Fri, Feb 20, 2026
[Archive]
Volume 20, Issue 3 (March 2022)
IJRM 2022, 20(3): 221-230 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hosseinisadat R, Saeed L, Ghasemirad A, Habibzadeh V, Safar Heidari S. Assessment of the effect of serum and follicular fluid vitamin D and glucose on assisted reproductive technique outcome: A cross-sectional study. IJRM 2022; 20 (3) :221-230
URL: http://ijrm.ir/article-1-2099-en.html
URL: http://ijrm.ir/article-1-2099-en.html
Robabe Hosseinisadat1 

 , Lida Saeed1
, Lida Saeed1 

 , Anis Ghasemirad *2
, Anis Ghasemirad *2 

 , Victoria Habibzadeh1
, Victoria Habibzadeh1 

 , Sedigheh Safar Heidari3
, Sedigheh Safar Heidari3 




 , Lida Saeed1
, Lida Saeed1 

 , Anis Ghasemirad *2
, Anis Ghasemirad *2 

 , Victoria Habibzadeh1
, Victoria Habibzadeh1 

 , Sedigheh Safar Heidari3
, Sedigheh Safar Heidari3 


1- Department of Obstetrics and Gynecology, School of Medicine, Kerman University of Medical Sciences, Kerman, Iran.
2- Department of Obstetrics and Gynecology, School of Medicine, Kerman University of Medical Sciences, Kerman, Iran. ,anisghasemirad1399@g,ail.com
3- Afzalipour Infertility Center, Kerman University of Medical Sciences, Kerman, Iran.
2- Department of Obstetrics and Gynecology, School of Medicine, Kerman University of Medical Sciences, Kerman, Iran. ,
3- Afzalipour Infertility Center, Kerman University of Medical Sciences, Kerman, Iran.
Full-Text [PDF 393 kb]
(1413 Downloads)
| Abstract (HTML) (2098 Views)
1. Introduction
Oocyte maturation depends on cytoplasmic and nuclear changes, and each defect in this process can reduce the chances of successful fertilization (1). Some studies have shown that a lack of many vitamins and nutrients can reduce the chances of successful natural fertility, and have confirmed the effect of vitamins and supplements on embryo development (2, 3). Follicular fluid is an important medium for the development of oocytes. Increasing or decreasing the structure of this fluid has negative effects on the oocyte and embryo morphology and quality (4).
Vitamin D is a fat-soluble steroid hormone which is essential for the production of sex hormones, estradiol and progesterone (5). Vitamin D connects to its receptor and then activates the molecular pathways in cells or tissues (6). There is a vitamin D receptor in the human ovaries, uterus, endometrium and placenta. Experiments have shown that vitamin D can support endometrium decidualization and regulatory factors for the expression of some genes such as Homeobox genes 10, and that it is important for embryo implantation. So vitamin D deficiency is associated with various reproduction problemes such as preeclampsia, insulin resistance, and gestational diabetes (5).
Research has shown that infertile women usually have lower levels of vitamin D (7). It was reported that, women who had ovulation defects were deficient in vitamin D, and that vitamin D can reduce inflammation in the body and help the fertility process (8). In another study showed that vitamin D levels in the follicular fluid had a significant positive effect on implantation and the pregnancy rate (9).
Previously it was showed that despite the positive effects of vitamin D as a hormone in the human body, its follicular fluid level did not have a significant effect on in vitro fertilization (IVF) outcomes (10). It was found that pregnancy rates were not associated with vitamin D levels in the follicular fluid. They declared that in women who has higher levels of vitamin D, we have a significant decrease in fertilization rate, while increasing the implantation rate (11).
Glucose in the follicular fluid is one of the most important substances supporting oocyte metabolism (12). Glucose is the main energy source for all follicullar cells including oocytes and other somatic cells (13). Glucose is one of the regulatory factors for the GH-IGF-1 pathway in a study on evaluting vitamin D and glucose levels in the follicular fluid of 101 women who underwent IVF, it was found that there were insufficient vitamin D levels in the follicular fluid of the infertile women. Also, the vitamin D levels in the follicular fluid had a significant negative association with embryo quality. They reported that the follicular fluid glucose level was higher when vitamin D levels were insufficient (14).
Therefore, it is necessary to study the hormonal and biochemical changes of follicular fluid. Studies have shown different results about the effect of vitamin D and glucose in the follicular fluid on fertility and assisted reproductive technique (ART) outcomes. The aim of this study was to measure the levels of vitamin D and glucose in the follicular fluid and serum of infertile wemen who underwent IVF and evaluate the effects of vitamin D and glucose on ART outcomes.
2. Material and Methods
In this cross-sectional study, 102 infertile women (aged 20-40 yr) who were candidates for IVF/intracytoplasmic sperm injection (ICSI) who referred to Afzalipour hospital of Kerman, Iran between March 2019 and February 2020 were enrolled.
Wemen were excluded if they had ovarian reserve depletion (defined as ≤ 3 oocytes with a conventional stimulation protocol, an antral follicular count of 5-7 follicles, Anti-Mullerian hormone levels of 0.5-1.1 ng/ml, or two episodes of polycystic ovarian response after maximal stimulation), reproductive anatomical defects, endocrine disorders, a history of kidney, liver or heart defects, vitamin D metabolism problems and/or a partner with severe male factor infertility.
All women were on an antagonist protocol for controlled ovarian hyperstimulation. Gonadotropin Cinal F (Cinal F, CinnaGen, Iran) was administered from day two of menstruation. The first dosage of gonadotropin measured based on the patients’ age and body weight (150-450 IU/day). On day 7 of the stimulation all patients monitored. When the dominant follicle size was 12 mm, the antagonist (Cetrorelix, Merck-Serono, Germany) was started. When follicles size was ≥ 18 mm in transvaginal ultrasonography imaging, final oocyte maturation was triggered by an intramuscular injection of 10,000 IU of human chorionic gonadotropin (pdpreg, Pooyesh Darou, Iran). After 34-36 hr, oocyte pickup was performed with vaginal ultrasound and general anesthesia. On the oocyte retrieval day, a sample was taken of peripheral blood for measuring vitamin D and fasting blood sugar (FBS). Then, follicular fluid containing oocytes was drained under a transvaginal ultrasound guide with general anesthesia. In the embryology lab, all oocytes were separated and the follicular fluid was transferred to the medical laboratory to determination of vitamin D and sugar level. Method for sample preparation was in the first of all centrifugation for 15 minutes at 1000 g and the seperated serum was kept at -20°C. For measurement of plasma and fulicular fluid vitamin D enzyme immunoassay (Immunodiagnostic Ltd, UK) was used. Serum and folicular fluid glucose levels were estimated with oxidase methods by using a spectrophotometer system (Olympus Life and Material Science Europa, Hamburg, Germany). Serum glucose levels were defined as normal at < 110 ng/ml and pre-diabetic at ≥ 110 ng/ml.
The oocytes were fertilized in the embryology lab byICSI and the number of fertilized oocytes was calculated two days after IVF/ICSI. Three days after oocyte retrieval, one or two high-quality embryos were transferred to the uterus. Implantation, chemical and clinical pregnancy rates, and abortion rates were assessed, and then the relationship between the serum and follicular fluid vitamin D and FBS levels, and the ART outcomes was assessed.
The implantation rate was measured based on the gestational sac in sonography per the number of embryos transferred. A positive chemical pregnancy was defined based on serum β-human chorionic gonadotropin two weeks after embryo transfer. A positive clinical pregnancy was defined by at least one gestational sac in the uterine cavity under sonography 2-3 wk after the positive chemical pregnancy. The abortion rate was defined as pregnancy losses earlier than 20 wk of gestation per positive chemical pregnancies.
