Tue, Apr 23, 2024
[Archive]
Volume 20, Issue 5 (May 2022)
IJRM 2022, 20(5): 389-398 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Nouri M, Mehrabani S, Firoozbakht H, Vatanian E, Abbasi H, Shirani M. The association between dietary fat and mineral intake with semen parameters: A cross-sectional study in infertile men. IJRM 2022; 20 (5) :389-398
URL: http://ijrm.ir/article-1-2204-en.html
URL: http://ijrm.ir/article-1-2204-en.html
Mehran Nouri1 

 , Sanaz Mehrabani2
, Sanaz Mehrabani2 

 , Hosein Firoozbakht3
, Hosein Firoozbakht3 

 , Elmira Vatanian4
, Elmira Vatanian4 

 , Homayoun Abbasi5
, Homayoun Abbasi5 

 , Mahsa Shirani *
, Mahsa Shirani * 

 6
6


 , Sanaz Mehrabani2
, Sanaz Mehrabani2 

 , Hosein Firoozbakht3
, Hosein Firoozbakht3 

 , Elmira Vatanian4
, Elmira Vatanian4 

 , Homayoun Abbasi5
, Homayoun Abbasi5 

 , Mahsa Shirani *
, Mahsa Shirani * 

 6
6
1- Students’ Research Committee, School of Nutrition and Food Science, Shiraz University of Medical Sciences, Shiraz, Iran. Department of Community Nutrition, School of Nutrition and Food Science, Shiraz University of Medical Sciences, Shiraz, Iran.
2- Department of Community Nutrition, School of Nutrition and Food Science, Food Security Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
3- Department of Nutrition, Science and Research Branch, Islamic Azad University, Tehran, Iran.
4- Department of Cellular and Molecular Nutrition, School of Nutritional Science and Dietetics, Tehran University of Medical Science, Tehran, Iran.
5- Isfahan Fertility and Infertility Center, Isfahan, Iran.
6- Department of Community Nutrition, School of Nutrition and Food Science, Food Security Research Center, Isfahan University of Medical Sciences, Isfahan, Iran. Students’ Research Committee, Isfahan University of Medical Sciences, Isfahan, Iran. , mahsa.shirani1373@yahoo.com
2- Department of Community Nutrition, School of Nutrition and Food Science, Food Security Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
3- Department of Nutrition, Science and Research Branch, Islamic Azad University, Tehran, Iran.
4- Department of Cellular and Molecular Nutrition, School of Nutritional Science and Dietetics, Tehran University of Medical Science, Tehran, Iran.
5- Isfahan Fertility and Infertility Center, Isfahan, Iran.
6- Department of Community Nutrition, School of Nutrition and Food Science, Food Security Research Center, Isfahan University of Medical Sciences, Isfahan, Iran. Students’ Research Committee, Isfahan University of Medical Sciences, Isfahan, Iran. , mahsa.shirani1373@yahoo.com
Full-Text [PDF 300 kb]
(692 Downloads)
| Abstract (HTML) (1021 Views)
1. Introduction
Over recent decades, infertility has been a significant issue in developing countries, affecting 15% of all couples falling within fertile age brackets (1). A meta-analysis estimated the infertility rate within the Iranian population to be about 10.9%. In approximately 40% of infertile couples, male infertility is a factor (2). Infertility is defined as the inability of a couple to conceive within 12 months or more of regular unprotected intercourse (3).
Infertility can be caused by various disorders including: anatomical or hormonal problems, genetic abnormalities, and infections. In addition, environmental factors such as air pollution, industrial chemicals, stress and depression, alcohol consumption, or smoking are all considered to be risk factors, which reduce sperm quality parameters in developed countries (4). Moreover, studies have shown a relationship between semen quality and lifestyle factors, including physical activity and diet (5).
Dietary fats are one of the dietary components that can contribute to infertility. Due to large amounts of unsaturated fatty acids in the membrane of sperm cells, the chance of lipid peroxidation in sperm cells and production of free radicals such as hydrogen peroxide, superoxide, and hydroxyl radicals is high (6, 7). Lipid peroxidation can drastically alter membrane integrity and permeability, alter cellular enzyme activation and cell apoptosis, and thus affect spermatozoa. As a result, sperm count and motility decrease and sperm shapes become abnormal (8).
Furthermore, over-consumption of saturated and trans-fatty acids can reduce sperm quality by increasing the rate of inflammation (9). Meanwhile, previous studies have shown that there is an important association between the intake of certain dietary minerals, zinc and iron, and semen quality (10-12). Although the association between dietary fat and the quality of sperm has been evaluated before, the results are controversial.
Therefore, this study aimed to evaluate the association between dietary fats and minerals with factors related to semen quantity and quality.
2. Materials and Methods
2.1. Study design
This cross-sectional study was performed on 260 infertile men referred to Isfahan Infertility Clinic in Isfahan, Iran, in June-August 2018. Participants were aged between 18-55 yr with a history of primary or secondary infertility in the last 5 yr. All participants voluntarily entered the study. Exclusion criteria were: a history of urinary infection, use of supplements, androgens, anticoagulants, cytotoxic drugs or immunosuppressant, and metabolic diseases such as diabetes or renal disease (13). Additionally, participants with incomplete information or caloric intakes outside the range of 800-4200 kcal/day were excluded from the study. Finally, the data for 260 cases were used in the study.
