Thu, Feb 19, 2026
[Archive]
Volume 20, Issue 3 (March 2022)
IJRM 2022, 20(3): 203-212 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Heidari Pebdeni P, Saffari F, Mirshekari T R, Ashourzadeh S, Taheri Soodejani M, Ahmadrajab R. Bacteriospermia and its association with seminal fluid parameters and infertility in infertile men, Kerman, Iran: A cross-sectional study. IJRM 2022; 20 (3) :203-212
URL: http://ijrm.ir/article-1-2028-en.html
URL: http://ijrm.ir/article-1-2028-en.html
Parastou Heidari Pebdeni1 

 , Fereshteh Saffari1
, Fereshteh Saffari1 

 , Toraj Reza Mirshekari2
, Toraj Reza Mirshekari2 

 , Sareh Ashourzadeh2
, Sareh Ashourzadeh2 

 , Moslem Taheri Soodejani3
, Moslem Taheri Soodejani3 

 , Roya Ahmadrajab *4
, Roya Ahmadrajab *4 




 , Fereshteh Saffari1
, Fereshteh Saffari1 

 , Toraj Reza Mirshekari2
, Toraj Reza Mirshekari2 

 , Sareh Ashourzadeh2
, Sareh Ashourzadeh2 

 , Moslem Taheri Soodejani3
, Moslem Taheri Soodejani3 

 , Roya Ahmadrajab *4
, Roya Ahmadrajab *4 


1- Department of Microbiology and Virology, School of Medicine, Kerman University of Medical Sciences, Kerman, Iran
2- Afzalipour Clinical Center for Infertility, Kerman University of Medical Sciences, Kerman, Iran
3- Department of Epidemiology, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, IR. Iran
4- Department of Microbiology and Virology, School of Medicine, Kerman University of Medical Sciences, Kerman, Iran ,ahmadrajabi3@yahoo.com
2- Afzalipour Clinical Center for Infertility, Kerman University of Medical Sciences, Kerman, Iran
3- Department of Epidemiology, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, IR. Iran
4- Department of Microbiology and Virology, School of Medicine, Kerman University of Medical Sciences, Kerman, Iran ,
Full-Text [PDF 489 kb]
(1589 Downloads)
| Abstract (HTML) (2392 Views)
1. Introduction
Sexually transmitted infections are of significant concern to investigators and clinicians of reproductive medicine (1). Even though the influence of certain genital bacterial infections on sperm function and whole spermatogenesis have been proposed, the role of these infections as the significant causes of male infertility is still controversial (2). The most prevalent sexually transmitted bacteria are Chlamydia trachomatis (C. trachomatis), Ureaplasma urealyticum (U. urealyticum), U. parvum, Mycoplasma hominis (M. hominis), and M. genitalium.
The prevalence of genital chlamydial infections has been estimated to be 50-70%. However, most infections are asymptomatic, and are neither diagnosed nor treated (2, 3). The impact of Chlamydia on semen parameters and its role in male infertility has been investigated in different studies (4, 5). However, no definite conclusion has been acquired. One study has shown that C. trachomatis infection is not associated with semen abnormalities, while another study has shown that contamination with C. trachomatis is associated with decreased sperm concentrations (6, 7).
Although genital mycoplasmas and ureaplasmas are natural residents of the male urethra, these microorganisms, particularly U. urealyticum, can contaminate seminal fluid during ejaculation and contribute to both genital infections and male infertility (8). However, the exact role of these agents in male infertility remains unknown (2). The study of Rybar and colleagues displayed that mycoplasma has the highest adverse effect on sperm quality, such as concentration, motility, and morphology (7). In contrast, in another study, genital Ureaplasma or Mycoplasma were not significantly related to semen abnormalities (8).
Determination of the possible relationship between infertility and the presence of C. trachomatis and genital ureaplasmas/mycoplasmas in the seminal fluid may have valuable consequences in the public health of different geographical areas (8). Due to the social and psychological effects and high costs of infertility treatments, accurate diagnosis and timely infection treatment are important to prevent future complications. So, screening strategies seems to be necessary.
To the best of our knowledge, no adequate research has been done for the frequencies of C. trachomatis, Ureaplasma speices (spp.) and Mycoplasma spp. infections in infertile men in Kerman, Iran. This research was proposed to determine the frequency of the above sexually transmitted bacteria in semen samples and investigate the relationship between the presence of these bacteria and semen quality using molecular assay.
2. Methods and Materials
2.1. Subjects
From July to December 2019, a total of 100 infertile men attending the Research and Clinical Centers for fertility in Kerman (southeast of Iran) were included in this cross-sectional study. "Infertility was defined as a failure to conceive after at least 12 months of unprotected sexual intercourse" (9). Inclusion criteria for male infertility were as follows: infertility without female factor subfertility, lack of hormonal abnormalities, and reproductive system abnormalities (varicocele, hydrocele, undescended testis, or inguinal hernia), the absence of clinical signs of genitourinary tract infections, and history of infertility and abnormal semen parameter. None of the participants had consumed any antibiotics for last 2 weeks (2, 9). As group control, semen specimens from 100 fertile men with normal semen parameters whose wives had non-assisted pregnancies in the past (2) were obtained from the same clinical centers during the same period.
For sample size estimating, the statistical formula was conducted which p, z 1- β, and z 1-α were 0.05, 0.84, and 1.96 respectively, and the sample size was determined about 100 (cases = 100, controls = 100).
2.2. Collection of semen specimens
Seminal fluids were collected either by self or assisted masturbation after 2-7 days of sexual abstinence. Before collecting the samples, participants were recommended to wash their hands and genital area with water and soap. Samples were collected in sterile plastic containers and transported to the microbiology laboratory within 1 hr. In the following, they were placed in the incubator for 15-30 min for liquefaction. They then were subjected to routine semen analysis and polymerase chain reaction (PCR) to detect mentioned bacteria.
2.3. Semen analysis
Semen analysis was performed according to world health organization guidelines to investigate the following parameters: appearance, liquefaction, semen volume, morphology, round cell, progressive motility, liquefaction, sperm concentration, and vitality.
2.4. DNA extraction and PCR
Bacterial DNA was extracted using an appropriate nucleic acid extraction kit (AmpliSens® RIBO-prep, Moscow, Russia) according to the manufacturer’s protocol for the isolation of bacterial DNA from clinical materials. The absorption of the extracted DNA was measured at 260 nm and 280 nm using NanoDrop NDe1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) to confirm the quality of the product. The extracted DNA was first examined for the human β- globin gene to check that there were no PCR inhibitors in the samples.
BgloF (5′- CAACTTCATCCACGTTCACC-3′) and BgloR (5′ GAAGAGCCAAGGACAGGTAC-3′) were used to amplify a 268-bp fragment in the exon 1 of the human β- globin (2). All β-globin-positive samples were examined for the presence of Ureaplasma spp., U. urealyticum, U. parvum, and M. hominis by conventional PCR (2, 10, 11) and semi-nested PCR for M. genitalium (12) and using an appropriate detection kit for C. trachomatis.
2.5. Detection of M. hominis and genital ureaplasmas by PCR
Amplification of ure A-B, multiple banded antigens (mba), and 16SrRNA by PCR assay were performed to identify Ureaplasma spp., U. urealyticum or U. parvum, and M. hominis, respectively. A total volume of 25-μl PCR mixture including 2 μl of bacterial DNA, 0.5 μl (5 pM) of each oligodeoxynucleotide primers, 12.5 μl of 2 × Master Mix Red (Ampliqon, Odensem, Denmark) was used for PCR. The primer quest software tool (http://www.ncbi.nlm.nih.gov/Gene) was used to check the specificity of primers shown in table I. After performing PCR according to the programs shown in table I, the PCR products were electrophoresed.
2.6. Detection of M. genitalium
The first conventional PCR was carried out for amplification 286-bp adhesion DNA fragment with the F1 and R1 primers and temperature program described in table I. Amplification of a 194-bp adhesion DNA fragment with F1 and R2 primers (Table I) was carried out by using semi-nested-PCR as described previously (12). Amplification was conducted with an initial denaturation at 95oC for 5 min, followed by 40 cycles at 94oC for 30 sec, 54oC for 45 sec, 72oC for 60 sec, and a final extension at 72oC for 5 min.
2.7. Detection of C. trachomatis
According to the manufacturer’s instructions, C. trachomatis was detected using an appropriate detection kit (Iranian Gene Fanavar Institute, Tehran, Iran).
2.8. Sequencing
The Sanger dideoxy chain termination technique on an Applied Biosystems 3730/3730Xl DNA Analyzer (Applied Biosystems, Foster City, CA, USA) by Bioneer Company was used for sequencing the PCR products. The primers used for sequencing were the same as PCR, except for C. trachomatis (Table I). Homology of the resultant sequences was checked using the NCBI-NIH BLAST search program.