2.1. Ethical considerations
This study was approved by the Ethical Committee of Kerman University of Medical Sciences, Kerman, Iran (Code: IR.KMU.REC.1397.513), and informed consent was obtained from all participants in this study.
2.2. Statistical analysis
The Statistical Package for the Social Sciences (SPSS) software version 20 (IBM Co., Illinois, USA) was used for the data analysis. The normal distribution of the data was examined by the Kolmogorov-Smirnov test. Student’s t test and proportional tests were performed for numerical variables and categorical variables, respectively. One-way ANOVA was used for assessing differences between the three groups of serum and follicular fluid vitamin D levels. The results were presented as mean ± standard deviation (SD) or frequency percentage (%). P-values less than 0.05 were considered statistically significant.
3. Results
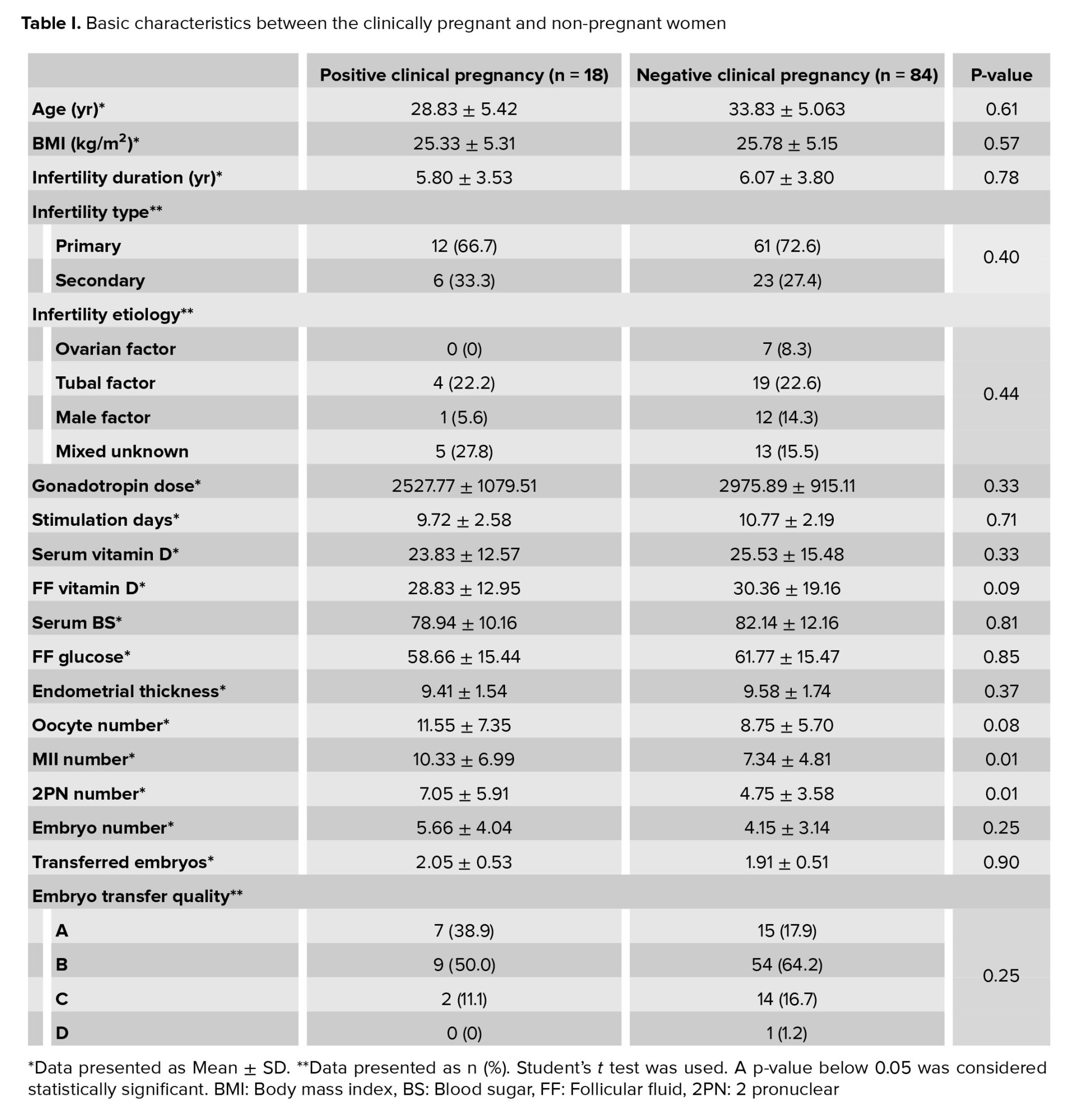
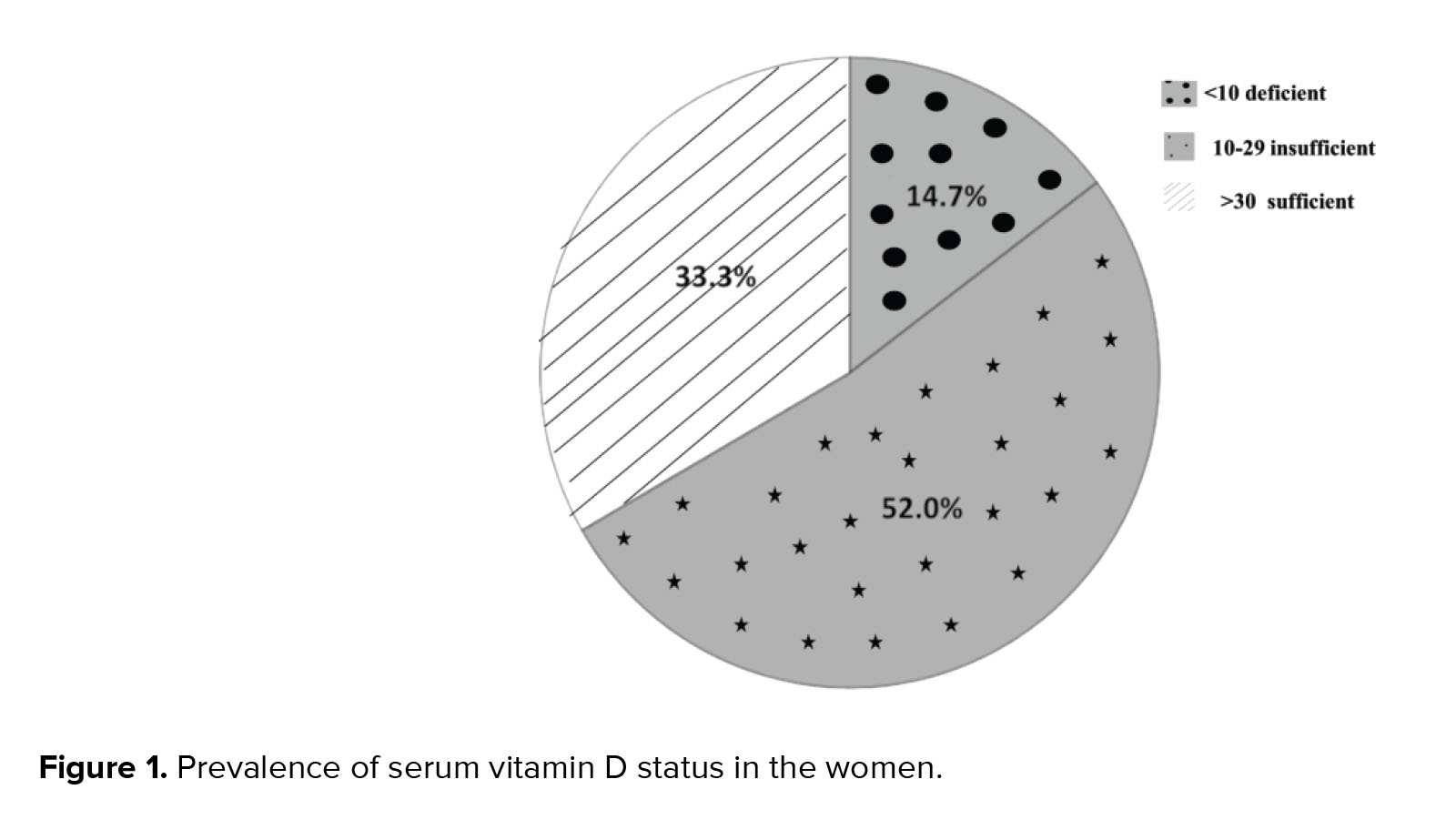
Data for 102 women were analyzed in the present study. From these 102 women, in 18 cases clinical pregnancy was positive and in 84 cases clinical pregnancy was negative. Basic characteristics, serum vitamin D, follicular vitamin D, FBS, follicular fluid glucose and ART outcomes in the women with positive and negative clinical pregnancy are summarized in table I. Comparison between some parameters such :as char:acteristics, serum vitamin D, follicular vitamin D, FBS and follicular fluid glucose in the two groups showed no significant difference. In terms of the ART outcomes, the numbers of matured oocytes and 2 pronucleus were significantly higher in the women with a positive clinical pregnancy (p = 0.01). But other parameters of ART outcomes were similar between the two groups (Table I). The serum vitamin D status of the cases showed that 66.7% of women were deficient or insufficient in vitamin D and only 33.3% had a sufficient status (Figure 1).
