Tue, Jul 15, 2025
[Archive]
Volume 20, Issue 4 (April 2022)
IJRM 2022, 20(4): 299-306 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Baranizadeh K, Bahmanzadeh M, Tavilani H, Ghiasvand T, Amiri I, Yavangi M et al . Evaluation of methylenetetrahydrofolate reductase and S-adenosyl-methionine level in male infertility: A case-control study. IJRM 2022; 20 (4) :299-306
URL: http://ijrm.ir/article-1-2185-en.html
URL: http://ijrm.ir/article-1-2185-en.html
Khadijeh Baranizadeh1 

 , Maryam Bahmanzadeh2
, Maryam Bahmanzadeh2 

 , Heidar Tavilani1
, Heidar Tavilani1 

 , Tayebeh Ghiasvand1
, Tayebeh Ghiasvand1 

 , Iraj Amiri2
, Iraj Amiri2 

 , Mahnaz Yavangi3
, Mahnaz Yavangi3 

 , Gholamreza Shafiee *4
, Gholamreza Shafiee *4 




 , Maryam Bahmanzadeh2
, Maryam Bahmanzadeh2 

 , Heidar Tavilani1
, Heidar Tavilani1 

 , Tayebeh Ghiasvand1
, Tayebeh Ghiasvand1 

 , Iraj Amiri2
, Iraj Amiri2 

 , Mahnaz Yavangi3
, Mahnaz Yavangi3 

 , Gholamreza Shafiee *4
, Gholamreza Shafiee *4 


1- Department of Clinical Biochemistry, Medicine School, Hamadan University of Medical Sciences, Hamadan, Iran.
2- Department of Anatomical Sciences, School of Medicine, Endometrium and Endometriosis Research Center, Hamadan University of Medical Sciences, Hamadan, Iran.
3- Department of Obstetrics and Gynecology, School of Medicine, Endometrium and Endometriosis Research Center, Fatemieh Hospital, Hamadan University of Medical Sciences, Hamadan, Iran.
4- Department of Clinical Biochemistry, Medicine School, Nutrition Health Research Center, Hamadan University of Medical Sciences, Hamadan, Iran. ,g.r_shafiee@yahoo.com
2- Department of Anatomical Sciences, School of Medicine, Endometrium and Endometriosis Research Center, Hamadan University of Medical Sciences, Hamadan, Iran.
3- Department of Obstetrics and Gynecology, School of Medicine, Endometrium and Endometriosis Research Center, Fatemieh Hospital, Hamadan University of Medical Sciences, Hamadan, Iran.
4- Department of Clinical Biochemistry, Medicine School, Nutrition Health Research Center, Hamadan University of Medical Sciences, Hamadan, Iran. ,
Keywords: Methylenetetrahydrofolate reductase, S-adenosylmethionine, Normozoospermia, Oligozoospermia, Folic acid.
Full-Text [PDF 324 kb]
(1274 Downloads)
| Abstract (HTML) (1681 Views)
1. Introduction
Infertility is the inability of a couple to get pregnant after one year of unprotected intercourse without contraceptive methods and despite adequate intercourse (1). Infertility is a disorder that affects about 30-50% of men in cases overall (2).
Defects in spermatogenesis are one of the causes of infertility, and folate is important in this process (3). Folate and B12 play the main role in the methylation of uracil to the production of thymine in the DNA structure (4). Methylenetetrahydrofolate reductase (MTHFR) is a key enzyme in the biochemical pathway of one-carbon metabolism (5) and the storage of methyl groups for DNA methylation (6).
DNA methylation is one of the important factors in regulating gene expression (7). This enzyme has enzyme committee number 1, 1, 99, 15, and catalyzes the reduction of 5, 10 MTHF to 5-methyltetrahydrofolate using nicotinamide adenine dinucleotide phosphate (NADPH), which is an irreversible reaction. The methyl group of 5-methyl tetrahydrofolate is transferred to homocysteine for producing methionine, and subsequently, methionine is used to form s-adenosine methionine (SAM) (8).
SAM acts as a methyl group donor for DNA methylation (9, 10). SAM is a methyl group source for thymidylate biosynthesis and SAM-dependent methylation (11). The methylation process is very important for the regulation of DNA transcription, histone modification, and stabilization of the genome, so it is tightly regulated (12). It seems that mutation or decreased activity of the MTHFR enzyme leads to a decrease in S-adenosylmethionine and DNA methylation, ultimately disrupting the spermatogenesis pathway (13).
Therefore, we evaluated MTHFR activity and the S-adenosylmethionine level in normozoospermic and oligozoospermic men.
2. Material and Methods
2.1. Collection of samples
This observational study recruited, semen samples of normozoospermic (n = 30) and oligozoospermic men (n = 30) from the endometrium and endometriosis center, Hamadan, Iran between May 2019 and August 2021. All subjects were evaluated using a questionnaire covering fertility parameters, medical history, and chronic diseases.
Participants with recognizable causes of male infertility such as obstructive oligozoospermia, varicocele, infections, and diabetes were excluded. Normozoospermic men were defined as samples with motility > 40%, morphology > 4%, and sperm concentration > 15 million/ml were included as normozoospermia, and samples low of these parameters were selected as oligozoospermia. Semen analysis was conducted according to the 2010 World Health Organization criteria (14). All semen samples were collected in sterile containers after 3-5 days of sexual abstinence. The samples were then incubated in a 37oC incubator for 30-40 min. Subsequently, semen liquid macroscopic tests were initially performed (15).
 ,
,  = 5%,
= 5%,  = 1.96,
= 1.96,  = 10%,
= 10%,  = 1.23
= 1.23
2.2. Sperm parameters
For grouping the individuals, semen fluid analysis was performed, and the parameters of sperm count, motility, and morphology were evaluated based on that, individuals were divided into 2 groups: normozoospermic and oligozoospermic.