2.9. Ethical considerations
The present study was approved by the Ethics Committee of the Research Council of Kerman University of Medical Sciences, Kerman, Iran (Code: IR.KMU.REC.1398.208). Written informed consent was obtained from all the participants.
2.10. Statistical analysis
Data analysis was done using IBM SPSS Statistics version 16 SPSS, version 16. Descriptive statistics were done by calculating mean, standard deviation, and frequency. Fisher's exact test was used to assess the relationship between the prevalence of some bacteria and infertility situations. The logistic regression was conducted to predict the impact of bacterial infections on male infertility. The significance level of 5% was considered in all statistical analysis.
3. Results
A total of 200 infertile and fertile men participated in this study. The mean age of infertile men was 35.33 yr (range 21-60; SD ± 7.38), while the mean age of fertile men was 34.29 yr (range 17-60, SD ± 7.3). Although the mean age of infertile men was 1.04 yr higher than those in the fertile group, this difference was not significant (p = 0.73).
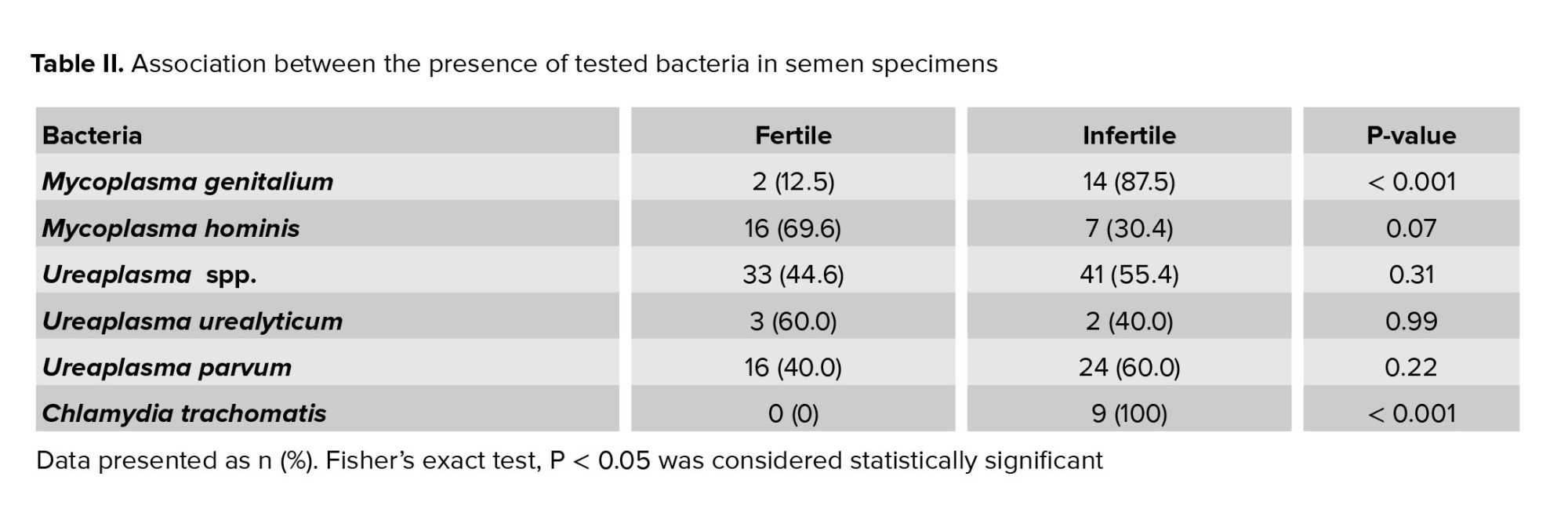
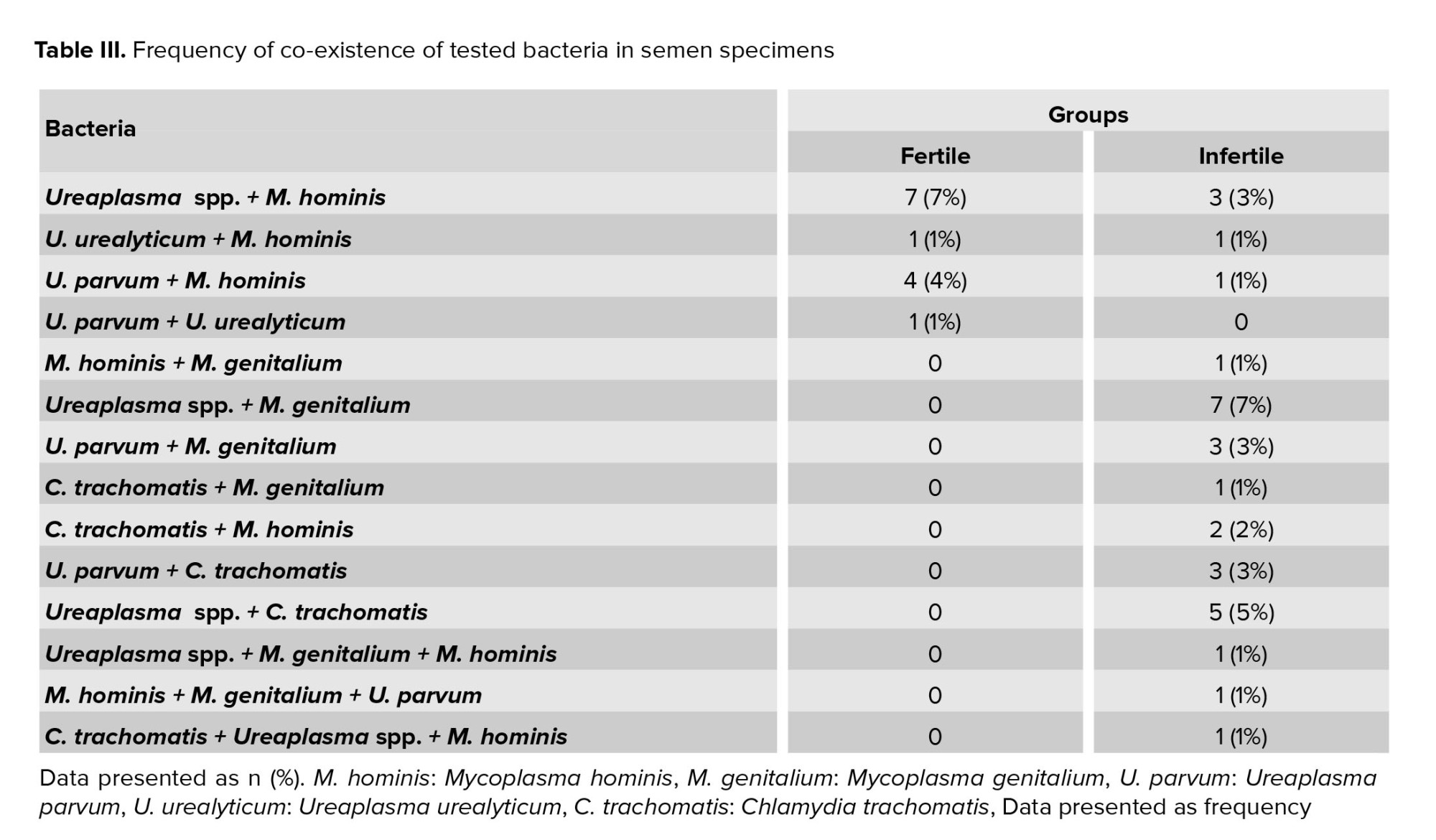
Sixty one percent of specimens (122 of 200) were positive for one or more studied bacteria. Overall 74% of total men carried Ureaplasma spp., of which 5% carried U. urealyticum (serovars 2, 10, 11, 12), 40% carried U. parvum (serovars 1, 3, 14), and the remained ones carried the other serovars that were not detectable by the used primers (Table II). Simultaneous infection with Ureaplasma spp. and M. genitalium was observed in 7% of infertile men (Table III).
There was a statistically significant difference only in the presence of M. genitalium and C. trachomatis in infertile men versus fertile ones (Table II).
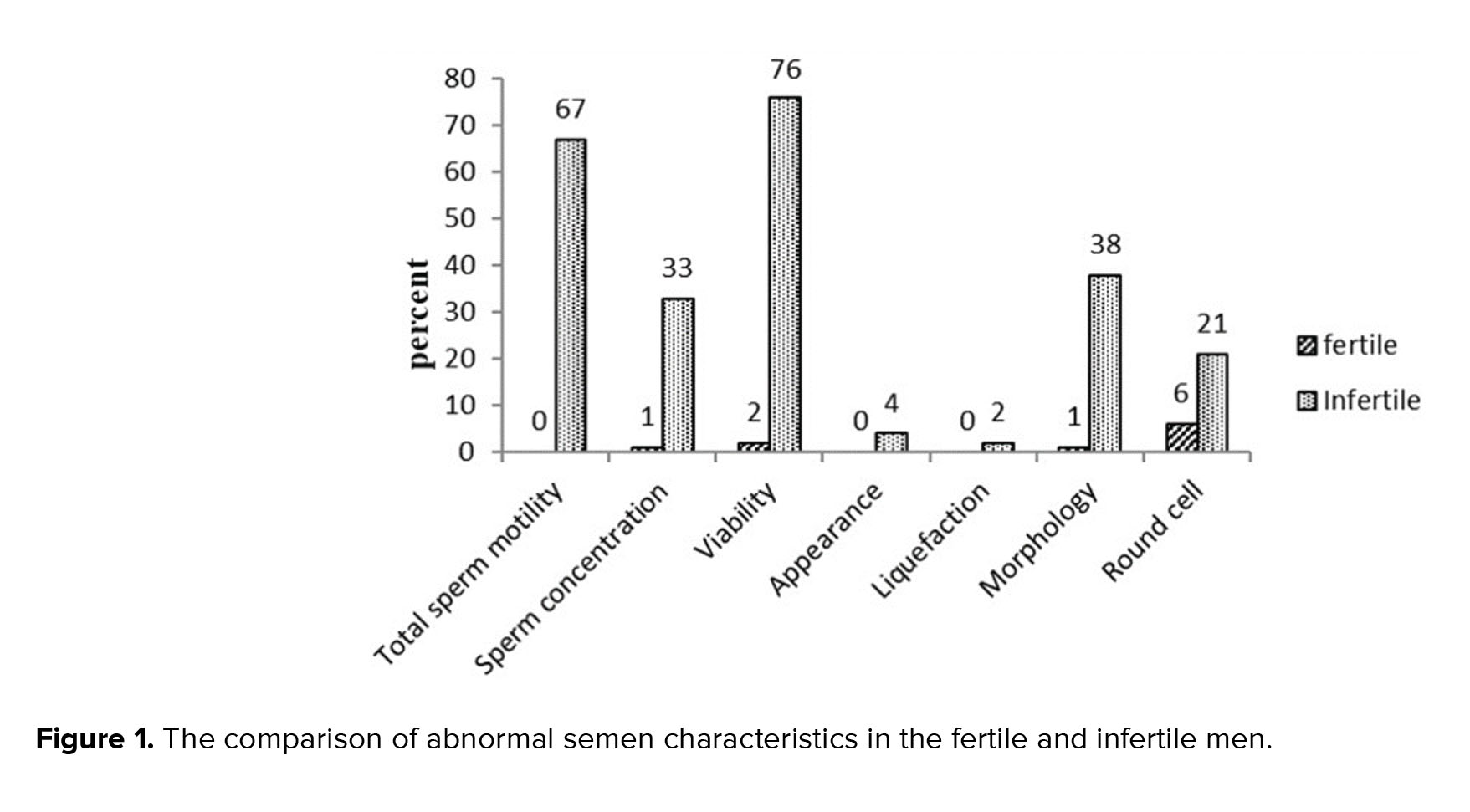