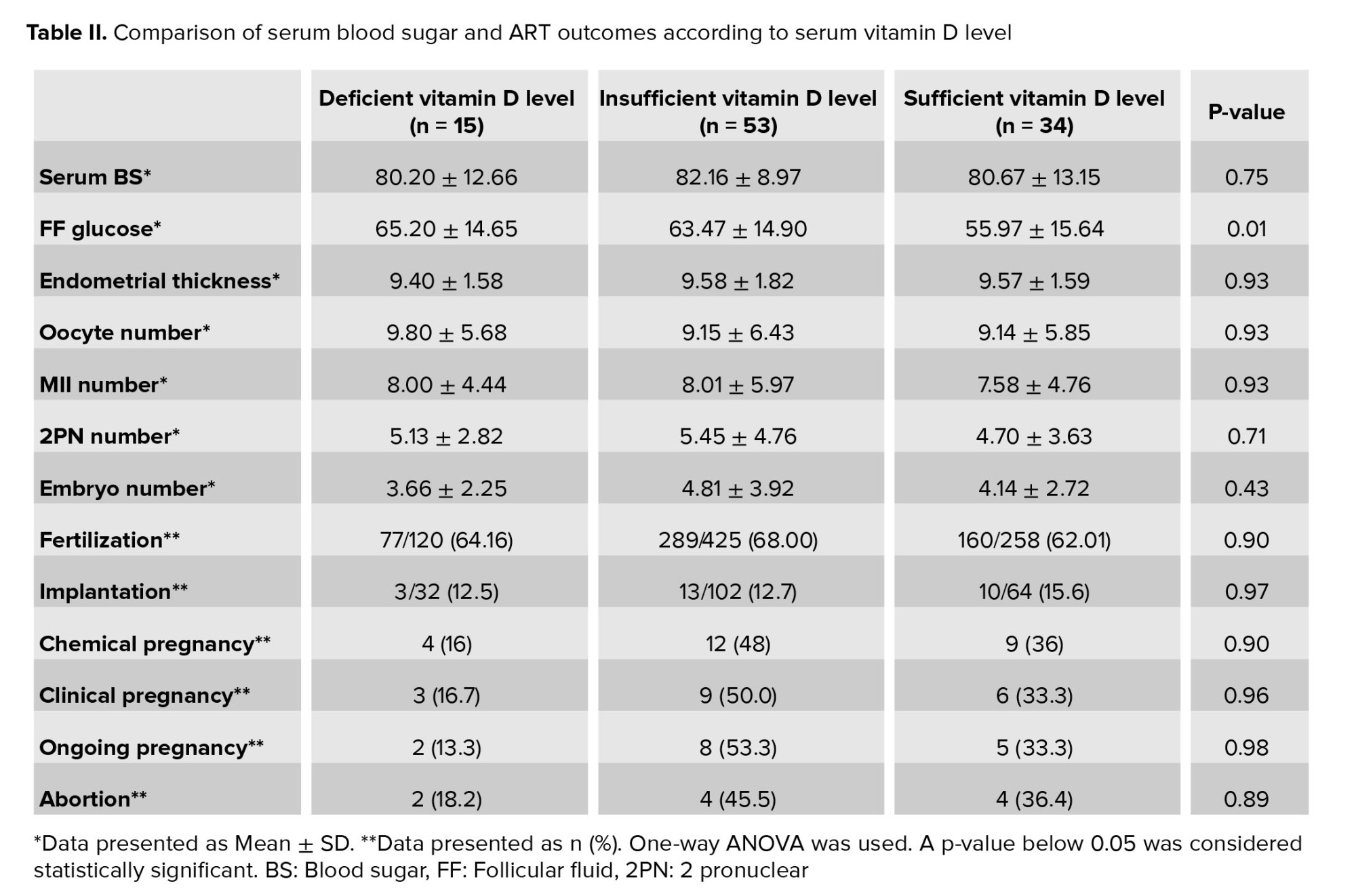
The comparison of glucose status and ART outcomes in women with deficient, insufficient, and sufficient vitamin D status is shown in table II. Based on vitamin D grouping, ART outcomes showed no significant difference. Follicular fluid glucose levels in women who were deficient, insufficient and sufficient in vitamin D were 65.20 ± 14.65, 63.47 ± 14.90 and 55.97 ± 15.64, respectively. Follicular fluid glucose levels were lower in women with sufficient vitamin D levels than in other groups and this difference was statistically significant (p = 0.01). In women with deficient serum vitamin D levels, the rates of chemical, clinical, and ongoing pregnancy were lower than in women with other vitamin D levels, but this difference was not statistically significant (Table II).
