Sun, May 5, 2024
[Archive]
Volume 20, Issue 2 (February 2022)
IJRM 2022, 20(2): 123-136 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ostovan F, Gol A, Javadi A. Protective properties of Rydingia persica in reproductive complications induced by diabetes in male rats: An experimental study. IJRM 2022; 20 (2) :123-136
URL: http://ijrm.ir/article-1-2134-en.html
URL: http://ijrm.ir/article-1-2134-en.html
1- Department of Biology, Faculty of Science, Shahid Bahonar University of Kerman, Kerman, Iran.
2- Department of Biology, Faculty of Science, Shahid Bahonar University of Kerman, Kerman, Iran. , agol@uk.ac.ir
3- Pathology Department, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Department of Biology, Faculty of Science, Shahid Bahonar University of Kerman, Kerman, Iran. , agol@uk.ac.ir
3- Pathology Department, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 4262 kb]
(815 Downloads)
| Abstract (HTML) (1073 Views)
1. Introduction
Diabetes mellitus (DM) is diagnosed based on a high serum glucose level due to a lack of insulin secretion (1), absence of insulin receptor responsiveness, or both. Overall, DM is one of the most important causes of death in developed countries and is responsible for 5% of deaths worldwide (2). DM can lead to many structural and functional effects such as neuropathy, nephropathy, retinopathy, and also disorders of the reproductive system (3, 4). Clinical and experimental investigations have revealed major changes in semen volume, spermatogenesis, sperm count, sperm motility, penile erection, ejaculation, serum luteinizing hormone (LH), follicle stimulating hormone, and testosterone levels as well as onset of puberty in diabetic subjects (5-7).
Oxidative stress (OS) is an important factor induced by hyperglycemia at the beginning and progression of DM complications. One of the main factors affecting the reproductive system in DM is the increased production of reactive oxygen species (ROS), which leads to protein and lipid oxidation (8). The balance between ROS and their scavengers is controlled tightly under physiological conditions. Defects in this balance can occur in metabolic disorders such as DM causing OS in the male reproductive system which can lead to a range of problems. Testicular oxidative damage can lead to biochemical and hormonal changes followed by a decrease in sexual libido and behavior (9-11).
In recent decades, some studies have shown the therapeutic effects of medicinal plants to control DM by modulating carbohydrate metabolism, repairing the pancreatic beta cell function and release of insulin, improving glucose utilization, and through their antioxidant properties (10, 12).
The aerial parts of Rydingia persica (Burm.f.) Scheen & V.A.Albert (R.P) - also known as Otostegia persica - have long been used as a medicinal herb in Iran and elsewhere in the Arab world and wider Asia as a way to alleviate various disorders (13). R.P is a medicinal plant containing polyphenolic compounds. These compounds are extensively distributed in different parts of the plant (14). R.P has some biological effects such as antioxidative, antidiabetic, anticancer, antiarthritic, and antimalarial effects (15, 16). Aerial parts of R.P contains components such as flavonoids, steroids, tannins, triterpenoids, and some essential mineral elements. Most of the mentioned compounds were demonstrated to possess strong antioxidant activity (17). Polyphenols may exert their protective effects through direct and indirect antioxidant activity. Direct effect is the result of in vitro metal chelation, ROS scavenging, lipid peroxidation inhibition, hydroperoxide formation reduction. The indirect effects are the result of modulation of pathways in cell signaling and gene expression, adjustment of defense enzymes, and alteration in nuclear histone acetylation (18).
This plant has been used for many varieties of disorders, including DM, but no studies have been done on its use for the reproductive complications of DM. We therefore decided to investigate the protective properties of the aerial parts of R.P against reproductive damage induced by DM by assessing the oxidative status and hormonal and histopathological changes in male rats.
2. Materials and Methods
This experimental study was carried out in the Department of Biology, Shahid Bahonar University of Kerman in November 2018.
2.1. Chemicals and devices
Streptozotocin (STZ, 18883-66-4), 5', 5'-dithiobis-2-nitrobenzoic acid (DTNB, 69-78-3), β-nicotinamide adenine dinucleotide 2´-phosphate (NADPH, 2646-71-1), riboflavin (83-88-5), L- glutathione oxidized (GSSG, 27025-41-8), glutathione reductase (9001-48-3), and xylol (1082972500) were purchased from Sigma Aldrich, USA. Potassium iodide (KI, 1.05040.1000), dipotassium hydrogen phosphate (K2HPO4, 1.05101.1000), potassium dihydrogen phosphate (KH2PO4, 1.04873.1000), sodium dihydrogen phosphate (NaH2PO4, 1.06346.1000), hydrogen peroxide (H2O2, 1.08600.1000), meta-phosphoric acid (1.00546.0100), eosin (1.15935.0025), trichloroacetic acid (TCA, 1.00807.0250), and glutathione (GSH, 1.04090.0005) were purchased from MERCK, Germany. Hematoxylin was purchased from Pouya Abzar (Azma, Iran) and formalin (37%, A-330) from Atlas Shimi, (Iran). The spectrophotometer (Cary 50) was purchased from Varian (Australia).
2.2. Preparation of Rydingia persica powder
The aerial parts of the R.P were collected from Birk Mountain, Saravan, in Sistan and Baluchestan province of Iran, and were taxonomically identified and approved by Dr. Mirtadzaddini, Biology Department of Faculty of Science, herbarium number (2388). The name of the plant was checked with dow.theplantlist.org. The R.P was powdered in an electrical grinder.
2.3. Animals
In this experimental study, 40 adult male Wister rats (230-270 gr, 12-wk old) were obtained from the animal house of Shahid Bahonar University of Kerman and were maintained under standard colony conditions with a 12-hr light/dark cycle at constant room temperature (23 ± 2oC) with free access to food and water.
2.4. Experimental design
Streptozotocin (STZ) (Sigma-Aldrich, USA 60 mg/kg body weight) was used for DM induction. STZ, dissolved in cold normal saline, was injected intraperitoneally. Seventy-two hr thereafter, the fasting blood glucose level was measured by a glucometer (Arkray Glucocard 01-mini glucometer, Japan), and the rats with a blood glucose over 300 mg/dl were allocated as diabetics. The study period was considered as 14 days according to a previous study (19).
The rats were divided into five groups (n = 8/each) and were fed orally for two wk:
Full-Text: (267 Views)
1. Introduction
Diabetes mellitus (DM) is diagnosed based on a high serum glucose level due to a lack of insulin secretion (1), absence of insulin receptor responsiveness, or both. Overall, DM is one of the most important causes of death in developed countries and is responsible for 5% of deaths worldwide (2). DM can lead to many structural and functional effects such as neuropathy, nephropathy, retinopathy, and also disorders of the reproductive system (3, 4). Clinical and experimental investigations have revealed major changes in semen volume, spermatogenesis, sperm count, sperm motility, penile erection, ejaculation, serum luteinizing hormone (LH), follicle stimulating hormone, and testosterone levels as well as onset of puberty in diabetic subjects (5-7).
Oxidative stress (OS) is an important factor induced by hyperglycemia at the beginning and progression of DM complications. One of the main factors affecting the reproductive system in DM is the increased production of reactive oxygen species (ROS), which leads to protein and lipid oxidation (8). The balance between ROS and their scavengers is controlled tightly under physiological conditions. Defects in this balance can occur in metabolic disorders such as DM causing OS in the male reproductive system which can lead to a range of problems. Testicular oxidative damage can lead to biochemical and hormonal changes followed by a decrease in sexual libido and behavior (9-11).
In recent decades, some studies have shown the therapeutic effects of medicinal plants to control DM by modulating carbohydrate metabolism, repairing the pancreatic beta cell function and release of insulin, improving glucose utilization, and through their antioxidant properties (10, 12).
The aerial parts of Rydingia persica (Burm.f.) Scheen & V.A.Albert (R.P) - also known as Otostegia persica - have long been used as a medicinal herb in Iran and elsewhere in the Arab world and wider Asia as a way to alleviate various disorders (13). R.P is a medicinal plant containing polyphenolic compounds. These compounds are extensively distributed in different parts of the plant (14). R.P has some biological effects such as antioxidative, antidiabetic, anticancer, antiarthritic, and antimalarial effects (15, 16). Aerial parts of R.P contains components such as flavonoids, steroids, tannins, triterpenoids, and some essential mineral elements. Most of the mentioned compounds were demonstrated to possess strong antioxidant activity (17). Polyphenols may exert their protective effects through direct and indirect antioxidant activity. Direct effect is the result of in vitro metal chelation, ROS scavenging, lipid peroxidation inhibition, hydroperoxide formation reduction. The indirect effects are the result of modulation of pathways in cell signaling and gene expression, adjustment of defense enzymes, and alteration in nuclear histone acetylation (18).
This plant has been used for many varieties of disorders, including DM, but no studies have been done on its use for the reproductive complications of DM. We therefore decided to investigate the protective properties of the aerial parts of R.P against reproductive damage induced by DM by assessing the oxidative status and hormonal and histopathological changes in male rats.
2. Materials and Methods
This experimental study was carried out in the Department of Biology, Shahid Bahonar University of Kerman in November 2018.
2.1. Chemicals and devices
Streptozotocin (STZ, 18883-66-4), 5', 5'-dithiobis-2-nitrobenzoic acid (DTNB, 69-78-3), β-nicotinamide adenine dinucleotide 2´-phosphate (NADPH, 2646-71-1), riboflavin (83-88-5), L- glutathione oxidized (GSSG, 27025-41-8), glutathione reductase (9001-48-3), and xylol (1082972500) were purchased from Sigma Aldrich, USA. Potassium iodide (KI, 1.05040.1000), dipotassium hydrogen phosphate (K2HPO4, 1.05101.1000), potassium dihydrogen phosphate (KH2PO4, 1.04873.1000), sodium dihydrogen phosphate (NaH2PO4, 1.06346.1000), hydrogen peroxide (H2O2, 1.08600.1000), meta-phosphoric acid (1.00546.0100), eosin (1.15935.0025), trichloroacetic acid (TCA, 1.00807.0250), and glutathione (GSH, 1.04090.0005) were purchased from MERCK, Germany. Hematoxylin was purchased from Pouya Abzar (Azma, Iran) and formalin (37%, A-330) from Atlas Shimi, (Iran). The spectrophotometer (Cary 50) was purchased from Varian (Australia).
2.2. Preparation of Rydingia persica powder
The aerial parts of the R.P were collected from Birk Mountain, Saravan, in Sistan and Baluchestan province of Iran, and were taxonomically identified and approved by Dr. Mirtadzaddini, Biology Department of Faculty of Science, herbarium number (2388). The name of the plant was checked with dow.theplantlist.org. The R.P was powdered in an electrical grinder.