Semen characteristics in all participants showed that 98% of them had normal semen appearance (gray or opalescent), and in 99.5% of the men, semen was liquefied within less than 60 min. Sperm morphology, concentration, viability, total sperm motility and the percent of round cells were also in the normal range in 80.5%, 83%, 61%, 66.5%, and 86.5% of specimens, respectively. Figure 1, shows the frequency of abnormal semen characteristics separately.
The semen variables in the fertile and infertile men are summarized in table IV. The mean values of volume, progressive motility, non-progressive motility, sperm concentration, total progressive motility, and viability were significantly lower in infertile men than fertile men.
We studied the effects of the presence of M. genitalium, M. hominis, Ureaplasma spp, U. urealyticum, U. parvum, and C. trachomatis on semen quality in infected versus uninfected infertile men. A statistically significant correlation was observed between infection with M. genitalium and the percentage of progressive sperm motility (22% in infected infertile men vs. 33% in uninfected infertile men, p = 0.04), infection with M. hominis, and semen volume (2.8 mL in infected infertile men vs. 3.27 mL in uninfected infertile men, p = 0.03) and infection with U. parvum and the normal form of sperm (67.5% in infected infertile men vs. 84.7% in uninfected infertile men, p = 0.02). Additionally, the correlation between infection with C. trachomatis and the progressive motility of sperm (15.67% in infected infertile men vs. 32.27% in uninfected infertile men, p = 0.03) and also the percentage of live sperm per milliliter was significant (22% in infected infertile men vs. 63.5% in uninfected infertile men, p = 0.03).
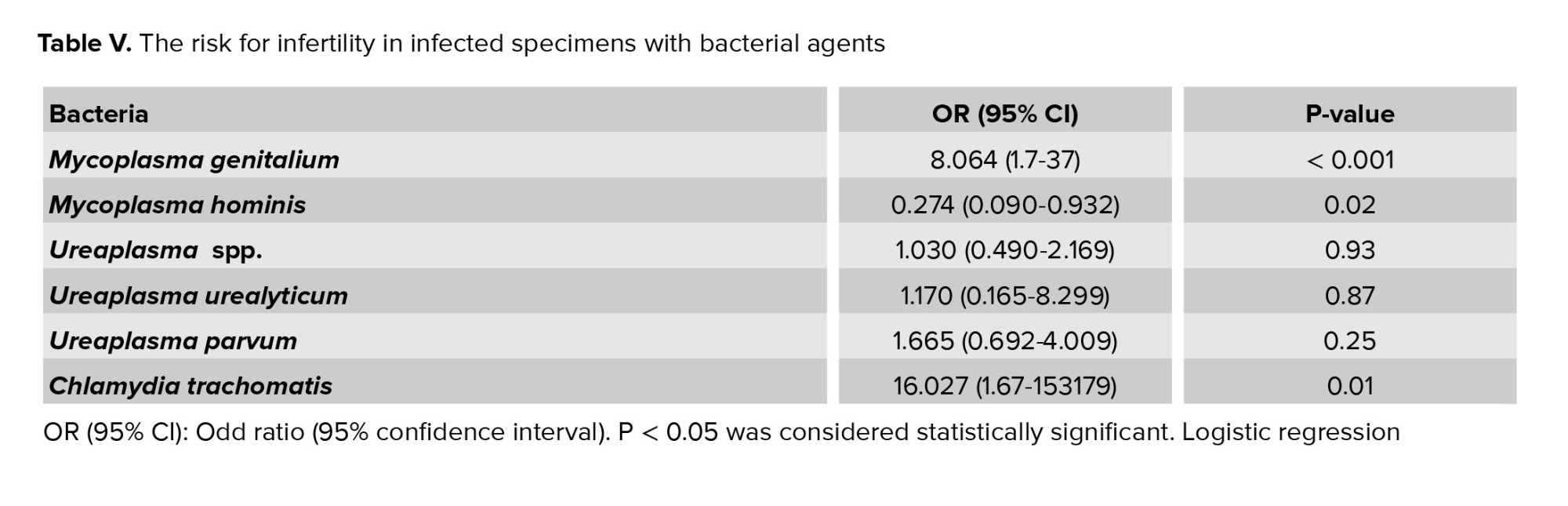
Logistic regression analysis (multivariate analysis) showed that M. genitalium and C. trachomatis were significantly associated with male infertility. So that the risk for infertility in infected men with M. genitalium was 8.06 times higher than non-infected ones, and in infected men with C. trachomatis, it was 16 times higher than non-infected individuals (OR = 16, CI: 1.67-153179) (Table V).