2.2. Sperm parameters
After 3 days of abstinence, semen samples were taken from participants. The samples were collected in sterile containers and placed at room temperature for 30 min to ensure liquidity. Analysis of the semen samples was done according to the 5th edition of the World Health Organization laboratory manual for human semen (14). 4 dependent semen parameters were measured: sperm concentration (SC); total sperm movement (TSM); normal sperm morphology (NSM); and seminal volume (SV). Abnormal semen parameters were defined as oligospermia: SC < 20 M/ml, TSM < 60%, sperm volume < 3 ml and NSM < 65% (13). As we were not able to analyze data with NSM < 65% in our study, the World Health Organization cut point of NSM < 4% was considered for normal morphology (13).
2.3. Assessment of dietary intakes
To assess dietary intakes of individuals, a validated detailed food frequency questionnaire was used in this study. It included 168 food items comprising the most common Iranian foods and has been previously shown to be valid and reproducible for use in Iranian adults (15).
For each food item, participants reported their average consumption during the last year in terms of frequency of use. This classification includes the following: ≥ 6 times a day, 3-5 times a day, 2-3 times a day, once a day, 5-6 times a week, 2-4 times a week, once a week, 1-3 times a month and < 1 a month. The selected group was then converted to the daily intake for each food item. Nutritionist IV for Windows software was used to determine micronutrient intakes for each participant.
2.4. Assessment of other variables
Demographic data, medical history, and intake of supplements were collected via a structured questionnaire for all participants. In this study, weight and height were measured with high accuracy and then body mass index was calculated according to the formula )kg/m2(.
2.5. Ethical considerations
The study was ethically approved by the Ethical Committee of Isfahan University of Medical Sciences, Isfahan, Iran (Code: IR.MUI.RESEARCH.REC.1397.232). All of the participants completed the informed consent form.
2.6. Statistical analysis
Statistical analyses were carried out using the Statistical Package for the Social Sciences (SPSS) for Windows software (version 20.0, SPSS Inc, Chicago, IL, USA). P-values < 0.05 were considered statistically significant. To describe quantitative variables, mean ± standard deviation (SD) and for the categorical outcome, frequency and percentage were used. Analysis of covariance (ANCOVA) was used to determine the association between semen parameters as dependent variables, and fats and minerals covariates as independent variables.
Multiple logistic regression (odds ratios [ORs] with 95% confidence intervals [CI]) was used to evaluate the relationship between dietary fat and mineral intakes with sperm quality parameters in the crude and adjusted models. In the adjusted model, potential confounding variables including age, BMI, education, total energy intake, alcohol, smoking, and vitamin-mineral use were justified by ANCOVA, and the first quartile was considered as the reference level.
3. Results
3.1. Baseline characteristics of participants
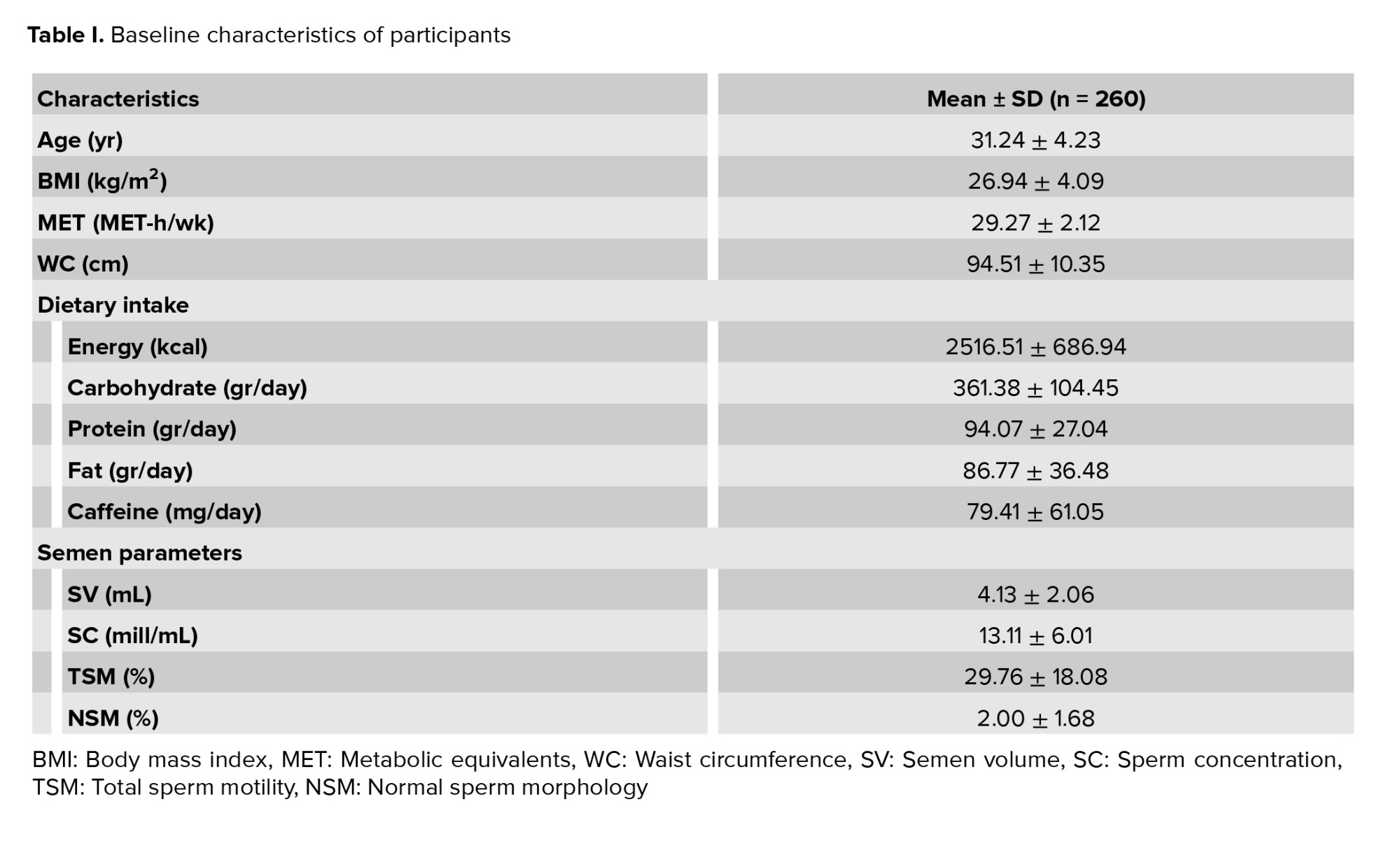
Finally, 260 participants aged between 18 and 55 yr were included in this study. 36% of the subjects were smokers, and 20% of participants used alcohol. Furthermore, 30% of participants used multivitamins. All of the participants had semen analyses performed and the semen analysis parameters included: the mean SC; SV; TSM; and NSM. Also, the dietary intake of participants is shown in table I.
