of EP were reviewed between March 2017 and March 2019 in hospitals affiliated to Shiraz University of Medical Sciences, Shiraz, Iran.
2.2. Inclusion and exclusion criteria
Women aged 15-44 yr who were diagnosed with EP were included. EP was diagnosed if the serum β-hCG level had a slower doubling time on serial measurement and the gestational sac was not seen in the uterine cavity by transvaginal sonography. The subjects who refused to complete their treatment in the current center or had incomplete historical data in the hospital records, were excluded.
2.3. Data collection
Data on participants’ demographic, medical and clinical characteristics (age, gestational age, size and location of ectopic mass, duration of hospital stay, serial β-hCG level, type of surgery, and history of EP, infertility, pelvic infection, and pelvic surgery were retrieved from hospital records. The cases were classified according to treatment methods and based on the success of the treatment protocols (response vs. failure).
Surgery was the first plan for the cases with: 1) severe free fluid in the abdominal pelvic cavity and who were unstable hemodynamically; 2) severe abdominal pain; 3) based on participant’s preference; or 4) contraindication for medical treatment. The remaining cases were treated by SD- or DD-MTX (50 mg/m2, injected intramuscularly; Austria, Ebewe company) on day one, or days one and four, respectively. If the β-hCG level declined ≥ 15% between days four and seven, the MTX therapy was considered to be successful; otherwise, the management was assumed to have failed. Some cases with the criteria of clinical stability were treated by expectant management.
2.4. Ethical considerations
The local Medical Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran approved the study protocol (Code: IR.SUMS.REC.1395.S940). All of the enrolled participants signed a written informed consent form after complete explanation of the study design.
2.5. Statistical analysis
The Statistical Package for the Social Sciences (SPSS) version 22 was used to analyze the data. One-way analysis of variance (ANOVA) was used to statistically compare the means of the four treatment groups. Tukey’s test was used for a pairwise comparison of all means. The difference between 2 groups was analyzed by independent sample t test. Chi-square test was performed to determine whether there were any statistically significant differences between the descriptive data. Quantitative data were expressed as mean ± SD and the qualitative data were represented as frequencies or percentages. A p-value < 0.05 was considered as significant. ROC curves were generated to assess the optimal cut-off value for the size of ectopic mass and the initial β-hCG that discriminated between DD-MTX and surgery management. The cut-off points were denoted by the value that had the largest sum of sensitivity and specificity.
3. Results
Among the 365 women with EP, 87 cases were treated by SD-MTX, 43 by DD-MTX, 154 by surgery, and 81 by expectant management.
According to our results, the most common site of EP was right and left adnexa and there were no significant differences between different treatments. The women who underwent surgery had the largest ectopic mass, the highest β-hCG level, and the shortest hospital stay (p < 0.01) (Table I).
Surgery had the highest success rate (99.35%) and DD-MTX had the lowest (72.09%) (Table I). Among the surgery group, the treatment failed for only one case who had laparoscopy-salpingostomy.
The ROC analysis is presented in figure 1. The areas under the curve for the size of ectopic mass and β-hCG level were statistically significant (p < 0.01). The ROC curves suggested that a size of ectopic mass > 34.50 mm with 42% sensitivity and 67% specificity, and a β-hCG level > 6419 mIU/ml with 47% sensitivity and 90% specificity were suitable cut-off points for selecting surgery as the treatment plan.
The results of comparison between the SD- and DD-MTX groups are summarized in table II. There were no significant differences in the size of ectopic mass, β-hCG level, duration to β-hCG < 5 mIU/ml or treatment success between the two groups. Hospital stays in the SD-MTX group were significantly shorter than in the DD-MTX group (p = 0.02) (Table II).
Within each group, the size of ectopic mass and β-hCG level of the women whose treatment was successful vs. in those whose treatment failed were compared (Table III). Only SD-MTX group represented a significant difference in β-hCG level between successful and failed treatment (p = 0.02).




4. Discussion
The current study was performed to compare different EP treatments including surgery, medical treatment and expectant management. EP treatment depends on the hemodynamic stability of patients, β-hCG levels, pregnancy planning, the size of the EP and the presence of fetal cardiac activity (12). Specialists have agreed that medical therapy is not recommended if the initial serum β-hCG values are < 1500 mIU/ml in women with unruptured tubal EP who may be treated by expectant management (13). MTX is a folic-acid antagonist that is accepted as a non-invasive effective treatment for cases with particular criteria (e.g. hemodynamic stability, fertility need) (14).
The SD-MTX method is the most commonly used protocol for cases with EP. The success rate of this protocol has been reported in previous studies to range between 52% and 94% (12, 15). In our study, the success of SD-MTX was 80.46%. Although there are no specific criteria used to recommend SD-MTX, the treatment success may be affected by β-hCG levels, positive fetal cardiac cavity, presence of a yolk sac, and the size of EP (16). It has been observed that the treatment success of the SD protocol decreases when the initial serum β-hCG values are > 2000 mIU/ml (17). Our results are in accordance with those reported in the literature. We demonstrated a significant difference in initial serum β-hCG values between the women who had a successful vs. failed SD treatment (1289.44 ± 1433.29 vs. 8110.21 ± 10060.76 (IQR = 7035) with p = 0.02). In this study, we did not find any significant difference in treatment success between SD and DD medical therapy which is in agreement with the findings reported in previous research. Therefore, we suggest that the SD-MTX protocol should be preferred since the use of the DD-MTX protocol may amplify the side effects, lengthen the hospital stay, cause more anxiety in patients, and also increase medical costs (18).