2.3. Sperm count and motility
The concentration and motility of spermatozoa were evaluated using a computer-assisted sperm analysis system by sperm test video software. At first, a 3 μl sample was loaded into a 20 μm slide at 37°C for analyses at 30 min intervals up to 180 min. Manual sampling was also performed to ensure the accuracy of the semen analyzer (18).
2.4. Sperm morphology
The sperm morphology was evaluated by quick-diff dye solutions. At first, a drop of the above samples was smeared then drying, and staining was performed. The dried slides were incubated in fixation solution for 75 sec, then in staining solution for 60 sec, and finally in detaining solution for 35 sec. After washing with distilled water and drying, their appearance was shown by microscope (15).
2.5. Preparation of seminal plasma
First, semen samples were centrifuged at 500 g for 10 min to separate the semen plasma; simultaneously were taken and were maintained at -20oC (19).
2.6. Seminal plasma measurement of MTHFR enzymatic activity
For detection of MTHFR enzymatic activity, seminal plasma was measured by a previous method with modifications (20). First, the 96 well plates were filled with 30 μL formaldehyde, 100 μL Tetrahydrofolate, and 200 μL phosphate buffer saline. The plate was incubated at 37°C for 5 min, and 200 μL flavine adenine dinucleotide and 200 μL ascorbic acid with 100 μL 2-mercaptoethanol were added to all wells, then 6 μL samples were added except the blank tube. The tubes were placed at 37°C for 5 min. Finally, 20 μL NADPH was added except the blanks, re-incubated at 37°C for 5 min and immediately the absorbance of the samples was measured at 340 nm by an enzyme-linked immunosorbent assay (ELISA) reader (Tecan Group Ltd, Männedorf-Switzerland).
2.7. Measurement of SAM
Semen SAM level was measured using sigma SAM ELISA kit. First, was added 40 µl of the semen sample and 10 µl of SAM-Antibody and 50 µl of streptavidin to the test well and was added 50 µl standard and 50 µl streptavidin to standard well. After the incubation of well sat 37oC for 60 min and washing 30 sec, 50 µl of chromogen A and 50 µl of chromogen B were added to each well and incubated at 37°C for 10 min. Finally, add 50 ml of stop solution was added to each well and measured absorbance at 450 nm by a SunriseTMELISA plate reader (Tecan Group Ltd, Männedorf-Switzerland).
2.8. Ethical considerations
Written informed consent was obtained from each participants. Approval was obtained from the Ethics Committee of Hamadan University of Medical Sciences, Hamadan, Iran (Code: IR.UMSHA.REC.1396.361).
2.9. Statistical analysis
Data were analyzed using statistical software by Statistical Package for the Social Sciences version 16.0 (SPSS Inc., Chicago, USA), and Shapiro-Wilks tests were used to determine the data normality. Sample's t test was used to analyze the data and compare 2 groups, and association tests were used to investigate the relationship between the variables. Results were presented as mean ± SD, and the p < 0.05 was considered significant.
3. Results
3.1. Demographic data
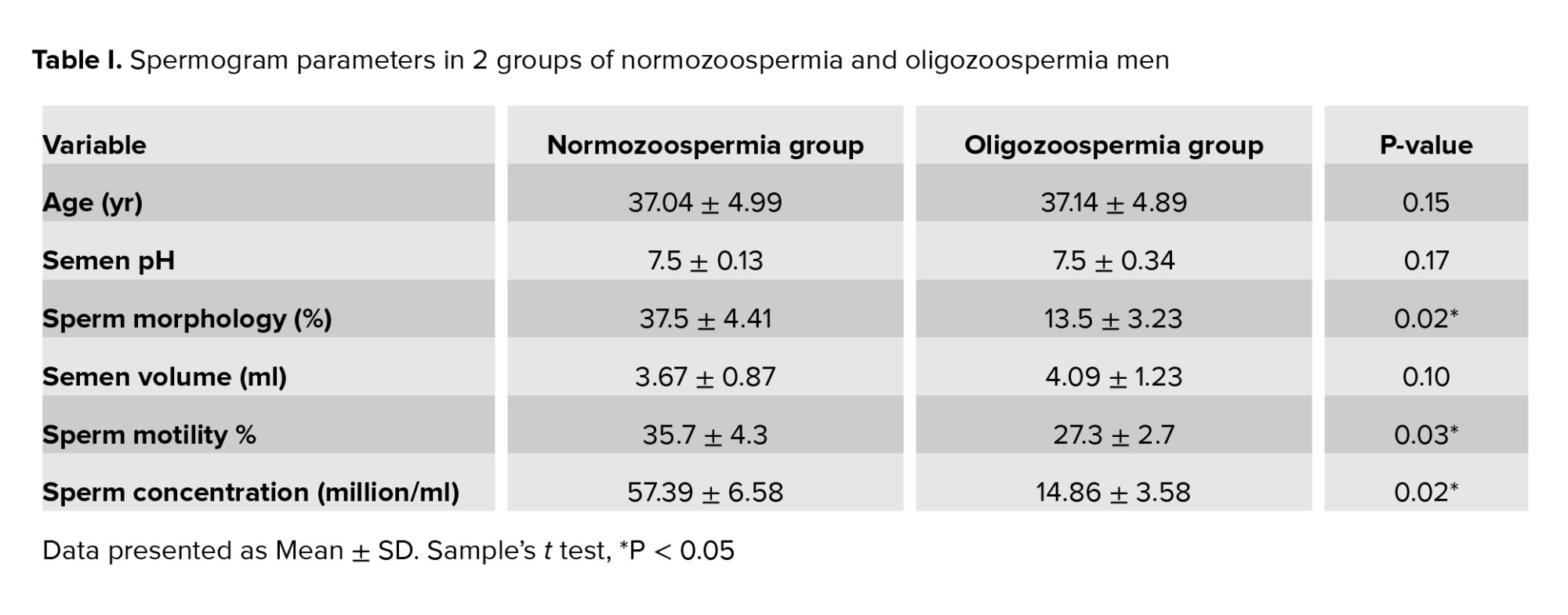
In the present study, it was found that the data were normal after Shapiro-Wilks tests analysis. The minimum age of men in the 2 groups was 32 yr, and a maximum of 42 yr and the mean age of the men was 37 yr, and the 2 study groups were matched by age (p = 0.15). The results of semen analysis are shown in table I. The 2 groups had a significant difference in sperm morphology (p = 0.02), concentration (p = 0.02) and motility (p = 0.03). Although the semen volume and pH were no significant difference in the 2 groups.
