Wed, Apr 24, 2024
[Archive]
Volume 20, Issue 7 (July 2022)
IJRM 2022, 20(7): 581-590 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Omar Hafizi E N, Abdul Rahim R, Mohamad Zon E, Ibrahim A. Clinical predictors of embryo quality among women of advanced age receiving intracytoplasmic sperm injection cycles in Malaysia: A cohort study. IJRM 2022; 20 (7) :581-590
URL: http://ijrm.ir/article-1-2147-en.html
URL: http://ijrm.ir/article-1-2147-en.html
1- Department of Obstetrics and Gynecology, Hospital Universiti Sains Malaysia, Health Campus, Kubang Kerian, Kelantan, Malaysia.
2- Department of Obstetrics and Gynecology, Hospital Universiti Sains Malaysia, Health Campus, Kubang Kerian, Kelantan, Malaysia. Department of Obstetrics and Gynecology, School of Medical Sciences, Health Campus, Universiti Sains Malaysia, Kubang Kerian, Kelantan, Malaysia.
3- Department of Obstetrics and Gynecology, Hospital Universiti Sains Malaysia, Health Campus, Kubang Kerian, Kelantan, Malaysia. Department of Obstetrics and Gynecology, School of Medical Sciences, Health Campus, Universiti Sains Malaysia, Kubang Kerian, Kelantan, Malaysia. , dradibah@usm.my
2- Department of Obstetrics and Gynecology, Hospital Universiti Sains Malaysia, Health Campus, Kubang Kerian, Kelantan, Malaysia. Department of Obstetrics and Gynecology, School of Medical Sciences, Health Campus, Universiti Sains Malaysia, Kubang Kerian, Kelantan, Malaysia.
3- Department of Obstetrics and Gynecology, Hospital Universiti Sains Malaysia, Health Campus, Kubang Kerian, Kelantan, Malaysia. Department of Obstetrics and Gynecology, School of Medical Sciences, Health Campus, Universiti Sains Malaysia, Kubang Kerian, Kelantan, Malaysia. , dradibah@usm.my
Keywords: Embryo development, Intracytoplasmic sperm injection, Ovarian hyperstimulation, Advanced age, Predictors.
Full-Text [PDF 313 kb]
(526 Downloads)
| Abstract (HTML) (810 Views)
1. Introduction
Advanced maternal age (AMA), defined as the age of women, that is, 35-45 yr, is associated with a decline in both ovarian reserve and competency of oocyte and embryo (1-3). Among these women, the success of any fertility treatment is low (4, 5). The declining reproductive capacity among AMA women is associated with a depletion of oocytes in ovaries. Several investigations have been proposed to evaluate ovarian reserve, which may be used to predict ovarian response to exogenous gonadotrophin stimuli (1, 6-13). The primary markers of ovarian reserve are age, basal follicle-stimulating hormone (FSH), anti-mullerian hormone, and antral follicle count.
Besides the depletion in ovarian reserve, an increase in the rate of spontaneous miscarriage is also observed in AMA women, mainly because of oocyte abnormalities. The meiotic spindle in the oocytes of women with AMA frequently has abnormalities in chromosome alignment and microtubular matrix composition. A higher rate of aneuploidy has been observed among AMA women, which is the primary contributor to the increase in spontaneous miscarriage and reduced live birth rate (14, 15).
Despite these known age-related fertility issues, > 70% of patients attending our infertility center are women of advanced age. Many studies have been done to identify predictors of in vitro fertilization (IVF) outcome. A meta-analysis involving 14 studies showed a negative association between cycle outcome with female age and basal FSH, and a positive association with number of retrieved oocytes and quality of transferred embryo (16). Female age has been shown to be the strongest predictor. AMA affects not only the quantity of oocytes but also its quality and subsequently developed embryos. Embryo quality is one of the decisive factors of a successful IVF/ intracytoplasmic sperm injection (ICSI) cycle. Presently, embryo quality is determined by its morphological appearance. Previous studies have identified multiple morphological kinetic markers that can predict embryo competence (1, 2). However, such identification requires technological competencies that may not readily be available in many centers, and in such centers, the use of clinical predictors of high-quality embryos would be especially beneficial in predicting the course of IVF cycles. In addition, these predictors can help physicians and couples to decide on the extent of treatment without unnecessarily increasing the cost. To date, predictors of embryo quality in women of advanced reproductive age are limited, and this is in contrast with data available on factors affecting live birth rates in these women.
The present study aimed to determine the clinical factors that contribute to the development of good-quality embryos among women of advanced age. We hypothesized the level of FSH, the total dose of gonadotrophins used as ovarian hyperstimulation, and the number and quality of oocytes and embryos could predict the quality of embryos. This study may not be a novel research. However, it may contribute to future meta-analysis or systemic review research on embryo quality.
2. Materials and Methods
This prospective, cohort study was conducted among women who received their first IVF/ICSI treatment. The present study was conducted over a period of 12 months, from January until December 2018.
2.1. Inclusion and exclusion criteria
All women aged 35-45 yr who received their first IVF/ICSI treatment were recruited for the present study. Women with severe endometriosis, polycystic ovarian syndrome, uncontrolled preexisting diabetes mellitus, underlying psychiatric disorders, recurrent miscarriage, antiphospholipid syndrome, personal or family history of inherited congenital disorders, or a partner with an abnormal seminal fluid analysis were excluded. A total of 941 oocytes were retrieved for study analysis.
2.2. Sample size calculation
Using the estimation of the mean formula to obtain good-quality embryos, a minimum of 190 oocytes and 38 embryos were needed to meet the 95% confidence interval. We retrieved a total of 934 oocytes from 124 women, of which only 734 oocytes were metaphase II (MII) normal oocytes which had undergone the ICSI procedure (Figure 1).