3.2. Correlation between sperm related parameters and dietary components
There was a significant association between dietary intakes of protein and sperm volume (p ˂ 0.001), dietary intakes of carbohydrates and SC (p = 0.01), and also dietary intakes of carbohydrates and sperm motility (p = 0.03, Table II).
3.3. Association between dietary mineral intakes and sperm parameters
In the adjusted model, the prevalence of abnormal SC was 5.23 times higher in the top quartile of calcium intake, compared with the bottom quartile (p = 0.03). Also, the prevalence of abnormal sperm morphology was 68% lower in the third quartile of calcium intake, compared with the first quartile (p = 0.03). Additionally, the prevalence of abnormal SC was 84% lower in the top quartile of folate intake in comparison to the bottom quartile (p = 0.007) and the prevalence of abnormal sperm morphology was 70% lower in the top quartile of folate intake, compared with the first quartile (p = 0.03). Also, the prevalence of abnormal SC was 72% lower in the top quartile of selenium intake in comparison to the bottom quartile (p = 0.04, Table III).
3.4. Association between dietary fat intakes and sperm parameters
As is shown in the adjusted model, the prevalence of abnormal SC was 5.6 and 4.8 times higher in the third quartile of cholesterol and saturated fatty acid ( SFA) intake, respectively, compared with the first quartile (p = 0.01). Additionally, the prevalence of abnormal sperm volume was 64% lower in the second quartile of linoleic acid intake than in the first quartile (p = 0.01) in the crude model; however, this was not significant in the adjusted model (Table IV).




4. Discussion
In this cross-sectional study on 260 infertile men, we found that calcium intake was reversely correlated with SC and positively related to NSM. Furthermore, folate intake showed a significant relationship with SC and morphology and there was a significant relationship between SC and selenium intake. In addition, there was a reverse association between cholesterol and SFA intake with SC.
The results of recent studies have both been in favor of, and contradict, the results of the present study. The possible effect of dairy foods and calcium on male fertility is highly controversial. Unlike the present study, the results of other studies have found no association between blood calcium concentration, the time of first serving, and the time of withdrawal from pregnancy, respectively (16, 17). In addition, one study found that serum calcium concentrations were in contrast to Ca2+ concentrations, which could be affected by blood pH and protein concentrations (16). Also, in plasma, calcium is available in 3 forms: ionized, complex, and bound protein. Among these, calcium ionization (Ca2+) is the physiologically active form, so measuring plasma Ca2+ concentration, as opposed to total plasma concentration, is an indicator of physiologically present calcium (18). In another study, no significant relationship was observed between calcium and sperm parameters (19). However, high-fat dairy products and cheese have been inversely related to sperm quality parameters (20), while low fat milk and skimmed milk were associated with better classical semen indicators. Consumption of low-fat and skimmed milk can be associated with higher levels of insulin and insulin-like growth factor-1 and thus spermatogenesis, while the reason for the adverse effect of high-fat dairy on infertility can be ascribed to the high content of SFA (21).