3.2. Semen MTHFR enzyme activity
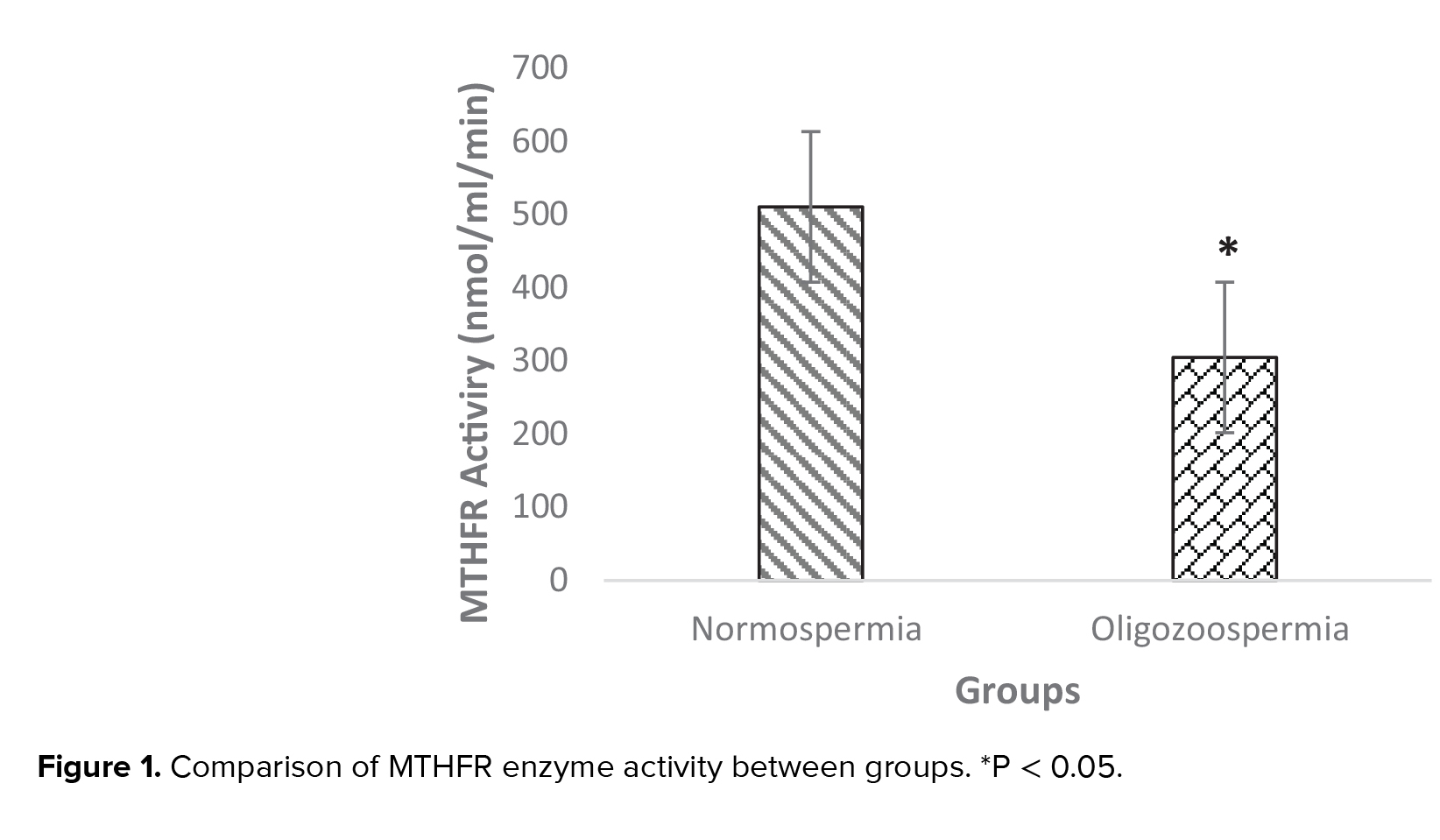
Our results showed that MTHFR activity in the normozoospermia group was 510.66 ± 43.86 nmol/ml/min, while in oligozoospermia, men were 304.9 ± 29.75 nmol/ml/min. The mean of MTHFR activity was significantly different between men with normozoospermia and oligozoospermia groups (p = 0.01) (Figure 1).