2.3. Ovarian stimulation and ICSI
All women in this study were stimulated with short gonadotrophin-releasing hormone (GnRH) antagonist protocol. Ovarian hyperstimulation was initiated using recombinant gonadotrophin, follitrophin beta (Puregon®, Organon Malaysia Sdn Bhd) and was dictated according to patients’ responses to stimulation, which was evaluated by serial vaginal ultrasound examinations (Acuson 50, Siemen Healthineers, Erlangen, Germany). When majority of the follicles reached 13-14 mm in diameter, GnRH antagonist, ganirelix (Orgalutron®, Vetter Pharma-Pertigung GmbH & Co. KG) was added at a dose of 250 μg daily. Once at least 3 leading follicles reached a size of 18 mm, 10,000 IU intramuscular human chorionic gonadotrophin (hCG; Pregnyl®, Merck Sharp & Dohme (Malaysia)) was administered. During this time, the number of mature follicles, endometrial thickness, and level of serum progesterone were determined. Oocyte retrieval was then performed 34-36 hr after the hCG administration. Using the total oocyte scoring system, the oocytes were scored as -1 (poor quality), 0 (almost normal), and +1 (normal) oocytes (17). The semen was prepared using density gradient method (18), and the most motile and normal sperm was selected for ICSI procedure.
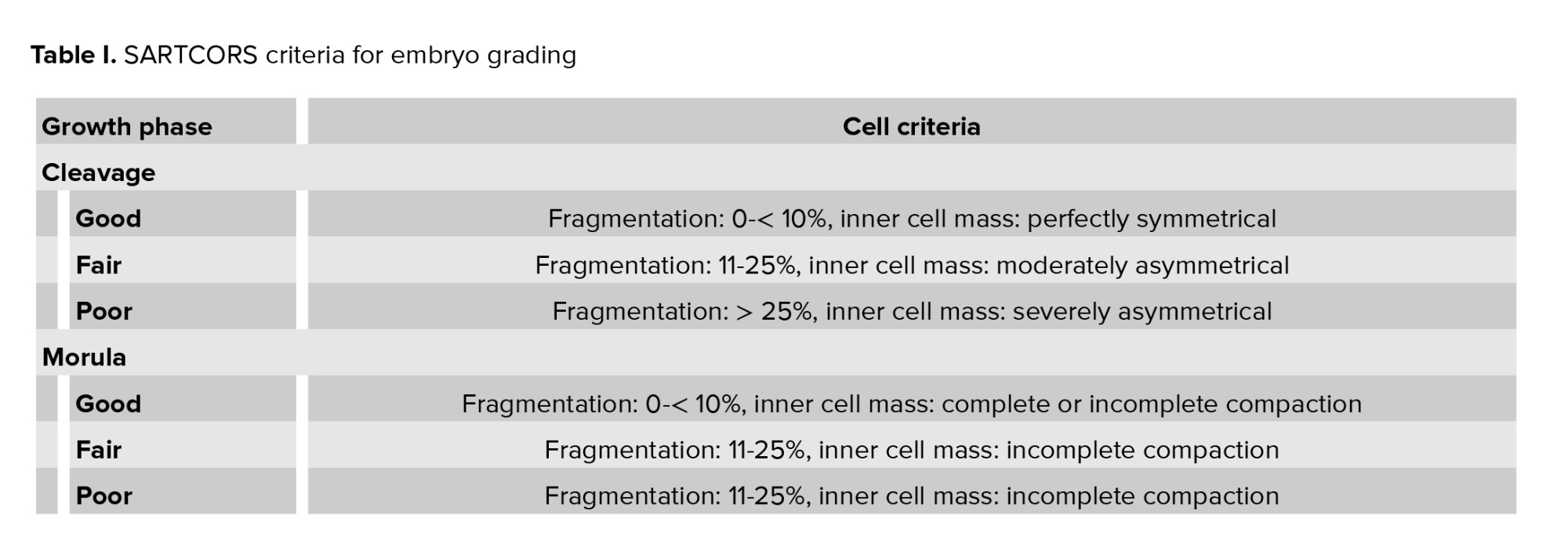
2.4. Evaluation of fertilization, cleavage, and embryo grading
Fertilization was determined 17 hr after ICSI. The fertilized oocytes (zygotes) were further incubated and re-examined for embryo grading 48 hr later during which the embryo was graded based on morphology using the standardized morphological grading system of the society of assisted reproductive techniques (SART) committee, also referred to as the SARTCORS criteria, as described in table I below (3). Based on these criteria, the embryos were graded as good-, fair-, or poor-quality embryos. To facilitate analysis, we grouped the fair-quality embryos with the good-quality embryos. Embryos were transferred on day 3 after oocyte retrieval. No more than 2 embryos were transferred at a time. Only good- and fair-quality embryos, up to a maximum of 2, were transferred in the present study. Embryos were expelled from the catheter, and the catheter was slowly withdrawn while maintaining a steady pressure on the plunger of the syringe. The catheter was then examined for complete expulsion confirmation. The luteal phase was supported with 100 mg micronized progesterone vaginal gel (Crinone® progesterone gel 8%, Merck Sdn Bhd), which was administered daily, and oral progestogen 10 mg taken twice a day.
2.5. Pregnancy confirmation
3 wk after the embryo transfer, 3 cc of venous blood was drawn to determine the level of beta human chorionic gonadotropin. A beta human chorionic gonadotropin of > 0.2 IU/L was considered a successful IVF cycle and was taken as a biochemical pregnancy. An ultrasound scan of the uterus was conducted 2 wk later to determine the presence of a gestational sac, which was considered a successful clinical pregnancy. Only successful clinical pregnancies were considered for analysis.

2.6. Ethical considerations
The study protocol was approved by the local Human Medical Research and Ethics Committee, Kelantan, Malaysia (Code: USM/JEPeM/16020055), which has adopted the ethical guidelines outlined by the Declaration of Helsinki. Written consent was obtained from all participants prior to study recruitment.