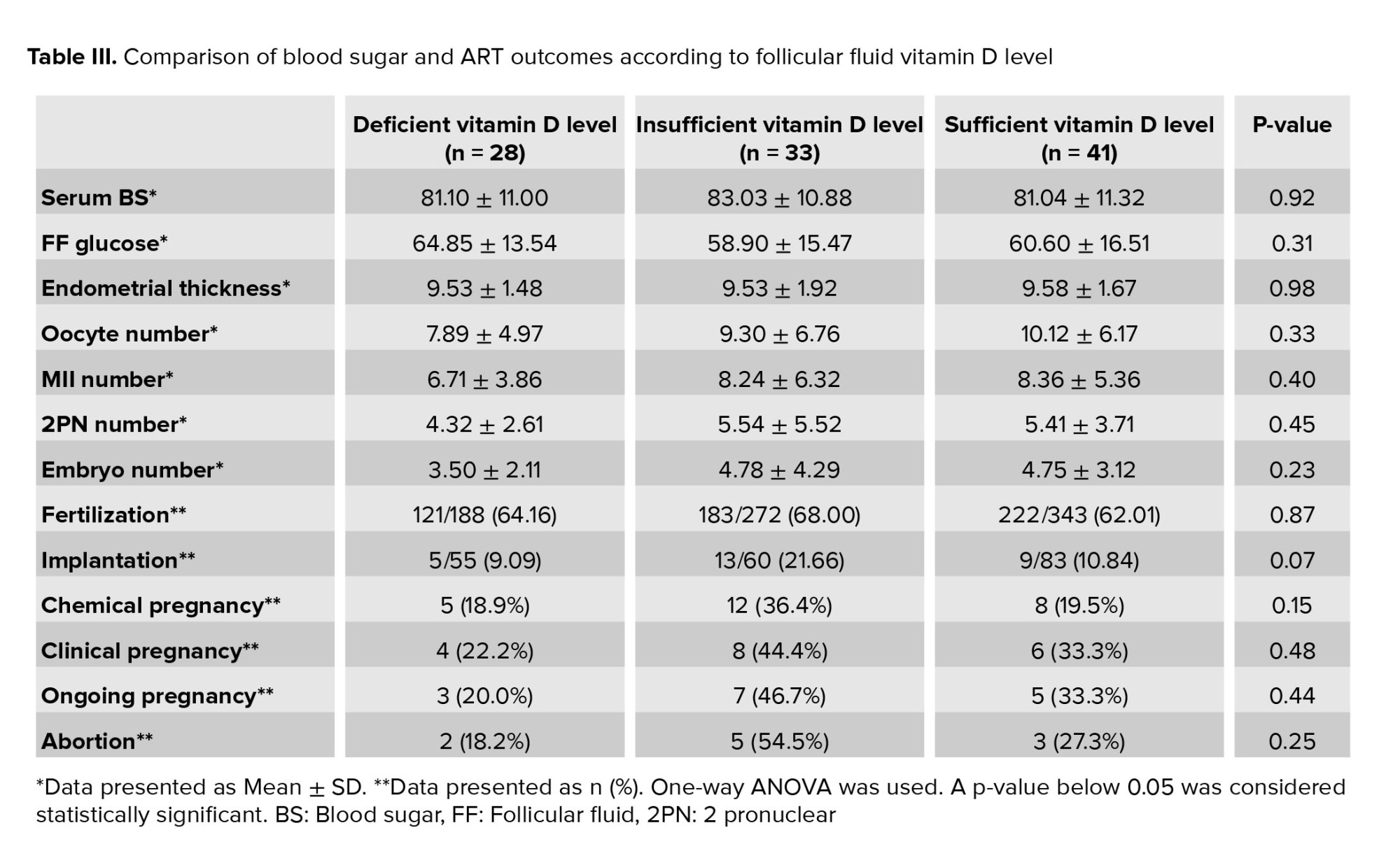
The assessment of study parameters based on follicular fluid vitamin D levels showed that there was no relationship between the three follicular fluid vitamin D levels and ART outcomes. In women who were deficient in follicular fluid vitamin D, the rates of chemical, clinical and ongoing pregnancy were lower than in other groups, but this was not statistically significant (Table III).
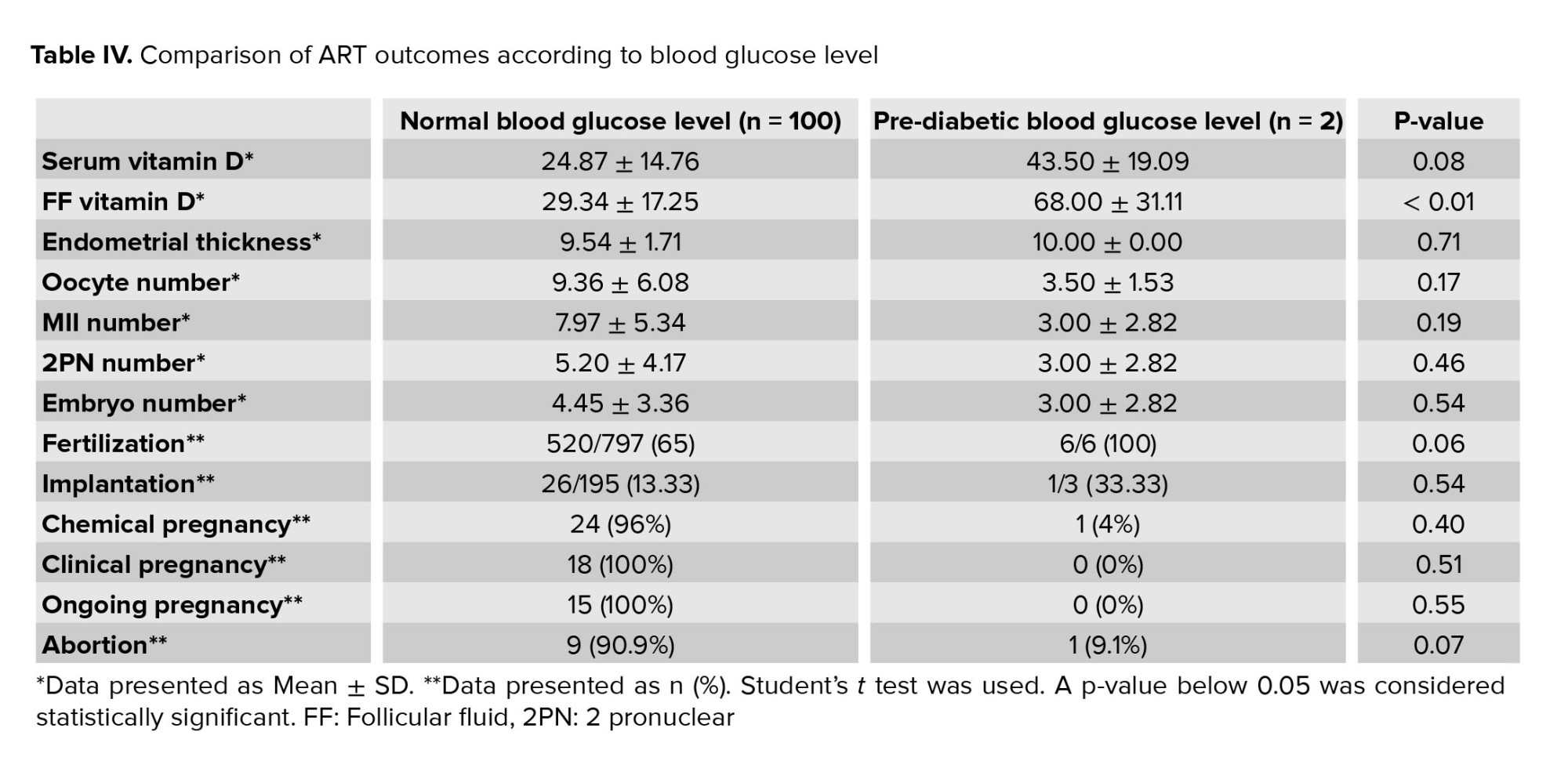
In table IV we summarized the serum and follicular fluid vitamin D levels and ART outcomes based on the FBS level. No significant difference was observed between the FBS levels and serum vitamin D or ART outcomes. The follicular fluid vitamin D level in women with normal and pre-diabetic FBS status was 29.34 ± 17.25 and 68.00 ± 31.11, respectively. In women with normal FBS levels, the level of follicular fluid vitamin D was significantly lower than in women with pre-diabetic FBS status (p < 0.01) (Table IV).