2.3. Animals
In this experimental study, 40 adult male Wister rats (230-270 gr, 12-wk old) were obtained from the animal house of Shahid Bahonar University of Kerman and were maintained under standard colony conditions with a 12-hr light/dark cycle at constant room temperature (23 ± 2oC) with free access to food and water.
2.4. Experimental design
Streptozotocin (STZ) (Sigma-Aldrich, USA 60 mg/kg body weight) was used for DM induction. STZ, dissolved in cold normal saline, was injected intraperitoneally. Seventy-two hr thereafter, the fasting blood glucose level was measured by a glucometer (Arkray Glucocard 01-mini glucometer, Japan), and the rats with a blood glucose over 300 mg/dl were allocated as diabetics. The study period was considered as 14 days according to a previous study (19).
The rats were divided into five groups (n = 8/each) and were fed orally for two wk:
- Group C: Control rats, animals received normal saline.
- Group D: Diabetic rats, animals received normal saline.
- Group D+R200: Diabetic rats received 200 mg/kg body weight R.P powder dissolved in normal saline.
- Group D+R400: Diabetic rats received 400 mg/kg body weight R.P powder dissolved in normal saline.
- Group D+R600: Diabetic rats received 600 mg/kg body weight R.P powder dissolved in normal saline.
On the 14th day of the experiment, the animals were decapitated, and blood serum for hormonal changes was collected. The testes and epididymis were removed, weighed, and then frozen until OS assay.
2.5. Serum preparation
Blood samples were collected and centrifuged at 3500 rpm for 15 min to obtain serum samples and then were stored at -20°C until biochemical analysis. Commercially available ELISA kits were used to estimate hormones and LH (Testosterone ELISA kit-DKO002, Diametra, Italy and rat LH ELISA kit-ER1123, Fine test, China, respectively). Assays were performed according to the manufacturers’ protocols.
2.6. OS markers measurement
To measure the malondialdehyde (MDA) concentration, 0.2 gr of the desired tissue. To measure the malondialdehyde (MDA) concentration, 0.2 gr of the desired tissue was homogenized in 5 ml of 0.1% trichloroacetic acid and the obtained solution was centrifuged for 10 minutes at 10,000 g at 4°C. Then, 1 ml of the supernatant was added to 20% trichloroacetic acid solution having 0.5% thiobarbituric acid. Finally, the mixture was heated for 30 at 95°C minutes and then cooled quickly in ice, and again was centrifuged for 10 minutes at 4000 g. Finally, the absorbance at 532 nm was read (20).
The amount of reduced glutathione (GSH) was measured using the total and oxidized glutathione (GSSG). Half a gr of the target tissue was homogenized in 2 ml of metaphosphoric acid. The resulting homogenate was centrifuged for 10 min at 10,000 g using a refrigerator centrifuge. For total glutathione, 100 μl of the supernatant solution was added to the test tube containing 700 μl of NADPH (0.3 mM), 100 μl of DTNB (6 mM) and 100 μl of distilled water. 10 μl of glutathione reductase was added to the solution after 3-4 min and the absorbance was read at 412 nm. To measure the amount of oxidized glutathione, 100 μl of the centrifuged extract from the previous step was added to a test tube containing 2-vinylpyridine and kept at room temperature for one hr. Then 0.3 mM of NADPH, 6 mM of DTNB and 10 μl of glutathione reductase enzyme were added. The absorption of the samples at 412 nm was read (21).
To prepare the tissue extract for measuring the antioxidant enzymes (catalase, glutathione peroxidase, superoxide dismutase, and glutathione reductase), 0.5 gr of the desired tissue was homogenized in 3 ml of 50 mM phosphate buffer and then the extract was centrifuged in a high-speed refrigerated centrifuge at 10,000 g for 10 min (22).
Catalase (CAT) activity was measured at 240 nm (23). The reaction mixture with a total volume of 3 ml consisted of 50 mM of potassium phosphate buffer with pH = 7, 15 mM of hydrogen peroxide (H2O2) and 100 μl of enzyme extract. The amount of H2O2 in the reaction mixture was calculated after one min, which indicated the activity of the enzyme catalase.
Glutathione peroxidase (GPx) activity was measured at 470 nm using guiacol, and the absorption of tetraguiacol which was formed from guiacol as a result of peroxidase activity was measured at 470 nm. The reaction mixture consisted of 2.77 ml of 50 mM phosphate buffer at pH = 7, 100 μl of 1% H2O2 and 100 μl of 4% guiacol (24).
The activity of the superoxide dismutase (SOD) enzyme was measured using the inhibition of NBT optical reduction reaction at 560 nm. The reaction mixture of the sample included 50 mM of phosphate buffer with pH = 7.8, 75 μM of NBT, 0.1 μM of Na-EDTA, 65 μM of riboflavin, 13 mM of methionine and 50 μl of enzyme extract (25).
To measure glutathione reductase (GR) activity, the reaction mixture contained 100 mM of sodium phosphate buffer (pH = 7.8), 0.5 mM of GSSG, 0.1 mM of NADPH and 50 μl of enzyme extract. The reaction was started by adding NADPH. The absorption of the samples was read for three min at a wavelength of 340 nm (26).
To prepare the tissue extract for measuring H2O2, 0.1 gr of the desired tissue was homogenized in 1 ml of 0.1% trichloroacetic acid and then the extract was centrifuged in a refrigerator centrifuge at a speed of 10,000 g for 10 min. Then 0.5 ml of the centrifuged solution was added to 10 mM of potassium phosphate buffer with pH = 7 and 1 M of potassium iodide, and the absorption was read at 390 nm (27).
2.7. Preparation of testes for histological studies
For histology of the testis, it was promptly fixed in 10% formaldehyde, buffered by a solution containing 54 mM of NaH2PO4 and 28 mM of Na2HPO4 (pH = 7.4). To complete the tissue fixation, a transverse section was made through the middle of the testis while immersed in the fixator. Thereafter, the tissues were put in paraffin and cut in slices (6-7 micrometer thick) and placed on the glass slides precoated with albumin. The samples were then deparaffinized with xylol, and finally for histological examination by light microscopy (Nikon, Y-THM, Japan) they were stained with hematoxylin and eosin.
Based on the following criteria, a grading score was applied from 1 to 10 for each tubule cross-section. Complete spermatogenesis with perfect tubules = 10, a lot of spermatozoa but disorganized spermatogenesis = 9, a few spermatozoa = 8, absence of spermatozoa but many spermatids = 7, a few spermatids = 6, absence of spermatozoa and spermatids but many spermatocytes = 5, a few spermatocytes = 4, only presence of spermatogonia = 3, absence of germ cells but presence of sertoli cells = 2, absence of both germ and sertoli cells = 1.
2.8. Ethical considerations
The study protocol and all animal procedures were approved by the educational assistant of Shahid Bahonar University of Kerman, Kerman, Iran (Code: IR.UK.VETMED.REC.1399.016).
2.9. Statistical analysis
SPSS (Statistical Package for the Social Sciences) version 20 (from I.B.M, U.S.A) was used for the statistical analysis. Data are presented as mean ± standard deviation (SD). Statistics are performed by one-way analysis of variance (ANOVA) and Tukey’s post hoc test, and significance was considered at p < 0.05.
3. Results
3.1. Glucose, testosterone and LH concentrations
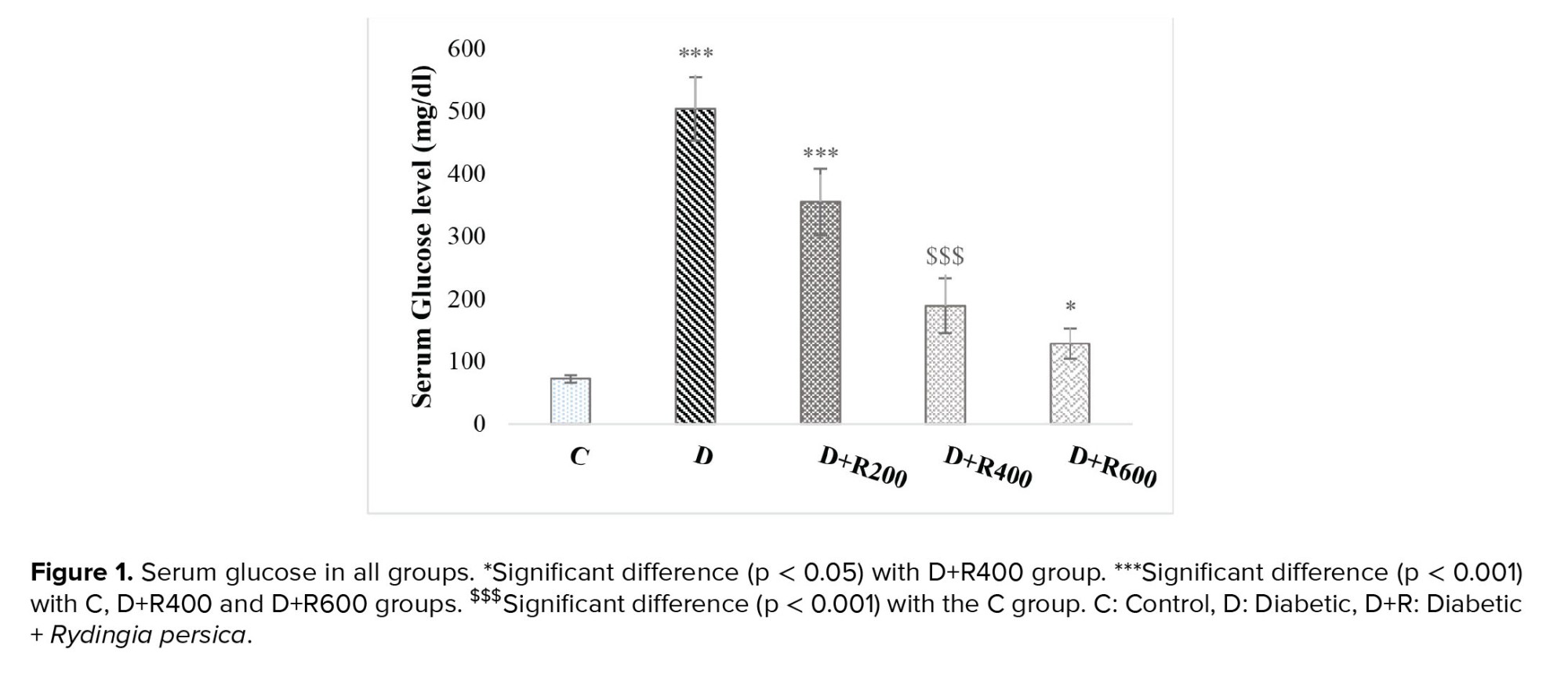
The results showed that the glucose concentration was significantly higher in the experimental groups compared to in the C group. The highest concentration was observed in group D and the lowest in group D+R600 (Figure 1).
