Thu, Apr 25, 2024
[Archive]
Volume 20, Issue 11 (November 2022)
IJRM 2022, 20(11): 893-914 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Amiri M, Bidhendi-Yarandi R, Fallahzadeh A, Marzban Z, Ramezani Tehrani F. Risk of endometrial, ovarian, and breast cancers in women with polycystic ovary syndrome: A systematic review and meta-analysis. IJRM 2022; 20 (11) :893-914
URL: http://ijrm.ir/article-1-2220-en.html
URL: http://ijrm.ir/article-1-2220-en.html
Mina Amiri1 

 , Razieh Bidhendi-Yarandi2
, Razieh Bidhendi-Yarandi2 

 , Aida Fallahzadeh3
, Aida Fallahzadeh3 

 , Zahra Marzban1
, Zahra Marzban1 

 , Fahimeh Ramezani Tehrani *
, Fahimeh Ramezani Tehrani * 

 4
4


 , Razieh Bidhendi-Yarandi2
, Razieh Bidhendi-Yarandi2 

 , Aida Fallahzadeh3
, Aida Fallahzadeh3 

 , Zahra Marzban1
, Zahra Marzban1 

 , Fahimeh Ramezani Tehrani *
, Fahimeh Ramezani Tehrani * 

 4
4
1- Reproductive Endocrinology Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Department of Biostatistics, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
3- Reproductive Endocrinology Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran. School of Medicine, Tehran University of Medical Science, Tehran, Iran.
4- Reproductive Endocrinology Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran. , fah.tehrani@gmail.com
2- Department of Biostatistics, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
3- Reproductive Endocrinology Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran. School of Medicine, Tehran University of Medical Science, Tehran, Iran.
4- Reproductive Endocrinology Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran. , fah.tehrani@gmail.com
Full-Text [PDF 1715 kb]
(576 Downloads)
| Abstract (HTML) (918 Views)
1. Introduction
Polycystic ovary syndrome (PCOS), one of the common abnormalities in reproductive age women (1), is defined by ovulation abnormalities, high levels of androgens, and polycystic ovaries in ultrasonography (2). PCOS is also associated with several conditions, such as glucose intolerance, diabetes, hypertension, central obesity, metabolic syndrome, and cardiovascular diseases (3-7).
The high prevalence of endometrial hyperplasia and carcinoma due to chronic anovulation, associated with prolonged exposure to unopposed estrogen has been long recognized. Moreover, PCOS complications, such as obesity, nulliparity, diabetes, and hypertension, are risk factors for endometrial carcinoma (8-10). In this regard, a population-based cohort study reported a 17-fold increase in the risk of endometrial cancer among women with PCOS, compared to those without PCOS (11). Several hypotheses have been proposed for the increased risk of ovarian cancer in PCOS women, such as anovulation, increased androgen exposure, and lack of progesterone (12). Another study found that the risk of ovarian cancer was 2.5 times higher in women with PCOS compared to healthy women (13). It is possible that the sustained elevation of serum estrogen levels may lead to the growth of hormone-sensitive tumors, such as breast cancer (14); therefore, anovulation can be considered as a critical risk factor in women with PCOS. Several studies have investigated the risk of breast cancer in women with PCOS; however, the actual risk remains unclear (11, 15-17). Although some studies have assessed the risk of endometrial, ovarian, and breast cancers in women with PCOS, their results are often conflicting, and the risk of these cancers in women with PCOS is still debated (11, 13, 15, 16, 18).
Hence, this study aims to conduct a meta-analysis of observational studies to investigate the association of PCOS with endometrial, ovarian, and breast cancers. Since the severity of PCOS manifestations can distort the results, we adjusted the results for PCOS diagnostic criteria in a meta-regression analysis.
2. Materials and Methods
This meta-analysis was designed according to the guidelines for the preferred reporting items for systematic reviews and meta-analyses (PRISMA) to assess the pooled odds ratio of endometrial cancer in women with PCOS, compared to healthy controls (19).
2.1. Search strategy
Data was searched using PubMed, Scopus, Web of Science, and Google Scholar for retrieving studies published up to April 2020 investigating gynecological cancers in women with PCOS.
2 reviewers (M.A. and A.F.) performed searches separately. Search on PubMed was performed initially, based on MESH terms using the following keywords: (‘polycystic ovary syndrome’ OR ‘PCOS’ OR ‘Stein-Leventhal Syndrome’) AND (‘breast cancer’ OR ‘breast tumor’ OR ‘breast neoplasm’ OR ‘breast carcinoma’ OR ‘Human Mammary Carcinoma’ OR ‘ovarian cancer’ OR ‘ovarian neoplasm’ OR ‘carcinoma’ OR ‘ovarian epithelial’ OR ‘ovary cancer’ OR ‘cancer of ovary’ OR ‘ovarian tumor’ OR ‘endometrial neoplasm’ OR ‘endometrial carcinoma’ OR ‘endometrial cancer’ OR ‘cancer of the endometrium’ OR ‘cancer, endometrium’ OR ‘carcinoma, endometrial’ OR ‘endometrial tumor’).
Search limitations were humans and English language publications. There was no time limitation. The same search strategy was applied for all databases, based on the titles, abstracts, and keywords. We applied a ‘pearl growing’ strategy. First, we obtained the full text of the studies, then the reference list of the studies was reviewed to prevent missing related articles.
2.2. Eligibility criteria
All types of observational studies, including case-control, cross-sectional, and cohort designs were eligible to be included in the meta-analysis. Studies needed to report raw data of events, odds ratio (OR), and relative risk to provide sufficient information to allow calculation. Any PCOS diagnosis criteria were eligible to be included, for example, Rotterdam, National Institute of Health, Androgen Excess Society, International Classification of Diseases, and also a self-reported questionnaire.
Exclusion criteria were: 1) studies assessing conditions, for example, polycystic ovary, androgen excess disease instead of PCOS, 2) without control groups, 3) with unreliable and incomplete results, and 4) assessing hyperplasia rather than cancer.
2.3. Study selection
All relevant studies assessing at least one of the cancers of endometrial, ovarian, and breast in women with PCOS were included in this meta-analysis.
Search results were screened based on the predefined eligibility criteria. All references were entered into EndNote software. The initial selection was based on article titles, and then a second selection based on abstracts was done and duplicates were deleted by one reviewer (A.F). Finally, the full text of selected articles was reviewed for data extraction. Disagreements were resolved by 2 other reviewers (M. A. and F.R.T.).
2.4. Data extraction
2 reviewers (M.A. and A.F.) extracted data from the full text of selected articles and checked twice to lessen errors. For each study, information such as author’s name, publications year, article title, design of the study, study population, number of outcomes, and unadjusted or adjusted OR, relative risk, or HR of the outcomes were extracted.
2.5. Quality assessment
M.A. and A.F. assessed the quality of the included studies and F.R.T. resolved any disagreements. Newcastle-Ottawa scale was applied for the quality assessment (20).
The high quality was defined when a study got ≥ 70% of the highest level of the Newcastle-Ottawa scale, moderate quality was defined when a study got 40-70%, and low quality was defined when a study got 20-40% and those with < 20% were defined as very low quality.
2.6. Bias assessment
Cochrane collaboration’s tools were applied to assess the risk of bias in each study (21).
2.7. Outcome measures
In this meta-analysis, outcomes of interest were endometrial cancer, ovarian cancer, and breast cancer.
2.8. Statistical analysis
This meta-analysis was conducted to obtain a pooled OR of endometrial, ovarian, and breast cancer in PCOS women. Heterogeneity was assessed via I-squared statistics and in the case of values with upper limits 50% random effect method was applied, otherwise the fixed-effect method.
To assess publication bias, Begg’s test was used (22); it was found significant for p < 0.05, and in this case trim and fill method was conducted to correct for publication bias by adding some study measures (23, 24). A funnel plot was also drawn to depict publication bias issues. A Forest plot was also drawn to summarize the result of each study’s effect sizes and its 95% confidence intervals.
In case of publication bias, forest plot corrected via trim and fill method was drawn. To estimate pooled ORs, we applied the ‘Meta-prop’ random effect method (25). The 95% prediction interval (95% PI) was estimated to evaluate clinical significance as compared to statistical significance for the pooled OR. Moreover, the random effect meta-regression model was fitted to assess the effects of PCOS diagnosis criteria and age on the results. We also run a sensitivity analysis to detect probable influential studies with a high risk of bias. Statistical analysis was conducted using STATA software (version 13; STATA, INC., college station, TX, USA).