4. Discussion
Vitamin D is a main factor for the regulation of many biological activities in the human body. Vitamin D receptors have been found in the female reproductive system including in the ovary, uterus, endometrium and placenta after successful pregnancy (3). Vitamin D in the female reproductive system can regulate some of the cascades in the hypothalamic pituitary-gonadal axis and can affect the progress of oocyte maturation, endometrial growth and receptivity, implantation development, and progress of pregnancy (15). So, vitamin D may be an influential factor for successful ART cycles.
The aim of our study was to evaluate the role of serum and follicular vitamin D levels in influencing ART outcomes. We found that there was no relationship between the serum and follicular fluid vitamin D levels, and the outcomes of ART. Women with the lower level of vitamin D had lower rates of chemical, clinical and ongoing pregnancy than the women with sufficient levels of vitamin D, but this difference was not statistically significant. Similarly to our results, it was reported that there was no association between serum or follicular fluid vitamin D levels and the outcomes of ART (10, 16-18). In contrast to our findings, some researcher reported that the women in their studies with vitamin D deficiency had lower rates of pregnancy than cases with sufficient vitamin D levels (7, 19-22).
The influence of serum and follicular fluid vitamin D has been considered for many yr. As we presented, some studies have suggested positive relationships between serum and follicular fluid vitamin D, and pregnancy rates, while some studies have not found such a relationship. This could be due to differences in the study design, case selection, infertility type, socioeconomic status, age, body mass index and/or laboratory tests.
It was reported that when vitamin D receptors were blocked in experimental animals, the oogenesis process was disrupted and fertility decreased (23). In another study it was demonstrated that in cases with serum vitamin D sufficiency the implantation rate was higher than in cases with serum vitamin D deficiency. They concluded that perhaps vitamin D is more influential in endometrial receptivity than the oogenesis process (20).
Two separate studies reported that in cases with vitamin D deficiency, some changes occurred in calcium-phosphate metabolism. This alteration can affect endometrial receptivity and ovary function (24, 25). In several experimental animal studies, scientifics found that hypocalcemia following vitamin D deficiency was responsible for decreased pregnancy rates and fertility (26, 27). Some studies have shown that calcium and vitamin D metabolism can induce aromatase gene expression (28, 29). Aromatase in the ovary and uterus induces molecular cascades leading to successful ovulation, endometrial growth, and implantation (30). So, alterations in normal vitamin D levels can change the calcium and aromatase sequence. This is an important factor for changes in fertility, ovulation, embryo formation, implantation, placenta formation, and pregnancy progression. Based on the above studies, serum vitamin D can play an important role in the female reproductive system, but the molecular process or main effective points are still unknown.
The results of the present study showed that in the serum vitamin D sufficient group, the level of follicular fluid glucose was significantly lower than in the deficient and insufficient groups. Anifandis and colleagues evaluated serum vitamin D in combination with glucose and their role in IVF outcomes. They reported that follicular fluid glucose was higher when vitamin D was insufficient (14), which is in accordance with our results.
Some studies have reported the role of vitamin D in inducing insulin secretion and controlling glucose metabolism. These have shown that vitamin D elevates insulin secretion (31, 32). It is possible that normal vitamin D levels promote insulin secretion and then insulin increases glucose utilization by oocytes and other cells in the follicular cavity, including granulosa and cumulus cells. Consequently, this could be responsible for the lower levels of follicular sugar in cases with sufficient vitamin D levels.
Also, in the present study, we assessed the FBS and follicular glucose and calculated the correlation between FBS and ART outcomes. The results showed that there was no correlation between FBS and follicular glucose, and ART outcomes. The relationship between FBS and follicular vitamin D was statistically significant. Several studies have focused on the role of blood sugar in the reproductive system. In cases with high levels of FBS and in diabetic cases, the rate of fertility is higher than in normal blood sugar cases (33, 34). Researchers have discussed that high sugar levels and low insulin levels may be responsible for defects in fertility and decreases in pregnancy rates (35, 36). There is no evidence demonstrating the relationship between FBS and follicular vitamin D. We suggest that the lower levels of follicular fluid vitamin D in cases with normal FBS may be due to sugar metabolism. Perhaps secreted insulin in cases with normal FBS can affect vitamin D metabolism in the follicular fluid. However, this theory needs more experimental and clinical studies to confirm the validity.
5. Conclusion
The results of this study demonstrated some evaluated parameters including serum vitamin D levels, follicular fluid vitamin D levels, FBS and follicular fluid sugar did not have any effect on ART outcomes. However, in women with lower levels of vitamin D in the blood and follicular fluid, the rate of pregnancy was lower than in the vitamin D sufficient group. We recommend that future studies have larger sample sizes and consider different infertility etiologies or vitamin D supplementation in infertile women to further understand the relationship between vitamin D, FBS or follicular fluid sugar and ART outcomes.
Acknowledgments
The authors thank all of the patients in the study and the staff of Afzalipor Infertility Center, Kerman University of Medical Sciences, Kerman, Iran, for their skillful technical assistance during the course of this study. The study was supported financially by the Research Deputy of Kerman University of Medical Sciences, Kerman, Iran.
Conflict of Interest
The authors declare that there is no conflict of interest.
Full-Text: (490 Views)
1. Introduction
Oocyte maturation depends on cytoplasmic and nuclear changes, and each defect in this process can reduce the chances of successful fertilization (1). Some studies have shown that a lack of many vitamins and nutrients can reduce the chances of successful natural fertility, and have confirmed the effect of vitamins and supplements on embryo development (2, 3). Follicular fluid is an important medium for the development of oocytes. Increasing or decreasing the structure of this fluid has negative effects on the oocyte and embryo morphology and quality (4).
Vitamin D is a fat-soluble steroid hormone which is essential for the production of sex hormones, estradiol and progesterone (5). Vitamin D connects to its receptor and then activates the molecular pathways in cells or tissues (6). There is a vitamin D receptor in the human ovaries, uterus, endometrium and placenta. Experiments have shown that vitamin D can support endometrium decidualization and regulatory factors for the expression of some genes such as Homeobox genes 10, and that it is important for embryo implantation. So vitamin D deficiency is associated with various reproduction problemes such as preeclampsia, insulin resistance, and gestational diabetes (5).
Research has shown that infertile women usually have lower levels of vitamin D (7). It was reported that, women who had ovulation defects were deficient in vitamin D, and that vitamin D can reduce inflammation in the body and help the fertility process (8). In another study showed that vitamin D levels in the follicular fluid had a significant positive effect on implantation and the pregnancy rate (9).
Previously it was showed that despite the positive effects of vitamin D as a hormone in the human body, its follicular fluid level did not have a significant effect on in vitro fertilization (IVF) outcomes (10). It was found that pregnancy rates were not associated with vitamin D levels in the follicular fluid. They declared that in women who has higher levels of vitamin D, we have a significant decrease in fertilization rate, while increasing the implantation rate (11).
Glucose in the follicular fluid is one of the most important substances supporting oocyte metabolism (12). Glucose is the main energy source for all follicullar cells including oocytes and other somatic cells (13). Glucose is one of the regulatory factors for the GH-IGF-1 pathway in a study on evaluting vitamin D and glucose levels in the follicular fluid of 101 women who underwent IVF, it was found that there were insufficient vitamin D levels in the follicular fluid of the infertile women. Also, the vitamin D levels in the follicular fluid had a significant negative association with embryo quality. They reported that the follicular fluid glucose level was higher when vitamin D levels were insufficient (14).