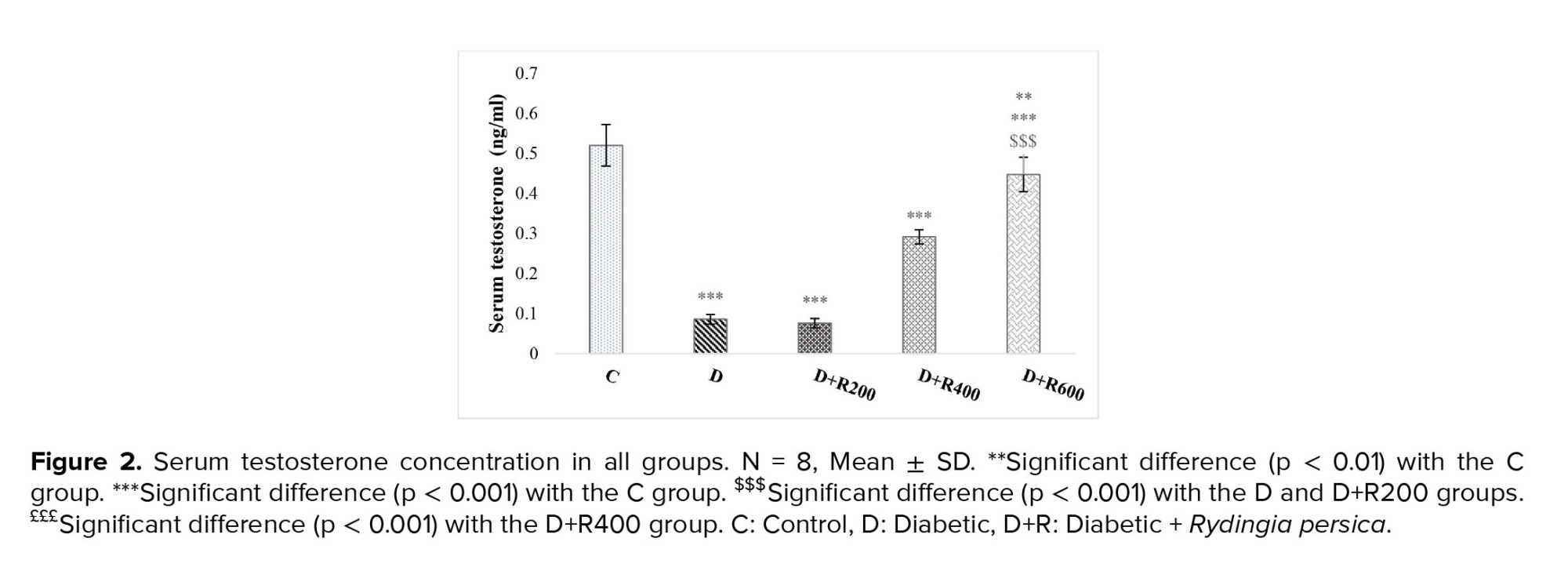
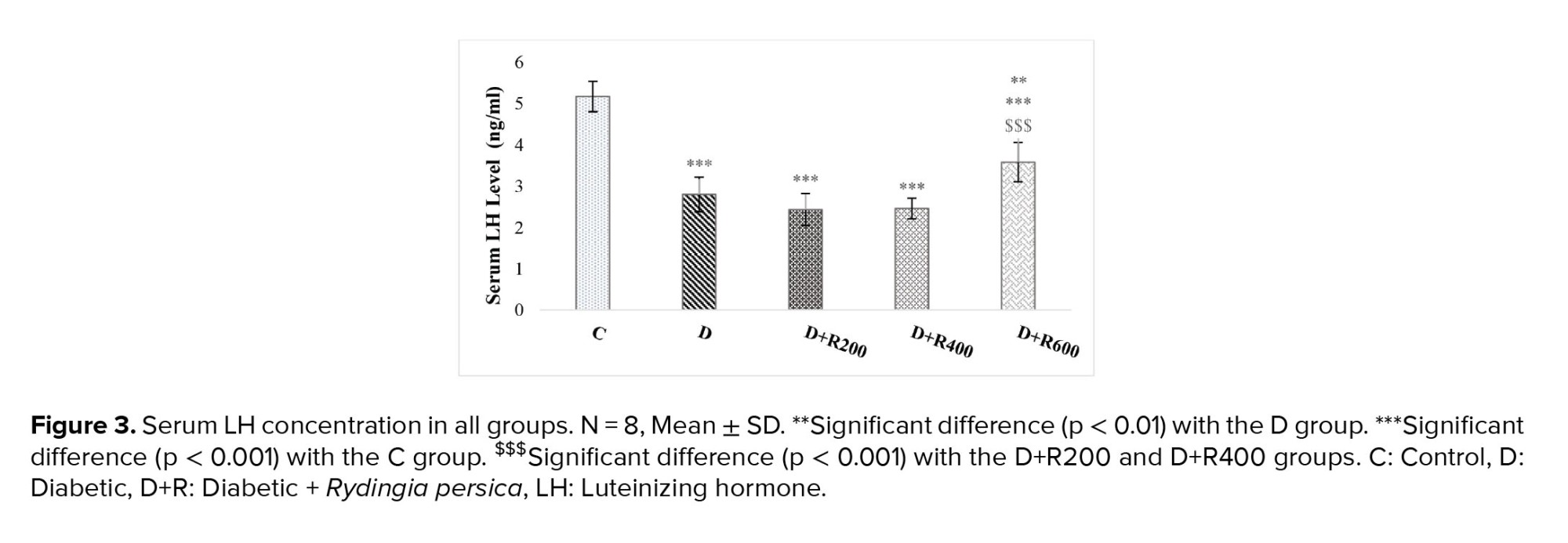
Figures 2 and 3 show that serum testosterone and LH concentration were significantly lower in the experimental groups compared to in the C group.
3.2. Effects in the testis tissue
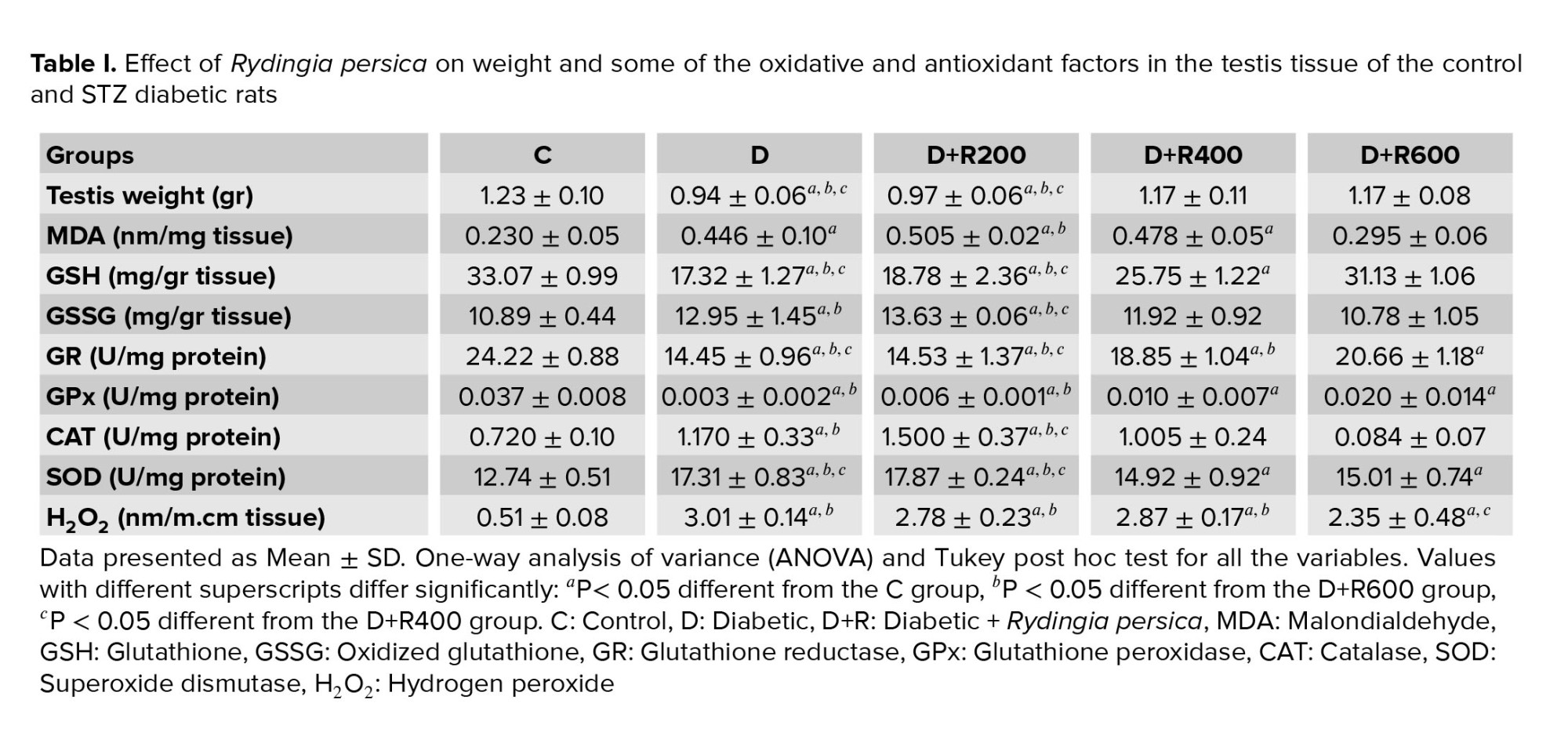
Table I shows that the testis weight in the D and D+R200 groups was significantly lower compared to in the C group. Also, the D+R400 and D+R600 groups showed a significantly higher weight compared to the D and D+R200 groups.
The MDA concentration in the testes in the D, D+R200 and D+R400 groups was also significantly higher compared to in the C group (p < 0.05 and p < 0.01, respectively). However, the D+R600 group showed a significantly lower level compared to the D+R200 group.
The GSH concentration in the testes in the D, D+R200 and D+R400 groups was significantly lower compared to in the C group. Also, the D+R400 and D+R600 groups showed a significantly higher concentration compared to the D and D+R200 groups. Furthermore, the D+R600 group had a significantly higher concentration compared to the D+R400 group.