3. Results
3.1. Search results, study selection, study characteristics, and quality assessment
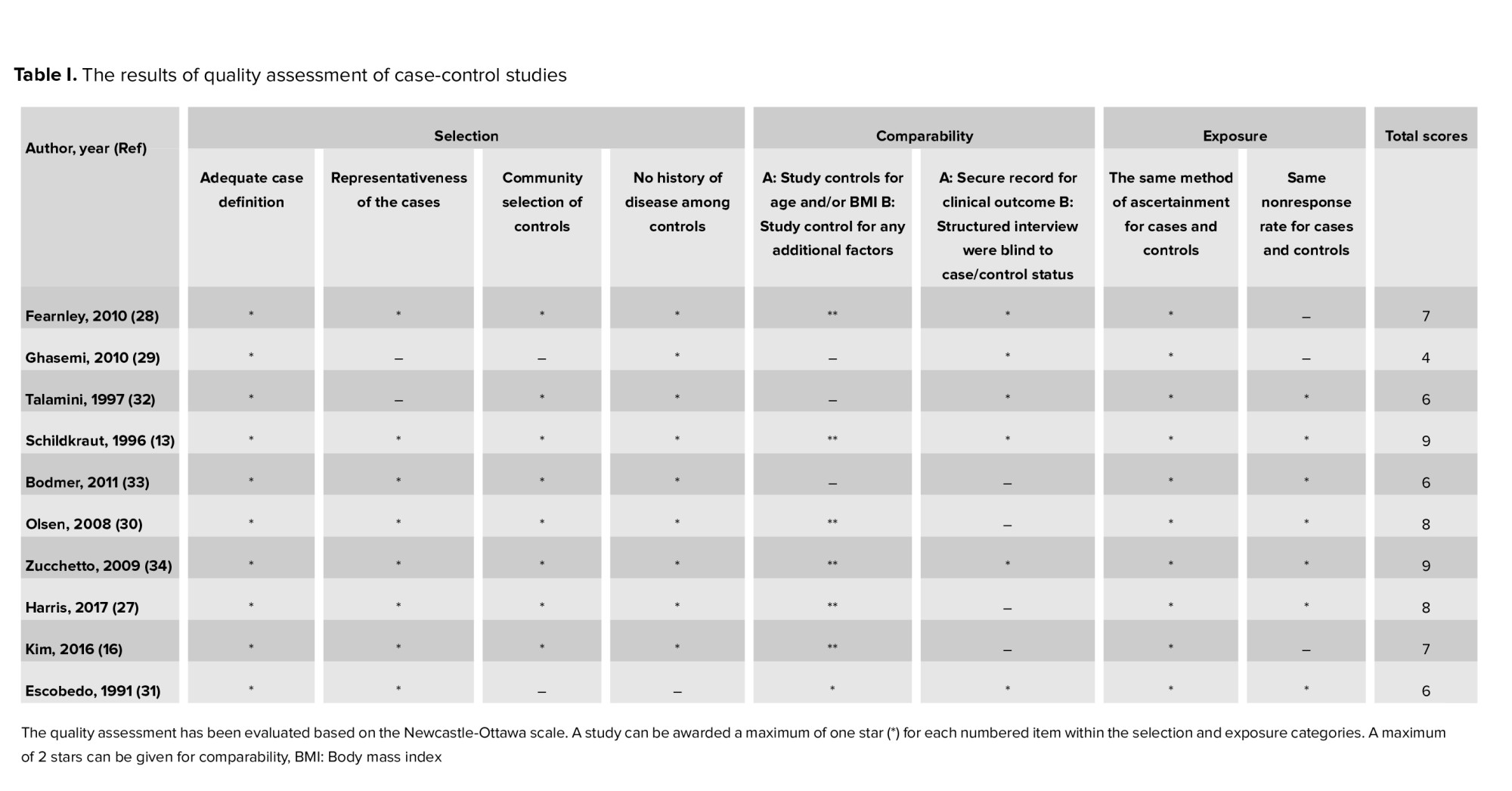
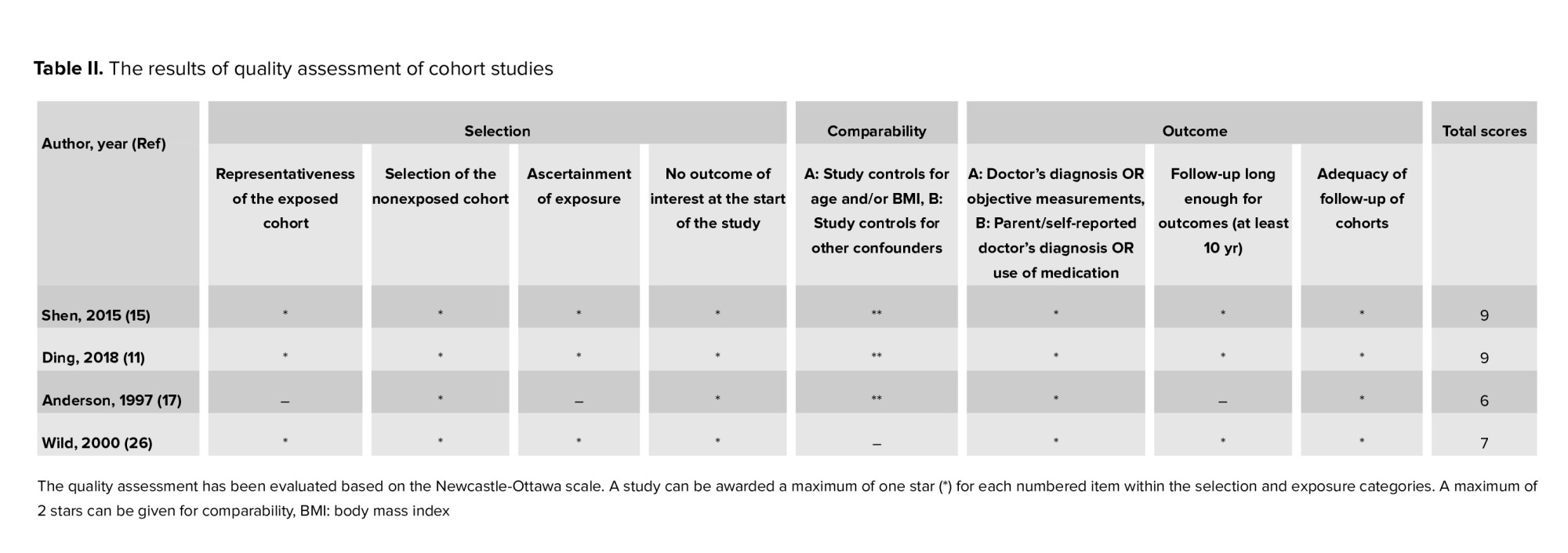
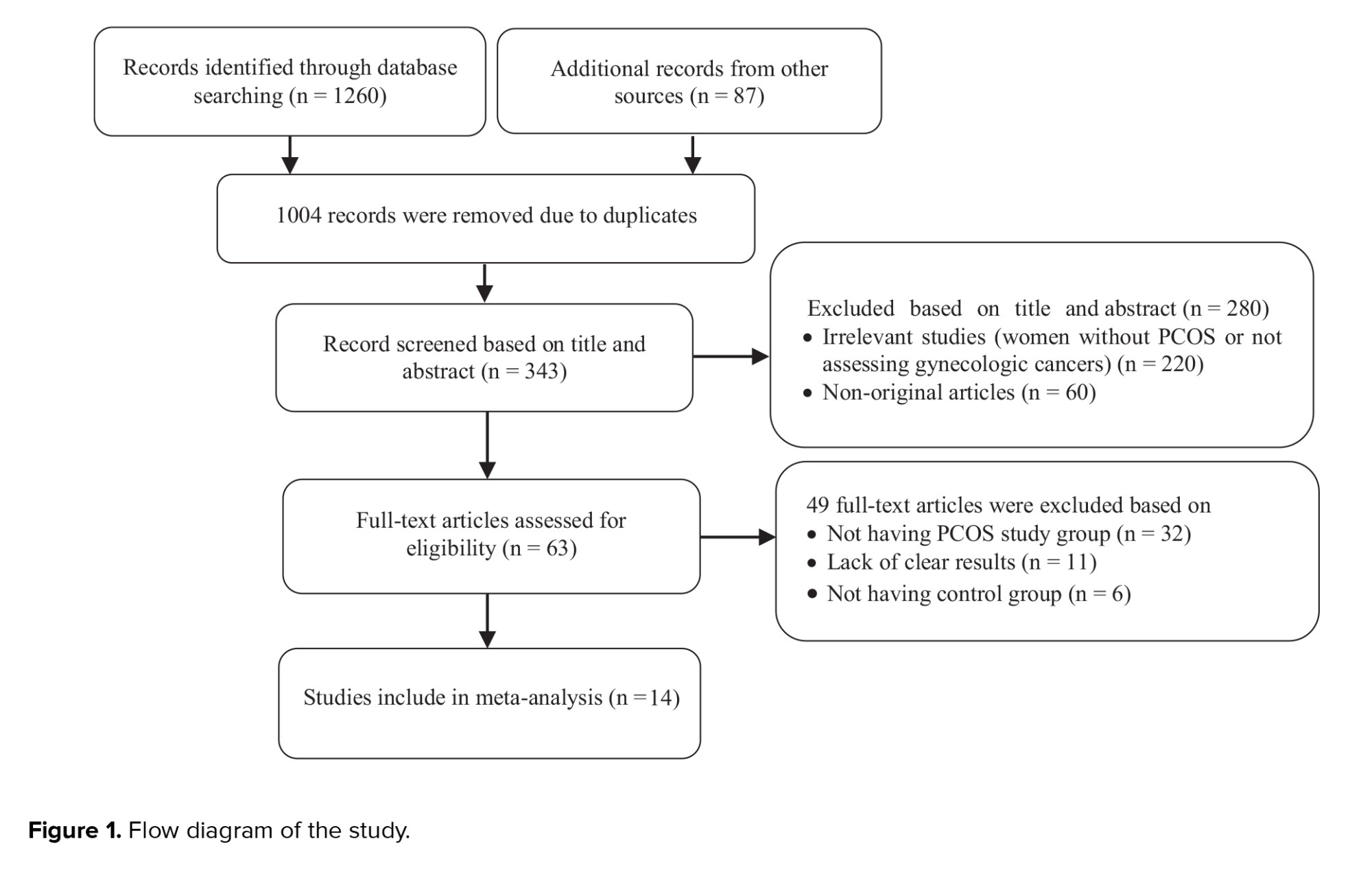
Figure 1 presents the flow diagram of the search strategy and study selection. Of 1347 records retrieved by searching the databases, a total of 14 studies, including 4 cohorts (11, 15, 17, 26) and 10 case-control (13, 16, 17, 27-34) studies, were selected for the final analysis. 9 studies (11, 13, 15-17, 26-28, 30, 34) were classified as high-quality, and 5 (17, 29, 31-33) as moderate-quality (Table I-II).
2 studies (11, 15) based on the international classification of diseases, 2 (16, 29) used the Rotterdam criteria, 1 (26) used the laparoscopic criteria, and 9 (13, 17, 27, 28, 30-34) reported no criteria. In addition, 7 (11, 15-17, 26, 29, 32) reported breast cancer, 6 (11, 13, 15, 27, 30, 33) reported ovarian cancer, and 6 (11, 15, 26, 28, 31, 34) reported endometrial cancer.
3.2. Meta-analysis and meta-regression of outcomes
In this meta-analysis, 12,955 women with PCOS and 118,481 controls were included. The pooled mean (95% CI) of age and body mass index of all study populations were 27.8 yr (95% CI: 27.8-27.9) and 26.5 kg/m2 (95% CI: 26.3-26.7), respectively. Table III presents the results of the meta-analysis and meta-regression.