2.7. Statistical analysis
The Statistical Package for the Social Sciences (IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp. IBM Corp. Released 2017) was used for data analysis. Study population and cycle characteristics were analyzed using descriptive statistics, in which numerical data were presented as mean and standard deviation, whereas categorical data were presented as a percentage. The association between age, duration of subfertility, body mass index (BMI), baseline FSH and luteinizing hormone (LH) levels, the total dose of gonadotrophin used, and a number of stimulated oocytes, mature oocytes, and total available embryos, with high-quality embryos, were analyzed using a simple linear regression test and multiple linear regression test. A p-value of < 0.05 was considered statistically significant.
The fertilization rate per cycle was determined as percentage of number of zygotes over total oocytes that underwent ICSI. The cleavage rate per cycle was determined as the percentage of cleaved embryos over total zygotes, and the pregnancy rate per transfer was determined as the percentage of successful clinical pregnancies over the number of transfers.
3. Results
3.1. Patient characteristics
A total of 941 stimulated oocytes were retrieved, of which 734 were mature and used for ICSI. The fertilization rate per cycle was 86.18%, and the cleavage rate per cycle was 97.83%. Out of the 586 available embryos, 288 (49.15%) were high-quality, while the rest were low-quality embryos (n = 298, 50.85%).
The implantation rate was 22.37%. There were no cases of miscarriage or ectopic pregnancy, resulting in a clinical pregnancy identical to the implantation rate of 22.37% per cycle. The baseline characteristics of the study population are summarized in table II. The mean maternal age of women undergoing ICSI treatment was 38.21 ± 2.78 yr. The average duration of subfertility was 7.43 ± 4.09 months. The mean BMI, baseline FSH, and baseline LH were 24.99 ± 4.75 kg/m2, 5.35 ± 2.50 mIU/m, and 5.18 ± 3.08 mIU/m, respectively. No significant difference was noted between the study characteristics and embryo quality groups, either high- or low-quality embryos.
3.2. Cycle characteristics and response to stimulation
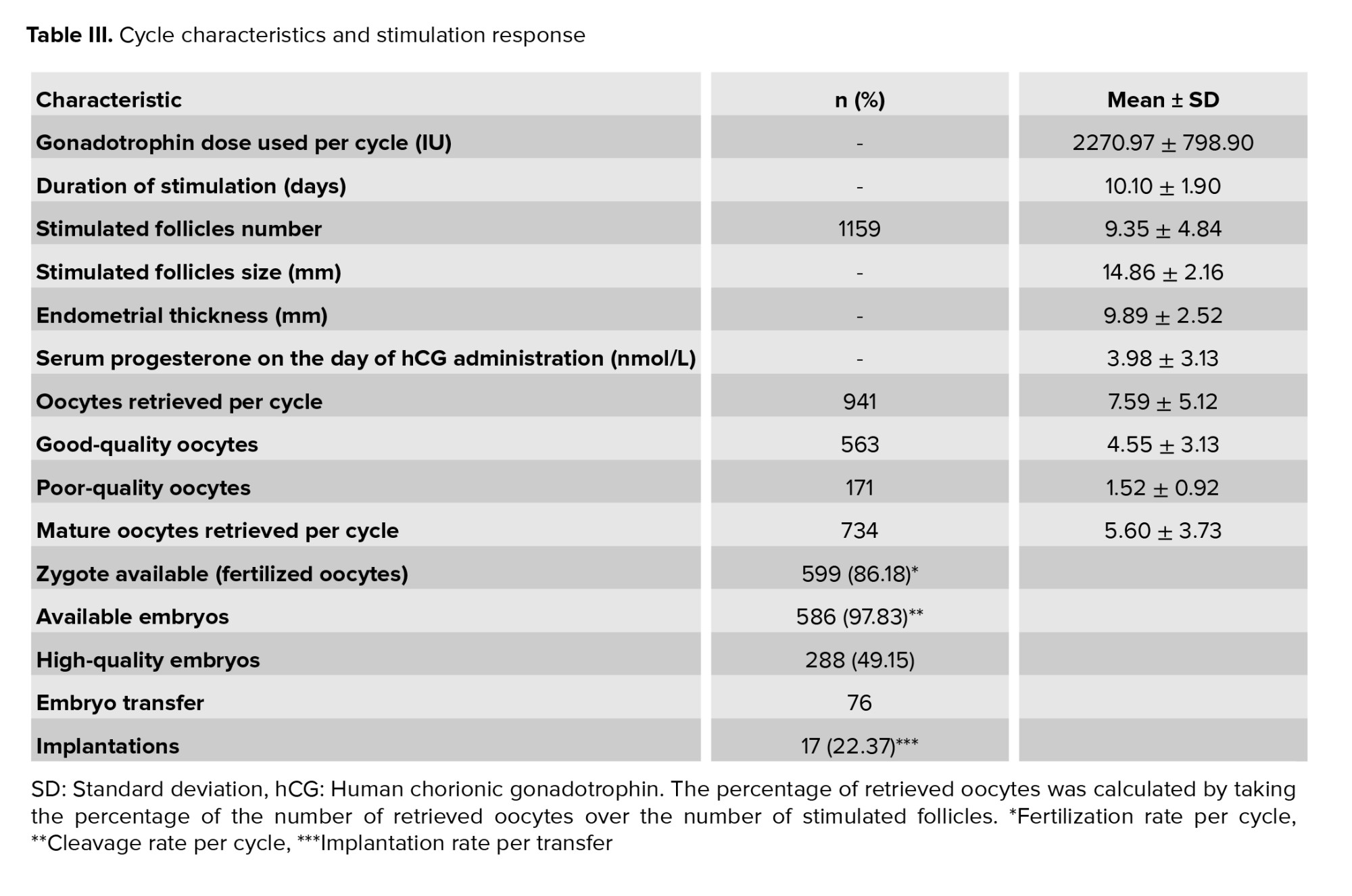
All patients received the same starting dose of gonadotrophin, 300 IU daily, for stimulation. The average dose of gonadotrophin used was 2270.97 ± 798.90 IU. A total of 1159 follicles were stimulated, with a mean 9.35 ± 4.84 per cycle, of which a mean of 7.59 ± 5.12 oocytes per cycle was retrieved. The endometrial thickness on hCG administration day, embryo quality, and fertilization, implantation, and clinical pregnancy rates are displayed in table III. The total dose of gonadotrophin used, size of the stimulated follicles, duration of stimulation, endometrial thickness, and level of progesterone on the day of hCG administration were comparable between the 2 groups (Table III).