Therefore, it is necessary to study the hormonal and biochemical changes of follicular fluid. Studies have shown different results about the effect of vitamin D and glucose in the follicular fluid on fertility and assisted reproductive technique (ART) outcomes. The aim of this study was to measure the levels of vitamin D and glucose in the follicular fluid and serum of infertile wemen who underwent IVF and evaluate the effects of vitamin D and glucose on ART outcomes.
2. Material and Methods
In this cross-sectional study, 102 infertile women (aged 20-40 yr) who were candidates for IVF/intracytoplasmic sperm injection (ICSI) who referred to Afzalipour hospital of Kerman, Iran between March 2019 and February 2020 were enrolled.
Wemen were excluded if they had ovarian reserve depletion (defined as ≤ 3 oocytes with a conventional stimulation protocol, an antral follicular count of 5-7 follicles, Anti-Mullerian hormone levels of 0.5-1.1 ng/ml, or two episodes of polycystic ovarian response after maximal stimulation), reproductive anatomical defects, endocrine disorders, a history of kidney, liver or heart defects, vitamin D metabolism problems and/or a partner with severe male factor infertility.
All women were on an antagonist protocol for controlled ovarian hyperstimulation. Gonadotropin Cinal F (Cinal F, CinnaGen, Iran) was administered from day two of menstruation. The first dosage of gonadotropin measured based on the patients’ age and body weight (150-450 IU/day). On day 7 of the stimulation all patients monitored. When the dominant follicle size was 12 mm, the antagonist (Cetrorelix, Merck-Serono, Germany) was started. When follicles size was ≥ 18 mm in transvaginal ultrasonography imaging, final oocyte maturation was triggered by an intramuscular injection of 10,000 IU of human chorionic gonadotropin (pdpreg, Pooyesh Darou, Iran). After 34-36 hr, oocyte pickup was performed with vaginal ultrasound and general anesthesia. On the oocyte retrieval day, a sample was taken of peripheral blood for measuring vitamin D and fasting blood sugar (FBS). Then, follicular fluid containing oocytes was drained under a transvaginal ultrasound guide with general anesthesia. In the embryology lab, all oocytes were separated and the follicular fluid was transferred to the medical laboratory to determination of vitamin D and sugar level. Method for sample preparation was in the first of all centrifugation for 15 minutes at 1000 g and the seperated serum was kept at -20°C. For measurement of plasma and fulicular fluid vitamin D enzyme immunoassay (Immunodiagnostic Ltd, UK) was used. Serum and folicular fluid glucose levels were estimated with oxidase methods by using a spectrophotometer system (Olympus Life and Material Science Europa, Hamburg, Germany). Serum glucose levels were defined as normal at < 110 ng/ml and pre-diabetic at ≥ 110 ng/ml.
The oocytes were fertilized in the embryology lab byICSI and the number of fertilized oocytes was calculated two days after IVF/ICSI. Three days after oocyte retrieval, one or two high-quality embryos were transferred to the uterus. Implantation, chemical and clinical pregnancy rates, and abortion rates were assessed, and then the relationship between the serum and follicular fluid vitamin D and FBS levels, and the ART outcomes was assessed.
The implantation rate was measured based on the gestational sac in sonography per the number of embryos transferred. A positive chemical pregnancy was defined based on serum β-human chorionic gonadotropin two weeks after embryo transfer. A positive clinical pregnancy was defined by at least one gestational sac in the uterine cavity under sonography 2-3 wk after the positive chemical pregnancy. The abortion rate was defined as pregnancy losses earlier than 20 wk of gestation per positive chemical pregnancies.
2.1. Ethical considerations
This study was approved by the Ethical Committee of Kerman University of Medical Sciences, Kerman, Iran (Code: IR.KMU.REC.1397.513), and informed consent was obtained from all participants in this study.
2.2. Statistical analysis
The Statistical Package for the Social Sciences (SPSS) software version 20 (IBM Co., Illinois, USA) was used for the data analysis. The normal distribution of the data was examined by the Kolmogorov-Smirnov test. Student’s t test and proportional tests were performed for numerical variables and categorical variables, respectively. One-way ANOVA was used for assessing differences between the three groups of serum and follicular fluid vitamin D levels. The results were presented as mean ± standard deviation (SD) or frequency percentage (%). P-values less than 0.05 were considered statistically significant.
3. Results
Data for 102 women were analyzed in the present study. From these 102 women, in 18 cases clinical pregnancy was positive and in 84 cases clinical pregnancy was negative. Basic characteristics, serum vitamin D, follicular vitamin D, FBS, follicular fluid glucose and ART outcomes in the women with positive and negative clinical pregnancy are summarized in table I. Comparison between some parameters such :as char:acteristics, serum vitamin D, follicular vitamin D, FBS and follicular fluid glucose in the two groups showed no significant difference. In terms of the ART outcomes, the numbers of matured oocytes and 2 pronucleus were significantly higher in the women with a positive clinical pregnancy (p = 0.01). But other parameters of ART outcomes were similar between the two groups (Table I). The serum vitamin D status of the cases showed that 66.7% of women were deficient or insufficient in vitamin D and only 33.3% had a sufficient status (Figure 1).
The comparison of glucose status and ART outcomes in women with deficient, insufficient, and sufficient vitamin D status is shown in table II. Based on vitamin D grouping, ART outcomes showed no significant difference. Follicular fluid glucose levels in women who were deficient, insufficient and sufficient in vitamin D were 65.20 ± 14.65, 63.47 ± 14.90 and 55.97 ± 15.64, respectively. Follicular fluid glucose levels were lower in women with sufficient vitamin D levels than in other groups and this difference was statistically significant (p = 0.01). In women with deficient serum vitamin D levels, the rates of chemical, clinical, and ongoing pregnancy were lower than in women with other vitamin D levels, but this difference was not statistically significant (Table II).
The assessment of study parameters based on follicular fluid vitamin D levels showed that there was no relationship between the three follicular fluid vitamin D levels and ART outcomes. In women who were deficient in follicular fluid vitamin D, the rates of chemical, clinical and ongoing pregnancy were lower than in other groups, but this was not statistically significant (Table III).
In table IV we summarized the serum and follicular fluid vitamin D levels and ART outcomes based on the FBS level. No significant difference was observed between the FBS levels and serum vitamin D or ART outcomes. The follicular fluid vitamin D level in women with normal and pre-diabetic FBS status was 29.34 ± 17.25 and 68.00 ± 31.11, respectively. In women with normal FBS levels, the level of follicular fluid vitamin D was significantly lower than in women with pre-diabetic FBS status (p < 0.01) (Table IV).





4. Discussion
Vitamin D is a main factor for the regulation of many biological activities in the human body. Vitamin D receptors have been found in the female reproductive system including in the ovary, uterus, endometrium and placenta after successful pregnancy (3). Vitamin D in the female reproductive system can regulate some of the cascades in the hypothalamic pituitary-gonadal axis and can affect the progress of oocyte maturation, endometrial growth and receptivity, implantation development, and progress of pregnancy (15). So, vitamin D may be an influential factor for successful ART cycles.
The aim of our study was to evaluate the role of serum and follicular vitamin D levels in influencing ART outcomes. We found that there was no relationship between the serum and follicular fluid vitamin D levels, and the outcomes of ART. Women with the lower level of vitamin D had lower rates of chemical, clinical and ongoing pregnancy than the women with sufficient levels of vitamin D, but this difference was not statistically significant. Similarly to our results, it was reported that there was no association between serum or follicular fluid vitamin D levels and the outcomes of ART (10, 16-18). In contrast to our findings, some researcher reported that the women in their studies with vitamin D deficiency had lower rates of pregnancy than cases with sufficient vitamin D levels (7, 19-22).