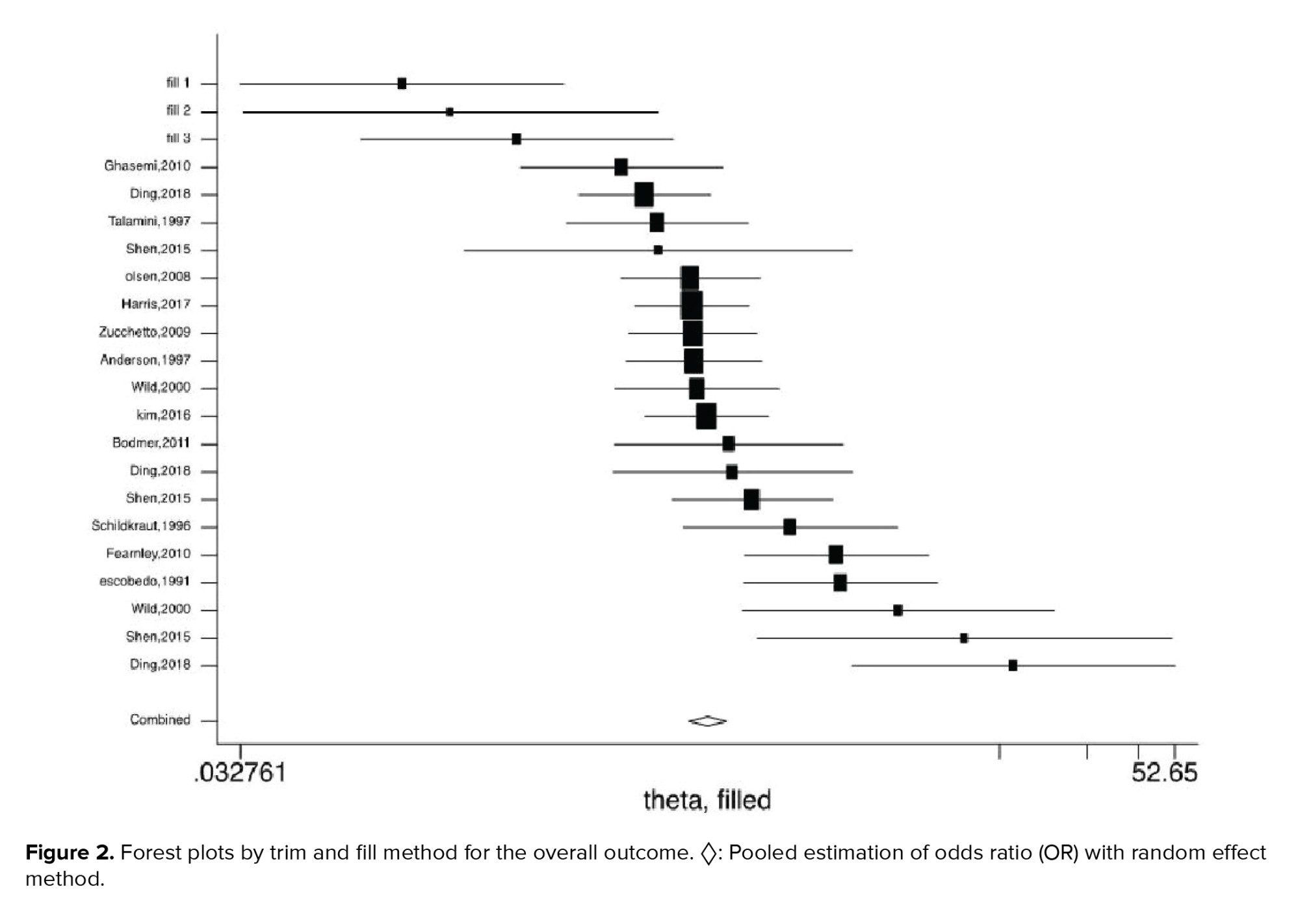
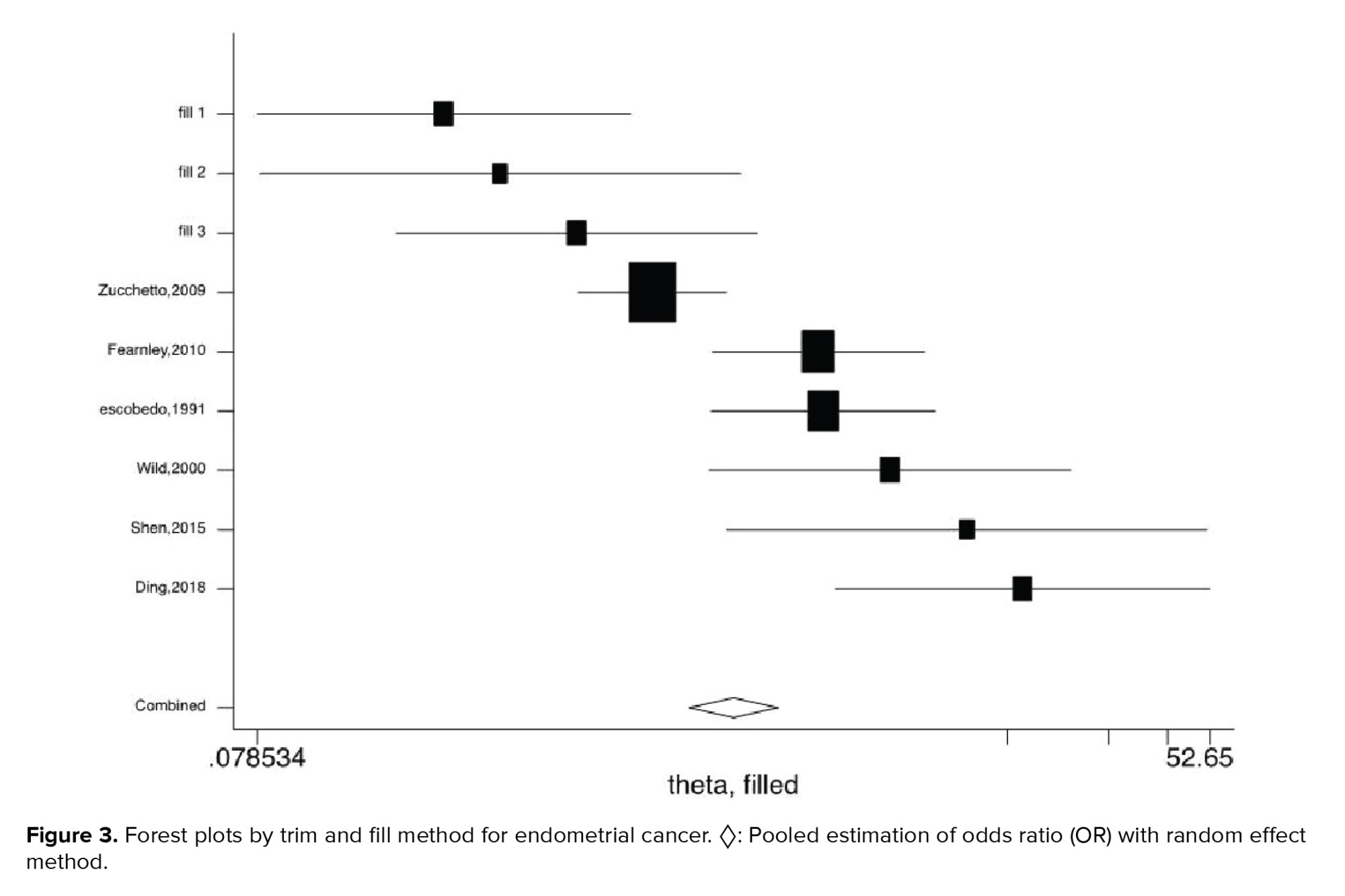
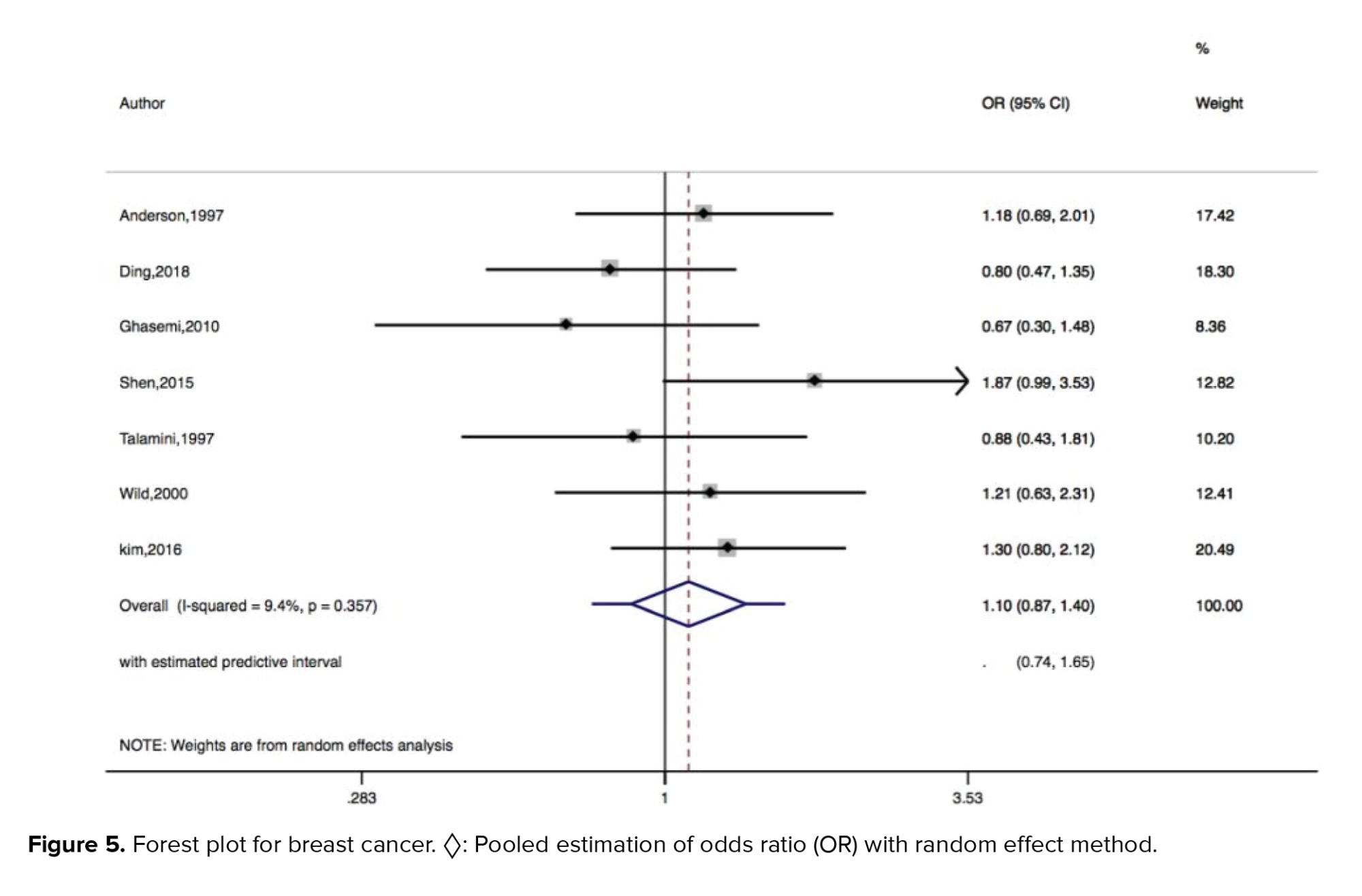
Figures 2-5 present the forest plots of the pooled ORs for the composite outcome (overall outcome) as well as separate outcomes, including endometrial, ovarian, and breast cancers in women with PCOS vs. the control group. The pooled OR of the composite outcome in women with PCOS was higher than that of women with no PCOS (pooled OR: 1.4, 95% CI: 1.0-1.9). The results also indicated that the pooled OR of endometrial and ovarian cancers in women with PCOS was higher than that of their counterparts (pooled OR: 2.2, 95% CI: 1.03-4.7 and pooled OR: 1.3, 95% CI: 1.0-1.8, respectively), whereas the pooled OR of breast cancer in women with PCOS was not significantly higher than controls.