3.3. Predictors of high-quality embryos
A correlation coefficient test as presented in table IV showed good correlations between the total stimulated follicles, retrieved oocytes, mature oocytes, high-quality oocytes, fertilized oocytes, and the obtained high-quality embryos (r = 0.297, 0.579, 0.634, 0.759, 0.908, and 0.917 respectively).
The single linear regression, which shows that the baseline LH, number of stimulated follicles, number of retrieved oocytes, number of mature oocytes retrieved, number of zygotes, and total number of available embryos can predict the development of high-quality embryos (p = 0.016, 0.001, < 0.001, 0.001, < 0.001, and < 0.001, respectively), is presented in table V. However, in the multiple linear regression analysis, only the number of retrieved oocytes and the number of available embryos could independently make such a prediction (Table V). 8 high-quality embryos were predicted to be produced for every 10 retrieved oocytes (p < 0.001). The number of mature oocytes could not significantly predict high-quality embryos. For every 10 available embryos, 9 were predicted to be of high quality (p < 0.001).


4. Discussion
Women’s age has been identified as a strong independent factor for the outcome of IVF. By the age of 35-39 yr, the rates of spontaneous cumulative pregnancy start to decline, and they approach almost zero immediately after 45 yr (1, 4, 6, 7, 19). The reason for this trend is believed to be the gradual depletion in the ovarian reserve associated with a progressive decrease in oocytes and embryo competence, which has become a great challenge for fertility physicians. In the present study, we evaluated the potential factors that could predict the quality of embryos among women of advanced age.
Currently, multiple systems to grade and rank embryos are available and these range from a simple grading system that assigns a single grade to account for all aspects of post-cleavage embryo appearance to more complex systems that use a formula to predict the likelihood of pregnancy based on embryo appearance and development (6, 8, 9, 19). In the present study, we used the SARTCORS grading system developed by the SART committee, which classifies embryos on a 3-point grading system (good, fair, and poor) based on their morphology (3).
At the beginning of the study, we hypothesized that embryo quality is affected by the level of FSH, the total dose of gonadotrophins used as ovarian hyperstimulation and the number and quality of oocytes, and the number of available embryos. In this study, although AMA is associated with reduced ovarian reserve, neither the FSH level nor the dosage of gonadotrophin used as ovarian stimulation could predict a high-quality embryo.
The present study demonstrated that embryo quality was affected by the number of retrieved oocytes and the number of available embryos. One can expect to have 8 high-quality embryos for every 10 retrieved oocytes. The number of retrieved oocytes decreased across various age groups and was highest among women younger than 30 yr (mean 18.5 ± 10.3 yr) and decreased significantly to 9.8 ± 6.7 for those 39 yr old and to 4.5 ± 2.3 for those older than 45 yr. In the present study, at a mean age of 38.21 ± 2.78 yr, the mean retrieved oocytes per cycle was 7.59 ± 5.12, which was about the expected rate.
Previous studies have indicated that age is predictive not only for the number of oocytes but also the number of MII oocytes, oocyte quality, and embryo quality (14-20). It was somewhat surprising to note that we had retrieved more good-quality oocytes (grade 1; mean 4.55 ± 3.13) than poor-quality oocytes (grades 2 and 3; mean 1.38 ± 2.82) from the population of the present study.
This could be because most of our patients were younger than 40 yr of age (mean 38.21 ± 2.78 yr). However, this study has demonstrated a significant association between good-quality oocytes and high-quality embryos as predicted.
In contrast to results reported previously (9, 11, 21, 22), we show that embryo quality was not affected by the number of MII oocytes, after stratification by multivariable regression test (r2 = 0.401, p = 0.653). We have also standardized the type and dosage of ovarian stimulating agents and embryo culture media and environment for our study population to make statistical analysis easier.
These factors could have contributed to the divergent findings of the present study. Many studies have tried to identify ways of increasing the number of stimulated oocytes from AMA patients (12-16, 21-23). In his review of ovarian stimulation regimens for poor responders to IVF, Blumenfeld has stated that the best treatment for these patients is egg donation (20); however, this method cannot be universally applied. In contrast, Huang et al. have suggested the use of the GnRH antagonist protocol in older poor responders and the GnRH agonist protocol in younger patients (24). Various other regimens have been proposed to improve ovarian response in poor responders, including the addition of LH (23, 25), androgen (15, 23, 25), and growth hormone (20, 26-28) to exogenous gonadotrophin during stimulation.
Increasing the number of available embryos could also increase the chance of obtaining a good-quality embryo, and embryo numbers can be improved by increasing the number of retrieved oocytes and by improving culture techniques. Much effort has been directed in improving culture techniques, including refining culture media and using insemination before the availability of time-lapsed incubators to minimize the temperature gradient during monitoring. Farhi and colleagues have reported a significant improvement in embryo quality when ICSI was implemented instead of IVF among AMA (7).
5. Conclusion
There is evidence that the embryo quality declines with maternal age. This study has shown that the chance of obtaining a high-quality embryo can be improved by increasing the number and quality of collected oocytes and the number of available embryos. This measure can be achieved by regulating the stimulation regimens and methods of embryo incubation.
Acknowledgments
The authors wish to thank the staff of hospital USM (HUSM) Fertility Unit for data collection, especially Mrs. Rohaini. They are also thankful to Associate Professor Dr. Wan Mohd. Zahiruddin Wan Mohd. for his help in statistical issue. This work was supported by USM Research University Grant, Kelantan, Malaysia (Individual) (RUI; grant no. 1001/PPSP/812205). Adibah Ibrahim is the principal investigator for the grant. The grant was one of incentive grants awarded to local researchers. The grant was used to support the running of study without having any influence or benefits from study outcome.