As seen in our study, selenium intake indicated a preservative effect against SC. For example, some minerals such as selenium and zinc may develop semen quality via their anti-inflammatory and protective roles against free radicals (22). There is a direct relation between the antioxidant status and the production of reactive oxygen species (ROS) in sperm (23). In addition, high concentrations of ROS negatively affect sperm DNA and, in turn, impact sperm motility, vitality and concentration, as well as potentially contributing to miscarriages and developmental abnormalities in offspring (24). Antioxidants are considered as ‘scavengers’ of ROS and their use has been studied as a treatment to neutralize the negative effect of high concentrations of ROS on semen parameters. A recent study also indicated the importance of selenium in completing sperm maturation and quality (25). Selenium, as a selenoprotein, is more involved in male reproductive function. Adequate selenium intake is important for normal spermatogenesis and sperm maturation (26).
Additionally, folate intake displayed significant correlations with SC and morphology in our study. In men with high folate intake, lower levels of aneuploid sperm have been reported, suggesting that this vitamin (found mainly in green leafy vegetables) may play a role in spermatogenesis due to its role in DNA retention, RNA transport, and protein synthesis (27). In addition, the beneficial effects of dietary antioxidants such as vitamin C, vitamin E, and folate on sperm motility have already been shown. The results of a randomized controlled trial showed that after treatment with sulfate and folic acid, the number of normal sperm increased in infertile and fertile men (28). In a clinical trial, analysis of DNA methylation in sperm with exposure to folic acid suggested the susceptibility of mice and humans in sequences of potential importance to germ cells and embryonic growth. This study also suggested hypomethylation of sperm DNA and involvement in the regulation of Methyl tetrahidrofolate reductase as a possible mechanism for folic acid supplementation for different species (29).
Furthermore, supporting the findings of our study, a previous study found an inverse relationship between dietary fat, especially cholesterol and SFA intake and SC. Trans-fatty acids, SFAs, and preservatives or hormonal residues such as xenobiotic or anabolic steroids may alter sperm quality (30). High SFA concentrations and low omega-3, poly-unsaturated fatty acids (PUFA) levels are associated with decreased fertility parameters (19). In an animal study, some dietary SFAs did not affect sperm quality parameters. However, one human study showed that higher levels of palmitic acid or stearic acid were present in the sperm of infertile men (31). Also, in our study, linoleic acid intake showed some preservative effects on sperm volume in the second quartile of intake. Several mechanisms have been proposed for the beneficial effects of PUFA and MUFA on sperm quality. Adipose tissue plays an important role in causing oxidative stress after an inflammatory disorder that may alter normal reproductive pathways and sperm activity (32, 33). In fact, under stress conditions, mitochondria are likely to cause a cascade of oxidative damage in the testicular environment. MUFA and PUFA fatty acids have protective effects against increased ROS levels due to their anti-inflammatory and antioxidant properties (34, 35).
4.1. Limitations
The present study, like other cross-sectional studies, is not able to definitively determine the cause-and-effect relationship between the variables and it is suggested that clinical trial or case-control studies be performed to confirm the results. Another limitation is that plasma or semen levels of minerals and fatty acids were not considered in this study. Finally, the 168-item food frequency questionnaire has some limitations in recording food intakes and relies on the individual's memory, and is not always as accurate when employed by the elderly or illiterate people; therefore, the use of 3-day food recall could be useful in future studies.
5. Conclusion
The results of the present study showed that high calcium intake was inversely related to SC and directly related to normal morphology. Also, folate and selenium intake showed protective effects on SC. On the other hand, the high levels of cholesterol and SFA intake showed an inverse relationship with SC.
Acknowledgments
We appreciate Isfahan infertility clinic, Isfahan, Iran. This study received no financial support.
Conflict of Interest
The authors declare that there is no conflict of interest.
Full-Text: (229 Views)
1. Introduction
Over recent decades, infertility has been a significant issue in developing countries, affecting 15% of all couples falling within fertile age brackets (1). A meta-analysis estimated the infertility rate within the Iranian population to be about 10.9%. In approximately 40% of infertile couples, male infertility is a factor (2). Infertility is defined as the inability of a couple to conceive within 12 months or more of regular unprotected intercourse (3).
Infertility can be caused by various disorders including: anatomical or hormonal problems, genetic abnormalities, and infections. In addition, environmental factors such as air pollution, industrial chemicals, stress and depression, alcohol consumption, or smoking are all considered to be risk factors, which reduce sperm quality parameters in developed countries (4). Moreover, studies have shown a relationship between semen quality and lifestyle factors, including physical activity and diet (5).
Dietary fats are one of the dietary components that can contribute to infertility. Due to large amounts of unsaturated fatty acids in the membrane of sperm cells, the chance of lipid peroxidation in sperm cells and production of free radicals such as hydrogen peroxide, superoxide, and hydroxyl radicals is high (6, 7). Lipid peroxidation can drastically alter membrane integrity and permeability, alter cellular enzyme activation and cell apoptosis, and thus affect spermatozoa. As a result, sperm count and motility decrease and sperm shapes become abnormal (8).