Moreover, the 95% PIs for the odds of endometrial cancer, ovarian cancer, breast cancer, and all cancer types were estimated in women with PCOS vs. their counterparts (95% PI: 0.33-9.5; 95% PI: 0.74-1.65; 95% PI: 0.89-1.98; and 95% PI: 0.57-4.5, respectively). The results of meta-regression analysis revealed that the diagnostic criteria of PCOS and age had no significant effects on the heterogeneity of the outcomes (Figure 6).
3.3. Publication bias, risk of bias, and sensitivity analysis
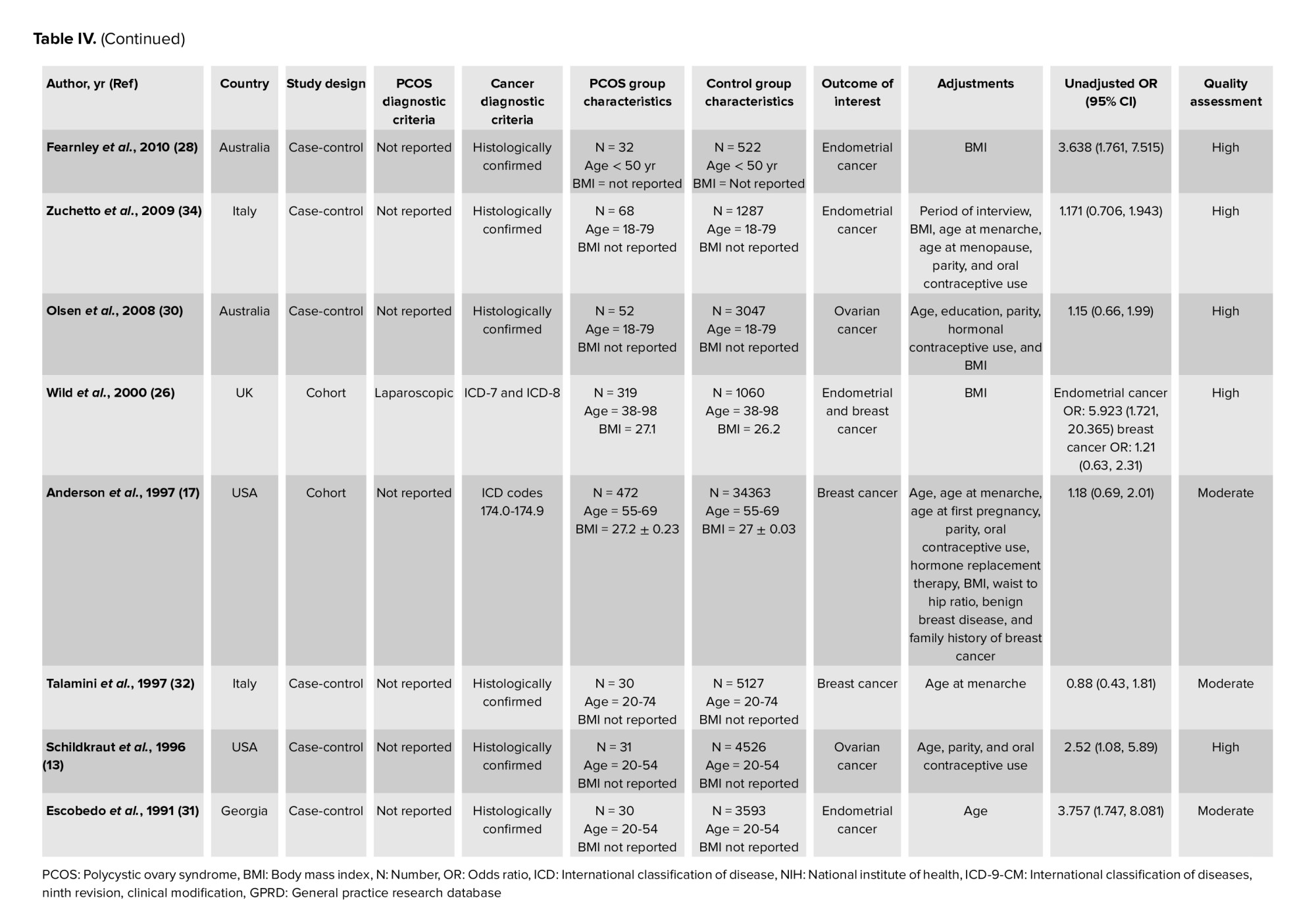
The results of Begg’s test showed a significant publication bias for endometrial cancer in both PCOS and non-PCOS groups and breast cancer in the PCOS group, which was adjusted by the trim and fill method (Table IV, Figure 7).
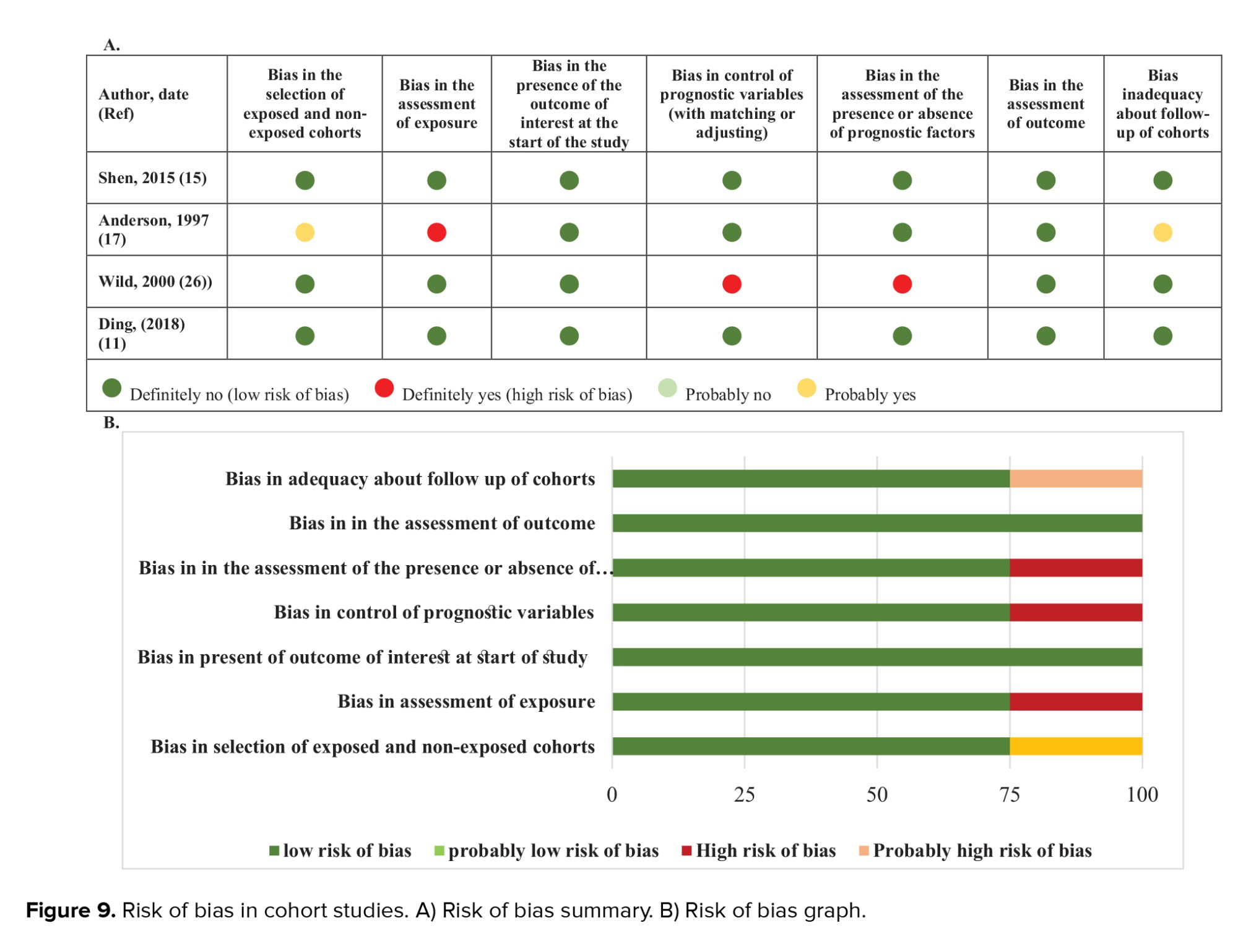
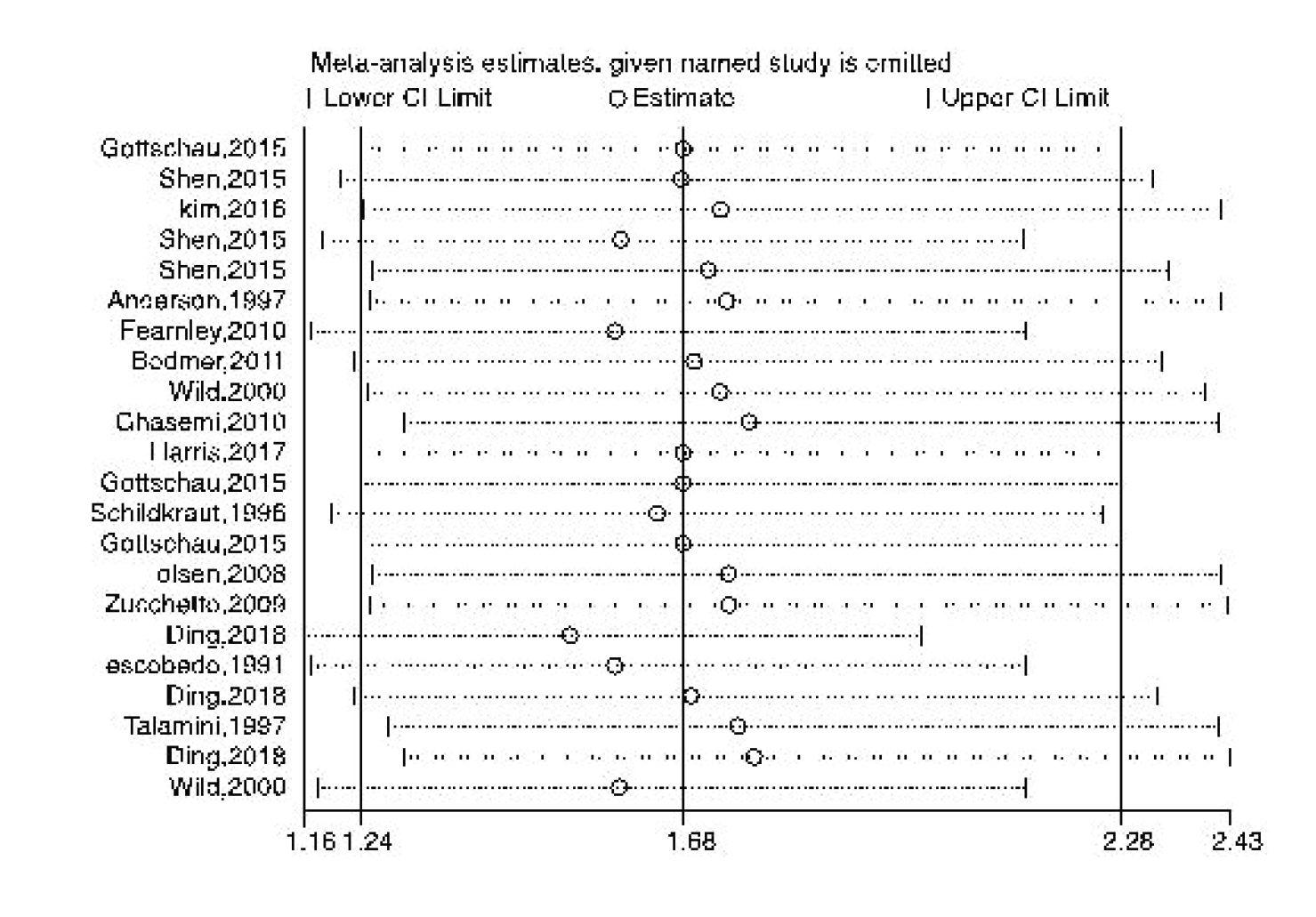
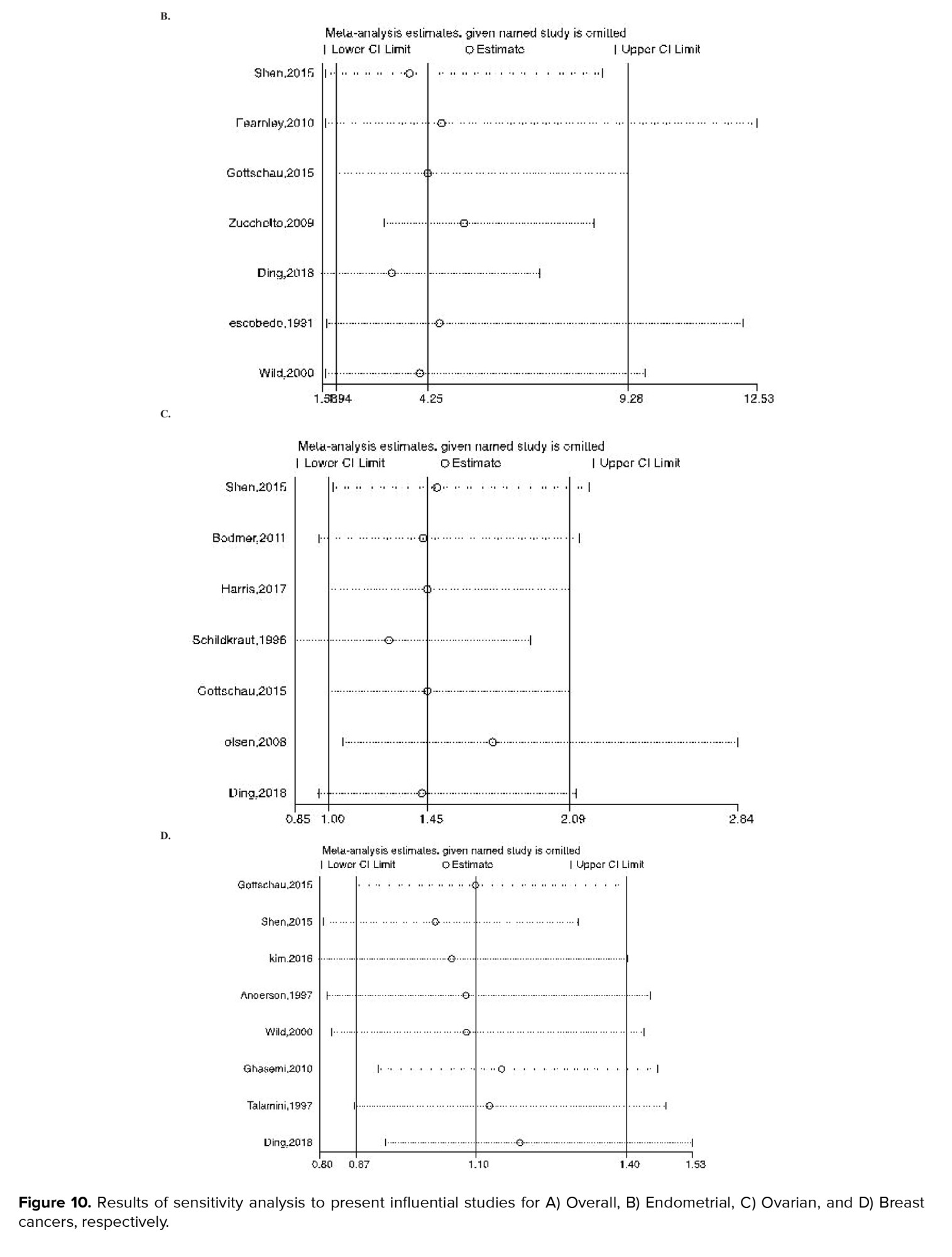
Figures 8-9 represent the risk of bias in the included studies. Most case-control studies had a low risk of bias in domains of sample selection and the primary outcome in the case and control groups, despite a high risk of bias in the assessment of exposure and control of the prognostic variable. In cohort studies, there was a low risk of bias in the adequacy of follow-ups, assessment of outcomes, selection of exposed and non-exposed cohorts, and presence of the outcome of interest at the onset of the study. Moreover, we found a high risk of bias in controlling for prognostic variables, assessment of exposure, and assessment of the presence or absence of prognostic factors. The sensitivity analysis of endometrial cancer, ovarian cancer, breast cancer, and all cancers showed that no study caused heterogeneity among the results (Figure 10).






4. Discussion
This meta-analysis was carried out to assess the association of PCOS with endometrial, ovarian, and breast cancers. The results revealed that PCOS was associated with an increased risk of endometrial and ovarian cancers, but not breast cancer.
It is known that in women with PCOS who have anovulatory menstrual cycles, progesterone, a key hormone in the endometrium against estrogen-driven growth, does not play a regulatory role. This can result in the development of endometrial hyperplasia and adenocarcinoma, mainly due to constant unopposed estrogen activity in the endometrium (35). In other words, an estrogen/progesterone imbalance may lead to endometrial hyperplasia increasing the risk of endometrial cancer in the long run (36). Also, the endometrium of women with PCOS, who received ovulation induction showed the downregulation of progesterone-regulated genes in the secretory phase, leading to progesterone resistance (37). Overall, hyperandrogenism is a clinical feature of PCOS (2). Besides, secretion of ovarian steroids, such as testosterone, is higher in women with endometrial cancer, compared to the healthy population (38). It is well documented that women with PCOS have increased levels of endometrial androgen receptors, compared to the fertile controls (39). Hypersecretion of luteinizing hormone is another feature of PCOS (40). This finding is important since the expression of luteinizing hormone receptors increase in women with anovulatory cycles and endometrial hyperplasia, and it may be associated with endometrial carcinogenesis (41). Insulin resistance is another common feature in many women with PCOS, which leads to compensatory hyperinsulinemia and a 4-fold increase in the prevalence of type II diabetes (40, 42). Hyperinsulinemia, caused by insulin resistance, promotes endometrial cell proliferation and increases the risk of endometrial cancer (14). Also, evidence suggests that the risk of endometrial cancer is higher in women with diabetes compared to those without diabetes (43). Other risk factors for endometrial cancer, such as obesity, nulliparity, and hypertension, are also associated with PCOS (14).