3.3. Semen SAM levels
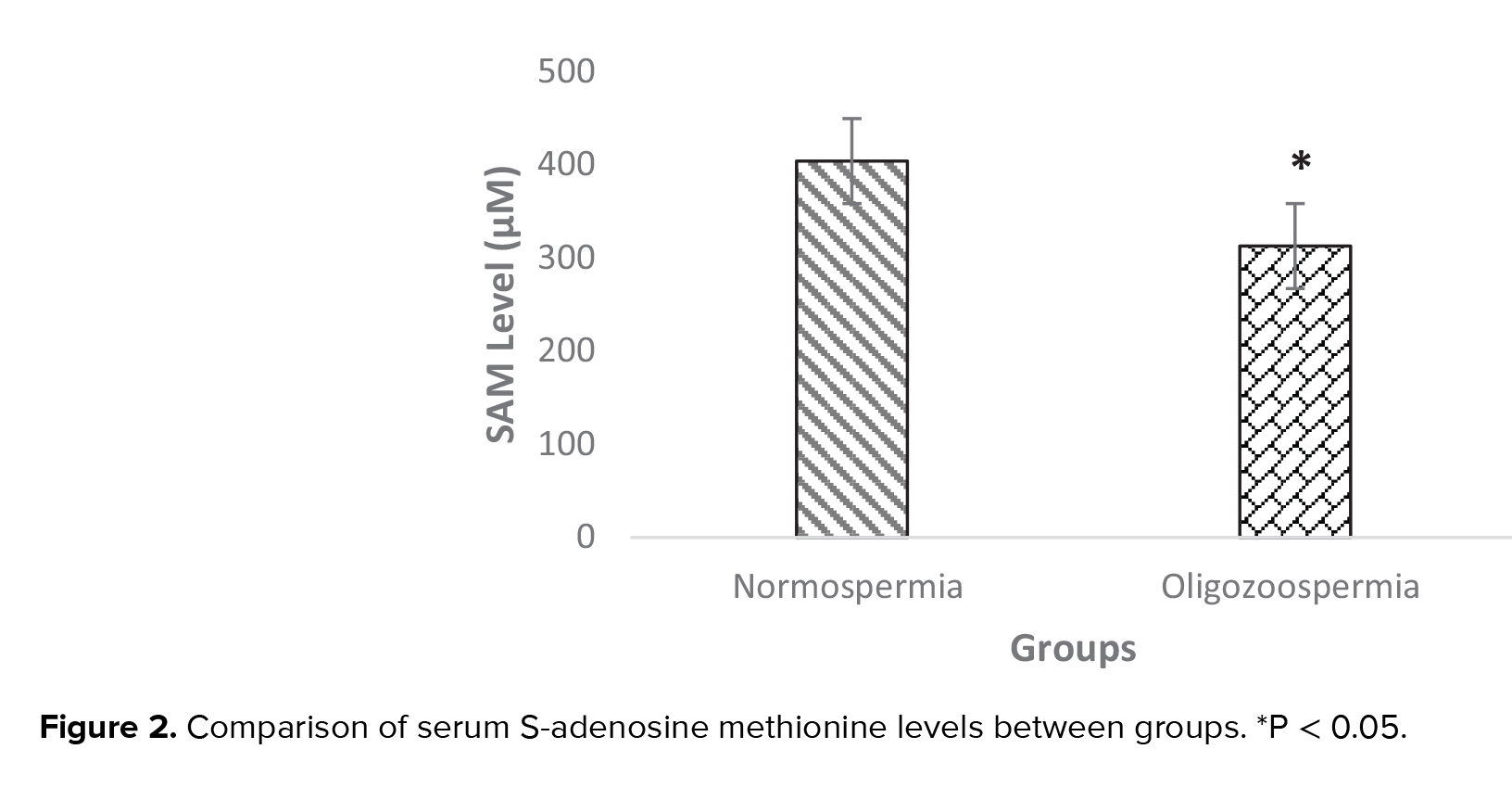
SAM level in the seminal plasma of oligozoospermia was 312.65 ± 80.27 µmol/l-1 and in normozoospermia men was 403.84 ± 86 µmol/l-1. SAM level was significantly lower in oligozoospermia men compared with normozoospermia men (p = 0.03) (Figure 2). According to association analysis, there was a positive association between MTHFR enzyme activity and SAM level in the normozoospermia group (p = 0.02, β = 0.67). Also, statistical analysis showed that in the oligozoospermia group, similar to normozoospermia men, there was a positive association between MTHFR enzyme activity and SAM level (p = 0.03, β = 0.54).
4. Discussion
The results of spermogram parameters showed that the mean of volume and motility, especially sperm concentration and morphology in the oligozoospermia group, were significantly lower than in the normozoospermia group. The results of S-adenosylmethionine measurement showed that although there was a significant relationship between SAM levels in the 2 groups, these results clearly show a low S-adenosylmethionine level in the oligozoospermia group. The amount of SAM and its effects on sperm methylation and spermatogenesis can be effective. Complete spermatogenesis leads to correct sperm morphology and motility and finally increases the concentration of normal sperm. This was consistent with previous studies in which there was a significant relationship between spermatogenesis and the folate metabolism pathway, such as methylation (5). A study found that SAM induces hypermethylation in male infertility, and the methylation was significantly associated with male infertility (9). The results of the present study in the folate-related methylation pathway showed that most individuals in the oligozoospermia group had significantly lower MTHFR enzymatic activity than the normozoospermia group. These results were comparable to previous studies, and a study by Chan and colleagues in 2010 revealed that in mice with the MTHFR gene defection, low quality in both semen and sperm was observed. So there was consequently a defect in the spermatogenesis pattern and testicular tissue growth (21). The MTHFR enzyme is a key enzyme in the folate metabolism pathway, and by using NADPH causes an irreversible reaction that converts 5, 10-methylenetetrahydrofolate to 5-methyltetrahydrofolate (21).