Conflict of Interest
The authors declare no conflict of interest with respect to the research, authorship, and publication of the manuscript.
Full-Text: (173 Views)
1. Introduction
Advanced maternal age (AMA), defined as the age of women, that is, 35-45 yr, is associated with a decline in both ovarian reserve and competency of oocyte and embryo (1-3). Among these women, the success of any fertility treatment is low (4, 5). The declining reproductive capacity among AMA women is associated with a depletion of oocytes in ovaries. Several investigations have been proposed to evaluate ovarian reserve, which may be used to predict ovarian response to exogenous gonadotrophin stimuli (1, 6-13). The primary markers of ovarian reserve are age, basal follicle-stimulating hormone (FSH), anti-mullerian hormone, and antral follicle count.
Besides the depletion in ovarian reserve, an increase in the rate of spontaneous miscarriage is also observed in AMA women, mainly because of oocyte abnormalities. The meiotic spindle in the oocytes of women with AMA frequently has abnormalities in chromosome alignment and microtubular matrix composition. A higher rate of aneuploidy has been observed among AMA women, which is the primary contributor to the increase in spontaneous miscarriage and reduced live birth rate (14, 15).
Despite these known age-related fertility issues, > 70% of patients attending our infertility center are women of advanced age. Many studies have been done to identify predictors of in vitro fertilization (IVF) outcome. A meta-analysis involving 14 studies showed a negative association between cycle outcome with female age and basal FSH, and a positive association with number of retrieved oocytes and quality of transferred embryo (16). Female age has been shown to be the strongest predictor. AMA affects not only the quantity of oocytes but also its quality and subsequently developed embryos. Embryo quality is one of the decisive factors of a successful IVF/ intracytoplasmic sperm injection (ICSI) cycle. Presently, embryo quality is determined by its morphological appearance. Previous studies have identified multiple morphological kinetic markers that can predict embryo competence (1, 2). However, such identification requires technological competencies that may not readily be available in many centers, and in such centers, the use of clinical predictors of high-quality embryos would be especially beneficial in predicting the course of IVF cycles. In addition, these predictors can help physicians and couples to decide on the extent of treatment without unnecessarily increasing the cost. To date, predictors of embryo quality in women of advanced reproductive age are limited, and this is in contrast with data available on factors affecting live birth rates in these women.
The present study aimed to determine the clinical factors that contribute to the development of good-quality embryos among women of advanced age. We hypothesized the level of FSH, the total dose of gonadotrophins used as ovarian hyperstimulation, and the number and quality of oocytes and embryos could predict the quality of embryos. This study may not be a novel research. However, it may contribute to future meta-analysis or systemic review research on embryo quality.
2. Materials and Methods
This prospective, cohort study was conducted among women who received their first IVF/ICSI treatment. The present study was conducted over a period of 12 months, from January until December 2018.
2.1. Inclusion and exclusion criteria
All women aged 35-45 yr who received their first IVF/ICSI treatment were recruited for the present study. Women with severe endometriosis, polycystic ovarian syndrome, uncontrolled preexisting diabetes mellitus, underlying psychiatric disorders, recurrent miscarriage, antiphospholipid syndrome, personal or family history of inherited congenital disorders, or a partner with an abnormal seminal fluid analysis were excluded. A total of 941 oocytes were retrieved for study analysis.
2.2. Sample size calculation
Using the estimation of the mean formula to obtain good-quality embryos, a minimum of 190 oocytes and 38 embryos were needed to meet the 95% confidence interval. We retrieved a total of 934 oocytes from 124 women, of which only 734 oocytes were metaphase II (MII) normal oocytes which had undergone the ICSI procedure (Figure 1).
2.3. Ovarian stimulation and ICSI
All women in this study were stimulated with short gonadotrophin-releasing hormone (GnRH) antagonist protocol. Ovarian hyperstimulation was initiated using recombinant gonadotrophin, follitrophin beta (Puregon®, Organon Malaysia Sdn Bhd) and was dictated according to patients’ responses to stimulation, which was evaluated by serial vaginal ultrasound examinations (Acuson 50, Siemen Healthineers, Erlangen, Germany). When majority of the follicles reached 13-14 mm in diameter, GnRH antagonist, ganirelix (Orgalutron®, Vetter Pharma-Pertigung GmbH & Co. KG) was added at a dose of 250 μg daily. Once at least 3 leading follicles reached a size of 18 mm, 10,000 IU intramuscular human chorionic gonadotrophin (hCG; Pregnyl®, Merck Sharp & Dohme (Malaysia)) was administered. During this time, the number of mature follicles, endometrial thickness, and level of serum progesterone were determined. Oocyte retrieval was then performed 34-36 hr after the hCG administration. Using the total oocyte scoring system, the oocytes were scored as -1 (poor quality), 0 (almost normal), and +1 (normal) oocytes (17). The semen was prepared using density gradient method (18), and the most motile and normal sperm was selected for ICSI procedure.
2.4. Evaluation of fertilization, cleavage, and embryo grading
Fertilization was determined 17 hr after ICSI. The fertilized oocytes (zygotes) were further incubated and re-examined for embryo grading 48 hr later during which the embryo was graded based on morphology using the standardized morphological grading system of the society of assisted reproductive techniques (SART) committee, also referred to as the SARTCORS criteria, as described in table I below (3). Based on these criteria, the embryos were graded as good-, fair-, or poor-quality embryos. To facilitate analysis, we grouped the fair-quality embryos with the good-quality embryos. Embryos were transferred on day 3 after oocyte retrieval. No more than 2 embryos were transferred at a time. Only good- and fair-quality embryos, up to a maximum of 2, were transferred in the present study. Embryos were expelled from the catheter, and the catheter was slowly withdrawn while maintaining a steady pressure on the plunger of the syringe. The catheter was then examined for complete expulsion confirmation. The luteal phase was supported with 100 mg micronized progesterone vaginal gel (Crinone® progesterone gel 8%, Merck Sdn Bhd), which was administered daily, and oral progestogen 10 mg taken twice a day.