The GSSG concentration in the testes of the D and D+R200 groups was significantly higher compared to in the C group. Also, the D+R600 group showed a significantly lower concentration compared to the D and D+R200 groups. The D+R400 group had a significantly lower concentration compared to the D+R200 group.
GR activity in the testes of the rats in the D, D+R200, D+R400 and D+R600 groups was significantly lower compared to in the C group. Also, the D+R400 and D+R600 groups showed a significantly higher level compared to the D and D+R200 groups. The D+R600 group showed significantly higher activity compared to the D+R400 group.
GPx activity in the testes of the D, D+R200, D+R400 and D+R600 groups was significantly lower compared to in the C group. Also, the D+R600 rats showed a significantly higher level of activity compared to the D and D+R200 rats.
CAT activity in the testes of the D and D+R200 groups was significantly higher compared to in the C group. Also, the D+R200 group showed a significantly higher level compared to the D+R400 and D+R600 groups.
SOD concentration in the testes of the D, D+R200, D+R400 and D+R600 rats was significantly higher compared to in the C group. The D+R400 and D+R600 groups had a significantly lower concentration compared to the D and D+R200 groups.
H2O2 concentration in the testes in the D, D+R200, D+R400 and D+R600 rats was significantly higher compared to in the C group. Moreover, the D+R600 group had a significantly lower concentration compared to the D, D+R200 and D+R400 groups.
3.3. Effects in the epididymis tissue
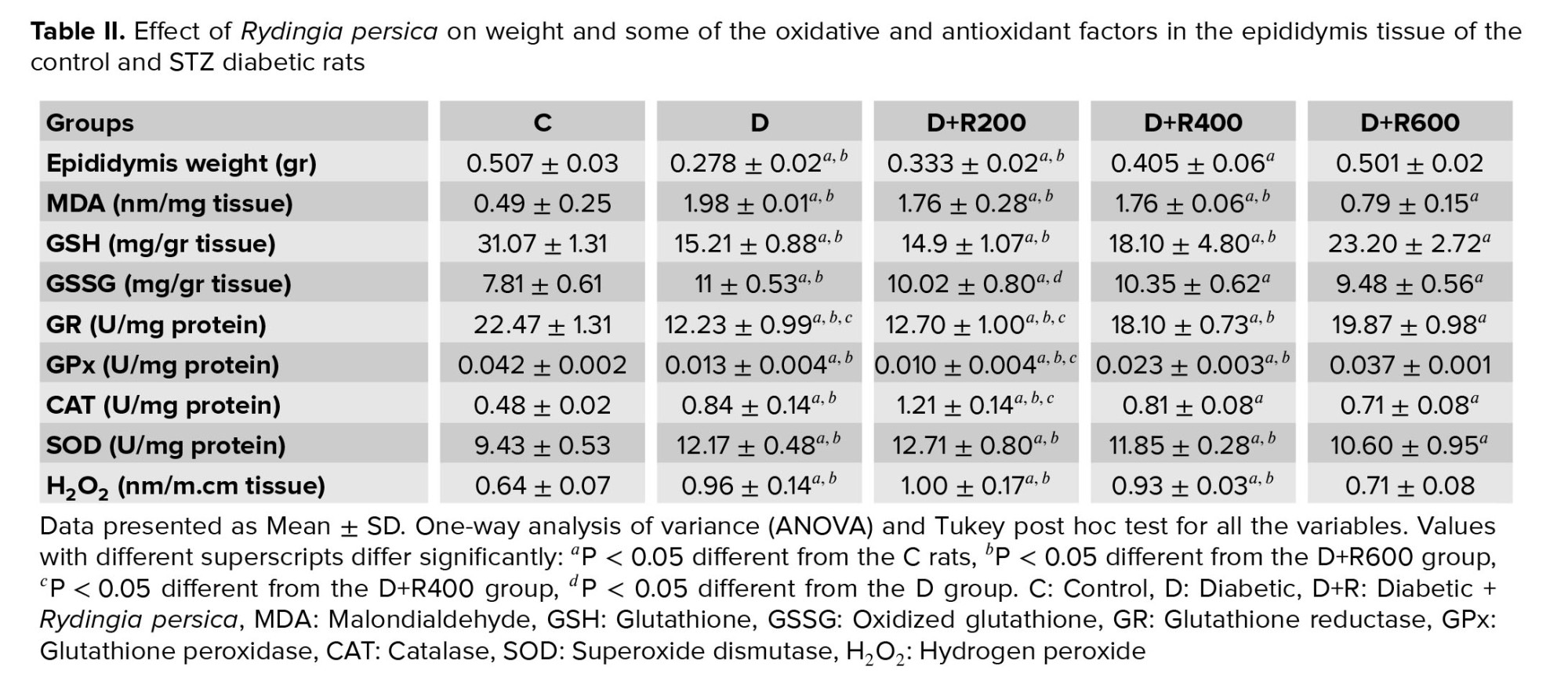
Table II shows that the epididymis weight in the D, D+R200 and D+R400 groups was significantly lower compared to in the C group. Also, the D+R600 group showed a significantly higher weight compared to the D and D+R200 groups. The D+R400 group had a significantly higher weight compared to the D and D+R200 groups.
The MDA concentration in the epididymis of the D, D+R200 and D+R400 rats was significantly higher compared to in the C rats, and the D+R600 group’s concentration was significantly higher compared to the C group. Also, the D+R600 group had a significantly lower concentration compared to the D, D+R200 and D+R400 groups.
The GSH concentration in the epididymis of the D, D+R200, D+R400 and D+R600 groups was significantly lower compared to in the C group. Also, the D+R600 group showed a significantly higher concentration compared to the D, D+R200 and D+R400 groups.
The GSSG concentration in the epididymis in the D, D+R200, D+R400 and D+R600 groups was a significantly higher level compared to in the C group. Also, the D+R600 and D+R200 groups showed a significantly lower concentration in comparison with the D group.
GR activity in the epididymis of the D, D+R200, D+R400 and D+R600 rats was significantly lower compared to in the C group. Also, the D+R400 and D+R600 rats showed a significantly higher level of activity compared to the D and D+R200 groups, and the D+R600 group demonstrated a significantly higher level compared to the D+R200 group.
GPx concentration in the epididymis of the D, D+R200 and D+R400 groups was significantly lower compared to in the C group. Also, the D+R600 group showed a significantly higher concentration compared to the D, D+R200 and D+R400 groups. The D+R400 group also had a significantly higher concentration compared to the D+R200 group.
CAT activity in the epididymis in the D, D+R200, D+R400 and D+R600 groups was significantly higher compared to in the C group. Also, the D+R400 and D+R600 groups showed significantly higher activity compared to the D+R200 group. The D+R200 and D+R600 groups had significantly lower activity compared to the D group.
SOD activity in the epididymis of the D, D+R200, D+R400 and D+R600 rats was significantly higher compared to in the C group. Also, the D+R600 group showed a significantly lower level compared to the D, D+R200 and D+R400 groups.
H2O2 concentration in the epididymis of the D, D+R200 and D+R400 groups showed a significantly higher level compared to the C group. Also, the D+R600 group had a significantly lower concentration compared to the D, D+R200 and D+R400 groups.
3.4. Histopathology
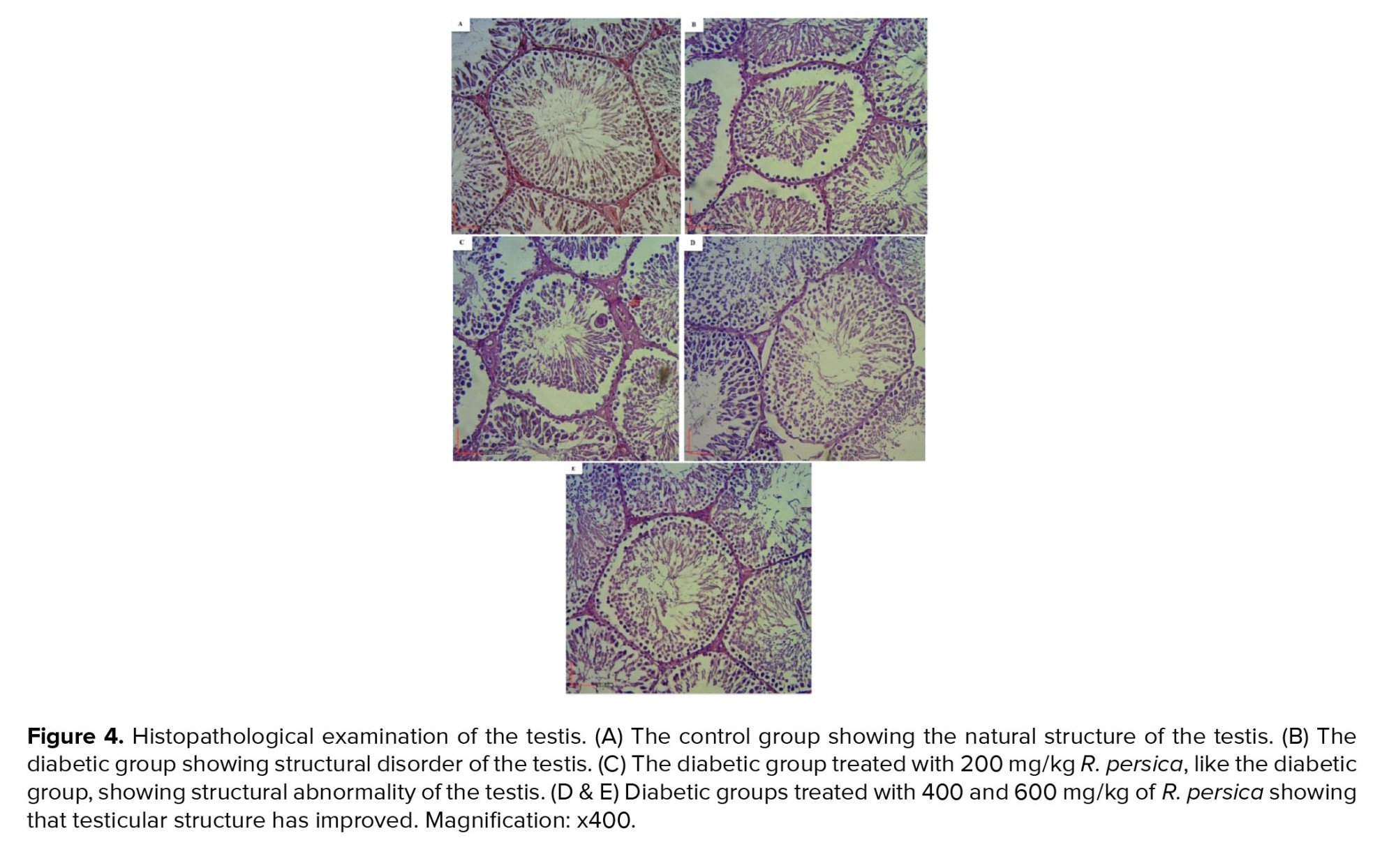
Figure 4 shows the histopathology of the testis. Examination of the C group did not show spermatogenic arrest, tubular atrophy, edema, or Leydig cell hyperplasia. The estimated Johnson score was about 9/10. The mean of the counted number of germ cells per same sized tubules was 230 per tubule (Figure 4A).