4. Discussion
There are many causes of male infertility, of which 8-35% are related to genital tract infections. Although sexually transmitted organisms, in particular, C. trachomatis, M. genitalium, M. hominis, U. parvum and U. urealyticum may affect spermatogenesis and subsequent fertility in men, the role of these bacteria as the important causes of male infertility is still a controversial issue (13-15). The majority of women and men who are infected with C. trachomatis, genital mycoplasma and genital Ureaplasma are not aware of their infection because they are asymptomatic. Therefore, detection of these agents is important (8, 16). This study provides information on the prevalence of the above-noted bacteria, their impact on fertility potential, and their association with quality of sperm among asymptomatic fertile and infertile men attending to Research and Clinical Centers of fertility in Kerman (southeast of Iran).
In Iran, the prevalence of C. trachomatis at low-risk people without any symptoms varies from 6.4-10.3% (17). In the present study, the frequency of C. trachomatis among the infertile group was 9% higher than previous reports from other countries (2, 6) and closely similar to the result reported from Ahvaz, Iran (18).
The prevalence of urogenital mycoplasmas in men varies between 2-44.3%. The prevalence of M. genitlium in our examined infertile men was almost similar to the previous study from Kerman, Iran (14%) (19), lower than reported in Tehran, Iran (20), and higher than Jordan (3.2%) (2), Kuwait (4.7%) (6), and Tunisia (4.8%) (8).
The high prevalence of C. trachomatis and genital mycoplasmas in infertile men compared with fertile men has been reported in several studies (2, 9, 21). Our research demonstrated a higher detection rate for C. trachomatis and M. genitalium in semen samples of infertile men than the control group. Similar to previous findings in Iran and the other countries (2, 18, 20-21), a significant difference was observed in the frequency of these microorganisms in the infertile and fertile groups (p < 0.001). However, these results are inconsistent with some studies that did not report any difference between these two groups (6, 22). These differences can be due to the diversity of the participants and type of samples, using techniques with different sensitivity and specificity, the cultural and geographical features of the countries, and multiple sexual partners (1, 18).
Our study demonstrated that the prevalence of Ureaplasma spp. in infertile men is high. Herein, we differentiate different Ureaplasmas spp. in infertile men, while most of the previous studies did not discriminate between U. urealyticum and U. parvum (9, 13). Our study indicated that U. parvum species was more common than U. urealyticum which agrees with the previously reported studies (2, 9) and is in contrast to other studies that found U. urealyticum species to be more common (8, 10). In our study, no statistically significant difference was found between infertile and fertile men in the prevalence of M. hominis and U. urealyticum (7% vs. 16%) and (2% vs. 3%), respectively. Our data with the previously reported studies (9, 23), while it is not in agreement with the study by Moosavian and co-workers (18).
The association between M. genitalium and male infertility has been investigated in a few studies, but, unlike our study these studies, did not have any control group (8, 24). Our data showed that the risk for infertility in infected men with M. genitalium is 8.06 times higher than in non-infected men (OR = 8.06, CI: 37-1.7). The effect of M. genitalium on the obstructive inflammatory process is far greater than its effect on functional parameters of semen (8). Although C. trachomatis attaches to the surface and the nucleolus of spermatozoa, its role in infertility is unclear (25). In our study, Logistic regression analysis showed that the risk of infertility in infected men with C. trachomatis is 16 times higher than in non-infected men.
Furthermore, we investigated the impact of infection on sperm quality. There are conflicting results in this field. Some studies have revealed no relationship between the presence of these bacteria and semen quality (8, 23). Nevertheless, others have proven the effectiveness of these bacteria on semen parameters (21, 26).
Our study was consistent with the latter groups, as it was shown that M. genitalium and M. hominis were associated with reducing progressive sperm motility and semen volume, respectively.
Also, similar to several studies, C. trachomatis was associated with reduced progressive sperm motility (1, 18, 27). In a study by Liu and co-workers, the comparison between C. trachomatis infected and non-infected specimens revealed that the prevalence of C. trachomatis infection was related to the reduction in sperm vitality, but the difference was not statistically significant (23). However, in our study, C. trachomatis was associated with reduced sperm vitality.
Mycoplasmas in urogenital tracts of infertile men may negatively affect semen volume, pH, sperm motility, morphology, concentration, and vitality (28). In this study, semen volume in infertile men infected with M. hominis significantly was low compared to non-infected infertile men.
One of the important agents in the natural fertilization cycles is progressive sperm motility. Similar to one study (9), Ureaplasma species had the same effect on semen quality except for U. parvum infection, which correlated with abnormal sperm morphology. The capability of attachment of bacteria to spermatozoa is different, and their influence on some of the seminal fluid parameters, host factors, and cellular interactions is various (18).
As the bacterial load may affect the outcome of the infection (9, 29), lack of knowledge about the bacterial load in infected specimens is of limitations in our study. Quantitative assessments either by quantitative culture or real-time PCR.
5. Conclusion
According to our results, C. trachomatis, genital mycoplasmas, and genital Ureaplasmas are widely present in infertile men in the Kerman, Iran. Because of the significant destructive effects of bacteriospermia on males' reproductive health, informing people about urogenital infections and protection ways, screening and treating asymptomatic cases seem to be indispensable. According to our results, the clinician should consider C. trachomatis and M. genitalium in men with decreased sperm progressive motility and viability during the infertility assessment.
Acknowledgments
This work was supported by the Research Council of Kerman University of Medical Sciences, Kerman, Iran (No. 98000206). The authors would like to thank Dr. Amjad Ahmadi (Microbiology Department, Kurdistan University of Medical Sciences, Iran) for kindly providing C. trachomatis L2 strain 434/Bu (ATCC VR-902B) as control positive.
Conflict of Interest
The authors declare that there is no conflict of interest.
Full-Text: (588 Views)
1. Introduction