The influence of serum and follicular fluid vitamin D has been considered for many yr. As we presented, some studies have suggested positive relationships between serum and follicular fluid vitamin D, and pregnancy rates, while some studies have not found such a relationship. This could be due to differences in the study design, case selection, infertility type, socioeconomic status, age, body mass index and/or laboratory tests.
It was reported that when vitamin D receptors were blocked in experimental animals, the oogenesis process was disrupted and fertility decreased (23). In another study it was demonstrated that in cases with serum vitamin D sufficiency the implantation rate was higher than in cases with serum vitamin D deficiency. They concluded that perhaps vitamin D is more influential in endometrial receptivity than the oogenesis process (20).
Two separate studies reported that in cases with vitamin D deficiency, some changes occurred in calcium-phosphate metabolism. This alteration can affect endometrial receptivity and ovary function (24, 25). In several experimental animal studies, scientifics found that hypocalcemia following vitamin D deficiency was responsible for decreased pregnancy rates and fertility (26, 27). Some studies have shown that calcium and vitamin D metabolism can induce aromatase gene expression (28, 29). Aromatase in the ovary and uterus induces molecular cascades leading to successful ovulation, endometrial growth, and implantation (30). So, alterations in normal vitamin D levels can change the calcium and aromatase sequence. This is an important factor for changes in fertility, ovulation, embryo formation, implantation, placenta formation, and pregnancy progression. Based on the above studies, serum vitamin D can play an important role in the female reproductive system, but the molecular process or main effective points are still unknown.
The results of the present study showed that in the serum vitamin D sufficient group, the level of follicular fluid glucose was significantly lower than in the deficient and insufficient groups. Anifandis and colleagues evaluated serum vitamin D in combination with glucose and their role in IVF outcomes. They reported that follicular fluid glucose was higher when vitamin D was insufficient (14), which is in accordance with our results.
Some studies have reported the role of vitamin D in inducing insulin secretion and controlling glucose metabolism. These have shown that vitamin D elevates insulin secretion (31, 32). It is possible that normal vitamin D levels promote insulin secretion and then insulin increases glucose utilization by oocytes and other cells in the follicular cavity, including granulosa and cumulus cells. Consequently, this could be responsible for the lower levels of follicular sugar in cases with sufficient vitamin D levels.
Also, in the present study, we assessed the FBS and follicular glucose and calculated the correlation between FBS and ART outcomes. The results showed that there was no correlation between FBS and follicular glucose, and ART outcomes. The relationship between FBS and follicular vitamin D was statistically significant. Several studies have focused on the role of blood sugar in the reproductive system. In cases with high levels of FBS and in diabetic cases, the rate of fertility is higher than in normal blood sugar cases (33, 34). Researchers have discussed that high sugar levels and low insulin levels may be responsible for defects in fertility and decreases in pregnancy rates (35, 36). There is no evidence demonstrating the relationship between FBS and follicular vitamin D. We suggest that the lower levels of follicular fluid vitamin D in cases with normal FBS may be due to sugar metabolism. Perhaps secreted insulin in cases with normal FBS can affect vitamin D metabolism in the follicular fluid. However, this theory needs more experimental and clinical studies to confirm the validity.
5. Conclusion
The results of this study demonstrated some evaluated parameters including serum vitamin D levels, follicular fluid vitamin D levels, FBS and follicular fluid sugar did not have any effect on ART outcomes. However, in women with lower levels of vitamin D in the blood and follicular fluid, the rate of pregnancy was lower than in the vitamin D sufficient group. We recommend that future studies have larger sample sizes and consider different infertility etiologies or vitamin D supplementation in infertile women to further understand the relationship between vitamin D, FBS or follicular fluid sugar and ART outcomes.
Acknowledgments
The authors thank all of the patients in the study and the staff of Afzalipor Infertility Center, Kerman University of Medical Sciences, Kerman, Iran, for their skillful technical assistance during the course of this study. The study was supported financially by the Research Deputy of Kerman University of Medical Sciences, Kerman, Iran.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Fertility & Infertility
References
1. Luciano AM, Franciosi F, Dieci C, Tessaro I, Terzaghi L, Modina S, et al. Large-scale chromatin structure and function changes during oogenesis: the interplay between oocyte and companion cumulus cells. Anim Reprod 2018; 11: 141-149.
2. Gaskins AJ, Chavarro JE. Diet and fertility: A review. Am J Obstet Gynecol 2018; 218: 379-389. [DOI:10.1016/j.ajog.2017.08.010] [PMID] [PMCID]
3. Fung JL, Hartman TJ, Schleicher RL, Goldman MB. Association of vitamin D intake and serum levels with fertility: Results from the lifestyle and fertility study. Fertil Steril 2017; 108: 302-311. [DOI:10.1016/j.fertnstert.2017.05.037] [PMID] [PMCID]
4. Bahadori MH, Sharami SH, Fakor F, Milani F, Pourmarzi D, Dalil-Heirati SF. Level of vitamin e in follicular fluid and serum and oocyte morphology and embryo quality in patients undergoing ivf treatment. J Family Reprod Health 2017; 11: 74-81.
5. Fichera M, Török P, Tesarik J, Della Corte L, Rizzo G, Garzon S, et al. Vitamin D, reproductive disorders and assisted reproduction: Evidences and perspectives. Int J Food Sci Nutr 2020; 71: 276-285. [DOI:10.1080/09637486.2019.1661978] [PMID]
6. Slominski AT, Brożyna AA, Zmijewski MA, Jóźwicki W, Jetten AM, Mason RS, et al. Vitamin D signaling and melanoma: Role of vitamin D and its receptors in melanoma progression and management. Lab Invest 2017; 97: 706-724. [DOI:10.1038/labinvest.2017.3] [PMID] [PMCID]
7. Eftekhar M, Mirhashemi ES, Molaei B, Pourmasumi S. Is there any association between vitamin D levels and polycystic ovary syndrome (PCOS) phenotypes? Arch Endocrinol Metab 2020; 64: 11-16. [DOI:10.20945/2359-3997000000177] [PMID]
8. Akl Sh, El-Mekkawi Sh, El-Kotb AM, Mostafa A. Role of vitamin D supplementation therapy on ovulation and insulin resistance in women with PCOS: A randomized controlled trial. Evid Based Womens Health J 2019; 9: 329-336. [DOI:10.21608/EBWHJ.2019.28635]
9. Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon Ch, et al. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril 2010; 94: 1314-1319. [DOI:10.1016/j.fertnstert.2009.05.019] [PMID] [PMCID]
10. Dehghani Firouzabadi R, Rahmani E, Rahsepar M, Mahdavi Firouzabadi M. Value of follicular fluid vitamin D in predicting the pregnancy rate in an IVF program. Arch Gynecol Obstet 2014; 289: 201-206. [DOI:10.1007/s00404-013-2959-9] [PMID]
11. Aleyasin A, Agha Hosseini M, Mahdavi A, Safdarian L, Fallahi P, Mohajeri MR, et al. Predictive value of the level of vitamin D in follicular fluid on the outcome of assisted reproductive technology. Eur J Obstet Gynecol Reprod Biol 2011; 159: 132-137. [DOI:10.1016/j.ejogrb.2011.07.006] [PMID]
12. Da Broi MG, Giorgi VSI, Wang F, Keefe DL, Albertini D, Navarro PA. Influence of follicular fluid and cumulus cells on oocyte quality: Clinical implications. J Assist Reprod Genet 2018; 35: 735-751. [DOI:10.1007/s10815-018-1143-3] [PMID] [PMCID]
13. Nandi S, Girish Kumar V, Manjunatha BM, Ramesh HS, Gupta PSP. Follicular fluid concentrations of glucose, lactate and pyruvate in buffalo and sheep, and their effects on cultured oocytes, granulosa and cumulus cells. Theriogenology 2008; 69: 186-196. [DOI:10.1016/j.theriogenology.2007.08.036] [PMID]
14. Anifandis GM, Dafopoulos K, Messini CI, Chalvatzas N, Liakos N, Pournaras S, et al. Prognostic value of follicular fluid 25-OH vitamin D and glucose levels in the IVF outcome. Reprod Biol Endocrinol 2010; 8: 91. [DOI:10.1186/1477-7827-8-91] [PMID] [PMCID]
15. Lee DM, Tajar A, Pye SR, Boonen S, Vanderschueren D, Bouillon R, et al. Association of hypogonadism with vitamin D status: the european male ageing study. Eur J Endocrinol 2012; 166: 77-85. [DOI:10.1530/EJE-11-0743] [PMID]
16. van de Vijver A, Drakopoulos P, Van Landuyt L, Vaiarelli A, Blockeel C, Santos-Ribeiro S, et al. Vitamin D deficiency and pregnancy rates following frozen-thawed embryo transfer: A prospective cohort study. Hum Reprod 2016; 31: 1749-1754. [DOI:10.1093/humrep/dew107] [PMID]
17. Franasiak JM, Molinaro ThA, Dubell EK, Scott KL, Ruiz AR, Forman EJ, et al. Vitamin D levels do not affect IVF outcomes following the transfer of euploid blastocysts. Am J Obstet Gynecol 2015; 212: 315. [DOI:10.1016/j.ajog.2014.09.029] [PMID]
18. Fabris A, Pacheco A, Cruz M, Puente JM, Fatemi H, Garcia-Velasco JA. Impact of circulating levels of total and bioavailable serum vitamin D on pregnancy rate in egg donation recipients. Fertil Steril 2014; 102: 1608-1612. [DOI:10.1016/j.fertnstert.2014.08.030] [PMID]
19. Garbedian K, Boggild M, Moody J, Liu KE. Effect of vitamin D status on clinical pregnancy rates following in vitro fertilization. CMAJ Open 2013; 1: E77-E82. [DOI:10.9778/cmajo.20120032] [PMID] [PMCID]
20. Rudick B, Ingles S, Chung K, Stanczyk F, Paulson R, Bendikson K. Characterizing the influence of vitamin D levels on IVF outcomes. Hum Reprod 2012; 27: 3321-3327. [DOI:10.1093/humrep/des280] [PMID]
21. Paffoni A, Ferrari S, Viganò P, Pagliardini L, Papaleo E, Candiani M, et al. Vitamin D deficiency and infertility: Insights from in vitro fertilization cycles. J Clin Endocrinol Metab 2014; 99: E2372-E2376. [DOI:10.1210/jc.2014-1802] [PMID]
22. Polyzos NP, Anckaert E, Guzman L, Schiettecatte J, Van Landuyt L, Camus M, et al. Vitamin D deficiency and pregnancy rates in women undergoing single embryo, blastocyst stage, transfer (SET) for IVF/ICSI. Hum Reprod 2014; 29: 2032-2040. [DOI:10.1093/humrep/deu156] [PMID]
23. Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet 1997; 16: 391-396. [DOI:10.1038/ng0897-391] [PMID]
24. Liao EY, Zhang ZhL, Xia WB, Lin H, Cheng Q, Wang L, et al. Calcifediol (25-hydroxyvitamin D) improvement and calcium-phosphate metabolism of alendronate sodium/vitamin D(3) combination in Chinese women with postmenopausal osteoporosis: A post hoc efficacy analysis and safety reappraisal. BMC Musculoskelet Disord 2018; 19: 210. [DOI:10.1186/s12891-018-2090-y] [PMID] [PMCID]
25. Harkness LS, Bonny AE. Calcium and vitamin D status in the adolescent: Key roles for bone, body weight, glucose tolerance, and estrogen biosynthesis. J Pediatr Adolesc Gynecol 2005; 18: 305-311. [DOI:10.1016/j.jpag.2005.06.002] [PMID]
26. Al-Harbi AN, Khan KhM, Rahman A. Developmental vitamin D deficiency affects spatial learning in Wistar rats. J Nutr 2017; 147: 1795-1805. [DOI:10.3945/jn.117.249953] [PMID]
27. Belenchia AM, Johnson SA, Kieschnick AC, Rosenfeld CS, Peterson CA. Time course of vitamin D depletion and repletion in reproductive-age female C57BL/6 mice. Comp Med 2017; 67: 483-490.
28. Grzesiak M, Burzawa G, Kurowska P, Blaszczyk K, Szlaga A, Blasiak A, et al. Altered vitamin D3 metabolism in the ovary and periovarian adipose tissue of rats with letrozole-induced PCOS. Histochem Cell Biol 2021; 155: 101-116. [DOI:10.1007/s00418-020-01928-z] [PMID] [PMCID]
29. Dupuis ML, Pagano MT, Pierdominici M, Ortona E. The role of vitamin D in autoimmune diseases: Could sex make the difference? Biol Sex Differ 2021; 12: 1-12. [DOI:10.1186/s13293-021-00358-3] [PMID] [PMCID]
30. Tosti C, Biscione A, Morgante G, Bifulco G, Luisi S, Petraglia F. Hormonal therapy for endometriosis: From molecular research to bedside. Eur J Obstet Gynecol Reprod Biol 2017; 209: 61-66. [DOI:10.1016/j.ejogrb.2016.05.032] [PMID]
31. Garbossa SG, Folli F. Vitamin D, sub-inflammation and insulin resistance: A window on a potential role for the interaction between bone and glucose metabolism. Rev Endocr Metab Disord 2017; 18: 243-258. [DOI:10.1007/s11154-017-9423-2] [PMID]
32. Osati S, Homayounfar R, Hajifaraji M. Metabolic effects of vitamin D supplementation in vitamin D deficient patients: A double-blind clinical trial. Diabetes Metab Syndr 2016; 10: S7-S10. [DOI:10.1016/j.dsx.2016.01.007] [PMID]
33. Kouhkan A, Khamseh ME, Moini A, Pirjani R, Arabipoor A, Zolfaghari Z, et al. Diagnostic accuracy of body mass index and fasting glucose for the prediction of gestational diabetes mellitus after assisted reproductive technology. Int J Fertil Steril 2019; 13: 32-37.
34. Tashrifi F, Haghani Nasimi O, Abdollahi M, Moradi B. Fasting blood sugar, glucose challenge test and one-two hour glucose tolerance test in diagnosis of gestational diabetes in women without risk factor. Iran J Diabetes Obesity 2018; 10: 106-108.
35. Zhang Ch, Yang Ch, Li N, Liu X, He J, Chen X, et al. Elevated insulin levels compromise endometrial decidualization in mice with decrease in uterine apoptosis in early-stage pregnancy. Arch Toxicol 2019; 93: 3601-3615. [DOI:10.1007/s00204-019-02601-8] [PMID]
36. Namvar Jahromi B, Dabbaghmanesh MH, Bakhshaie P, Parsanezhad ME, Anvar Z, Alborzi M, et al. Assessment of oxytocin level, glucose metabolism components and cutoff values for oxytocin and anti-mullerian hormone in infertile PCOS women. Taiwan J Obstet Gynecol 2018; 57: 555-559. [DOI:10.1016/j.tjog.2018.06.015] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