2.5. Pregnancy confirmation
3 wk after the embryo transfer, 3 cc of venous blood was drawn to determine the level of beta human chorionic gonadotropin. A beta human chorionic gonadotropin of > 0.2 IU/L was considered a successful IVF cycle and was taken as a biochemical pregnancy. An ultrasound scan of the uterus was conducted 2 wk later to determine the presence of a gestational sac, which was considered a successful clinical pregnancy. Only successful clinical pregnancies were considered for analysis.


2.6. Ethical considerations
The study protocol was approved by the local Human Medical Research and Ethics Committee, Kelantan, Malaysia (Code: USM/JEPeM/16020055), which has adopted the ethical guidelines outlined by the Declaration of Helsinki. Written consent was obtained from all participants prior to study recruitment.
2.7. Statistical analysis
The Statistical Package for the Social Sciences (IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp. IBM Corp. Released 2017) was used for data analysis. Study population and cycle characteristics were analyzed using descriptive statistics, in which numerical data were presented as mean and standard deviation, whereas categorical data were presented as a percentage. The association between age, duration of subfertility, body mass index (BMI), baseline FSH and luteinizing hormone (LH) levels, the total dose of gonadotrophin used, and a number of stimulated oocytes, mature oocytes, and total available embryos, with high-quality embryos, were analyzed using a simple linear regression test and multiple linear regression test. A p-value of < 0.05 was considered statistically significant.
The fertilization rate per cycle was determined as percentage of number of zygotes over total oocytes that underwent ICSI. The cleavage rate per cycle was determined as the percentage of cleaved embryos over total zygotes, and the pregnancy rate per transfer was determined as the percentage of successful clinical pregnancies over the number of transfers.
3. Results
3.1. Patient characteristics
A total of 941 stimulated oocytes were retrieved, of which 734 were mature and used for ICSI. The fertilization rate per cycle was 86.18%, and the cleavage rate per cycle was 97.83%. Out of the 586 available embryos, 288 (49.15%) were high-quality, while the rest were low-quality embryos (n = 298, 50.85%).
The implantation rate was 22.37%. There were no cases of miscarriage or ectopic pregnancy, resulting in a clinical pregnancy identical to the implantation rate of 22.37% per cycle. The baseline characteristics of the study population are summarized in table II. The mean maternal age of women undergoing ICSI treatment was 38.21 ± 2.78 yr. The average duration of subfertility was 7.43 ± 4.09 months. The mean BMI, baseline FSH, and baseline LH were 24.99 ± 4.75 kg/m2, 5.35 ± 2.50 mIU/m, and 5.18 ± 3.08 mIU/m, respectively. No significant difference was noted between the study characteristics and embryo quality groups, either high- or low-quality embryos.
3.2. Cycle characteristics and response to stimulation
All patients received the same starting dose of gonadotrophin, 300 IU daily, for stimulation. The average dose of gonadotrophin used was 2270.97 ± 798.90 IU. A total of 1159 follicles were stimulated, with a mean 9.35 ± 4.84 per cycle, of which a mean of 7.59 ± 5.12 oocytes per cycle was retrieved. The endometrial thickness on hCG administration day, embryo quality, and fertilization, implantation, and clinical pregnancy rates are displayed in table III. The total dose of gonadotrophin used, size of the stimulated follicles, duration of stimulation, endometrial thickness, and level of progesterone on the day of hCG administration were comparable between the 2 groups (Table III).
3.3. Predictors of high-quality embryos
A correlation coefficient test as presented in table IV showed good correlations between the total stimulated follicles, retrieved oocytes, mature oocytes, high-quality oocytes, fertilized oocytes, and the obtained high-quality embryos (r = 0.297, 0.579, 0.634, 0.759, 0.908, and 0.917 respectively).
The single linear regression, which shows that the baseline LH, number of stimulated follicles, number of retrieved oocytes, number of mature oocytes retrieved, number of zygotes, and total number of available embryos can predict the development of high-quality embryos (p = 0.016, 0.001, < 0.001, 0.001, < 0.001, and < 0.001, respectively), is presented in table V. However, in the multiple linear regression analysis, only the number of retrieved oocytes and the number of available embryos could independently make such a prediction (Table V). 8 high-quality embryos were predicted to be produced for every 10 retrieved oocytes (p < 0.001). The number of mature oocytes could not significantly predict high-quality embryos. For every 10 available embryos, 9 were predicted to be of high quality (p < 0.001).



4. Discussion
Women’s age has been identified as a strong independent factor for the outcome of IVF. By the age of 35-39 yr, the rates of spontaneous cumulative pregnancy start to decline, and they approach almost zero immediately after 45 yr (1, 4, 6, 7, 19). The reason for this trend is believed to be the gradual depletion in the ovarian reserve associated with a progressive decrease in oocytes and embryo competence, which has become a great challenge for fertility physicians. In the present study, we evaluated the potential factors that could predict the quality of embryos among women of advanced age.
Currently, multiple systems to grade and rank embryos are available and these range from a simple grading system that assigns a single grade to account for all aspects of post-cleavage embryo appearance to more complex systems that use a formula to predict the likelihood of pregnancy based on embryo appearance and development (6, 8, 9, 19). In the present study, we used the SARTCORS grading system developed by the SART committee, which classifies embryos on a 3-point grading system (good, fair, and poor) based on their morphology (3).
At the beginning of the study, we hypothesized that embryo quality is affected by the level of FSH, the total dose of gonadotrophins used as ovarian hyperstimulation and the number and quality of oocytes, and the number of available embryos. In this study, although AMA is associated with reduced ovarian reserve, neither the FSH level nor the dosage of gonadotrophin used as ovarian stimulation could predict a high-quality embryo.