Furthermore, over-consumption of saturated and trans-fatty acids can reduce sperm quality by increasing the rate of inflammation (9). Meanwhile, previous studies have shown that there is an important association between the intake of certain dietary minerals, zinc and iron, and semen quality (10-12). Although the association between dietary fat and the quality of sperm has been evaluated before, the results are controversial.
Therefore, this study aimed to evaluate the association between dietary fats and minerals with factors related to semen quantity and quality.
2. Materials and Methods
2.1. Study design
This cross-sectional study was performed on 260 infertile men referred to Isfahan Infertility Clinic in Isfahan, Iran, in June-August 2018. Participants were aged between 18-55 yr with a history of primary or secondary infertility in the last 5 yr. All participants voluntarily entered the study. Exclusion criteria were: a history of urinary infection, use of supplements, androgens, anticoagulants, cytotoxic drugs or immunosuppressant, and metabolic diseases such as diabetes or renal disease (13). Additionally, participants with incomplete information or caloric intakes outside the range of 800-4200 kcal/day were excluded from the study. Finally, the data for 260 cases were used in the study.
2.2. Sperm parameters
After 3 days of abstinence, semen samples were taken from participants. The samples were collected in sterile containers and placed at room temperature for 30 min to ensure liquidity. Analysis of the semen samples was done according to the 5th edition of the World Health Organization laboratory manual for human semen (14). 4 dependent semen parameters were measured: sperm concentration (SC); total sperm movement (TSM); normal sperm morphology (NSM); and seminal volume (SV). Abnormal semen parameters were defined as oligospermia: SC < 20 M/ml, TSM < 60%, sperm volume < 3 ml and NSM < 65% (13). As we were not able to analyze data with NSM < 65% in our study, the World Health Organization cut point of NSM < 4% was considered for normal morphology (13).
2.3. Assessment of dietary intakes
To assess dietary intakes of individuals, a validated detailed food frequency questionnaire was used in this study. It included 168 food items comprising the most common Iranian foods and has been previously shown to be valid and reproducible for use in Iranian adults (15).
For each food item, participants reported their average consumption during the last year in terms of frequency of use. This classification includes the following: ≥ 6 times a day, 3-5 times a day, 2-3 times a day, once a day, 5-6 times a week, 2-4 times a week, once a week, 1-3 times a month and < 1 a month. The selected group was then converted to the daily intake for each food item. Nutritionist IV for Windows software was used to determine micronutrient intakes for each participant.
2.4. Assessment of other variables
Demographic data, medical history, and intake of supplements were collected via a structured questionnaire for all participants. In this study, weight and height were measured with high accuracy and then body mass index was calculated according to the formula )kg/m2(.
2.5. Ethical considerations
The study was ethically approved by the Ethical Committee of Isfahan University of Medical Sciences, Isfahan, Iran (Code: IR.MUI.RESEARCH.REC.1397.232). All of the participants completed the informed consent form.
2.6. Statistical analysis
Statistical analyses were carried out using the Statistical Package for the Social Sciences (SPSS) for Windows software (version 20.0, SPSS Inc, Chicago, IL, USA). P-values < 0.05 were considered statistically significant. To describe quantitative variables, mean ± standard deviation (SD) and for the categorical outcome, frequency and percentage were used. Analysis of covariance (ANCOVA) was used to determine the association between semen parameters as dependent variables, and fats and minerals covariates as independent variables.
Multiple logistic regression (odds ratios [ORs] with 95% confidence intervals [CI]) was used to evaluate the relationship between dietary fat and mineral intakes with sperm quality parameters in the crude and adjusted models. In the adjusted model, potential confounding variables including age, BMI, education, total energy intake, alcohol, smoking, and vitamin-mineral use were justified by ANCOVA, and the first quartile was considered as the reference level.
3. Results
3.1. Baseline characteristics of participants
Finally, 260 participants aged between 18 and 55 yr were included in this study. 36% of the subjects were smokers, and 20% of participants used alcohol. Furthermore, 30% of participants used multivitamins. All of the participants had semen analyses performed and the semen analysis parameters included: the mean SC; SV; TSM; and NSM. Also, the dietary intake of participants is shown in table I.
3.2. Correlation between sperm related parameters and dietary components
There was a significant association between dietary intakes of protein and sperm volume (p ˂ 0.001), dietary intakes of carbohydrates and SC (p = 0.01), and also dietary intakes of carbohydrates and sperm motility (p = 0.03, Table II).
3.3. Association between dietary mineral intakes and sperm parameters
In the adjusted model, the prevalence of abnormal SC was 5.23 times higher in the top quartile of calcium intake, compared with the bottom quartile (p = 0.03). Also, the prevalence of abnormal sperm morphology was 68% lower in the third quartile of calcium intake, compared with the first quartile (p = 0.03). Additionally, the prevalence of abnormal SC was 84% lower in the top quartile of folate intake in comparison to the bottom quartile (p = 0.007) and the prevalence of abnormal sperm morphology was 70% lower in the top quartile of folate intake, compared with the first quartile (p = 0.03). Also, the prevalence of abnormal SC was 72% lower in the top quartile of selenium intake in comparison to the bottom quartile (p = 0.04, Table III).