Surgery is approved as the first option for women who present with signs of tubal rupture and probable intra-abdominal bleeding (19). We found that the women who were selected for surgery had significantly larger ectopic masses and shorter hospital stays. According to the results of this study, we recommend surgery for cases with an ectopic mass size > 34.5 mm or a β-hCG level > 6419 mIU/ml.
Our findings are compatible with previous studies which reported that a high failure rate of medical therapy occurred if the size of the EP mass was > 35 mm (20, 21). Previously it was reported that a serum β-hCG level < 5000 mIU/ml could be considered for successful medical treatment (1, 22). In the current study, the predictive cut-off value for successful DD-MTX was ≤ 6419 mIU/ml), which was higher than prior studies.
In this study, among the management groups, surgery had a 99.35% success rate and so was the most effective method, and DD-MTX, with a 72.09% success rate, was the least. Previous studies have found a success rate from 72-87% for SD- and DD-MTX treatment (23, 24). Some studies have reported that the success rates of DD and multiple dose MTX regimens are higher than that of SD (24); and Song et al., in a prospective study, showed that the SD-MTX method, with the option of second dose administration for the cases with treatment failure, could be an appropriate medical treatment of EP for eligible women (23). In our study, women who were given SD-MTX and DD-MTX treatment did not significantly differ in their size of ectopic mass, β-hCG level or the treatment success; furthermore, the hospital stays in the SD-MTX group were significantly shorter than in the DD-MTX group. We conclude that SD-MTX is preferable to DD-MTX for eligible cases.
The limitation of this study was that characteristics like former EP in the same location, child-bearing characteristics and others that may influence the specialist’s opinion on treatment method were not examined. It is recommended to categorize the patients according to different criteria in further studies.
5. Conclusion
Based on our results, surgery is suggested as the best option for cases with a size of ectopic mass > 34.50 mm in diameter or a β-hCG level > 6419 mIU/ml. SD-MTX had a higher success rate and shorter duration of hospitalization compared to the DD-MTX protocol, and so was found to be an efficient and safe alternative. However, further randomized clinical trials with larger sample sizes are required to confirm the current results.
Acknowledgments
This article was partially extracted from the M.D. thesis done by Dr. Sana Atbaei with the grant number of 95-01-50-12789 and financially supported by the Vice Chancellor for Research of Shiraz University of Medical Sciences, Shiraz, Iran.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Pregnancy Health
References
1. Tas EE, Akcay GFY, Avsar AF. Single-dose methotrexate for the treatment of ectopic pregnancy: Our experience from 2010 to 2015. Pak J Med Sci 2017; 33: 13-17. [DOI:10.12669/pjms.331.11238] [PMID] [PMCID]
2. Shrestha J, Saha R. Comparison of laparoscopy and laparotomy in the surgical management of ectopic pregnancy. J Coll Physicians Surg Pak 2012; 22: 760-764.
3. Guven ESG, Dilbaz S, Dilbaz B, Ozdemir DS, Akdag D, Haberal A. Comparison of the effect of single-dose and multiple-dose methotrexate therapy on tubal patency. Fertil Steril 2007; 88: 1288-1292. [DOI:10.1016/j.fertnstert.2006.12.059] [PMID]
4. Sendy F, AlShehri E, AlAjmi A, Bamanie E, Appani S, Shams T. Failure rate of single dose methotrexate in managment of ectopic pregnancy. Obstet Gynecol Int 2015; 2015: 902426. [DOI:10.1155/2015/902426] [PMID] [PMCID]
5. Karasu Y, Akselim B. Feasibility of single-incision laparoscopy for ruptured ectopic pregnancies with hemoperitoneum. Minim Invasive Ther Allied Technol 2019; 28: 46-50. [DOI:10.1080/13645706.2018.1470538] [PMID]
6. Mol F, Mol BW, Ankum WM, Van der Veen F, Hajenius PJ. Current evidence on surgery, systemic methotrexate and expectant management in the treatment of tubal ectopic pregnancy: A systematic review and meta-analysis. Hum Reprod Update 2008; 14: 309-319. [DOI:10.1093/humupd/dmn012] [PMID]