Our results showed that the risk of endometrial cancer was 2.2 times higher in women with PCOS than the controls. In agreement with our findings, a meta-analysis of 4r studies suggested that women with PCOS were 3 times more likely to develop endometrial cancer, compared to those without PCOS (14). Likewise, another meta-analysis of 5 studies showed a 3-fold increase in the risk of endometrial cancer in PCOS women compared to the general population (44). Similarly, a meta-analysis of 11 studies demonstrated that the risk of endometrial cancer was nearly 3 times higher in PCOS women compared to their counterparts (45).
It is well-documented that ovulation is an etiological cause of ovarian cancer; however, it cannot be the only contributor to the pathogenesis of this cancer (12). Although the risk of ovarian cancer in women with PCOS is expected to be low due to anovulation, it is higher than that of healthy women (6). Hormonal mechanisms are hypothesized to be involved in the etiology of ovarian cancer (46). Increased androgen exposure in women with PCOS has been hypothesized to be associated with an increased risk of ovarian cancer (12). This association could be explained by evidence regarding the presence of androgen receptors on healthy ovarian cells, as well as benign and borderline tumors (12). Moreover, it has been shown that higher androgen levels during pregnancy were associated with an increased risk of borderline serous and mucinous tumors (47, 48). Hyperinsulinemia and the resulting increase in insulin-like growth factor-1, which plays an important role in tumorigenesis, have also been suggested as the main mechanism (49).
The present study indicates that the risk of ovarian cancer is 1.3 times higher in PCOS women than their counterparts. In line with our results, a meta-analysis conducted in 2009 (14) reported that women with PCOS are twice more likely to develop ovarian cancer, compared to those without PCOS. Our results are also consistent with the findings reported by another meta-analysis, which showed a 2.5-fold increase in the risk of ovarian cancer in women with PCOS aged < 54 yr, compared to those with no PCOS; however, no significant association was detected between PCOS and ovarian cancer before excluding women aged over 54 yr (45).
There is a complex relationship between PCOS and breast cancer, as the consequences of PCOS have been associated with both the increased and decreased risk of breast cancer (18). The anovulatory cycle and infertility are among the characteristics of PCOS, suggested to decrease the risk of breast cancer (50). However, obesity is a major risk factor for breast cancer in both post and premenopausal women, and as mentioned previously, it is also a common finding in PCOS women (51). This reduction in the risk of breast cancer among women with ovulatory disorders is because of luteal phase deficiency in the menstrual cycle; therefore, the levels of estrogen and progesterone do not increase. Also, it is well-documented that breast cancer cell proliferation is higher during the luteal phase (18, 52). Moreover, hyperinsulinemia has been suggested as an independent risk factor for breast cancer (53). The correlation between androgen excess and the pathogenesis of PCOS is still controversial; however, androgens seem to trigger the development of estrogen-receptor (ER)-negative breast cancer (54, 55). Advanced maternal age during the first pregnancy and nulliparity are also important risk factors for breast cancer (56).