3.4. Association between dietary fat intakes and sperm parameters
As is shown in the adjusted model, the prevalence of abnormal SC was 5.6 and 4.8 times higher in the third quartile of cholesterol and saturated fatty acid (





4. Discussion
In this cross-sectional study on 260 infertile men, we found that calcium intake was reversely correlated with SC and positively related to NSM. Furthermore, folate intake showed a significant relationship with SC and morphology and there was a significant relationship between SC and selenium intake. In addition, there was a reverse association between cholesterol and SFA intake with SC.
The results of recent studies have both been in favor of, and contradict, the results of the present study. The possible effect of dairy foods and calcium on male fertility is highly controversial. Unlike the present study, the results of other studies have found no association between blood calcium concentration, the time of first serving, and the time of withdrawal from pregnancy, respectively (16, 17). In addition, one study found that serum calcium concentrations were in contrast to Ca2+ concentrations, which could be affected by blood pH and protein concentrations (16). Also, in plasma, calcium is available in 3 forms: ionized, complex, and bound protein. Among these, calcium ionization (Ca2+) is the physiologically active form, so measuring plasma Ca2+ concentration, as opposed to total plasma concentration, is an indicator of physiologically present calcium (18). In another study, no significant relationship was observed between calcium and sperm parameters (19). However, high-fat dairy products and cheese have been inversely related to sperm quality parameters (20), while low fat milk and skimmed milk were associated with better classical semen indicators. Consumption of low-fat and skimmed milk can be associated with higher levels of insulin and insulin-like growth factor-1 and thus spermatogenesis, while the reason for the adverse effect of high-fat dairy on infertility can be ascribed to the high content of SFA (21).
As seen in our study, selenium intake indicated a preservative effect against SC. For example, some minerals such as selenium and zinc may develop semen quality via their anti-inflammatory and protective roles against free radicals (22). There is a direct relation between the antioxidant status and the production of reactive oxygen species (ROS) in sperm (23). In addition, high concentrations of ROS negatively affect sperm DNA and, in turn, impact sperm motility, vitality and concentration, as well as potentially contributing to miscarriages and developmental abnormalities in offspring (24). Antioxidants are considered as ‘scavengers’ of ROS and their use has been studied as a treatment to neutralize the negative effect of high concentrations of ROS on semen parameters. A recent study also indicated the importance of selenium in completing sperm maturation and quality (25). Selenium, as a selenoprotein, is more involved in male reproductive function. Adequate selenium intake is important for normal spermatogenesis and sperm maturation (26).
Additionally, folate intake displayed significant correlations with SC and morphology in our study. In men with high folate intake, lower levels of aneuploid sperm have been reported, suggesting that this vitamin (found mainly in green leafy vegetables) may play a role in spermatogenesis due to its role in DNA retention, RNA transport, and protein synthesis (27). In addition, the beneficial effects of dietary antioxidants such as vitamin C, vitamin E, and folate on sperm motility have already been shown. The results of a randomized controlled trial showed that after treatment with sulfate and folic acid, the number of normal sperm increased in infertile and fertile men (28). In a clinical trial, analysis of DNA methylation in sperm with exposure to folic acid suggested the susceptibility of mice and humans in sequences of potential importance to germ cells and embryonic growth. This study also suggested hypomethylation of sperm DNA and involvement in the regulation of Methyl tetrahidrofolate reductase as a possible mechanism for folic acid supplementation for different species (29).
Furthermore, supporting the findings of our study, a previous study found an inverse relationship between dietary fat, especially cholesterol and SFA intake and SC. Trans-fatty acids, SFAs, and preservatives or hormonal residues such as xenobiotic or anabolic steroids may alter sperm quality (30). High SFA concentrations and low omega-3, poly-unsaturated fatty acids (PUFA) levels are associated with decreased fertility parameters (19). In an animal study, some dietary SFAs did not affect sperm quality parameters. However, one human study showed that higher levels of palmitic acid or stearic acid were present in the sperm of infertile men (31). Also, in our study, linoleic acid intake showed some preservative effects on sperm volume in the second quartile of intake. Several mechanisms have been proposed for the beneficial effects of PUFA and MUFA on sperm quality. Adipose tissue plays an important role in causing oxidative stress after an inflammatory disorder that may alter normal reproductive pathways and sperm activity (32, 33). In fact, under stress conditions, mitochondria are likely to cause a cascade of oxidative damage in the testicular environment. MUFA and PUFA fatty acids have protective effects against increased ROS levels due to their anti-inflammatory and antioxidant properties (34, 35).
4.1. Limitations
The present study, like other cross-sectional studies, is not able to definitively determine the cause-and-effect relationship between the variables and it is suggested that clinical trial or case-control studies be performed to confirm the results. Another limitation is that plasma or semen levels of minerals and fatty acids were not considered in this study. Finally, the 168-item food frequency questionnaire has some limitations in recording food intakes and relies on the individual's memory, and is not always as accurate when employed by the elderly or illiterate people; therefore, the use of 3-day food recall could be useful in future studies.