In addition, examination of the D group showed testicular parenchyma with spermatogenic arrest (1+), seminiferous tubular atrophy (3+) and thickening of the basal lamina (2+). It also did not show Leydig cell hyperplasia or edema. The Johnson score was 5/10 basal on the morphological scoring system. The mean of the counted number of germ cells per same sized tubules was 190 per tubule (Figure 4B).
The D+R200 group showed testicular parenchyma with spermatogenic arrest (1+), seminiferous tubular atrophy (2+) and thickening of the basal lamina (2+). It also did not show Leydig cell hyperplasia but moderate interstitial edema was observed (2+). The Johnson score was 5/10 basal on the morphological scoring system. The mean of the counted number of germ cells per same sized tubule was 185.1 per tubule (Figure 4C).
Microscopic examination of the D+R400 group showed testicular parenchyma with spermatogenic arrest (1+), seminiferous tubular atrophy (2+) and thickening of the basal lamina (1+). In this group, Leydig cell hyperplasia was not observed but the examination did show mild interstitial edema (1+). The Johnson score was 6/10 basal on the morphological scoring system. The mean of the counted number of germ cells per same sized tubule was 197.7 per tubule (Figure 4D).
Furthermore, microscopic examination of the D+R600 group did not show spermatogenic arrest or thickening of the basal lamina, but showed atrophy of the seminiferous tubules, Leydig cell hyperplasia, and mild interstitial edema (1+). The Johnson score was 7/10 basal on the morphological scoring system. The mean of the counted number of germ cells per same sized tubule was 212.5 per tubule (Figure 4E).
4. Discussion
In this study, the diabetic group showed increased serum glucose, decreased serum LH and testosterone, decreased testis and epididymis weight, and finally, it showed changes in all variables assayed related to OS, indicating DM-induced OS. Also, diabetic rats treated with R.P with a dose of 600 mg/kg, and in some cases the dose of 400 mg/kg, showed changes in measured variables toward the control group, indicating the protective effect of R.P against OS, testosterone and LH changes.
One study has emphasized the connection between DM and OS and the resulting complications. DM-induced OS can be the result of increased glucose auto-oxidation from hyperglycemia and protein glycation followed by oxidative degradation of glycated proteins (28). It has been reported that the MDA level in the tissues and blood of diabetic rats is increased due to both lipid peroxidation and reduced antioxidant activity (29, 30). In this study, testis, and epididymis MDA levels were significantly higher in the diabetic group. Glutathione plays an important role in cellular defense against ROS. We saw a significant depletion of GSH in the testis and epididymis of diabetic rats, which is a sign of OS (31). In the present study, lower levels of GSH and higher levels of GSSG in the diabetic rats probably were related to the lower activity of GR in both the testis and epididymis of diabetic rats as also described in another study (31). We observed higher activity of SOD and CAT in the diabetic group. As it has already been shown, SOD converts superoxide anion to H2O2 which in turn is converted to H2O by CAT (32, 33).
Increased activity of CAT in the heart and liver and decreased activity in the kidney of diabetic rats have already been shown (34). In another study, DM led to increased CAT and SOD activity in the liver and serum of diabetic rats (35). Also, it was shown that CAT activity increased in the testis, epididymis and liver of diabetic rats (33, 36). In addition, increased SOD and CAT enzyme expression with hyperglycemia have been demonstrated (37). So, the increased activity of SOD and CAT observed in the present study can be interpreted as a compensatory response against hyperglycemia-induced OS.
We saw decreased serum testosterone in the diabetic group. Leydig cell membrane injury induced by OS can be one explanation for this (15). It can also be the result of steroidogenic defects in the Leydig cells in DM (16). Moreover, the mechanism by which testosterone is reduced in DM may be the direct effect of glucose or its metabolism. It has been reported that insulin stimulates the hypothalamus and hypophysis for secretion of follicle stimulating hormone and LH; therefore, DM leads to a reduction in these two hormones and thereby disturbs spermatogenesis and testosterone production (38). In the present study, diabetic rats treated with R.P with the dose of 400 or 600 mg/kg showed changes in the measured variables toward the control group, indicating the protective effect of R.P against OS. Honey has been shown to reverse the reduced LH and testosterone in diabetic animals toward the control group (39). In our study, the D+R400 group did not display any effects on LH concentration, but showed increased testosterone concentration, while for the D+R600 group, effects both on LH and testosterone were observed. The reason for this could be that at a dose of 600 mg/kg, R.P could have some effect on hypophysis, but at dose of 400 mg/kg, R.P had its effect directly on the testis (40).
The histological examinations of the testis showed that in diabetic rats, spermatogenesis was impaired compared to in Control rats. These histological changes included a decreased number of spermatogonia, atrophy of the seminiferous tubules, thickening of the basal lamina, hypoplasia of the Leydig cells, and presence of edema in the diabetic testes. In the present study, the Johnson scores of the diabetic rats were 5, indicating testicular damage. Our results are in agreement with a study that showed DM causes cell death through apoptosis leading to testicular dysfunction, seminiferous tubule atrophy, reduction in tubule's diameter, and diminished spermatogenetic cell series (41). Basal lamina thickness has a crucial role in spermatogenesis and its increase results in diminished sperm production and decreased in the size of seminiferous tubules (42). In the histopathological examination of the epididymis, we observed a decrease in sperm density, and atrophy and vacuolation of the epithelium in the diabetic group, which is consistent with another study (43). However, treatment with R.P had a protective effect on the studied tissues, which is consistent with biochemical data.
R.P is used widely by the people of Baluch who live in the southeastern part of Iran. It has some antioxidant, antidiabetic, antimicrobial, anti-inflammatory and antimalarial properties (14). The hypoglycemic effect of R.P may be due to the presence of one or more antidiabetic components which demonstrate synergistic properties (44). Phytochemical screening of the R.P crude extract have revealed the presence of flavonoids, phenolic acids, and steroids/ triterpenoids (44, 45).
Flavonoids have been shown to work as antidiabetic compounds, because they possess multiple characteristics that promote both glucose-lowering actions (insulin-mimetic) and insulin secretion (insulin secretagogue) (46). Two flavonoids, morin and quercetin, both present in R.P, have been shown to have strong antioxidant activity, which is equel to BHA and stronger than alpha tocopherol (47). Quercetin strongly inhibits the transport of glucose and fructose through intestinal cells’ GLUT2 (48). Another study showed it potentiates insulin secretion induced by glucose and has a protective action against beta-cell oxidative injury induced by H2O2 (49). The effects of quercetin administrated intraperitoneally was analyzed (10 or 15 mg/kg body weight) for 10 days in control and diabetic rats which significantly decreased glucose levels in the diabetic rats. Quercetin increases the glucokinase activity, leading to this effect (50). Also it has been shown that quercetin has a similar effect as metformin (51).
The antioxidant activity of morin has been reported to be effective against OS in several disease profiles (52-54). Administration of morin has been reported to improve the pathological condition of hyperglycemia, glucose intolerance, lipid peroxidation, and insulin resistance (52, 55). Improvement in insulin receptor signaling and reduction of hyperglycemia and lipid deposits in the liver of diabetic rats has been reported, as well as antidiabetic and antioxidant effects, which directly supports the notion of the antidiabetic activity of morin (56, 57). In another study, morin administration was shown to increase insulin receptor activation and decrease gluconeogenesis potency (58). The effect of morin has been found to be similar to the conventionally used antidiabetic drug Glibenclamide. Moreover, monoterpene derivatives in the R.P flower are other compounds with antioxidant activity (59).
Therefore, it can be suggested that the reason for the improvement in the hyperglycemic, oxidative, and antioxidant status observed in the rats treated with R.P powder was due to the insulin-like activity and antidiabetic properties of R.P.
5. Conclusion
Our findings showed that R.P powder in doses of 600 or 400 mg/kg body weight had protective effects against DM by reducing hyperglycemia, defending against OS, and restoring hormonal and testicular histological changes.
Acknowledgements
The Ph.D. dissertation was financially supported by Shahid Bahonar University of Kerman, Kerman, Iran. The researchers are grateful to Dr. SM. Mirtadzaddini in the Department of Biology, Faculty of Science, in Shahid Bahonar University of Kerman for his invaluable assistance in the plant identification.
Conflict of Interest
The authors declare that there is no conflict of interest.
2.5. Serum preparation
Blood samples were collected and centrifuged at 3500 rpm for 15 min to obtain serum samples and then were stored at -20°C until biochemical analysis. Commercially available ELISA kits were used to estimate hormones and LH (Testosterone ELISA kit-DKO002, Diametra, Italy and rat LH ELISA kit-ER1123, Fine test, China, respectively). Assays were performed according to the manufacturers’ protocols.
2.6. OS markers measurement
To measure the malondialdehyde (MDA) concentration, 0.2 gr of the desired tissue. To measure the malondialdehyde (MDA) concentration, 0.2 gr of the desired tissue was homogenized in 5 ml of 0.1% trichloroacetic acid and the obtained solution was centrifuged for 10 minutes at 10,000 g at 4°C. Then, 1 ml of the supernatant was added to 20% trichloroacetic acid solution having 0.5% thiobarbituric acid. Finally, the mixture was heated for 30 at 95°C minutes and then cooled quickly in ice, and again was centrifuged for 10 minutes at 4000 g. Finally, the absorbance at 532 nm was read (20).
The amount of reduced glutathione (GSH) was measured using the total and oxidized glutathione (GSSG). Half a gr of the target tissue was homogenized in 2 ml of metaphosphoric acid. The resulting homogenate was centrifuged for 10 min at 10,000 g using a refrigerator centrifuge. For total glutathione, 100 μl of the supernatant solution was added to the test tube containing 700 μl of NADPH (0.3 mM), 100 μl of DTNB (6 mM) and 100 μl of distilled water. 10 μl of glutathione reductase was added to the solution after 3-4 min and the absorbance was read at 412 nm. To measure the amount of oxidized glutathione, 100 μl of the centrifuged extract from the previous step was added to a test tube containing 2-vinylpyridine and kept at room temperature for one hr. Then 0.3 mM of NADPH, 6 mM of DTNB and 10 μl of glutathione reductase enzyme were added. The absorption of the samples at 412 nm was read (21).