The present study demonstrated that embryo quality was affected by the number of retrieved oocytes and the number of available embryos. One can expect to have 8 high-quality embryos for every 10 retrieved oocytes. The number of retrieved oocytes decreased across various age groups and was highest among women younger than 30 yr (mean 18.5 ± 10.3 yr) and decreased significantly to 9.8 ± 6.7 for those 39 yr old and to 4.5 ± 2.3 for those older than 45 yr. In the present study, at a mean age of 38.21 ± 2.78 yr, the mean retrieved oocytes per cycle was 7.59 ± 5.12, which was about the expected rate.
Previous studies have indicated that age is predictive not only for the number of oocytes but also the number of MII oocytes, oocyte quality, and embryo quality (14-20). It was somewhat surprising to note that we had retrieved more good-quality oocytes (grade 1; mean 4.55 ± 3.13) than poor-quality oocytes (grades 2 and 3; mean 1.38 ± 2.82) from the population of the present study.
This could be because most of our patients were younger than 40 yr of age (mean 38.21 ± 2.78 yr). However, this study has demonstrated a significant association between good-quality oocytes and high-quality embryos as predicted.
In contrast to results reported previously (9, 11, 21, 22), we show that embryo quality was not affected by the number of MII oocytes, after stratification by multivariable regression test (r2 = 0.401, p = 0.653). We have also standardized the type and dosage of ovarian stimulating agents and embryo culture media and environment for our study population to make statistical analysis easier.
These factors could have contributed to the divergent findings of the present study. Many studies have tried to identify ways of increasing the number of stimulated oocytes from AMA patients (12-16, 21-23). In his review of ovarian stimulation regimens for poor responders to IVF, Blumenfeld has stated that the best treatment for these patients is egg donation (20); however, this method cannot be universally applied. In contrast, Huang et al. have suggested the use of the GnRH antagonist protocol in older poor responders and the GnRH agonist protocol in younger patients (24). Various other regimens have been proposed to improve ovarian response in poor responders, including the addition of LH (23, 25), androgen (15, 23, 25), and growth hormone (20, 26-28) to exogenous gonadotrophin during stimulation.
Increasing the number of available embryos could also increase the chance of obtaining a good-quality embryo, and embryo numbers can be improved by increasing the number of retrieved oocytes and by improving culture techniques. Much effort has been directed in improving culture techniques, including refining culture media and using insemination before the availability of time-lapsed incubators to minimize the temperature gradient during monitoring. Farhi and colleagues have reported a significant improvement in embryo quality when ICSI was implemented instead of IVF among AMA (7).
5. Conclusion
There is evidence that the embryo quality declines with maternal age. This study has shown that the chance of obtaining a high-quality embryo can be improved by increasing the number and quality of collected oocytes and the number of available embryos. This measure can be achieved by regulating the stimulation regimens and methods of embryo incubation.
Acknowledgments
The authors wish to thank the staff of hospital USM (HUSM) Fertility Unit for data collection, especially Mrs. Rohaini. They are also thankful to Associate Professor Dr. Wan Mohd. Zahiruddin Wan Mohd. for his help in statistical issue. This work was supported by USM Research University Grant, Kelantan, Malaysia (Individual) (RUI; grant no. 1001/PPSP/812205). Adibah Ibrahim is the principal investigator for the grant. The grant was one of incentive grants awarded to local researchers. The grant was used to support the running of study without having any influence or benefits from study outcome.
Conflict of Interest
The authors declare no conflict of interest with respect to the research, authorship, and publication of the manuscript.
Type of Study: Original Article |
Subject:
Assisted Reproductive Technologies
References
1. Cimadomo D, Fabozzi G, Vaiarelli A, Ubaldi N, Ubaldi FM, Rienzi L. Impact of maternal age on oocyte and embryo competence. Front Endocrinol (Lausanne) 2018; 9: 327. [DOI:10.3389/fendo.2018.00327] [PMID] [PMCID]
2. Ubaldi FM, Cimadomo D, Vaiarelli A, Fabozzi G, Venturella R, Maggiulli R, et al. Advanced maternal age in IVF: Still a challenge? The present and the future of its treatment. Front Endocrinol (Lausanne) 2019; 10: 94. [DOI:10.3389/fendo.2019.00094] [PMID] [PMCID]
3. Racowsky C, Vernon M, Mayer J, Ball GD, Behr B, Pomeroy KO, et al. Standardization of grading embryo morphology. J Assist Reprod Genet 2010; 27: 437-439. [DOI:10.1007/s10815-010-9443-2] [PMID] [PMCID]
4. Barberet J, Bruno C, Valot E, Antunes-Nunes C, Jonval L, Chammas J, et al. Can novel early non-invasive biomarkers of embryo quality be identified with time-lapse imaging to predict live birth? Hum Reprod 2019; 34: 1439-1449. [DOI:10.1093/humrep/dez085] [PMID] [PMCID]
5. Tan TY, Lau SK, Loh SF, Tan HH. Female ageing and reproductive outcome in assisted reproduction cycles. Singapore Med J 2014; 55: 305-309. [DOI:10.11622/smedj.2014081] [PMID] [PMCID]