Sexually transmitted infections are of significant concern to investigators and clinicians of reproductive medicine (1). Even though the influence of certain genital bacterial infections on sperm function and whole spermatogenesis have been proposed, the role of these infections as the significant causes of male infertility is still controversial (2). The most prevalent sexually transmitted bacteria are Chlamydia trachomatis (C. trachomatis), Ureaplasma urealyticum (U. urealyticum), U. parvum, Mycoplasma hominis (M. hominis), and M. genitalium.
The prevalence of genital chlamydial infections has been estimated to be 50-70%. However, most infections are asymptomatic, and are neither diagnosed nor treated (2, 3). The impact of Chlamydia on semen parameters and its role in male infertility has been investigated in different studies (4, 5). However, no definite conclusion has been acquired. One study has shown that C. trachomatis infection is not associated with semen abnormalities, while another study has shown that contamination with C. trachomatis is associated with decreased sperm concentrations (6, 7).
Although genital mycoplasmas and ureaplasmas are natural residents of the male urethra, these microorganisms, particularly U. urealyticum, can contaminate seminal fluid during ejaculation and contribute to both genital infections and male infertility (8). However, the exact role of these agents in male infertility remains unknown (2). The study of Rybar and colleagues displayed that mycoplasma has the highest adverse effect on sperm quality, such as concentration, motility, and morphology (7). In contrast, in another study, genital Ureaplasma or Mycoplasma were not significantly related to semen abnormalities (8).
Determination of the possible relationship between infertility and the presence of C. trachomatis and genital ureaplasmas/mycoplasmas in the seminal fluid may have valuable consequences in the public health of different geographical areas (8). Due to the social and psychological effects and high costs of infertility treatments, accurate diagnosis and timely infection treatment are important to prevent future complications. So, screening strategies seems to be necessary.
To the best of our knowledge, no adequate research has been done for the frequencies of C. trachomatis, Ureaplasma speices (spp.) and Mycoplasma spp. infections in infertile men in Kerman, Iran. This research was proposed to determine the frequency of the above sexually transmitted bacteria in semen samples and investigate the relationship between the presence of these bacteria and semen quality using molecular assay.
2. Methods and Materials
2.1. Subjects
From July to December 2019, a total of 100 infertile men attending the Research and Clinical Centers for fertility in Kerman (southeast of Iran) were included in this cross-sectional study. "Infertility was defined as a failure to conceive after at least 12 months of unprotected sexual intercourse" (9). Inclusion criteria for male infertility were as follows: infertility without female factor subfertility, lack of hormonal abnormalities, and reproductive system abnormalities (varicocele, hydrocele, undescended testis, or inguinal hernia), the absence of clinical signs of genitourinary tract infections, and history of infertility and abnormal semen parameter. None of the participants had consumed any antibiotics for last 2 weeks (2, 9). As group control, semen specimens from 100 fertile men with normal semen parameters whose wives had non-assisted pregnancies in the past (2) were obtained from the same clinical centers during the same period.
For sample size estimating, the statistical formula was conducted which p, z 1- β, and z 1-α were 0.05, 0.84, and 1.96 respectively, and the sample size was determined about 100 (cases = 100, controls = 100).
2.2. Collection of semen specimens
Seminal fluids were collected either by self or assisted masturbation after 2-7 days of sexual abstinence. Before collecting the samples, participants were recommended to wash their hands and genital area with water and soap. Samples were collected in sterile plastic containers and transported to the microbiology laboratory within 1 hr. In the following, they were placed in the incubator for 15-30 min for liquefaction. They then were subjected to routine semen analysis and polymerase chain reaction (PCR) to detect mentioned bacteria.
2.3. Semen analysis
Semen analysis was performed according to world health organization guidelines to investigate the following parameters: appearance, liquefaction, semen volume, morphology, round cell, progressive motility, liquefaction, sperm concentration, and vitality.
2.4. DNA extraction and PCR
Bacterial DNA was extracted using an appropriate nucleic acid extraction kit (AmpliSens® RIBO-prep, Moscow, Russia) according to the manufacturer’s protocol for the isolation of bacterial DNA from clinical materials. The absorption of the extracted DNA was measured at 260 nm and 280 nm using NanoDrop NDe1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) to confirm the quality of the product. The extracted DNA was first examined for the human β- globin gene to check that there were no PCR inhibitors in the samples.
BgloF (5′- CAACTTCATCCACGTTCACC-3′) and BgloR (5′ GAAGAGCCAAGGACAGGTAC-3′) were used to amplify a 268-bp fragment in the exon 1 of the human β- globin (2). All β-globin-positive samples were examined for the presence of Ureaplasma spp., U. urealyticum, U. parvum, and M. hominis by conventional PCR (2, 10, 11) and semi-nested PCR for M. genitalium (12) and using an appropriate detection kit for C. trachomatis.
2.5. Detection of M. hominis and genital ureaplasmas by PCR
Amplification of ure A-B, multiple banded antigens (mba), and 16SrRNA by PCR assay were performed to identify Ureaplasma spp., U. urealyticum or U. parvum, and M. hominis, respectively. A total volume of 25-μl PCR mixture including 2 μl of bacterial DNA, 0.5 μl (5 pM) of each oligodeoxynucleotide primers, 12.5 μl of 2 × Master Mix Red (Ampliqon, Odensem, Denmark) was used for PCR. The primer quest software tool (http://www.ncbi.nlm.nih.gov/Gene) was used to check the specificity of primers shown in table I. After performing PCR according to the programs shown in table I, the PCR products were electrophoresed.
2.6. Detection of M. genitalium
The first conventional PCR was carried out for amplification 286-bp adhesion DNA fragment with the F1 and R1 primers and temperature program described in table I. Amplification of a 194-bp adhesion DNA fragment with F1 and R2 primers (Table I) was carried out by using semi-nested-PCR as described previously (12). Amplification was conducted with an initial denaturation at 95oC for 5 min, followed by 40 cycles at 94oC for 30 sec, 54oC for 45 sec, 72oC for 60 sec, and a final extension at 72oC for 5 min.
2.7. Detection of C. trachomatis
According to the manufacturer’s instructions, C. trachomatis was detected using an appropriate detection kit (Iranian Gene Fanavar Institute, Tehran, Iran).
2.8. Sequencing
The Sanger dideoxy chain termination technique on an Applied Biosystems 3730/3730Xl DNA Analyzer (Applied Biosystems, Foster City, CA, USA) by Bioneer Company was used for sequencing the PCR products. The primers used for sequencing were the same as PCR, except for C. trachomatis (Table I). Homology of the resultant sequences was checked using the NCBI-NIH BLAST search program.