Despite the mentioned mechanisms, we found no significant association between PCOS and breast cancer. Similar to our results, a meta-analysis showed that women with PCOS were not exposed to a higher risk of breast cancer, compared to those without PCOS (14). Likewise, another meta-analysis of 8 studies found no significant association between PCOS and breast cancer (57). Similarly, a recent meta-analysis reported no significant association between PCOS and breast cancer (45). However, more comprehensive prospective cohort studies are needed to examine the association between PCOS and breast cancer and to identify the involved mechanisms.
In our meta-analysis, the risk of bias assessment revealed the high risk of bias for some included studies, especially case-control studies, which might influence our results. To detect these possible effects, we performed a sensitivity analysis. However, no significant studies were found, which could cause heterogeneity.
The most important strength of our study was including a larger number of studies, especially cohort studies. Unlike previous meta-analyses, we did not include studies investigating women with polycystic ovarian morphology, who were not diagnosed with PCOS (58-60); this may increase the accuracy of our findings.
On the other hand, the main limitation of this meta-analysis was that a large number of studies did not report the mean age and details of diagnostic criteria for PCOS. However, after considering the PCOS diagnostic criteria and the mean age through meta-regression, we found that these variables were not significant sources of heterogeneity. Also, in most previous studies, cases of self-reported PCOS have effects on the exactness and validity of the results. Even though in this meta-analysis we tried to lessen all possible biases, it should be considered that there was significant heterogeneity in most of the outcomes. Since this heterogeneity could be due to variations in PCOS phenotypes and diagnostic criteria, we considered the results for PCOS diagnostic criteria via meta-regression. However, our findings showed that the diagnostic criteria of PCOS exerted no significant effects on the heterogeneity of the outcomes.
Moreover, a large number of studies, identified through our database search, did not have any control groups, which could limit the number of studies eligible for our analysis. While some potential confounders, such as body mass index, might affect the results, we could not consider these variables due to the paucity of data. Although pooling fully adjusted ORs can provide more real effect sizes, we considered unadjusted ORs, since included studies had not adjusted the same confounders that may lead to further bias for summary effect size in meta-analyses, especially for weak or medium associations so that the direction of causal inference would be even reversed (61). Also, we could not consider family history, lifestyle, medication use (e.g., metformin or oral contraceptive pills), and many other conditions, which can play important roles in the development of cancer. Ovarian cancer consists of distinct histotypes; however, we combined them due to the paucity of data. Moreover, considering the borderline statistical significance of the results, we estimated the clinical significance by measuring PIs, which showed no clinical significance, possibly due to the limited number of included studies. All of these limitations should be considered in interpreting the results.
5. Conclusion
This study indicated the increased risk of endometrial and ovarian cancers in women with PCOS. Therefore, screening programs for early detection of these cancers, especially in women with PCOS, can be considered an important strategy for improving their survival.
Acknowledgments
This project was supported by the Research Institute for Endocrine Sciences of the Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code: IR.SBMU.ENDOCRINE.REC.1398.131). Authors wish to acknowledge the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Conflict of Interest
The authors have no conflict of interest to declare.
Full-Text: (70 Views)
1. Introduction
Polycystic ovary syndrome (PCOS), one of the common abnormalities in reproductive age women (1), is defined by ovulation abnormalities, high levels of androgens, and polycystic ovaries in ultrasonography (2). PCOS is also associated with several conditions, such as glucose intolerance, diabetes, hypertension, central obesity, metabolic syndrome, and cardiovascular diseases (3-7).
The high prevalence of endometrial hyperplasia and carcinoma due to chronic anovulation, associated with prolonged exposure to unopposed estrogen has been long recognized. Moreover, PCOS complications, such as obesity, nulliparity, diabetes, and hypertension, are risk factors for endometrial carcinoma (8-10). In this regard, a population-based cohort study reported a 17-fold increase in the risk of endometrial cancer among women with PCOS, compared to those without PCOS (11). Several hypotheses have been proposed for the increased risk of ovarian cancer in PCOS women, such as anovulation, increased androgen exposure, and lack of progesterone (12). Another study found that the risk of ovarian cancer was 2.5 times higher in women with PCOS compared to healthy women (13). It is possible that the sustained elevation of serum estrogen levels may lead to the growth of hormone-sensitive tumors, such as breast cancer (14); therefore, anovulation can be considered as a critical risk factor in women with PCOS. Several studies have investigated the risk of breast cancer in women with PCOS; however, the actual risk remains unclear (11, 15-17). Although some studies have assessed the risk of endometrial, ovarian, and breast cancers in women with PCOS, their results are often conflicting, and the risk of these cancers in women with PCOS is still debated (11, 13, 15, 16, 18).
Hence, this study aims to conduct a meta-analysis of observational studies to investigate the association of PCOS with endometrial, ovarian, and breast cancers. Since the severity of PCOS manifestations can distort the results, we adjusted the results for PCOS diagnostic criteria in a meta-regression analysis.
2. Materials and Methods
This meta-analysis was designed according to the guidelines for the preferred reporting items for systematic reviews and meta-analyses (PRISMA) to assess the pooled odds ratio of endometrial cancer in women with PCOS, compared to healthy controls (19).
2.1. Search strategy
Data was searched using PubMed, Scopus, Web of Science, and Google Scholar for retrieving studies published up to April 2020 investigating gynecological cancers in women with PCOS.
2 reviewers (M.A. and A.F.) performed searches separately. Search on PubMed was performed initially, based on MESH terms using the following keywords: (‘polycystic ovary syndrome’ OR ‘PCOS’ OR ‘Stein-Leventhal Syndrome’) AND (‘breast cancer’ OR ‘breast tumor’ OR ‘breast neoplasm’ OR ‘breast carcinoma’ OR ‘Human Mammary Carcinoma’ OR ‘ovarian cancer’ OR ‘ovarian neoplasm’ OR ‘carcinoma’ OR ‘ovarian epithelial’ OR ‘ovary cancer’ OR ‘cancer of ovary’ OR ‘ovarian tumor’ OR ‘endometrial neoplasm’ OR ‘endometrial carcinoma’ OR ‘endometrial cancer’ OR ‘cancer of the endometrium’ OR ‘cancer, endometrium’ OR ‘carcinoma, endometrial’ OR ‘endometrial tumor’).
Search limitations were humans and English language publications. There was no time limitation. The same search strategy was applied for all databases, based on the titles, abstracts, and keywords. We applied a ‘pearl growing’ strategy. First, we obtained the full text of the studies, then the reference list of the studies was reviewed to prevent missing related articles.
2.2. Eligibility criteria
All types of observational studies, including case-control, cross-sectional, and cohort designs were eligible to be included in the meta-analysis. Studies needed to report raw data of events, odds ratio (OR), and relative risk to provide sufficient information to allow calculation. Any PCOS diagnosis criteria were eligible to be included, for example, Rotterdam, National Institute of Health, Androgen Excess Society, International Classification of Diseases, and also a self-reported questionnaire.
Exclusion criteria were: 1) studies assessing conditions, for example, polycystic ovary, androgen excess disease instead of PCOS, 2) without control groups, 3) with unreliable and incomplete results, and 4) assessing hyperplasia rather than cancer.
2.3. Study selection
All relevant studies assessing at least one of the cancers of endometrial, ovarian, and breast in women with PCOS were included in this meta-analysis.
Search results were screened based on the predefined eligibility criteria. All references were entered into EndNote software. The initial selection was based on article titles, and then a second selection based on abstracts was done and duplicates were deleted by one reviewer (A.F). Finally, the full text of selected articles was reviewed for data extraction. Disagreements were resolved by 2 other reviewers (M. A. and F.R.T.).
2.4. Data extraction
2 reviewers (M.A. and A.F.) extracted data from the full text of selected articles and checked twice to lessen errors. For each study, information such as author’s name, publications year, article title, design of the study, study population, number of outcomes, and unadjusted or adjusted OR, relative risk, or HR of the outcomes were extracted.
2.5. Quality assessment
M.A. and A.F. assessed the quality of the included studies and F.R.T. resolved any disagreements. Newcastle-Ottawa scale was applied for the quality assessment (20).
The high quality was defined when a study got ≥ 70% of the highest level of the Newcastle-Ottawa scale, moderate quality was defined when a study got 40-70%, and low quality was defined when a study got 20-40% and those with < 20% were defined as very low quality.
2.6. Bias assessment
Cochrane collaboration’s tools were applied to assess the risk of bias in each study (21).
2.7. Outcome measures
In this meta-analysis, outcomes of interest were endometrial cancer, ovarian cancer, and breast cancer.
2.8. Statistical analysis
This meta-analysis was conducted to obtain a pooled OR of endometrial, ovarian, and breast cancer in PCOS women. Heterogeneity was assessed via I-squared statistics and in the case of values with upper limits 50% random effect method was applied, otherwise the fixed-effect method.
To assess publication bias, Begg’s test was used (22); it was found significant for p < 0.05, and in this case trim and fill method was conducted to correct for publication bias by adding some study measures (23, 24). A funnel plot was also drawn to depict publication bias issues. A Forest plot was also drawn to summarize the result of each study’s effect sizes and its 95% confidence intervals.
In case of publication bias, forest plot corrected via trim and fill method was drawn. To estimate pooled ORs, we applied the ‘Meta-prop’ random effect method (25). The 95% prediction interval (95% PI) was estimated to evaluate clinical significance as compared to statistical significance for the pooled OR. Moreover, the random effect meta-regression model was fitted to assess the effects of PCOS diagnosis criteria and age on the results. We also run a sensitivity analysis to detect probable influential studies with a high risk of bias. Statistical analysis was conducted using STATA software (version 13; STATA, INC., college station, TX, USA).
3. Results
3.1. Search results, study selection, study characteristics, and quality assessment
Figure 1 presents the flow diagram of the search strategy and study selection. Of 1347 records retrieved by searching the databases, a total of 14 studies, including 4 cohorts (11, 15, 17, 26) and 10 case-control (13, 16, 17, 27-34) studies, were selected for the final analysis. 9 studies (11, 13, 15-17, 26-28, 30, 34) were classified as high-quality, and 5 (17, 29, 31-33) as moderate-quality (Table I-II).
2 studies (11, 15) based on the international classification of diseases, 2 (16, 29) used the Rotterdam criteria, 1 (26) used the laparoscopic criteria, and 9 (13, 17, 27, 28, 30-34) reported no criteria. In addition, 7 (11, 15-17, 26, 29, 32) reported breast cancer, 6 (11, 13, 15, 27, 30, 33) reported ovarian cancer, and 6 (11, 15, 26, 28, 31, 34) reported endometrial cancer.