SAM supplies the methyl group is in the cell, which is more important for DNA methylation and regulation of genes involved in spermatogenesis (13). Also, vitamin B12 is a critical coenzyme for the methylation of homocysteine to methionine with the conversion of 5-MTHF to THF (4, 11). Therefore, every change in enzymes involved in folate metabolism, such as MTHFR, may lead to male infertility (22, 23). The results of many studies showed that men with the G1793AMTHFR gene had an inverse relationship with sperm defects (24-28). Other studies found that infertile subjects receiving high doses of folic acid showed no improvement in the disease due to low levels of MTHFR enzyme protein and sperm DNA hypomethylation (29, 30). The results also showed that the MTHFR plays an important role in many diseases, including cardiovascular disease, nervous system diseases, especially central nervous system development in the fetal stage, diabetes, cancer, liver disease, and inflammatory diseases (31, 32). A similar study showed that the mutation MTHFR genotypes had a significant association with diseases such as pregnancy and infertility (33, 34). As noted in most studies, only the MTHFR gene has been investigated, so one of the innovations of the present study is to measure the activity of this enzyme by a manual method because the enzyme activity as a functional form can be more valuable for use in future diagnostic and therapeutic studies.
To conclude, there was a positive association between SAM levels and MTHFR activity in the 2 groups. It means that with increasing MTHFR activity, the amount of SAM increases. MTHFR activity and SAM levels similar to sperm parameters in the normozoospermia group were more than that seen in the oligozoospermia group. This indicates that MTHFR and its product SAM can be more effective in sperm development and complete spermatogenesis.
5. Conclusion
In the present study, it was observed that the level of SAM in the normozoospermia group was higher than in the oligozoospermia group. It was also found that the rate of MTHFR activity in normozoospermia individuals was significantly higher than oligozoospermia group. MTHFR activity and its product, SAM, play a role in sperm evolution, morphology, and motility. It seems that the activity of the MTHFR enzyme is an enzyme involved in folic acid metabolism and may be an important factor in spermatogenesis.
Acknowledgments
The authors wish to acknowledge Hamadan University of Medical Sciences, Hamedan, Iran for supporting this study (Project NO: 9605103136).
Conflicts of Interest
The authors declare no conflict of interest.
Full-Text: (304 Views)
1. Introduction
Infertility is the inability of a couple to get pregnant after one year of unprotected intercourse without contraceptive methods and despite adequate intercourse (1). Infertility is a disorder that affects about 30-50% of men in cases overall (2).
Defects in spermatogenesis are one of the causes of infertility, and folate is important in this process (3). Folate and B12 play the main role in the methylation of uracil to the production of thymine in the DNA structure (4). Methylenetetrahydrofolate reductase (MTHFR) is a key enzyme in the biochemical pathway of one-carbon metabolism (5) and the storage of methyl groups for DNA methylation (6).
DNA methylation is one of the important factors in regulating gene expression (7). This enzyme has enzyme committee number 1, 1, 99, 15, and catalyzes the reduction of 5, 10 MTHF to 5-methyltetrahydrofolate using nicotinamide adenine dinucleotide phosphate (NADPH), which is an irreversible reaction. The methyl group of 5-methyl tetrahydrofolate is transferred to homocysteine for producing methionine, and subsequently, methionine is used to form s-adenosine methionine (SAM) (8).
SAM acts as a methyl group donor for DNA methylation (9, 10). SAM is a methyl group source for thymidylate biosynthesis and SAM-dependent methylation (11). The methylation process is very important for the regulation of DNA transcription, histone modification, and stabilization of the genome, so it is tightly regulated (12). It seems that mutation or decreased activity of the MTHFR enzyme leads to a decrease in S-adenosylmethionine and DNA methylation, ultimately disrupting the spermatogenesis pathway (13).
Therefore, we evaluated MTHFR activity and the S-adenosylmethionine level in normozoospermic and oligozoospermic men.
2. Material and Methods
2.1. Collection of samples
This observational study recruited, semen samples of normozoospermic (n = 30) and oligozoospermic men (n = 30) from the endometrium and endometriosis center, Hamadan, Iran between May 2019 and August 2021. All subjects were evaluated using a questionnaire covering fertility parameters, medical history, and chronic diseases.
Participants with recognizable causes of male infertility such as obstructive oligozoospermia, varicocele, infections, and diabetes were excluded. Normozoospermic men were defined as samples with motility > 40%, morphology > 4%, and sperm concentration > 15 million/ml were included as normozoospermia, and samples low of these parameters were selected as oligozoospermia. Semen analysis was conducted according to the 2010 World Health Organization criteria (14). All semen samples were collected in sterile containers after 3-5 days of sexual abstinence. The samples were then incubated in a 37oC incubator for 30-40 min. Subsequently, semen liquid macroscopic tests were initially performed (15).
The number of samples required for this study was calculated based on the dependent variable of plasma S-adenosylmethionine concentration. The sample size was calculated based on the deviation of the criteria obtained from previous studies and using the following formula (16, 17):
2.2. Sperm parameters
For grouping the individuals, semen fluid analysis was performed, and the parameters of sperm count, motility, and morphology were evaluated based on that, individuals were divided into 2 groups: normozoospermic and oligozoospermic.
2.3. Sperm count and motility
The concentration and motility of spermatozoa were evaluated using a computer-assisted sperm analysis system by sperm test video software. At first, a 3 μl sample was loaded into a 20 μm slide at 37°C for analyses at 30 min intervals up to 180 min. Manual sampling was also performed to ensure the accuracy of the semen analyzer (18).
2.4. Sperm morphology
The sperm morphology was evaluated by quick-diff dye solutions. At first, a drop of the above samples was smeared then drying, and staining was performed. The dried slides were incubated in fixation solution for 75 sec, then in staining solution for 60 sec, and finally in detaining solution for 35 sec. After washing with distilled water and drying, their appearance was shown by microscope (15).
2.5. Preparation of seminal plasma
First, semen samples were centrifuged at 500 g for 10 min to separate the semen plasma; simultaneously were taken and were maintained at -20oC (19).