To prepare the tissue extract for measuring the antioxidant enzymes (catalase, glutathione peroxidase, superoxide dismutase, and glutathione reductase), 0.5 gr of the desired tissue was homogenized in 3 ml of 50 mM phosphate buffer and then the extract was centrifuged in a high-speed refrigerated centrifuge at 10,000 g for 10 min (22).
Catalase (CAT) activity was measured at 240 nm (23). The reaction mixture with a total volume of 3 ml consisted of 50 mM of potassium phosphate buffer with pH = 7, 15 mM of hydrogen peroxide (H2O2) and 100 μl of enzyme extract. The amount of H2O2 in the reaction mixture was calculated after one min, which indicated the activity of the enzyme catalase.
Glutathione peroxidase (GPx) activity was measured at 470 nm using guiacol, and the absorption of tetraguiacol which was formed from guiacol as a result of peroxidase activity was measured at 470 nm. The reaction mixture consisted of 2.77 ml of 50 mM phosphate buffer at pH = 7, 100 μl of 1% H2O2 and 100 μl of 4% guiacol (24).
The activity of the superoxide dismutase (SOD) enzyme was measured using the inhibition of NBT optical reduction reaction at 560 nm. The reaction mixture of the sample included 50 mM of phosphate buffer with pH = 7.8, 75 μM of NBT, 0.1 μM of Na-EDTA, 65 μM of riboflavin, 13 mM of methionine and 50 μl of enzyme extract (25).
To measure glutathione reductase (GR) activity, the reaction mixture contained 100 mM of sodium phosphate buffer (pH = 7.8), 0.5 mM of GSSG, 0.1 mM of NADPH and 50 μl of enzyme extract. The reaction was started by adding NADPH. The absorption of the samples was read for three min at a wavelength of 340 nm (26).
To prepare the tissue extract for measuring H2O2, 0.1 gr of the desired tissue was homogenized in 1 ml of 0.1% trichloroacetic acid and then the extract was centrifuged in a refrigerator centrifuge at a speed of 10,000 g for 10 min. Then 0.5 ml of the centrifuged solution was added to 10 mM of potassium phosphate buffer with pH = 7 and 1 M of potassium iodide, and the absorption was read at 390 nm (27).
2.7. Preparation of testes for histological studies
For histology of the testis, it was promptly fixed in 10% formaldehyde, buffered by a solution containing 54 mM of NaH2PO4 and 28 mM of Na2HPO4 (pH = 7.4). To complete the tissue fixation, a transverse section was made through the middle of the testis while immersed in the fixator. Thereafter, the tissues were put in paraffin and cut in slices (6-7 micrometer thick) and placed on the glass slides precoated with albumin. The samples were then deparaffinized with xylol, and finally for histological examination by light microscopy (Nikon, Y-THM, Japan) they were stained with hematoxylin and eosin.
Based on the following criteria, a grading score was applied from 1 to 10 for each tubule cross-section. Complete spermatogenesis with perfect tubules = 10, a lot of spermatozoa but disorganized spermatogenesis = 9, a few spermatozoa = 8, absence of spermatozoa but many spermatids = 7, a few spermatids = 6, absence of spermatozoa and spermatids but many spermatocytes = 5, a few spermatocytes = 4, only presence of spermatogonia = 3, absence of germ cells but presence of sertoli cells = 2, absence of both germ and sertoli cells = 1.
2.8. Ethical considerations
The study protocol and all animal procedures were approved by the educational assistant of Shahid Bahonar University of Kerman, Kerman, Iran (Code: IR.UK.VETMED.REC.1399.016).
2.9. Statistical analysis
SPSS (Statistical Package for the Social Sciences) version 20 (from I.B.M, U.S.A) was used for the statistical analysis. Data are presented as mean ± standard deviation (SD). Statistics are performed by one-way analysis of variance (ANOVA) and Tukey’s post hoc test, and significance was considered at p < 0.05.
3. Results
3.1. Glucose, testosterone and LH concentrations
The results showed that the glucose concentration was significantly higher in the experimental groups compared to in the C group. The highest concentration was observed in group D and the lowest in group D+R600 (Figure 1).
Figures 2 and 3 show that serum testosterone and LH concentration were significantly lower in the experimental groups compared to in the C group.
3.2. Effects in the testis tissue
Table I shows that the testis weight in the D and D+R200 groups was significantly lower compared to in the C group. Also, the D+R400 and D+R600 groups showed a significantly higher weight compared to the D and D+R200 groups.
The MDA concentration in the testes in the D, D+R200 and D+R400 groups was also significantly higher compared to in the C group (p < 0.05 and p < 0.01, respectively). However, the D+R600 group showed a significantly lower level compared to the D+R200 group.
The GSH concentration in the testes in the D, D+R200 and D+R400 groups was significantly lower compared to in the C group. Also, the D+R400 and D+R600 groups showed a significantly higher concentration compared to the D and D+R200 groups. Furthermore, the D+R600 group had a significantly higher concentration compared to the D+R400 group.
The GSSG concentration in the testes of the D and D+R200 groups was significantly higher compared to in the C group. Also, the D+R600 group showed a significantly lower concentration compared to the D and D+R200 groups. The D+R400 group had a significantly lower concentration compared to the D+R200 group.
GR activity in the testes of the rats in the D, D+R200, D+R400 and D+R600 groups was significantly lower compared to in the C group. Also, the D+R400 and D+R600 groups showed a significantly higher level compared to the D and D+R200 groups. The D+R600 group showed significantly higher activity compared to the D+R400 group.
GPx activity in the testes of the D, D+R200, D+R400 and D+R600 groups was significantly lower compared to in the C group. Also, the D+R600 rats showed a significantly higher level of activity compared to the D and D+R200 rats.
CAT activity in the testes of the D and D+R200 groups was significantly higher compared to in the C group. Also, the D+R200 group showed a significantly higher level compared to the D+R400 and D+R600 groups.
SOD concentration in the testes of the D, D+R200, D+R400 and D+R600 rats was significantly higher compared to in the C group. The D+R400 and D+R600 groups had a significantly lower concentration compared to the D and D+R200 groups.
H2O2 concentration in the testes in the D, D+R200, D+R400 and D+R600 rats was significantly higher compared to in the C group. Moreover, the D+R600 group had a significantly lower concentration compared to the D, D+R200 and D+R400 groups.
3.3. Effects in the epididymis tissue
Table II shows that the epididymis weight in the D, D+R200 and D+R400 groups was significantly lower compared to in the C group. Also, the D+R600 group showed a significantly higher weight compared to the D and D+R200 groups. The D+R400 group had a significantly higher weight compared to the D and D+R200 groups.
The MDA concentration in the epididymis of the D, D+R200 and D+R400 rats was significantly higher compared to in the C rats, and the D+R600 group’s concentration was significantly higher compared to the C group. Also, the D+R600 group had a significantly lower concentration compared to the D, D+R200 and D+R400 groups.
The GSH concentration in the epididymis of the D, D+R200, D+R400 and D+R600 groups was significantly lower compared to in the C group. Also, the D+R600 group showed a significantly higher concentration compared to the D, D+R200 and D+R400 groups.
The GSSG concentration in the epididymis in the D, D+R200, D+R400 and D+R600 groups was a significantly higher level compared to in the C group. Also, the D+R600 and D+R200 groups showed a significantly lower concentration in comparison with the D group.
GR activity in the epididymis of the D, D+R200, D+R400 and D+R600 rats was significantly lower compared to in the C group. Also, the D+R400 and D+R600 rats showed a significantly higher level of activity compared to the D and D+R200 groups, and the D+R600 group demonstrated a significantly higher level compared to the D+R200 group.
GPx concentration in the epididymis of the D, D+R200 and D+R400 groups was significantly lower compared to in the C group. Also, the D+R600 group showed a significantly higher concentration compared to the D, D+R200 and D+R400 groups. The D+R400 group also had a significantly higher concentration compared to the D+R200 group.
CAT activity in the epididymis in the D, D+R200, D+R400 and D+R600 groups was significantly higher compared to in the C group. Also, the D+R400 and D+R600 groups showed significantly higher activity compared to the D+R200 group. The D+R200 and D+R600 groups had significantly lower activity compared to the D group.
SOD activity in the epididymis of the D, D+R200, D+R400 and D+R600 rats was significantly higher compared to in the C group. Also, the D+R600 group showed a significantly lower level compared to the D, D+R200 and D+R400 groups.
H2O2 concentration in the epididymis of the D, D+R200 and D+R400 groups showed a significantly higher level compared to the C group. Also, the D+R600 group had a significantly lower concentration compared to the D, D+R200 and D+R400 groups.
3.4. Histopathology
Figure 4 shows the histopathology of the testis. Examination of the C group did not show spermatogenic arrest, tubular atrophy, edema, or Leydig cell hyperplasia. The estimated Johnson score was about 9/10. The mean of the counted number of germ cells per same sized tubules was 230 per tubule (Figure 4A).
In addition, examination of the D group showed testicular parenchyma with spermatogenic arrest (1+), seminiferous tubular atrophy (3+) and thickening of the basal lamina (2+). It also did not show Leydig cell hyperplasia or edema. The Johnson score was 5/10 basal on the morphological scoring system. The mean of the counted number of germ cells per same sized tubules was 190 per tubule (Figure 4B).
The D+R200 group showed testicular parenchyma with spermatogenic arrest (1+), seminiferous tubular atrophy (2+) and thickening of the basal lamina (2+). It also did not show Leydig cell hyperplasia but moderate interstitial edema was observed (2+). The Johnson score was 5/10 basal on the morphological scoring system. The mean of the counted number of germ cells per same sized tubule was 185.1 per tubule (Figure 4C).
Microscopic examination of the D+R400 group showed testicular parenchyma with spermatogenic arrest (1+), seminiferous tubular atrophy (2+) and thickening of the basal lamina (1+). In this group, Leydig cell hyperplasia was not observed but the examination did show mild interstitial edema (1+). The Johnson score was 6/10 basal on the morphological scoring system. The mean of the counted number of germ cells per same sized tubule was 197.7 per tubule (Figure 4D).
Furthermore, microscopic examination of the D+R600 group did not show spermatogenic arrest or thickening of the basal lamina, but showed atrophy of the seminiferous tubules, Leydig cell hyperplasia, and mild interstitial edema (1+). The Johnson score was 7/10 basal on the morphological scoring system. The mean of the counted number of germ cells per same sized tubule was 212.5 per tubule (Figure 4E).