2.9. Ethical considerations
The present study was approved by the Ethics Committee of the Research Council of Kerman University of Medical Sciences, Kerman, Iran (Code: IR.KMU.REC.1398.208). Written informed consent was obtained from all the participants.
2.10. Statistical analysis
Data analysis was done using IBM SPSS Statistics version 16 SPSS, version 16. Descriptive statistics were done by calculating mean, standard deviation, and frequency. Fisher's exact test was used to assess the relationship between the prevalence of some bacteria and infertility situations. The logistic regression was conducted to predict the impact of bacterial infections on male infertility. The significance level of 5% was considered in all statistical analysis.
3. Results
A total of 200 infertile and fertile men participated in this study. The mean age of infertile men was 35.33 yr (range 21-60; SD ± 7.38), while the mean age of fertile men was 34.29 yr (range 17-60, SD ± 7.3). Although the mean age of infertile men was 1.04 yr higher than those in the fertile group, this difference was not significant (p = 0.73).
Sixty one percent of specimens (122 of 200) were positive for one or more studied bacteria. Overall 74% of total men carried Ureaplasma spp., of which 5% carried U. urealyticum (serovars 2, 10, 11, 12), 40% carried U. parvum (serovars 1, 3, 14), and the remained ones carried the other serovars that were not detectable by the used primers (Table II). Simultaneous infection with Ureaplasma spp. and M. genitalium was observed in 7% of infertile men (Table III).
There was a statistically significant difference only in the presence of M. genitalium and C. trachomatis in infertile men versus fertile ones (Table II).
Semen characteristics in all participants showed that 98% of them had normal semen appearance (gray or opalescent), and in 99.5% of the men, semen was liquefied within less than 60 min. Sperm morphology, concentration, viability, total sperm motility and the percent of round cells were also in the normal range in 80.5%, 83%, 61%, 66.5%, and 86.5% of specimens, respectively. Figure 1, shows the frequency of abnormal semen characteristics separately.
The semen variables in the fertile and infertile men are summarized in table IV. The mean values of volume, progressive motility, non-progressive motility, sperm concentration, total progressive motility, and viability were significantly lower in infertile men than fertile men.
We studied the effects of the presence of M. genitalium, M. hominis, Ureaplasma spp, U. urealyticum, U. parvum, and C. trachomatis on semen quality in infected versus uninfected infertile men. A statistically significant correlation was observed between infection with M. genitalium and the percentage of progressive sperm motility (22% in infected infertile men vs. 33% in uninfected infertile men, p = 0.04), infection with M. hominis, and semen volume (2.8 mL in infected infertile men vs. 3.27 mL in uninfected infertile men, p = 0.03) and infection with U. parvum and the normal form of sperm (67.5% in infected infertile men vs. 84.7% in uninfected infertile men, p = 0.02). Additionally, the correlation between infection with C. trachomatis and the progressive motility of sperm (15.67% in infected infertile men vs. 32.27% in uninfected infertile men, p = 0.03) and also the percentage of live sperm per milliliter was significant (22% in infected infertile men vs. 63.5% in uninfected infertile men, p = 0.03).
Logistic regression analysis (multivariate analysis) showed that M. genitalium and C. trachomatis were significantly associated with male infertility. So that the risk for infertility in infected men with M. genitalium was 8.06 times higher than non-infected ones, and in infected men with C. trachomatis, it was 16 times higher than non-infected individuals (OR = 16, CI: 1.67-153179) (Table V).