2.6. Seminal plasma measurement of MTHFR enzymatic activity
For detection of MTHFR enzymatic activity, seminal plasma was measured by a previous method with modifications (20). First, the 96 well plates were filled with 30 μL formaldehyde, 100 μL Tetrahydrofolate, and 200 μL phosphate buffer saline. The plate was incubated at 37°C for 5 min, and 200 μL flavine adenine dinucleotide and 200 μL ascorbic acid with 100 μL 2-mercaptoethanol were added to all wells, then 6 μL samples were added except the blank tube. The tubes were placed at 37°C for 5 min. Finally, 20 μL NADPH was added except the blanks, re-incubated at 37°C for 5 min and immediately the absorbance of the samples was measured at 340 nm by an enzyme-linked immunosorbent assay (ELISA) reader (Tecan Group Ltd, Männedorf-Switzerland).
2.7. Measurement of SAM
Semen SAM level was measured using sigma SAM ELISA kit. First, was added 40 µl of the semen sample and 10 µl of SAM-Antibody and 50 µl of streptavidin to the test well and was added 50 µl standard and 50 µl streptavidin to standard well. After the incubation of well sat 37oC for 60 min and washing 30 sec, 50 µl of chromogen A and 50 µl of chromogen B were added to each well and incubated at 37°C for 10 min. Finally, add 50 ml of stop solution was added to each well and measured absorbance at 450 nm by a SunriseTMELISA plate reader (Tecan Group Ltd, Männedorf-Switzerland).
2.8. Ethical considerations
Written informed consent was obtained from each participants. Approval was obtained from the Ethics Committee of Hamadan University of Medical Sciences, Hamadan, Iran (Code: IR.UMSHA.REC.1396.361).
2.9. Statistical analysis
Data were analyzed using statistical software by Statistical Package for the Social Sciences version 16.0 (SPSS Inc., Chicago, USA), and Shapiro-Wilks tests were used to determine the data normality. Sample's t test was used to analyze the data and compare 2 groups, and association tests were used to investigate the relationship between the variables. Results were presented as mean ± SD, and the p < 0.05 was considered significant.
3. Results
3.1. Demographic data
In the present study, it was found that the data were normal after Shapiro-Wilks tests analysis. The minimum age of men in the 2 groups was 32 yr, and a maximum of 42 yr and the mean age of the men was 37 yr, and the 2 study groups were matched by age (p = 0.15). The results of semen analysis are shown in table I. The 2 groups had a significant difference in sperm morphology (p = 0.02), concentration (p = 0.02) and motility (p = 0.03). Although the semen volume and pH were no significant difference in the 2 groups.
3.2. Semen MTHFR enzyme activity
Our results showed that MTHFR activity in the normozoospermia group was 510.66 ± 43.86 nmol/ml/min, while in oligozoospermia, men were 304.9 ± 29.75 nmol/ml/min. The mean of MTHFR activity was significantly different between men with normozoospermia and oligozoospermia groups (p = 0.01) (Figure 1).
3.3. Semen SAM levels
SAM level in the seminal plasma of oligozoospermia was 312.65 ± 80.27 µmol/l-1 and in normozoospermia men was 403.84 ± 86 µmol/l-1. SAM level was significantly lower in oligozoospermia men compared with normozoospermia men (p = 0.03) (Figure 2). According to association analysis, there was a positive association between MTHFR enzyme activity and SAM level in the normozoospermia group (p = 0.02, β = 0.67). Also, statistical analysis showed that in the oligozoospermia group, similar to normozoospermia men, there was a positive association between MTHFR enzyme activity and SAM level (p = 0.03, β = 0.54).



4. Discussion
The results of spermogram parameters showed that the mean of volume and motility, especially sperm concentration and morphology in the oligozoospermia group, were significantly lower than in the normozoospermia group. The results of S-adenosylmethionine measurement showed that although there was a significant relationship between SAM levels in the 2 groups, these results clearly show a low S-adenosylmethionine level in the oligozoospermia group. The amount of SAM and its effects on sperm methylation and spermatogenesis can be effective. Complete spermatogenesis leads to correct sperm morphology and motility and finally increases the concentration of normal sperm. This was consistent with previous studies in which there was a significant relationship between spermatogenesis and the folate metabolism pathway, such as methylation (5). A study found that SAM induces hypermethylation in male infertility, and the methylation was significantly associated with male infertility (9). The results of the present study in the folate-related methylation pathway showed that most individuals in the oligozoospermia group had significantly lower MTHFR enzymatic activity than the normozoospermia group. These results were comparable to previous studies, and a study by Chan and colleagues in 2010 revealed that in mice with the MTHFR gene defection, low quality in both semen and sperm was observed. So there was consequently a defect in the spermatogenesis pattern and testicular tissue growth (21). The MTHFR enzyme is a key enzyme in the folate metabolism pathway, and by using NADPH causes an irreversible reaction that converts 5, 10-methylenetetrahydrofolate to 5-methyltetrahydrofolate (21).
SAM supplies the methyl group is in the cell, which is more important for DNA methylation and regulation of genes involved in spermatogenesis (13). Also, vitamin B12 is a critical coenzyme for the methylation of homocysteine to methionine with the conversion of 5-MTHF to THF (4, 11). Therefore, every change in enzymes involved in folate metabolism, such as MTHFR, may lead to male infertility (22, 23). The results of many studies showed that men with the G1793AMTHFR gene had an inverse relationship with sperm defects (24-28). Other studies found that infertile subjects receiving high doses of folic acid showed no improvement in the disease due to low levels of MTHFR enzyme protein and sperm DNA hypomethylation (29, 30). The results also showed that the MTHFR plays an important role in many diseases, including cardiovascular disease, nervous system diseases, especially central nervous system development in the fetal stage, diabetes, cancer, liver disease, and inflammatory diseases (31, 32). A similar study showed that the mutation MTHFR genotypes had a significant association with diseases such as pregnancy and infertility (33, 34). As noted in most studies, only the MTHFR gene has been investigated, so one of the innovations of the present study is to measure the activity of this enzyme by a manual method because the enzyme activity as a functional form can be more valuable for use in future diagnostic and therapeutic studies.