3.2. Meta-analysis and meta-regression of outcomes
In this meta-analysis, 12,955 women with PCOS and 118,481 controls were included. The pooled mean (95% CI) of age and body mass index of all study populations were 27.8 yr (95% CI: 27.8-27.9) and 26.5 kg/m2 (95% CI: 26.3-26.7), respectively. Table III presents the results of the meta-analysis and meta-regression.
Figures 2-5 present the forest plots of the pooled ORs for the composite outcome (overall outcome) as well as separate outcomes, including endometrial, ovarian, and breast cancers in women with PCOS vs. the control group. The pooled OR of the composite outcome in women with PCOS was higher than that of women with no PCOS (pooled OR: 1.4, 95% CI: 1.0-1.9). The results also indicated that the pooled OR of endometrial and ovarian cancers in women with PCOS was higher than that of their counterparts (pooled OR: 2.2, 95% CI: 1.03-4.7 and pooled OR: 1.3, 95% CI: 1.0-1.8, respectively), whereas the pooled OR of breast cancer in women with PCOS was not significantly higher than controls.
Moreover, the 95% PIs for the odds of endometrial cancer, ovarian cancer, breast cancer, and all cancer types were estimated in women with PCOS vs. their counterparts (95% PI: 0.33-9.5; 95% PI: 0.74-1.65; 95% PI: 0.89-1.98; and 95% PI: 0.57-4.5, respectively). The results of meta-regression analysis revealed that the diagnostic criteria of PCOS and age had no significant effects on the heterogeneity of the outcomes (Figure 6).
3.3. Publication bias, risk of bias, and sensitivity analysis
The results of Begg’s test showed a significant publication bias for endometrial cancer in both PCOS and non-PCOS groups and breast cancer in the PCOS group, which was adjusted by the trim and fill method (Table IV, Figure 7).
Figures 8-9 represent the risk of bias in the included studies. Most case-control studies had a low risk of bias in domains of sample selection and the primary outcome in the case and control groups, despite a high risk of bias in the assessment of exposure and control of the prognostic variable. In cohort studies, there was a low risk of bias in the adequacy of follow-ups, assessment of outcomes, selection of exposed and non-exposed cohorts, and presence of the outcome of interest at the onset of the study. Moreover, we found a high risk of bias in controlling for prognostic variables, assessment of exposure, and assessment of the presence or absence of prognostic factors. The sensitivity analysis of endometrial cancer, ovarian cancer, breast cancer, and all cancers showed that no study caused heterogeneity among the results (Figure 10).
















4. Discussion
This meta-analysis was carried out to assess the association of PCOS with endometrial, ovarian, and breast cancers. The results revealed that PCOS was associated with an increased risk of endometrial and ovarian cancers, but not breast cancer.
It is known that in women with PCOS who have anovulatory menstrual cycles, progesterone, a key hormone in the endometrium against estrogen-driven growth, does not play a regulatory role. This can result in the development of endometrial hyperplasia and adenocarcinoma, mainly due to constant unopposed estrogen activity in the endometrium (35). In other words, an estrogen/progesterone imbalance may lead to endometrial hyperplasia increasing the risk of endometrial cancer in the long run (36). Also, the endometrium of women with PCOS, who received ovulation induction showed the downregulation of progesterone-regulated genes in the secretory phase, leading to progesterone resistance (37). Overall, hyperandrogenism is a clinical feature of PCOS (2). Besides, secretion of ovarian steroids, such as testosterone, is higher in women with endometrial cancer, compared to the healthy population (38). It is well documented that women with PCOS have increased levels of endometrial androgen receptors, compared to the fertile controls (39). Hypersecretion of luteinizing hormone is another feature of PCOS (40). This finding is important since the expression of luteinizing hormone receptors increase in women with anovulatory cycles and endometrial hyperplasia, and it may be associated with endometrial carcinogenesis (41). Insulin resistance is another common feature in many women with PCOS, which leads to compensatory hyperinsulinemia and a 4-fold increase in the prevalence of type II diabetes (40, 42). Hyperinsulinemia, caused by insulin resistance, promotes endometrial cell proliferation and increases the risk of endometrial cancer (14). Also, evidence suggests that the risk of endometrial cancer is higher in women with diabetes compared to those without diabetes (43). Other risk factors for endometrial cancer, such as obesity, nulliparity, and hypertension, are also associated with PCOS (14).
Our results showed that the risk of endometrial cancer was 2.2 times higher in women with PCOS than the controls. In agreement with our findings, a meta-analysis of 4r studies suggested that women with PCOS were 3 times more likely to develop endometrial cancer, compared to those without PCOS (14). Likewise, another meta-analysis of 5 studies showed a 3-fold increase in the risk of endometrial cancer in PCOS women compared to the general population (44). Similarly, a meta-analysis of 11 studies demonstrated that the risk of endometrial cancer was nearly 3 times higher in PCOS women compared to their counterparts (45).
It is well-documented that ovulation is an etiological cause of ovarian cancer; however, it cannot be the only contributor to the pathogenesis of this cancer (12). Although the risk of ovarian cancer in women with PCOS is expected to be low due to anovulation, it is higher than that of healthy women (6). Hormonal mechanisms are hypothesized to be involved in the etiology of ovarian cancer (46). Increased androgen exposure in women with PCOS has been hypothesized to be associated with an increased risk of ovarian cancer (12). This association could be explained by evidence regarding the presence of androgen receptors on healthy ovarian cells, as well as benign and borderline tumors (12). Moreover, it has been shown that higher androgen levels during pregnancy were associated with an increased risk of borderline serous and mucinous tumors (47, 48). Hyperinsulinemia and the resulting increase in insulin-like growth factor-1, which plays an important role in tumorigenesis, have also been suggested as the main mechanism (49).
The present study indicates that the risk of ovarian cancer is 1.3 times higher in PCOS women than their counterparts. In line with our results, a meta-analysis conducted in 2009 (14) reported that women with PCOS are twice more likely to develop ovarian cancer, compared to those without PCOS. Our results are also consistent with the findings reported by another meta-analysis, which showed a 2.5-fold increase in the risk of ovarian cancer in women with PCOS aged < 54 yr, compared to those with no PCOS; however, no significant association was detected between PCOS and ovarian cancer before excluding women aged over 54 yr (45).
There is a complex relationship between PCOS and breast cancer, as the consequences of PCOS have been associated with both the increased and decreased risk of breast cancer (18). The anovulatory cycle and infertility are among the characteristics of PCOS, suggested to decrease the risk of breast cancer (50). However, obesity is a major risk factor for breast cancer in both post and premenopausal women, and as mentioned previously, it is also a common finding in PCOS women (51). This reduction in the risk of breast cancer among women with ovulatory disorders is because of luteal phase deficiency in the menstrual cycle; therefore, the levels of estrogen and progesterone do not increase. Also, it is well-documented that breast cancer cell proliferation is higher during the luteal phase (18, 52). Moreover, hyperinsulinemia has been suggested as an independent risk factor for breast cancer (53). The correlation between androgen excess and the pathogenesis of PCOS is still controversial; however, androgens seem to trigger the development of estrogen-receptor (ER)-negative breast cancer (54, 55). Advanced maternal age during the first pregnancy and nulliparity are also important risk factors for breast cancer (56).
Despite the mentioned mechanisms, we found no significant association between PCOS and breast cancer. Similar to our results, a meta-analysis showed that women with PCOS were not exposed to a higher risk of breast cancer, compared to those without PCOS (14). Likewise, another meta-analysis of 8 studies found no significant association between PCOS and breast cancer (57). Similarly, a recent meta-analysis reported no significant association between PCOS and breast cancer (45). However, more comprehensive prospective cohort studies are needed to examine the association between PCOS and breast cancer and to identify the involved mechanisms.
In our meta-analysis, the risk of bias assessment revealed the high risk of bias for some included studies, especially case-control studies, which might influence our results. To detect these possible effects, we performed a sensitivity analysis. However, no significant studies were found, which could cause heterogeneity.
The most important strength of our study was including a larger number of studies, especially cohort studies. Unlike previous meta-analyses, we did not include studies investigating women with polycystic ovarian morphology, who were not diagnosed with PCOS (58-60); this may increase the accuracy of our findings.
On the other hand, the main limitation of this meta-analysis was that a large number of studies did not report the mean age and details of diagnostic criteria for PCOS. However, after considering the PCOS diagnostic criteria and the mean age through meta-regression, we found that these variables were not significant sources of heterogeneity. Also, in most previous studies, cases of self-reported PCOS have effects on the exactness and validity of the results. Even though in this meta-analysis we tried to lessen all possible biases, it should be considered that there was significant heterogeneity in most of the outcomes. Since this heterogeneity could be due to variations in PCOS phenotypes and diagnostic criteria, we considered the results for PCOS diagnostic criteria via meta-regression. However, our findings showed that the diagnostic criteria of PCOS exerted no significant effects on the heterogeneity of the outcomes.
Moreover, a large number of studies, identified through our database search, did not have any control groups, which could limit the number of studies eligible for our analysis. While some potential confounders, such as body mass index, might affect the results, we could not consider these variables due to the paucity of data. Although pooling fully adjusted ORs can provide more real effect sizes, we considered unadjusted ORs, since included studies had not adjusted the same confounders that may lead to further bias for summary effect size in meta-analyses, especially for weak or medium associations so that the direction of causal inference would be even reversed (61). Also, we could not consider family history, lifestyle, medication use (e.g., metformin or oral contraceptive pills), and many other conditions, which can play important roles in the development of cancer. Ovarian cancer consists of distinct histotypes; however, we combined them due to the paucity of data. Moreover, considering the borderline statistical significance of the results, we estimated the clinical significance by measuring PIs, which showed no clinical significance, possibly due to the limited number of included studies. All of these limitations should be considered in interpreting the results.
5. Conclusion
This study indicated the increased risk of endometrial and ovarian cancers in women with PCOS. Therefore, screening programs for early detection of these cancers, especially in women with PCOS, can be considered an important strategy for improving their survival.
Acknowledgments
This project was supported by the Research Institute for Endocrine Sciences of the Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code: IR.SBMU.ENDOCRINE.REC.1398.131). Authors wish to acknowledge the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Conflict of Interest
The authors have no conflict of interest to declare.
Type of Study: Original Article |
Subject:
Reproductive Endocrinology
References
1. 1. March WA, Moore VM, Willson KJ, Phillips DIW, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 2010; 25: 544-551.
2. 2. Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Positions statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An androgen excess society guideline. J Clin Endocrinol Metab 2006; 91: 4237-4245.
3. 3. Ramezani Tehrani F, Amiri M, Behboudi-Gandevani S, Bidhendi-Yarandi R, Carmina E. Cardiovascular events among reproductive and menopausal age women with polycystic ovary syndrome: A systematic review and meta-analysis. Gynecol Endocrinol 2020; 36: 12-23.
4. 4. Burghen GA, Givens JR, Kitabchi AE. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab 1980; 50: 113-116.