7. Cheng X, Tian X, Yan Zh, Jia M, Deng J, Wang Y, et al. Comparison of the fertility outcome of salpingotomy and salpingectomy in women with tubal pregnancy: A systematic review and meta-analysis. PLoS One 2016; 11: e0152343. [DOI:10.1371/journal.pone.0152343] [PMID] [PMCID]
8. Snyman L, Makulana T, Makin JD. A randomised trial comparing laparoscopy with laparotomy in the management of women with ruptured ectopic pregnancy. S Afr Med J 2017; 107: 258-263. [DOI:10.7196/SAMJ.2017.v107i3.11447] [PMID]
9. Ozyuncu O, Tanacan A, Duru SA, Beksac MS. Methotrexate therapy for ectopic pregnancies: A tertiary center experience. Rev Bras Ginecol Obstet 2018; 40: 680-685. [DOI:10.1055/s-0038-1675807] [PMID]
10. Boey O, Van Hooland S, Woestenburg A, Van der Niepen P, Verbeelen D. Methotrexate should not be used for patients with end-stage kidney disease. Acta Clin Belg 2006; 61: 166-169. [DOI:10.1179/acb.2006.028] [PMID]
11. Pulatoglu C, Dogan O, Basbug A, Kaya AE, Yildiz A, Temizkan O. Predictive factors of methotrexate treatment success in ectopic pregnancy: A single-center tertiary study. North Clin Istanb 2018; 5: 227-231. [DOI:10.14744/nci.2017.04900] [PMID] [PMCID]
12. Inal ZO, Inal HA. Comparison of four methods of treating ectopic pregnancy: A retrospective cohort study. Geburtshilfe Frauenheilkd 2018; 78: 70-77. [DOI:10.1055/s-0043-122151] [PMID] [PMCID]
13. Nadim B, Leonardi M, Infante F, Lattouf I, Reid Sh, Condous G. Rationalizing the management of pregnancies of unknown location: Diagnostic accuracy of human chorionic gonadotropin ratio‐based decision tree compared with the risk prediction model M4. Acta Obstet Gynecol Scand 2020; 99: 381-390. [DOI:10.1111/aogs.13752] [PMID]
14. Athanasiou AI, Athanasiou A, Spandorfer SD. Review of ectopic pregnancy treatment for IVF patients. J Immunol Sci 2020; 4: 1-5. [DOI:10.29245/2578-3009/2019/1.1181]
15. Hakim H, Yaich R, Halouani S, Jouou S, Arfaoudi R, Rachdi R. Non-surgical management of ectopic pregnancies. J Gynecol Oncol 2019; 2: 1013.
16. Leonardi M, Allison E, Lu Ch, Nadim B, Condous G. Prognostic accuracy of a novel methotrexate protocol for the resolution of tubal ectopic pregnancies. Eur J Obstet Gynecol Reprod Biol 2020; 247: 186-190. [DOI:10.1016/j.ejogrb.2020.02.029] [PMID]
17. Alsammani MA, Moona NA. Predictors of success of a single-dose methotrexate in the treatment of ectopic pregnancy. J Obstet Gynaecol India 2016; 66: 233-238. [DOI:10.1007/s13224-014-0668-3] [PMID] [PMCID]
18. Song T, Kim MK, Kim ML, Lung YW, Yun BS, Seong SJ. Single-dose versus two-dose administration of methotrexate for the treatment of ectopic pregnancy: A randomized controlled trial. Hum Reprod 2015; 31: 332-338. [DOI:10.1093/humrep/dev312] [PMID]
19. Brincat M, Bryant-Smith A, Holland TK. The diagnosis and management of interstitial ectopic pregnancies: A review. Gynecol Surg 2019; 16: 2. [DOI:10.1186/s10397-018-1054-4]
20. Alsammani MA, Moona NA. Predictors of success of a single-dose methotrexate in the treatment of ectopic pregnancy. J Obstet Gynecol India 2016; 66: 233-238. [DOI:10.1007/s13224-014-0668-3] [PMID] [PMCID]
21. Kimiaei P, Khani Z, Marefian A, Gholampour Ghavamabadi M, Salimnejad M. The importance of gestational sac size of ectopic pregnancy in response to single-dose methotrexate. ISRN Obstet Gynecol 2013; 2013: 269425. [DOI:10.1155/2013/269425] [PMID] [PMCID]
22. Sowter MC, Farquhar CM, Petrie KJ, Gudex G. A randomised trial comparing single dose systemic methotrexate and laparoscopic surgery for the treatment of unruptured tubal pregnancy. BJOG 2001; 108: 192-203.
https://doi.org/10.1111/j.1471-0528.2001.00038.x
https://doi.org/10.1016/S0306-5456(00)00037-1
https://doi.org/10.1111/j.1471-0528.2001.00037.x [DOI:10.1016/S0306-5456(00)00038-3]
23. Song T, Kim MK, Kim ML, Jung YW, Yun BS, Seong SJ. Single-dose versus two-dose administration of methotrexate for the treatment of ectopic pregnancy: A randomized controlled trial. Hum Reprod 2016; 31: 332-338. [DOI:10.1093/humrep/dev312] [PMID]
24. Alur-Gupta S, Cooney LG, Senapati S, Sammel MD, Barnhart KT. Two-dose versus single-dose methotrexate for treatment of ectopic pregnancy: A meta-analysis. Am J Obstet Gynecol 2019; 221: 95-108. [DOI:10.1016/j.ajog.2019.01.002] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