To conclude, there was a positive association between SAM levels and MTHFR activity in the 2 groups. It means that with increasing MTHFR activity, the amount of SAM increases. MTHFR activity and SAM levels similar to sperm parameters in the normozoospermia group were more than that seen in the oligozoospermia group. This indicates that MTHFR and its product SAM can be more effective in sperm development and complete spermatogenesis.
5. Conclusion
In the present study, it was observed that the level of SAM in the normozoospermia group was higher than in the oligozoospermia group. It was also found that the rate of MTHFR activity in normozoospermia individuals was significantly higher than oligozoospermia group. MTHFR activity and its product, SAM, play a role in sperm evolution, morphology, and motility. It seems that the activity of the MTHFR enzyme is an enzyme involved in folic acid metabolism and may be an important factor in spermatogenesis.
Acknowledgments
The authors wish to acknowledge Hamadan University of Medical Sciences, Hamedan, Iran for supporting this study (Project NO: 9605103136).
Conflicts of Interest
The authors declare no conflict of interest.
Type of Study: Original Article |
Subject:
Fertility & Infertility
References
1. Tahmasbpour E, Balasubramanian D, Agarwal A. A multi-faceted approach to understanding male infertility: Gene mutations, molecular defects and assisted reproductive techniques (ART). J Assist Reprod Genet 2014; 31: 1115-1137. [DOI:10.1007/s10815-014-0280-6] [PMID] [PMCID]
2. Machado Gava M, de Oliveira Chagas E, Bianco B, Christofolini DM, Pompeo ACL, Glina S, et al. Methylenetetrahydrofolate reductase polymorphisms are related to male infertility in Brazilian men. Genet Test Mol Biomarkers 2011; 15: 153-157. [DOI:10.1089/gtmb.2010.0128] [PMID]
3. Gunes S, Arslan MA, Hekim GNT, Asci R. The role of epigenetics in idiopathic male infertility. J Assist Reprod Genet 2016; 33: 553-569. [DOI:10.1007/s10815-016-0682-8] [PMID] [PMCID]
4. Singh K, Jaiswal D. One-carbon metabolism, spermatogenesis, and male infertility. Reprod Sci 2012; 20: 622-630. [DOI:10.1177/1933719112459232] [PMID]
5. Rajender S, Avery K, Agarwal A. Epigenetics, spermatogenesis and male infertility. Mutat Res 2011; 727: 62-71. [DOI:10.1016/j.mrrev.2011.04.002] [PMID]
6. Stuppia L, Franzago M, Ballerini P, Gatta V, Antonucci I. Epigenetics and male reproduction: The consequences of paternal lifestyle on fertility, embryo development, and children lifetime health. Clin Epigenetics 2015; 7: 120-127. [DOI:10.1186/s13148-015-0155-4] [PMID] [PMCID]
7. Karimian M, Hosseinzadeh Colagar A. Methionine synthase A2756G transition might be a risk factor for male infertility: Evidences from seven case-control studies. Mol Cell Endocrinol 2016; 425: 1-10. [DOI:10.1016/j.mce.2016.02.012] [PMID]
8. Mazloomi S, Alimohammadi Sh, Khodadadi I, Ghiasvand T, Shafiee Gh. Evaluation of methylene tetrahydrofolate reductase (MTHFR) activity and the levels of homocysteine and malondialdehyde (MDA) in the serum of women with preeclampsia. Clin Exp Hypertens 2020; 42: 590-594. [DOI:10.1080/10641963.2020.1739700] [PMID]
9. Wu W, Shen O, Qin Y, Niu X, Lu C, Xia Y, et al. Idiopathic male infertility is strongly associated with aberrant promoter methylation of methylenetetrahydrofolate reductase (MTHFR). PloS One 2010; 5: e13884. [DOI:10.1371/journal.pone.0013884] [PMID] [PMCID]
10. Botezatu A, Socolov R, Socolov D, Iancu IV, Anton G. Methylation pattern of methylene tetrahydrofolate reductase and small nuclear ribonucleoprotein polypeptide N promoters in oligoasthenospermia: A case-control study. Reprod Biomed Online 2014; 28: 225-231. [DOI:10.1016/j.rbmo.2013.10.010] [PMID]
11. Huang L, Zhang J, Hayakawa T, Tsuge H. Assays of methylenetetrahydrofolate reductase and methionine synthase activities by monitoring 5-methyltetrahydrofolate and tetrahydrofolate using high-performance liquid chromatography with fluorescence detection. Anal Biochem 2011; 299: 253-259. [DOI:10.1006/abio.2001.5421] [PMID]
12. Ali F, Zeb F, Almajwal A, Fatima S, Wu X. Relationship of nutrigenomics and aging: Involvement of DNA methylation. J Nutr Inter Metab 2019; 16; 100098. [DOI:10.1016/j.jnim.2019.100098]
13. Kobayashi H, Sato A, Otsu E, Hiura H, Tomatsu C, Utsunomiya T, et al. Aberrant DNA methylation of imprinted loci in sperm from oligospermic patients. Hum Mol Genet 2007; 16: 2542-2551. [DOI:10.1093/hmg/ddm187] [PMID]
14. Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World health organization reference values for human semen characteristics. Hum Reprod Update 2010; 16: 231-245.