4. Discussion
In this study, the diabetic group showed increased serum glucose, decreased serum LH and testosterone, decreased testis and epididymis weight, and finally, it showed changes in all variables assayed related to OS, indicating DM-induced OS. Also, diabetic rats treated with R.P with a dose of 600 mg/kg, and in some cases the dose of 400 mg/kg, showed changes in measured variables toward the control group, indicating the protective effect of R.P against OS, testosterone and LH changes.
One study has emphasized the connection between DM and OS and the resulting complications. DM-induced OS can be the result of increased glucose auto-oxidation from hyperglycemia and protein glycation followed by oxidative degradation of glycated proteins (28). It has been reported that the MDA level in the tissues and blood of diabetic rats is increased due to both lipid peroxidation and reduced antioxidant activity (29, 30). In this study, testis, and epididymis MDA levels were significantly higher in the diabetic group. Glutathione plays an important role in cellular defense against ROS. We saw a significant depletion of GSH in the testis and epididymis of diabetic rats, which is a sign of OS (31). In the present study, lower levels of GSH and higher levels of GSSG in the diabetic rats probably were related to the lower activity of GR in both the testis and epididymis of diabetic rats as also described in another study (31). We observed higher activity of SOD and CAT in the diabetic group. As it has already been shown, SOD converts superoxide anion to H2O2 which in turn is converted to H2O by CAT (32, 33).
Increased activity of CAT in the heart and liver and decreased activity in the kidney of diabetic rats have already been shown (34). In another study, DM led to increased CAT and SOD activity in the liver and serum of diabetic rats (35). Also, it was shown that CAT activity increased in the testis, epididymis and liver of diabetic rats (33, 36). In addition, increased SOD and CAT enzyme expression with hyperglycemia have been demonstrated (37). So, the increased activity of SOD and CAT observed in the present study can be interpreted as a compensatory response against hyperglycemia-induced OS.
We saw decreased serum testosterone in the diabetic group. Leydig cell membrane injury induced by OS can be one explanation for this (15). It can also be the result of steroidogenic defects in the Leydig cells in DM (16). Moreover, the mechanism by which testosterone is reduced in DM may be the direct effect of glucose or its metabolism. It has been reported that insulin stimulates the hypothalamus and hypophysis for secretion of follicle stimulating hormone and LH; therefore, DM leads to a reduction in these two hormones and thereby disturbs spermatogenesis and testosterone production (38). In the present study, diabetic rats treated with R.P with the dose of 400 or 600 mg/kg showed changes in the measured variables toward the control group, indicating the protective effect of R.P against OS. Honey has been shown to reverse the reduced LH and testosterone in diabetic animals toward the control group (39). In our study, the D+R400 group did not display any effects on LH concentration, but showed increased testosterone concentration, while for the D+R600 group, effects both on LH and testosterone were observed. The reason for this could be that at a dose of 600 mg/kg, R.P could have some effect on hypophysis, but at dose of 400 mg/kg, R.P had its effect directly on the testis (40).
The histological examinations of the testis showed that in diabetic rats, spermatogenesis was impaired compared to in Control rats. These histological changes included a decreased number of spermatogonia, atrophy of the seminiferous tubules, thickening of the basal lamina, hypoplasia of the Leydig cells, and presence of edema in the diabetic testes. In the present study, the Johnson scores of the diabetic rats were 5, indicating testicular damage. Our results are in agreement with a study that showed DM causes cell death through apoptosis leading to testicular dysfunction, seminiferous tubule atrophy, reduction in tubule's diameter, and diminished spermatogenetic cell series (41). Basal lamina thickness has a crucial role in spermatogenesis and its increase results in diminished sperm production and decreased in the size of seminiferous tubules (42). In the histopathological examination of the epididymis, we observed a decrease in sperm density, and atrophy and vacuolation of the epithelium in the diabetic group, which is consistent with another study (43). However, treatment with R.P had a protective effect on the studied tissues, which is consistent with biochemical data.
R.P is used widely by the people of Baluch who live in the southeastern part of Iran. It has some antioxidant, antidiabetic, antimicrobial, anti-inflammatory and antimalarial properties (14). The hypoglycemic effect of R.P may be due to the presence of one or more antidiabetic components which demonstrate synergistic properties (44). Phytochemical screening of the R.P crude extract have revealed the presence of flavonoids, phenolic acids, and steroids/ triterpenoids (44, 45).
Flavonoids have been shown to work as antidiabetic compounds, because they possess multiple characteristics that promote both glucose-lowering actions (insulin-mimetic) and insulin secretion (insulin secretagogue) (46). Two flavonoids, morin and quercetin, both present in R.P, have been shown to have strong antioxidant activity, which is equel to BHA and stronger than alpha tocopherol (47). Quercetin strongly inhibits the transport of glucose and fructose through intestinal cells’ GLUT2 (48). Another study showed it potentiates insulin secretion induced by glucose and has a protective action against beta-cell oxidative injury induced by H2O2 (49). The effects of quercetin administrated intraperitoneally was analyzed (10 or 15 mg/kg body weight) for 10 days in control and diabetic rats which significantly decreased glucose levels in the diabetic rats. Quercetin increases the glucokinase activity, leading to this effect (50). Also it has been shown that quercetin has a similar effect as metformin (51).
The antioxidant activity of morin has been reported to be effective against OS in several disease profiles (52-54). Administration of morin has been reported to improve the pathological condition of hyperglycemia, glucose intolerance, lipid peroxidation, and insulin resistance (52, 55). Improvement in insulin receptor signaling and reduction of hyperglycemia and lipid deposits in the liver of diabetic rats has been reported, as well as antidiabetic and antioxidant effects, which directly supports the notion of the antidiabetic activity of morin (56, 57). In another study, morin administration was shown to increase insulin receptor activation and decrease gluconeogenesis potency (58). The effect of morin has been found to be similar to the conventionally used antidiabetic drug Glibenclamide. Moreover, monoterpene derivatives in the R.P flower are other compounds with antioxidant activity (59).
Therefore, it can be suggested that the reason for the improvement in the hyperglycemic, oxidative, and antioxidant status observed in the rats treated with R.P powder was due to the insulin-like activity and antidiabetic properties of R.P.
5. Conclusion
Our findings showed that R.P powder in doses of 600 or 400 mg/kg body weight had protective effects against DM by reducing hyperglycemia, defending against OS, and restoring hormonal and testicular histological changes.
Acknowledgements
The Ph.D. dissertation was financially supported by Shahid Bahonar University of Kerman, Kerman, Iran. The researchers are grateful to Dr. SM. Mirtadzaddini in the Department of Biology, Faculty of Science, in Shahid Bahonar University of Kerman for his invaluable assistance in the plant identification.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Endocrinology
References
1. Brownlee M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005; 54: 1615-1625. [DOI:10.2337/diabetes.54.6.1615] [PMID]
2. Roglic G, Unwin N, Bennett PH, Mathers C, Tuomilehto J, Nag S, et al. The burden of mortality attributable to diabetes: Realistic estimates for the year 2000. Diabetes Care 2005; 28: 2130-2135. [DOI:10.2337/diacare.28.9.2130] [PMID]
3. Aitken RJ, Clarkson JS, Fishel S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod 1989; 41: 183-197. [DOI:10.1095/biolreprod41.1.183] [PMID]
4. Sexton WJ, Jarow JP. Effect of diabetes mellitus upon male reproductive function. Urology 1997; 49: 508-513. [DOI:10.1016/S0090-4295(96)00573-0]
5. Meyer K, Deutscher J, Anil M, Berthold A, Bartsch M, Kiess W. Serum androgen levels in adolescents with type 1 diabetes: Relationship to pubertal stage and metabolic control. J Endocrinol Invest 2000; 23: 362-368. [DOI:10.1007/BF03343739] [PMID]
6. Johar DR, Bernstein LH. Biomarkers of stress-mediated metabolic deregulation in diabetes mellitus. Diabetes Res Clin Pract 2017; 126: 222-229. [DOI:10.1016/j.diabres.2017.02.023] [PMID]
7. Guneli E, Tugyan K, Ozturk H, Gumustekin M, Cilaker S, Uysal N. Effect of melatonin on testicular damage in streptozotocin-induced diabetes rats. Eur Surg Res 2008; 40: 354-360. [DOI:10.1159/000118032] [PMID]
8. Jiang X, Zhang Ch, Xin Y, Huang Zh, Tan Y, Huang Y, et al. Protective effect of FGF21 on type 1 diabetes-induced testicular apoptotic cell death probably via both mitochondrial- and endoplasmic reticulum stress-dependent pathways in the mouse model. Toxicol Lett 2013; 219: 65-76. [DOI:10.1016/j.toxlet.2013.02.022] [PMID]
9. Baccetti B, Marca A, Piomboni P, Capitani S, Bruni E, Petraglia F, et al. Insulin-dependent diabetes in men is associated with hypothalamo-pituitary derangement and with impairment in semen quality. Hum Reprod 2002; 17: 2673-2677. [DOI:10.1093/humrep/17.10.2673] [PMID]
10. Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes 1991; 40: 405-412.
https://doi.org/10.2337/diabetes.40.4.405 [DOI:10.2337/diab.40.4.405] [PMID]
11. Khaksar Z, Jelodar Gh, Hematian H, Poorahmadi M. Alterations in gonadal and testicular cells of male fetuses and neonates of diabetic rats. Comparat Clin Pathol 2013; 22: 1111-1115. [DOI:10.1007/s00580-012-1537-z]
12. Shapiro K, Gong WC. Natural products used for diabetes. J Am Pharm Assoc 2002; 42: 217-226. [DOI:10.1331/108658002763508515] [PMID]
13. Asgarpanah J, Mohammadi Motamed S. A review on phytochemistry and pharmacology of otostegia persica (Burm. f.) boiss. J Med Plant 2013; 12: 8.
14. Sadeghi Z, Akaberi M, Valizadeh J. Otostegia persica (Lamiaceae): A review on its ethnopharmacology, phytochemistry, and pharmacology. Avicenna J Phytomed 2014; 4: 79-88.
15. Bahmanzadeh M, Vahidinia A, Mehdinejadiani Sh, Shokri S, Alizadeh Z. Dietary supplementation with astaxanthin may ameliorate sperm parameters and DNA integrity in streptozotocin-induced diabetic rats. Clin Exp Reprod Med 2016; 43: 90-96. [DOI:10.5653/cerm.2016.43.2.90] [PMID] [PMCID]
16. La Vignera S, Condorelli R, Vicari E, D'Agata R, Calogero AE. Diabetes mellitus and sperm parameters. J Androl 2012; 33: 145-153. [DOI:10.2164/jandrol.111.013193] [PMID]
17. Akbartabar Toori M, Joodi B, Sadeghi H, Sadeghi H, Jafari M, Talebianpoor MSh, et al. Hepatoprotective activity of aerial parts of otostegia persica against carbon tetrachloride-induced liver damage in rats. Avicenna J Phytomed 2015; 5: 238-246.