4. Discussion
There are many causes of male infertility, of which 8-35% are related to genital tract infections. Although sexually transmitted organisms, in particular, C. trachomatis, M. genitalium, M. hominis, U. parvum and U. urealyticum may affect spermatogenesis and subsequent fertility in men, the role of these bacteria as the important causes of male infertility is still a controversial issue (13-15). The majority of women and men who are infected with C. trachomatis, genital mycoplasma and genital Ureaplasma are not aware of their infection because they are asymptomatic. Therefore, detection of these agents is important (8, 16). This study provides information on the prevalence of the above-noted bacteria, their impact on fertility potential, and their association with quality of sperm among asymptomatic fertile and infertile men attending to Research and Clinical Centers of fertility in Kerman (southeast of Iran).
In Iran, the prevalence of C. trachomatis at low-risk people without any symptoms varies from 6.4-10.3% (17). In the present study, the frequency of C. trachomatis among the infertile group was 9% higher than previous reports from other countries (2, 6) and closely similar to the result reported from Ahvaz, Iran (18).
The prevalence of urogenital mycoplasmas in men varies between 2-44.3%. The prevalence of M. genitlium in our examined infertile men was almost similar to the previous study from Kerman, Iran (14%) (19), lower than reported in Tehran, Iran (20), and higher than Jordan (3.2%) (2), Kuwait (4.7%) (6), and Tunisia (4.8%) (8).
The high prevalence of C. trachomatis and genital mycoplasmas in infertile men compared with fertile men has been reported in several studies (2, 9, 21). Our research demonstrated a higher detection rate for C. trachomatis and M. genitalium in semen samples of infertile men than the control group. Similar to previous findings in Iran and the other countries (2, 18, 20-21), a significant difference was observed in the frequency of these microorganisms in the infertile and fertile groups (p < 0.001). However, these results are inconsistent with some studies that did not report any difference between these two groups (6, 22). These differences can be due to the diversity of the participants and type of samples, using techniques with different sensitivity and specificity, the cultural and geographical features of the countries, and multiple sexual partners (1, 18).
Our study demonstrated that the prevalence of Ureaplasma spp. in infertile men is high. Herein, we differentiate different Ureaplasmas spp. in infertile men, while most of the previous studies did not discriminate between U. urealyticum and U. parvum (9, 13). Our study indicated that U. parvum species was more common than U. urealyticum which agrees with the previously reported studies (2, 9) and is in contrast to other studies that found U. urealyticum species to be more common (8, 10). In our study, no statistically significant difference was found between infertile and fertile men in the prevalence of M. hominis and U. urealyticum (7% vs. 16%) and (2% vs. 3%), respectively. Our data with the previously reported studies (9, 23), while it is not in agreement with the study by Moosavian and co-workers (18).
The association between M. genitalium and male infertility has been investigated in a few studies, but, unlike our study these studies, did not have any control group (8, 24). Our data showed that the risk for infertility in infected men with M. genitalium is 8.06 times higher than in non-infected men (OR = 8.06, CI: 37-1.7). The effect of M. genitalium on the obstructive inflammatory process is far greater than its effect on functional parameters of semen (8). Although C. trachomatis attaches to the surface and the nucleolus of spermatozoa, its role in infertility is unclear (25). In our study, Logistic regression analysis showed that the risk of infertility in infected men with C. trachomatis is 16 times higher than in non-infected men.
Furthermore, we investigated the impact of infection on sperm quality. There are conflicting results in this field. Some studies have revealed no relationship between the presence of these bacteria and semen quality (8, 23). Nevertheless, others have proven the effectiveness of these bacteria on semen parameters (21, 26).
Our study was consistent with the latter groups, as it was shown that M. genitalium and M. hominis were associated with reducing progressive sperm motility and semen volume, respectively.
Also, similar to several studies, C. trachomatis was associated with reduced progressive sperm motility (1, 18, 27). In a study by Liu and co-workers, the comparison between C. trachomatis infected and non-infected specimens revealed that the prevalence of C. trachomatis infection was related to the reduction in sperm vitality, but the difference was not statistically significant (23). However, in our study, C. trachomatis was associated with reduced sperm vitality.
Mycoplasmas in urogenital tracts of infertile men may negatively affect semen volume, pH, sperm motility, morphology, concentration, and vitality (28). In this study, semen volume in infertile men infected with M. hominis significantly was low compared to non-infected infertile men.
One of the important agents in the natural fertilization cycles is progressive sperm motility. Similar to one study (9), Ureaplasma species had the same effect on semen quality except for U. parvum infection, which correlated with abnormal sperm morphology. The capability of attachment of bacteria to spermatozoa is different, and their influence on some of the seminal fluid parameters, host factors, and cellular interactions is various (18).
As the bacterial load may affect the outcome of the infection (9, 29), lack of knowledge about the bacterial load in infected specimens is of limitations in our study. Quantitative assessments either by quantitative culture or real-time PCR.
5. Conclusion
According to our results, C. trachomatis, genital mycoplasmas, and genital Ureaplasmas are widely present in infertile men in the Kerman, Iran. Because of the significant destructive effects of bacteriospermia on males' reproductive health, informing people about urogenital infections and protection ways, screening and treating asymptomatic cases seem to be indispensable. According to our results, the clinician should consider C. trachomatis and M. genitalium in men with decreased sperm progressive motility and viability during the infertility assessment.
Acknowledgments
This work was supported by the Research Council of Kerman University of Medical Sciences, Kerman, Iran (No. 98000206). The authors would like to thank Dr. Amjad Ahmadi (Microbiology Department, Kurdistan University of Medical Sciences, Iran) for kindly providing C. trachomatis L2 strain 434/Bu (ATCC VR-902B) as control positive.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Fertility & Infertility
References
1. Sellami H, Znazen A, Sellami A, Mnif H, Louati N, Zarrouk SB, et al. Molecular detection of Chlamydia trachomatis and other sexually transmitted bacteria in semen of male partners of infertile couples in Tunisia: The effect on semen parameters and spermatozoa apoptosis markers. PLoS One 2014; 9: e98903. [DOI:10.1371/journal.pone.0098903] [PMID] [PMCID]