https://doi.org/10.1093/humupd/dmp048 [DOI:10.1093/humupd/dmq020] [PMID]
15. Khabour OF, Al-azzam AM, Alfaouri AA, Zayed F. Association of polymorphisms in DAZL gene with male infertility. J Adv Med Med Res 2013; 3: 41-48. [DOI:10.9734/BJMMR/2013/2009]
16. Zalata A, El-Baz A, Othman G, Hassan A, Mostaf T. Seminal plasma S-adenosylmethionine and S-adenosylhomocysteine associations in infertile men. Hum Androl 2011; 1: 103-107. [DOI:10.1097/01.XHA.0000407148.53472.9a]
17. Rosner B. Fundamentas of biostatistics. 4th Ed. Belmont: Duxbury Press; 1995: 283.
18. Kuo-Kuang Lee R, Tseng HCh, Hwu YM, Fan ChCh, Lin MH, Yu JJ, et al. Expression of cystatin C in the female reproductive tract and its effect on human sperm capacitation. Reprod Biol Endocrinol 2018; 16: 1-10. [DOI:10.1186/s12958-018-0327-0] [PMID] [PMCID]
19. Ghiasvand T, Goodarzi MT, Shafiee Gh, Zamani A, Karimi J, Ghorbani M, et al. Association between seminal plasma neopterin and oxidative stress in male infertility: A case-control study. Int J Reprod BioMed 2018; 16: 93-100. [DOI:10.29252/ijrm.16.2.93] [PMID] [PMCID]
20. Klenova EM, Morse HC, Ohlsson R, Lobanenkov VV. The novel BORIS + CTCF gene family is uniquely involved in the epigenetics of normal biology and cancer. Semin Cancer Biol 2020; 12: 399-414. [DOI:10.1016/S1044-579X(02)00060-3]
21. Chan D, Cushnie DW, Neaga OR, Lawrance AK, Rozen R, Trasler JM. Strain-specific defects in testicular development and sperm epigenetic patterns in 5, 10-methylenetetrahydrofolate reductase-deficient mice. Endocrinology 2010; 151: 3363-3373. [DOI:10.1210/en.2009-1340] [PMID]
22. Nikzad H, Karimian M, Sareban K, Khoshsokhan M, Hosseinzadeh Colagar A. MTHFR-Ala222Val and male infertility: A study in Iranian men, an updated meta-analysis and an in silico-analysis. Reprod BioMed Online 2015; 31: 668-680. [DOI:10.1016/j.rbmo.2015.07.015] [PMID]
23. Karimian M, Hosseinzadeh Colagar A. Association of C677T transition of the human methylenetetrahydrofolate reductase (MTHFR) gene with male infertility. Reprod Fertil Dev 2016; 28: 785-794. [DOI:10.1071/RD14186] [PMID]
24. Filippo G, Rossella C, Laura M, Angela A, Rosita A, Aldo E, et al. Epigenetics of male fertility: Effects on assisted reproductive techniques. World J Mens Health 2019; 37: 148-156. [DOI:10.5534/wjmh.180071] [PMID] [PMCID]
25. Swayne BG, Kawata A, Behan NA, Williams A, Wade MG, Macfarlane AJ, et al. Investigating the effects of dietary folic acid on sperm count, DNA damage and mutation in Balb/c mice. Mutat Res 2012; 737: 1-7. [DOI:10.1016/j.mrfmmm.2012.07.002] [PMID]
26. Gupta N, Sarkar S, David A, Gangwar PK, Gupta R, Khanna G, et al. Significant impact of the MTHFR polymorphisms and haplotypes on male infertility risk. PloS One 2013; 8: e69180. [DOI:10.1371/journal.pone.0069180] [PMID] [PMCID]
27. Mfady DS, Sadiq MF, Khabour OF, Fararjeh AS, Abu-Awad A, Khader Y. Associations of variants in MTHFR and MTRR genes with male infertility in the Jordanian population. Gene 2014; 536: 40-44. [DOI:10.1016/j.gene.2013.12.001] [PMID]
28. Karimian M, Hosseinzadeh Colagar A. Human MTHFR-G1793A transition may be a protective mutation against male infertility: A genetic association study and in silico analysis. Hum Fertil 2018; 21: 128-136. [DOI:10.1080/14647273.2017.1298161] [PMID]
29. Aarabi M, Christensen KE, Chan D, Leclerc D, Landry M, Ly L, et al. Testicular MTHFR deficiency may explain sperm DNA hypomethylation associated with high dose folic acid supplementation. Hum Mol Genet 2018; 27: 1123-1135. [DOI:10.1093/hmg/ddy021] [PMID] [PMCID]
30. Wen-Jie H, Xi-Lan L, Jun-Tao L, Jian-Min Z. Effects of folic acid on oligozoospermia with MTHFR polymorphisms in term of seminal parameters, DNA fragmentation, and live birth rate: A double-blind, randomized, placebo-controlledtrial. Andrology 2020; 8: 110-116. [DOI:10.1111/andr.12652] [PMID]
31. Ahmadi MSZ, Bahadori M. [The study of GPx1 Pro198Leu lolymorphism in idiopathic male infertility]. Sci J Hamadan Univ Med Sci 2015; 22: 76-82. (in Persian)
32. Yi-Le WC, Shan-Shan L, Feng-Feng G, Fang F, Zhen-Zhong Q, Xiu-Xiu D, et al. Association between methylenetetrahydrofolate reductase (MTHFR)C677T/A1298C polymorphisms and essential hypertension. Metabolism 2014; 63: 1503-1511. [DOI:10.1016/j.metabol.2014.10.001] [PMID]
33. Kulac T, Hekim N, Kocamanoglu F, Beyaz C, Gunes S, Asci R. Methylation patterns of methylenetetrahydrofolate reductase gene promoter in infertile males. Andrologia 2021; 53: e13942-e13945. [DOI:10.1111/and.13942] [PMID]
34. Servy EJ, Jacquesson-Fournols L, Cohen M, Menezo YJR. MTHFR isoform carriers. 5-MTHF (5-methyl tetrahydrofolate) vs folic acid: A key to pregnancy outcome: A case series. J Assist Reprod Genet 2018; 35: 1431-1435. [DOI:10.1007/s10815-018-1225-2] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