5. Conclusion
The results of the present study showed that high calcium intake was inversely related to SC and directly related to normal morphology. Also, folate and selenium intake showed protective effects on SC. On the other hand, the high levels of cholesterol and SFA intake showed an inverse relationship with SC.
Acknowledgments
We appreciate Isfahan infertility clinic, Isfahan, Iran. This study received no financial support.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Fertility & Infertility
References
1. Benedetti S, Tagliamonte MC, Catalani S, Primiterra M, Canestrari F, De Stefani S, et al. Differences in blood and semen oxidative status in fertile and infertile men, and their relationship with sperm quality. Reprod Biomed Online 2012; 25: 300-306. [DOI:10.1016/j.rbmo.2012.05.011] [PMID]
2. Aflakseir A, Mahdiyar M. The role of religious coping strategies in predicting depression among a sample of women with fertility problems in Shiraz. J Reprod Infertil 2016; 17: 117-122.
3. Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. The international committee for monitoring assisted reproductive technology (ICMART) and the world health organization (WHO) revised glossary on ART terminology, 2009. Hum Reprod 2009; 24: 2683-2687. [DOI:10.1093/humrep/dep343] [PMID]
4. Karayiannis D, Kontogianni MD, Mendorou C, Douka L, Mastrominas M, Yiannakouris N. Association between adherence to the Mediterranean diet and semen quality parameters in male partners of couples attempting fertility. Hum Reprod 2017; 32: 215-222. [DOI:10.1093/humrep/dew288] [PMID]
5. Gabrielsen JS, Tanrikut C. Chronic exposures and male fertility: The impacts of environment, diet, and drug use on spermatogenesis. Andrology 2016; 4: 648-661. [DOI:10.1111/andr.12198] [PMID]
6. Lancellotti TES, Boarelli PV, Monclus MA, Cabrillana ME, Clementi MA, Espínola LS, et al. Hypercholesterolemia impaired sperm functionality in rabbits. PLoS One 2010; 5: e13457. [DOI:10.1371/journal.pone.0013457] [PMID] [PMCID]
7. Dadkhah H, Kazemi A, Nasr-Isfahani MH, Ehsanpour S. The relationship between the amount of saturated fat intake and semen quality in men. Iran J Nurs Midwif Res 2017; 22: 46-50. [DOI:10.4103/1735-9066.202067] [PMID] [PMCID]
8. Eslamian Gh, Amirjannati N, Rashidkhani B, Sadeghi MR, Baghestani AR, Hekmatdoost A. Dietary fatty acid intakes and asthenozoospermia: A case-control study. Fertil Steril 2015; 103: 190-198. [DOI:10.1016/j.fertnstert.2014.10.010] [PMID]
9. Attaman JA, Toth TL, Furtado J, Campos H, Hauser R, Chavarro JE. Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod 2012; 27: 1466-1474. [DOI:10.1093/humrep/des065] [PMID] [PMCID]
10. Moslemi MK, Tavanbakhsh S. Selenium-vitamin E supplementation in infertile men: Effects on semen parameters and pregnancy rate. Int J Gen Med 2011; 4: 99-104. [DOI:10.2147/IJGM.S16275] [PMID] [PMCID]
11. Ammar O, Houas Z, Mehdi M. The association between iron, calcium, and oxidative stress in seminal plasma and sperm quality. Environ Sci Pollut Res Int 2019; 26: 14097-14105. [DOI:10.1007/s11356-019-04575-7] [PMID]
12. Fallah A, Mohammad-Hasani A, Colagar AH. Zinc is an essential element for male fertility: A review of Zn roles in men's health, germination, sperm quality, and fertilization. J Reprod Infertil 2018; 19: 69-81.