6. Cetinkaya MB, Siano LJ, Benadiva C, Sakkas D, Patrizio P. Reproductive outcome of women 43 years and beyond undergoing ART treatment with their own oocytes in two Connecticut university programs. J Assist Reprod Genet 2013; 30: 673-678. [DOI:10.1007/s10815-013-9981-5] [PMID] [PMCID]
7. Farhi J, Cohen K, Mizrachi Y, Weissman A, Raziel A, Orvieto R. Should ICSI be implemented during IVF to all advanced-age patients with non-male factor subfertility? Reprod Biol Endocrinol 2019; 17: 30. [DOI:10.1186/s12958-019-0474-y] [PMID] [PMCID]
8. Liu K, Case A, Reproductive Endocrinology, and Infertility Committee. Advanced reproductive age and fertility. J Obstet Gynaecol Can 2011; 33: 1165-1175. [DOI:10.1016/S1701-2163(16)35087-3]
9. Choi HW, Park YS, Lee SH, Lim CK, Seo JT, Yang KM. Effects of maternal age on embryo quality and pregnancy outcomes using testicular sperm with intracytoplasmic sperm injection. Clin Exp Reprod Med 2016; 43: 221-227. [DOI:10.5653/cerm.2016.43.4.221] [PMID] [PMCID]
10. Tsafrir A, Simon A, Revel A, Reubinoff B, Lewin A, Laufer N. Retrospective analysis of 1217 IVF cycles in women aged 40 years and older. Reprod Biomed Online 2007; 14: 348-355. [DOI:10.1016/S1472-6483(10)60878-4]
11. Keefe D, Kumar M, Kalmbach K. Oocyte competency is the key to embryo potential. Fertil Steril 2015; 103: 317-322. [DOI:10.1016/j.fertnstert.2014.12.115] [PMID]
12. Jeve YB, Bhandari HM. Effective treatment protocol for poor ovarian response: A systematic review and meta-analysis. J Hum Reprod Sci 2016; 9: 70-81. [DOI:10.4103/0974-1208.183515] [PMID] [PMCID]
13. Keay SD, Lenton EA, Cooke ID, Hull MG, Jenkins JM. Low-dose dexamethasone augments the ovarian response to exogenous gonadotrophins leading to a reduction in cycle cancellation rate in a standard IVF programme. Hum Reprod 2001; 16: 1861-1865. [DOI:10.1093/humrep/16.9.1861] [PMID]
14. Kyrou D, Kolibianakis EM, Venetis CA, Papanikolaou EG, Bontis J, Tarlatzis BC. How to improve the probability of pregnancy in poor responders undergoing in vitro fertilization: A systematic review and meta-analysis. Fertil Steril 2009; 91: 749-766. [DOI:10.1016/j.fertnstert.2007.12.077] [PMID]
15. Sunkara SK, Pundir J, Khalaf Y. Effect of androgen supplementation or modulation on ovarian stimulation outcome in poor responders: A meta-analysis. Reprod BioMed Online 2011; 22: 545-555. [DOI:10.1016/j.rbmo.2011.01.015] [PMID]
16. van Loendersloot LL, van Wely M, Limpens J, Bossuyt PMM, Repping S, van der Veen F. Predictive factors in in vitro fertilization (IVF): A systematic review and meta-analysis. Hum Reprod Update 2010; 16: 577-589. [DOI:10.1093/humupd/dmq015] [PMID]
17. Lazzaroni-Tealdi E, Barad DH, Albertini DF, Yu Y, Kushnir VA, Russell H, et al. Oocyte scoring enhances embryo-scoring in predicting pregnancy chances with IVF where it counts most. PLoS One 2015; 10: e0143632. [DOI:10.1371/journal.pone.0143632] [PMID] [PMCID]
18. Pousette A, Akerlöf E, Rosenborg L, Fredricsson B. Increase in progressive motility and improved morphology of human spermatozoa following their migration through Percoll gradients. Int J Androl 1986; 9: 1-13. [DOI:10.1111/j.1365-2605.1986.tb00862.x] [PMID]
19. Tan TY, Lau SK, Loh SF, Tan HH. Female ageing and reproductive outcome in assisted reproduction cycles. Singapore Med J 2014; 55: 305-309. [DOI:10.11622/smedj.2014081] [PMID] [PMCID]
20. Zheng H, Chen Sh, Du H, Ling J, Wu Y, Liu H, et al. Ovarian response prediction in controlled ovarian stimulation for IVF using anti-Müllerian hormone in Chinese women: A retrospective cohort study. Medicine 2017; 96: e6495. [DOI:10.1097/MD.0000000000006495] [PMID] [PMCID]
21. Yan JH, Wu KL, Tang R, Ding LL, Chen ZJ. Effect of maternal age on the outcomes of in vitro fertilization and embryo transfer (IVF-ET). Sci China Life Sci 2012; 55: 694-698. [DOI:10.1007/s11427-012-4357-0] [PMID]
22. Polyzos NP, Devroey P. A systematic review of randomized trials for the treatment of poor ovarian responders: Is there any light at the end of the tunnel? Fertil Steril 2011; 96: 1058-1061. [DOI:10.1016/j.fertnstert.2011.09.048] [PMID]
23. Polyzos NP, Nwoye M, Corona R, Blockeel C, Stoop D, Haentjens P, et al. Live birth rates in Bologna poor responders treated with ovarian stimulation for IVF/ICSI. Reprod Biomed Online 2014; 28: 469-474. [DOI:10.1016/j.rbmo.2013.11.010] [PMID]
24. Huang M-Ch, Tzeng Sh-L, Lee Ch-I, Chen H-H, Huang Ch-Ch, Lee T-H, et al. GnRH agonist long protocol versus GnRH antagonist protocol for various aged patients with diminished ovarian reserve: A retrospective study. PLoS One 2018; 13: e0207081. [DOI:10.1371/journal.pone.0207081] [PMID] [PMCID]
25. Zhang Y, Zhang Ch, Shu J, Guo J, Chang H-M, Leung PCK, et al. Adjuvant treatment strategies in ovarian stimulation for poor responders undergoing IVF: A systematic review and network meta-analysis. Hum Reprod Update 2020; 26: 247-263. [DOI:10.1093/humupd/dmz046] [PMID]
26. Meldrum DR, Casper RF, Diez-Juan A, Simon C, Domar AD, Frydman R. Aging and the environment affect gamete and embryo potential: Can we intervene? Fertil Steril 2016; 105: 548-559. [DOI:10.1016/j.fertnstert.2016.01.013] [PMID]
27. Colaco S, Sakkas D. Paternal factors contributing to embryo quality. J Assist Reprod Genet 2018; 35: 1953-1968. [DOI:10.1007/s10815-018-1304-4] [PMID] [PMCID]
28. Li J, Chen Q, Wang J, Huang G, Ye H. Does growth hormone supplementation improve oocyte competence and IVF outcomes in patients with poor embryonic development? A randomized controlled trial. BMC Pregnancy Childbirth 2020; 20: 310. [DOI:10.1186/s12884-020-03004-9] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