5. 5. Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 1989; 38: 1165-1174.
6. 6. Daniilidis A, Dinas K. Long term health consequences of polycystic ovarian syndrome: A review analysis. Hippokratia 2009; 13: 90-92.
7. 7. Cheung LP, Ma RC, Lam PM, Lok IH, Haines CJ, So WY, et al. Cardiovascular risks and metabolic syndrome in Hong Kong Chinese women with polycystic ovary syndrome. Hum Reprod 2008; 23: 1431-1438.
8. 8. Balen A. Polycystic ovary syndrome and cancer. Hum Reprod Update 2001; 7: 522-525.
9. 9. Carmina E, Lobo RA. Polycystic ovary syndrome (PCOS): Arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab 1999; 84: 1897-1899.
10. 10. Gibson M. Reproductive health and polycystic ovary syndrome. Am J Med 1995; 98: 67-75.
11. 11. Ding D-Ch, Chen W, Wang J-H, Lin Sh-Z. Association between polycystic ovarian syndrome and endometrial, ovarian, and breast cancer: A population-based cohort study in Taiwan. Medicine 2018; 97: e12608.
12. 12. Risch HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. J Natl Cancer Inst 1998; 90: 1774-1786.
13. 13. Schildkraut JM, Schwingl PJ, Bastos E, Evanoff A, Hughes C. Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstet Gynecol 1996; 88: 554-559.
14. 14. Chittenden BG, Fullerton G, Maheshwari A, Bhattacharya S. Polycystic ovary syndrome and the risk of gynaecological cancer: A systematic review. Reprod Biomed Online 2009; 19: 398-405.
15. 15. Shen Ch-Ch, Yang AC, Hung J-H, Hu L-Y, Tsai Sh-J. A nationwide population-based retrospective cohort study of the risk of uterine, ovarian and breast cancer in women with polycystic ovary syndrome. Oncologist 2015; 20: 45-49.
16. 16. Kim J, Mersereau JE, Khankari N, Bradshaw PT, McCullough LE, Cleveland R, et al. Polycystic ovarian syndrome (PCOS), related symptoms/sequelae, and breast cancer risk in a population-based case-control study. Cancer Causes Control 2016; 27: 403-414.
17. 17. Anderson KE, Sellers TA, Chen PL, Rich SS, Hong CP, Folsom AR. Association of Stein-Leventhal syndrome with the incidence of postmenopausal breast carcinoma in a large prospective study of women in Iowa. Cancer 1997; 79: 494-499.
18. 18. Harris HR, Terry KL. Polycystic ovary syndrome and risk of endometrial, ovarian, and breast cancer: A systematic review. Fertil Res Pract 2016; 2: 14.
19. 19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg 2010; 8: 336-341.
20. 20. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603-605.
21. 21. Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928.
22. 22. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088-1101.
23. 23. Duval S, Tweedie R. Trim and fill: A simple funnel‐plot-based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000; 56: 455-463.
24. 24. Mavridis D, Salanti G. How to assess publication bias: Funnel plot, trim-and-fill method and selection models. Evid Based Ment Health 2014; 17: 30.
25. 25. Nyaga VN, Arbyn M, Aerts M. Metaprop: A Stata command to perform meta-analysis of binomial data. Arch Public Health 2014; 72: 39.
26. 26. Wild S, Pierpoint T, Jacobs H, McKeigue P. Long-term consequences of polycystic ovary syndrome: Results of a 31 year follow-up study. Hum Fertil 2000; 3: 101-105.
27. 27. Harris HR, Titus LJ, Cramer DW, Terry KL. Long and irregular menstrual cycles, polycystic ovary syndrome, and ovarian cancer risk in a population-based case-control study. Int J Cancer 2017; 140: 285-291.
28. 28. Fearnley EJ, Marquart L, Spurdle AB, Weinstein P, Webb PM, Australian ovarian cancer study group and australian national endometrial cancer study group. Polycystic ovary syndrome increases the risk of endometrial cancer in women aged less than 50 years: An Australian case-control study. Cancer Causes Control 2010; 21: 2303-2308.
29. 29. Ghasemi N, Mortazavizadeh MR, Khorasani Gerdekoohi A. Frequency of poly cystic ovary syndrome in patients with premenopausal breast cancer. Iran J Reprod Med 2010; 8: 86-90.
30. 30. Olsen CM, Green AC, Nagle CM, Jordan SJ, Whiteman DC, Bain CJ, et al. Epithelial ovarian cancer: Testing the 'androgens hypothesis'. Endocr Relat Cancer 2008; 15: 1061-1068.
31. 31. Escobedo LG, Lee NC, Peterson HB, Wingo PA. Infertility-associated endometrial cancer risk may be limited to specific subgroups of infertile women. Obstet Gynecol 1991; 77: 124-128.
32. 32. Talamini R, Franceschi S, Favero A, Negri E, Parazzini F, La Vecchia C. Selected medical conditions and risk of breast cancer. Br J Cancer 1997; 75: 1699-1703.
33. 33. Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin and the risk of ovarian cancer: A case-control analysis. Gynecol Oncol 2011; 123: 200-204.
34. 34. Zucchetto A, Serraino D, Polesel J, Negri E, De Paoli A, Dal Maso L, et al. Hormone-related factors and gynecological conditions in relation to endometrial cancer risk. Eur J Cancer Prev 2009; 18: 316-321.
35. 35. Lopes IMRS, Baracat MCP, Simoes Mde J, Simoes RS, Baracat EC, Soares Jr JM. Endometrium in women with polycystic ovary syndrome during the window of implantation. Rev Assoc Med Bras 2011; 57: 702-709.
36. 36. Schindler AE. Progestogen deficiency and endometrial cancer risk. Maturitas 2009; 62: 334-337.
37. 37. Savaris RF, Groll JM, Young SL, DeMayo FJ, Jeong JW, Hamilton AE, et al. Progesterone resistance in PCOS endometrium: A microarray analysis in clomiphene citrate-treated and artificial menstrual cycles. J Clin Endocrinol Metab 2011; 96: 1737-1746.
38. 38. Nagmani M, Hannigan EV, Dillard JR EA, Dinh TV. Ovarian steroid secretion in postmenopausal women with and without endometrial cancer. J Clin Endocrinol Metab 1986; 62: 508-512.
39. 39. Apparao KBC, Lovely LP, Gui Y, Lininger RA, Lessey BA. Elevated endometrial androgen receptor expression in women with polycystic ovarian syndrome. Biol Reprod 2002; 66: 297-304.
40. 40. Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nat Rev Endocrinol 2011; 7: 219-231.
41. 41. Konishi I, Koshiyama M, Mandai M, Kuroda H, Yamamoto S, Nanbu K, et al. Increased expression of LH/hCG receptors in endometrial hyperplasia and carcinoma in anovulatory women. Gynecol Oncol 1997; 65: 273-280.
42. 42. Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod Update 2010; 16: 347-363.
43. 43. Saed L, Varse F, Baradaran HR, Moradi Y, Khateri S, Friberg E, et al. The effect of diabetes on the risk of endometrial cancer: An updated a systematic review and meta-analysis. BMC Cancer 2019; 19: 527.
44. 44. Haoula Z, Salman M, Atiomo W. Evaluating the association between endometrial cancer and polycystic ovary syndrome. Hum Reprod 2012; 27: 1327-1331.
45. 45. Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod Update 2014; 20: 748-758.
46. 46. Helzlsouer KJ, Alberg AJ, Gordon GB, Longcope C, Bush TL, Hoffman SC, et al. Serum gonadotropins and steroid hormones and the development of ovarian cancer. JAMA 1995; 274: 1926-1930.
47. 47. Schock H, Surcel H-M, Zeleniuch-Jacquotte A, Grankvist K, Lakso H-A, Fortner RT, et al. Early pregnancy sex steroids and maternal risk of epithelial ovarian cancer. Endocr Relat Cancer 2014; 21: 831-844.
48. 48. Butler M, Ricciardelli C, Tilley W, Hickey T. Androgen receptor protein levels are significantly reduced in serous ovarian carcinomas compared with benign or borderline disease but are not altered by cancer stage or metastatic progression. Horm Cancer 2013; 4: 154-164.
49. 49. Lee J-Y, Jeon I, Kim JW, Song Y-S, Yoon JM, Park SM. Diabetes mellitus and ovarian cancer risk: A systematic review and meta-analysis of observational studies. Int J Gynecol Cancer 2013; 23: 402-412.
50. 50. Terry KL, Willett WC, Rich-Edwards JW, Michels KB. A prospective study of infertility due to ovulatory disorders, ovulation induction, and incidence of breast cancer. Arch Intern Med 2006; 166: 2484-2489.
51. 51. Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin 2017; 67: 378-397.
52. 52. Meyer JS. Cell proliferation in normal human breast ducts, fibroadenomas, and other ductal hyperplasias measured by nuclear labeling with tritiated thymidine. Effects of menstrual phase, age, and oral contraceptive hormones. Hum Pathol 1977; 8: 67-81.
53. 53. Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst 2009; 101: 48-60.
54. 54. Secreto G, Girombelli A, Krogh V. Androgen excess in breast cancer development: Implications for prevention and treatment. Endocr Relat Cancer 2019; 26: R81-R94.
55. 55. Carvalho MJ, Subtil S, Rodrigues A, Oliveira J, Figueiredo-Dias M. Controversial association between polycystic ovary syndrome and breast cancer. Eur J Obstet Gynecol Reprod Biol 2019; 243: 125-132.
56. 56. Sun Y-Sh, Zhao Zh, Yang Zh-N, Xu F, Lu H-J, Zhu Zh-Y, et al. Risk factors and preventions of breast cancer. Int J Biol Sci 2017; 13: 1387-1397.
57. 57. Shobeiri F, Jenabi E. The association between polycystic ovary syndrome and breast cancer: A meta-analysis. Obstet Gynecol Sci 2016; 59: 367-372.
58. 58. Gammon MD, Thompson WD. Polycystic ovaries and the risk of breast cancer. Am J Epidemiol 1991; 134: 818-824.
59. 59. Pillay OC, Te Fong LFW, Crow JC, Benjamin E, Mould T, Atiomo W, et al. The association between polycystic ovaries and endometrial cancer. Hum Reprod 2005; 21: 924-929.
60. 60. Baron JA, Weiderpass E, Newcomb PA, Stampfer M, Titus-Ernstoff L, Egan KM, et al. Metabolic disorders and breast cancer risk (United States). Cancer Causes Control 2001; 12: 875-880.
61. 61. Liu T, Nie X, Wu Z, Zhang Y, Feng G, Cai S, et al. Can statistic adjustment of OR minimize the potential confounding bias for meta-analysis of case-control study? A secondary data analysis. BMC Med Res Method 2017; 17: 179.
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