13. Biswas TK, Pandit S, Mondal S, Biswas SK, Jana U, Ghosh T, et al. Clinical evaluation of spermatogenic activity of processed Shilajit in oligospermia. Andrologia 2010; 42: 48-56. [DOI:10.1111/j.1439-0272.2009.00956.x] [PMID]
14. Morselli MG, Colombo M, Faustini M, Luvoni GC. Morphological indices for canine spermatozoa based on the World Health Organization laboratory manual for human semen. Reprod Domest Anim 2019; 54: 949-955. [DOI:10.1111/rda.13440] [PMID]
15. Mirmiran P, Hosseini Esfahani F, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr 2010; 13: 654-662. [DOI:10.1017/S1368980009991698] [PMID]
16. Wilhelm AL, Maquivar MG, Bas S, Brick TA, Weiss WP, Bothe H, et al. Effect of serum calcium status at calving on survival, health, and performance of postpartum Holstein cows and calves under certified organic management. J Dairy Sci 2017; 100: 3059-3067. [DOI:10.3168/jds.2016-11743] [PMID]
17. Chamberlin WG, Middleton JR, Spain JN, Johnson GC, Ellersieck MR, Pithua P. Subclinical hypocalcemia, plasma biochemical parameters, lipid metabolism, postpartum disease, and fertility in postparturient dairy cows. J Dairy Sci 2013; 96: 7001-7013. [DOI:10.3168/jds.2013-6901] [PMID]
18. Rosol TJ, Capen C. Calcium-regulating hormones and diseases of abnormal mineral (calcium, phosphorus, magnesium) metabolism. In: Kaneko JJ, Harvey JW, Bruss ML. Clinical biochemistry of domestic animals. 5th Ed. San Diego: Academic Press; 1997. [DOI:10.1016/B978-012396305-5/50024-5]
19. Eslamian G, Amirjannati N, Rashidkhani B, Sadeghi MR, Hekmatdoost A. Nutrient patterns and asthenozoospermia: A case-control study. Andrologia 2017; 49: e12624. [DOI:10.1111/and.12624] [PMID]
20. Afeiche M, Williams PL, Mendiola J, Gaskins AJ, Jørgensen N, Swan SH, et al. Dairy food intake in relation to semen quality and reproductive hormone levels among physically active young men. Hum Reprod 2013; 28: 2265-2275. [DOI:10.1093/humrep/det133] [PMID] [PMCID]
21. Afeiche MC, Bridges ND, Williams PL, Gaskins AJ, Tanrikut C, Petrozza JC, et al. Dairy intake and semen quality among men attending a fertility clinic. Fertil Steril 2014; 101: 1280-1287. [DOI:10.1016/j.fertnstert.2014.02.003] [PMID] [PMCID]
22. Ross C, Morriss A, Khairy M, Khalaf Y, Braude P, Coomarasamy A, et al. A systematic review of the effect of oral antioxidants on male infertility. Reprod Biomed Online 2010; 20: 711-723. [DOI:10.1016/j.rbmo.2010.03.008] [PMID]
23. Showell MG, Mackenzie‐Proctor R, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev 2014; 12: CD007411. [DOI:10.1002/14651858.CD007411.pub3] [PMID]
24. Aitken RJ, Gibb Z, Baker MA, Drevet J, Gharagozloo P. Causes and consequences of oxidative stress in spermatozoa. Reprod Fertil Dev 2016; 28: 1-10. [DOI:10.1071/RD15325] [PMID]
25. Gharagozloo P, Aitken RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod 2011; 26: 1628-1640. [DOI:10.1093/humrep/der132] [PMID]
26. Ahsan U, Kamran Z, Raza I, Ahmad S, Babar W, Riaz M, et al. Role of selenium in male reproduction: A review. Anim Reprod Sci 2014; 146: 55-62. [DOI:10.1016/j.anireprosci.2014.01.009] [PMID]
27. Molloy AM. Genetic aspects of folate metabolism. Subcell Biochecm 2012; 56: 105-130. [DOI:10.1007/978-94-007-2199-9_7] [PMID]
28. Wong WY, Merkus HMWM, Thomas ChMG, Menkveld R, Zielhuis GA, Steegers-Theunissen RPM. Effects of folic acid and zinc sulfate on male factor subfertility: A double-blind, randomized, placebo-controlled trial. Fertil Steril 2002; 77: 491-498. [DOI:10.1016/S0015-0282(01)03229-0]
29. Aarabi M, Christensen KE, Chan D, Leclerc D, Landry M, Ly L, et al. Testicular MTHFR deficiency may explain sperm DNA hypomethylation associated with high dose folic acid supplementation. Hum Mol Genet 2018; 27: 1123-1135. [DOI:10.1093/hmg/ddy021] [PMID] [PMCID]
30. Chavarro JE, Mínguez-Alarcón L, Mendiola J, Cutillas-Tolín A, López-Espín JJ, Torres-Cantero AM. Trans fatty acid intake is inversely related to total sperm count in young healthy men. Hum Reprod 2014; 29: 429-440.
https://doi.org/10.1093/humrep/deu059 [DOI:10.1093/humrep/det464] [PMID]
31. Fair S, Doyle DN, Diskin MG, Hennessy AA, Kenny DA. The effect of dietary n-3 polyunsaturated fatty acids supplementation of rams on semen quality and subsequent quality of liquid stored semen. Theriogenology 2014; 81: 210-219. [DOI:10.1016/j.theriogenology.2013.09.002] [PMID]
32. Bachir BG, Jarvi K. Infectious, inflammatory, and immunologic conditions resulting in male infertility. Urol Clin North Am 2014; 41: 67-81. [DOI:10.1016/j.ucl.2013.08.008] [PMID]
33. Morielli T, O'Flaherty C. Oxidative stress impairs function and increases redox protein modifications in human spermatozoa. Reproduction 2015; 149: 113-123. [DOI:10.1530/REP-14-0240] [PMID] [PMCID]
34. Ferramosca A, Moscatelli N, Di Giacomo M, Zara V. Dietary fatty acids influence sperm quality and function. Andrology 2017; 5: 423-430. [DOI:10.1111/andr.12348] [PMID]
35. Gürler H, Calisici O, Calisici D, Bollwein H. Effects of feeding omega-3-fatty acids on fatty acid composition and quality of bovine sperm and on antioxidative capacity of bovine seminal plasma. Anim Reprod Sci 2015; 160: 97-104. [DOI:10.1016/j.anireprosci.2015.07.010] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