18. Mitjavila M, Moreno JJ. The effects of polyphenols on oxidative stress and the arachidonic acid cascade. Implications for the prevention/treatment of high prevalence diseases. Biochem Pharmacol 2012; 84: 1113-1122. [DOI:10.1016/j.bcp.2012.07.017] [PMID]
19. Ebokaiwe AP, Ijomone OM, Osawe ShO, Chukwu ChJ, Ejike ChECC, Zhang G, et al. Alteration in sperm characteristics, endocrine balance and redox status in rats rendered diabetic by streptozotocin treatment: Attenuating role of Loranthus micranthus. Redox Rep 2018; 23: 194-205. [DOI:10.1080/13510002.2018.1540675] [PMID] [PMCID]
20. Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 1968; 125: 189-198. [DOI:10.1016/0003-9861(68)90654-1]
21. Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 1980; 106: 207-212. [DOI:10.1016/0003-2697(80)90139-6]
22. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248-254. [DOI:10.1016/0003-2697(76)90527-3]
23. Dhindsa RS, Matowe W. Drought tolerance in two mosses: Correlated with enzymatic defence against lipid peroxidation. J Exp Botany 1981; 32: 79-91. [DOI:10.1093/jxb/32.1.79]
24. Plewa MJ, Smith SR, Wagner ED. Diethyldithiocarbamate suppresses the plant activation of aromatic amines into mutagens by inhibiting tobacco cell peroxidase. Mutat Res 1991; 247: 57-64. [DOI:10.1016/0027-5107(91)90033-K]
25. Giannopolitis CN, Ries SK. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 1977; 59: 309-314. [DOI:10.1104/pp.59.2.309] [PMID] [PMCID]
26. Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976; 133: 21-25. [DOI:10.1007/BF00386001] [PMID]
27. Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci 2000; 151: 59-66. [DOI:10.1016/S0168-9452(99)00197-1]
28. Bruce CR, Carey AL, Hawley JA, Febbraio MA. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: Evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes 2003; 52: 2338-2345. [DOI:10.2337/diabetes.52.9.2338] [PMID]
29. Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H. Glucose toxicity in beta-cells: Type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes 2003; 52: 581-587. [DOI:10.2337/diabetes.52.3.581] [PMID]
30. Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 1997; 46: 1733-1742.
https://doi.org/10.2337/diab.46.11.1733 [DOI:10.2337/diabetes.46.11.1733] [PMID]
31. Paolisso G, Giugliano D, Pizza G, Gambardella A, Tesauro P, Varricchio M, et al. Glutathione infusion potentiates glucose-induced insulin secretion in aged patients with impaired glucose tolerance. Diabetes Care 1992; 15: 1-7. [DOI:10.2337/diacare.15.1.1] [PMID]
32. Hsieh Y, Guan Y, Tu C, Bratt PJ, Angerhofer A, Lepock JR, et al. Probing the active site of human manganese superoxide dismutase: The role of glutamine 143. Biochemistry 1998; 37: 4731-4739. [DOI:10.1021/bi972395d] [PMID]
33. Ostovan F, Gol A, Javadi AR. Investigating the effects of citrullus colocynthis pulp on oxidative stress in testes and epididymis in streptozotocin-induced diabetic male rats. Int J Reprod Biomed 2017; 15: 41-48. [DOI:10.29252/ijrm.15.1.41] [PMID] [PMCID]
34. Kakkar R, Kalra J, Mantha SV, Prasad K. Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol Cell Biochem 1995; 151: 113-119. [DOI:10.1007/BF01322333] [PMID]
35. Cho SY, Park JY, Park EM, Choi MS, Lee MK, Jeon SM, et al. Alternation of hepatic antioxidant enzyme activities and lipid profile in streptozotocin-induced diabetic rats by supplementation of dandelion water extract. Clin Chim Acta 2002; 317: 109-117. [DOI:10.1016/S0009-8981(01)00762-8]
36. Ostovan F, Olomi H, Gol A. [The citrullus colocynthis pulp antioxidant activity on oxidative stress factors of liver in streptozotocin-induced diabetic rats]. Physiol Pharmacol 2014; 17: 388-398. (in Persian)
37. Ceriello A, dello Russo P, Amstad P, Cerutti P. High glucose induces antioxidant enzymes in human endothelial cells in culture: Evidence linking hyperglycemia and oxidative stress. Diabetes 1996; 45: 471-477.
https://doi.org/10.2337/diab.45.4.471 [DOI:10.2337/diabetes.45.4.471] [PMID]
38. Mohamed NA, Abdel Gawad HS. Taurine dietary supplementation attenuates brain, thyroid, testicular disturbances and oxidative stress in streptozotocin-induced diabetes mellitus in male rats. Beni-Suef Univ J Basic Appl Sci 2017; 6: 247-252. [DOI:10.1016/j.bjbas.2017.04.006]
39. Nasrolahi O, Khaneshi F, Rahmani F, Razi M. Honey and metformin ameliorated diabetes-induced damages in testes of rat; correlation with hormonal changes. Iran J Reprod Med 2013; 11: 1013-1020.
40. Nna VU, Abu Bakar AB, Mohamed M. Diabetes mellitus-induced male reproductive impairment: the role of natural products: A review. J Appl Pharm Sci 2017; 7: 233-242.
41. Cameron DF, Murray FT, Drylie DD. Interstitial compartment pathology and spermatogenic disruption in testes from impotent diabetic men. Anat Rec 1985; 213: 53-62. [DOI:10.1002/ar.1092130108] [PMID]
42. Kehinde EO, Anim JT, Mojiminiyi OA, Al‐Awadi F, Shihab‐Eldeen A, Omu AE, et al. Allopurinol provides long‐term protection for experimentally induced testicular torsion in a rabbit model. BJU Int 2005; 96: 175-180.
https://doi.org/10.1111/j.1464-410X.2005.05590.x [DOI:10.1111/j.1464-410X.2005.05924_5.x] [PMID]
43. Cheng Y, Yang ZhL, Shi J, Yang J, Zhao J, He Y, et al. Total flavonoids of Epimedium ameliorates testicular damage in streptozotocin‐induced diabetic rats by suppressing inflammation and oxidative stress. Environ Toxicol 2020; 35: 268-276. [DOI:10.1002/tox.22864] [PMID]
44. Tofighi Z, Alipour F, Hadavinia H, Abdollahi M, Hadjiakhoondi A, Yassa N. Effective antidiabetic and antioxidant fractions of otostegia persica extract and their constituents. Pharm Biol 2014; 52: 961-966. [DOI:10.3109/13880209.2013.874463] [PMID]
45. Shewamene Z, Abdelwuhab MB, Birhanu Z. Methanolic leaf exctract of otostegia integrifolia benth reduces blood glucose levels in diabetic, glucose loaded and normal rodents. BMC Complement Altern Med 2015; 15: 19. [DOI:10.1186/s12906-015-0535-5] [PMID] [PMCID]
46. Pereira DF, Cazarolli LH, Lavado C, Mengatto V, Figueiredo MSRB, Guedes A, et al. Effects of flavonoids on α-glucosidase activity: Potential targets for glucose homeostasis. Nutrition 2011; 27: 1161-1167. [DOI:10.1016/j.nut.2011.01.008] [PMID]
47. Sharififar F, Yasa N, Shafiei A. Antioxidant activity of Otostegia persica (Labiatae) and its constituents. Iranian Journal of Pharmaceutical Research 2003; 2: 235-239.
48. Kwon O, Eck P, Chen S, Corpe CP, Lee JH, Kruhlak M, et al. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J 2007; 21: 366-377. [DOI:10.1096/fj.06-6620com] [PMID]
49. Youl E, Bardy G, Magous R, Cros G, Sejalon F, Virsolvy A, et al. Quercetin potentiates insulin secretion and protects INS‐1 pancreatic β‐cells against oxidative damage via the ERK1/2 pathway. Br J Pharmacol 2010; 161: 799-814. [DOI:10.1111/j.1476-5381.2010.00910.x] [PMID] [PMCID]
50. Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol 2003; 135: 357-364. [DOI:10.1016/S1532-0456(03)00140-6]
51. Kannappan S, Anuradha C. Insulin sensitizing actions of fenugreek seed polyphenols, quercetin and metformin in a rat model. Indian J Med Res 2009; 129: 401-408.
52. Ola MS, Aleisa AM, Al-Rejaie SS, Abuohashish HM, Parmar MY, Alhomida AS, et al. Flavonoid, morin inhibits oxidative stress, inflammation and enhances neurotrophic support in the brain of streptozotocin-induced diabetic rats. Neurol Sci 2014; 35: 1003-1008. [DOI:10.1007/s10072-014-1628-5] [PMID]
53. Chen YT, Zheng RL, Jia ZJ, Ju Y. Flavonoids as superoxide scavengers and antioxidants. Free Radic Biol Med 1990; 9: 19-21. [DOI:10.1016/0891-5849(90)90045-K]
54. Subash S, Subramanian P. Effect of morin on the levels of circulatory liver markers and redox status in experimental chronic hyperammonaemic rats. Singapore Med J 2008; 49: 650-655.
55. Paoli P, Cirri P, Caselli A, Ranaldi F, Bruschi G, Santi A, et al. The insulin-mimetic effect of morin: A promising molecule in diabetes treatment. Biochim Biophys Acta 2013; 1830: 3102-3111. [DOI:10.1016/j.bbagen.2013.01.017] [PMID]
56. Basha SKh, Kumari VS. In vitro antidiabetic activity of psidium guajava leaves extracts. Asian Pac J Trop Dis 2012; 2: S98-S100. [DOI:10.1016/S2222-1808(12)60131-5]
57. Oh WK, Lee ChH, Lee MS, Bae EY, Sohn ChB, Oh H, et al. Antidiabetic effects of extracts from psidium guajava. J Ethnopharmacol 2005; 96: 411-415. [DOI:10.1016/j.jep.2004.09.041] [PMID]
58. Vinayagam R, Xu B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutr Metab 2015; 12: 60. [DOI:10.1186/s12986-015-0057-7] [PMID] [PMCID]
59. AlSharari ShD, Al-Rejaie SS, Abuohashish HM, Aleisa AM, Parmar MY, Ahmed MM. Ameliorative potential of morin in streptozotocin-induced neuropathic pain in rats. Trop J Pharm Res 2014; 13: 1429-1436. [DOI:10.4314/tjpr.v13i9.8]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