2. Abusarah EA, Awwad ZM, Charvalos E, Shehabi AA. Molecular detection of potential sexually transmitted pathogens in semen and urine specimens of infertile and fertile males. Diagn Microbiol Infect Dis 2013; 77: 283-286. [DOI:10.1016/j.diagmicrobio.2013.05.018] [PMID]
3. Nwankwo EO, Sadiq MN. Prevalence of Chlamydia trachomatis infection among patients attending infertility and sexually transmitted diseases clinic (STD) in Kano, North Western Nigeria. Afr Health Sci 2014; 14: 672-678. [DOI:10.4314/ahs.v14i3.24] [PMID] [PMCID]
4. Eley A, Pacey AA, Galdiero M, Galdiero M, Galdiero F. Can Chlamydia trachomatis directly damage your sperm? Lancet Infect Dis 2005; 5: 53-57. [DOI:10.1016/S1473-3099(04)01254-X]
5. Ahmadi MH, Mirsalehian A, Bahador A. Prevalence of genital Chlamydia trachomatis in Iran: A systematic review and meta-analysis. Pathog Glob Health 2015; 109: 290-299. [DOI:10.1179/2047773215Y.0000000033] [PMID] [PMCID]
6. Al‐Sweih NA, Al‐Fadli AH, Omu AE, Rotimi VO. Prevalence of Chlamydia trachomatis, Mycoplasma hominis, Mycoplasma genitalium and Ureaplasma urealyticum infections and seminal quality in infertile and fertile men in Kuwait. J Androl 2012; 33: 1323-1329. [DOI:10.2164/jandrol.111.013821] [PMID]
7. Rybar R, Prinosilova P, Kopecka V, Hlavicova J, Veznik Z, Zajicova A, et al. The effect of bacterial contamination of semen on sperm chromatin integrity and standard semen parameters in men from infertile couples. Andrologia 2012; 44: 410-418. [DOI:10.1111/j.1439-0272.2011.01198.x] [PMID]
8. Gdoura R, Kchaou W, Ammar‐Keskes L, Chakroun N, Sellemi A, Znazen A, et al. Assessment of Chlamydia trachomatis, Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis and Mycoplasma genitalium in semen and first void urine specimens of asymptomatic male partners of infertile couples. J Androl 2008; 29: 198-206. [DOI:10.2164/jandrol.107.003566] [PMID]
9. Zhou YH, Ma HX, Shi XX, Liu Y. Ureaplasma spp. in male infertility and its relationship with semen quality and seminal plasma components. J Microbiol Immunol Infect 2018; 51: 778-783. [DOI:10.1016/j.jmii.2016.09.004] [PMID]
10. Zeighami H, Peerayeh SN, Yazdi RS, Sorouri R. Prevalence of Ureaplasma urealyticum and Ureaplasma parvum in semen of infertile and healthy men. Int J STD AIDS 2009; 20: 387-390. [DOI:10.1258/ijsa.2008.008334] [PMID]
11. Maleki S, Motamedi H, Moosavian SM, Shahbaziyan N. Frequency of Mycoplasma hominis and Ureaplasma urealyticum in females with urogenital infections and habitual abortion history in Ahvaz, Iran; using multiplex PCR. Jundishapur J Microbiol 2013; 6: 1-5. [DOI:10.5812/jjm.10088]
12. Ramazanzadeh R, Khodabandehloo M, Farhadifar F, Rouhi S, Ahmadi A, Menbari S, et al. A case-control study on the relationship between Mycoplasma genitalium infection in women with normal pregnancy and spontaneous abortion using polymerase chain reaction. Osong Public Health Res Perspect 2016; 7: 334-338. [DOI:10.1016/j.phrp.2016.07.001] [PMID] [PMCID]
13. Huang C, Zhu HL, Xu KR, Wang SY, Fan LQ, Zhu WB. Mycoplasma and ureaplasma infection and male infertility: A systematic review and meta‐analysis. Andrology 2015; 3: 809-816. [DOI:10.1111/andr.12078] [PMID]
14. Bachir BG, Jarvi K. Infectious, inflammatory, and immunologic conditions resulting in male infertility. Urol Clin North Am 2014; 41: 67-81. [DOI:10.1016/j.ucl.2013.08.008] [PMID]
15. Ahmadi MH, Mirsalehian A, Bahador A. Prevalence of urogenital Mycoplasmas in Iran and their effects on fertility potential: A systematic review and meta-analysis. Iran J Public Health 2016; 45: 409-422.
16. Ahmadi A, Ramazanzadeh R, Sayehmiri K, Sayehmiri F, Amirmozafari N. Association of Chlamydia trachomatis infections with preterm delivery: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2018; 18: 240-246. [DOI:10.1186/s12884-018-1868-0] [PMID] [PMCID]
17. Janghorban R, Azarkish F. An overview on sexually transmitted infections in Iran. Int J Reprod Contracept Obstet Gynecol 2016; 5: 585-595. [DOI:10.18203/2320-1770.ijrcog20160557]
18. Moosavian M, Ghadiri A, Amirzadeh S, Rashno M, Afzali M, Ahmadi K. Investigating Chlamydia trachomatis and genital Mycoplasma prevalence and apoptosis markers in infertile and fertile couples. Jundishapur J Microbiol 2019; 12: e84954. [DOI:10.5812/jjm.84954]
19. Mohseni Moghadam N, Kheirkhah B, Mirshekari TR, Fasihi Harandi M, Tafsiri E. Isolation and molecular identification of mycoplasma genitalium from the secretion of genital tract in infertile male and female. Iran J Reprod Med 2014; 12: 601-608.
20. Safavifar F, Bandehpour M, Hosseiny SJ, Khorramizadeh MR, Shahverdi A, Kazemi B. Mycoplasma Infection in pyospermic infertile and healthy fertile men. Novel Biomed 2015; 3: 25-29.
21. Lee JSh, Kim KT, Lee HS, Yang KM, Seo JT, Choe JH. Concordance of Ureaplasma urealyticum and Mycoplasma hominis in infertile couples: Impact on semen parameters. Urology 2013; 81: 1219-1224. [DOI:10.1016/j.urology.2013.02.044] [PMID]
22. Günyeli İ, Abike F, Dünder İ, Aslan C, Tapısız ÖL, Temizkan O, et al. Chlamydia, Mycoplasma and Ureaplasma infections in infertile couples and effects of these infections on fertility. Arch Gynecol Obstet 2011; 283: 379-385. [DOI:10.1007/s00404-010-1726-4] [PMID]
23. Liu J, Wang Q, Ji X, Guo S, Dai Y, Zhang Z, et al. Prevalence of Ureaplasma urealyticum, Mycoplasma hominis, Chlamydia trachomatis infections, and semen quality in infertile and fertile men in China. Urology 2014; 83: 795-799. [DOI:10.1016/j.urology.2013.11.009] [PMID]
24. Gdoura R, Kchaou W, Chaari C, Znazen A, Keskes L, Rebai T, et al. Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis and Mycoplasma genitalium infections and semen quality of infertile men. BMC Infect Dis 2007; 7: 129-137. [DOI:10.1186/1471-2334-7-129] [PMID] [PMCID]
25. Plecko V, Zele-Starcevic L, Tripkovic V, Skerlev M, Ljubojevic S, Plesko S, et al. Unusually low prevalence of Mycoplasma genitalium in urine samples from infertile men and healthy controls: A prevalence study. BMJ Open 2014; 4: e005372. [DOI:10.1136/bmjopen-2014-005372] [PMID] [PMCID]
26. Ouzounova-Raykova V, Ouzounova I, Mitov I. Chlamydia trachomatis infection as a problem among male partners of infertile couples. Andrologia 2009; 41: 14-19. [DOI:10.1111/j.1439-0272.2008.00881.x] [PMID]
27. Mazzoli S, Cai T, Addonisio P, Bechi A, Mondaini N, Bartoletti R. Chlamydia trachomatis infection is related to poor semen quality in young prostatitis patients. Eur Urol 2010; 57: 708-714. [DOI:10.1016/j.eururo.2009.05.015] [PMID]
28. Rose BI, Scott B. Sperm motility, morphology, hyperactivation, and ionophore-induced acrosome reactions after overnight incubation with Mycoplasmas. Fertil Steril 1994; 61: 341-348. [DOI:10.1016/S0015-0282(16)56529-7]
29. Deguchi T, Shimada Y, Horie K, Mizutani K, Seike K, Tsuchiya T, et al. Bacterial loads of Ureaplasma parvum contributes to the development of inflammatory responses in the male urethra. Int J STD AIDS 2015; 26: 1035-1039. [DOI:10.1177/0956462414565796] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





