Thu, Apr 25, 2024
[Archive]
Volume 20, Issue 6 (June 2022)
IJRM 2022, 20(6): 433-446 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shahmoradi A, Aghaei A, Ghaderi K, Rezaei M J, Azarnezhad A. A meta-analysis of the association of ApaI, BsmI, FokI, and TaqI polymorphisms in the vitamin D receptor gene with the risk of polycystic ovary syndrome in the Eastern Mediterranean Regional Office population. IJRM 2022; 20 (6) :433-446
URL: http://ijrm.ir/article-1-2233-en.html
URL: http://ijrm.ir/article-1-2233-en.html
1- Department of Laboratory Medicine, Faculty of Paramedical, Kurdistan University of Medical Sciences, Sanandaj, Iran.
2- Social Determinants of Health Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences, Sanandaj, Iran.
3- Department of Anatomical Sciences, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran.
4- Student Research Committee, Kurdistan University of Medical Sciences, Sanandaj, Iran. , azarnezhad@gmail.com
2- Social Determinants of Health Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences, Sanandaj, Iran.
3- Department of Anatomical Sciences, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran.
4- Student Research Committee, Kurdistan University of Medical Sciences, Sanandaj, Iran. , azarnezhad@gmail.com
Full-Text [PDF 3058 kb]
(782 Downloads)
| Abstract (HTML) (1298 Views)
Full-Text: (282 Views)
- Introduction
Polycystic ovary syndrome (PCOS) is one of the most common syndromes in reproductive-age people, distinguished by clinical characteristics such as prolonged anovulation, menstrual dysfunction, and polycystic ovaries (1, 2). According to the National Institutes of Health 1990 criteria and the Rotterdam 2003 criteria, the combined prevalence of PCOS is ~4-21% worldwide (3). Women with PCOS have a high probability of type 2 diabetes mellitus and cardiovascular disease (4, 5). Patients with PCOS have an impaired glucose tolerance, insulin resistance, hyperinsulinemia, and obesity (6, 7). However, the exact etiology and underlying pathological mechanisms of PCOS are not clear.
Interactions between genetic and environmental factors are believed to play a significant role in the incidence and progression of PCOS (8, 9, 10). Insulin resistance and hyperinsulinemia are recurrent metabolic disturbances in PCOS. Research demonstrates that levels of vitamin D could be related to hormonal and metabolic conditions (11). Vitamin D is converted to 1, 25-dihydroxycholecalciferol in the kidneys and liver (12, 13). Lines of evidence suggest a significant relationship of vitamin D levels with the pathogenesis, signs, and symptoms of PCOS (14). A recent meta-analysis identified more considerable variation in serum 25-hydroxyvitamin D, total cholesterol, serum insulin, low-density lipoprotein cholesterol, and triglycerides in women with PCOS than in healthy subjects (15).
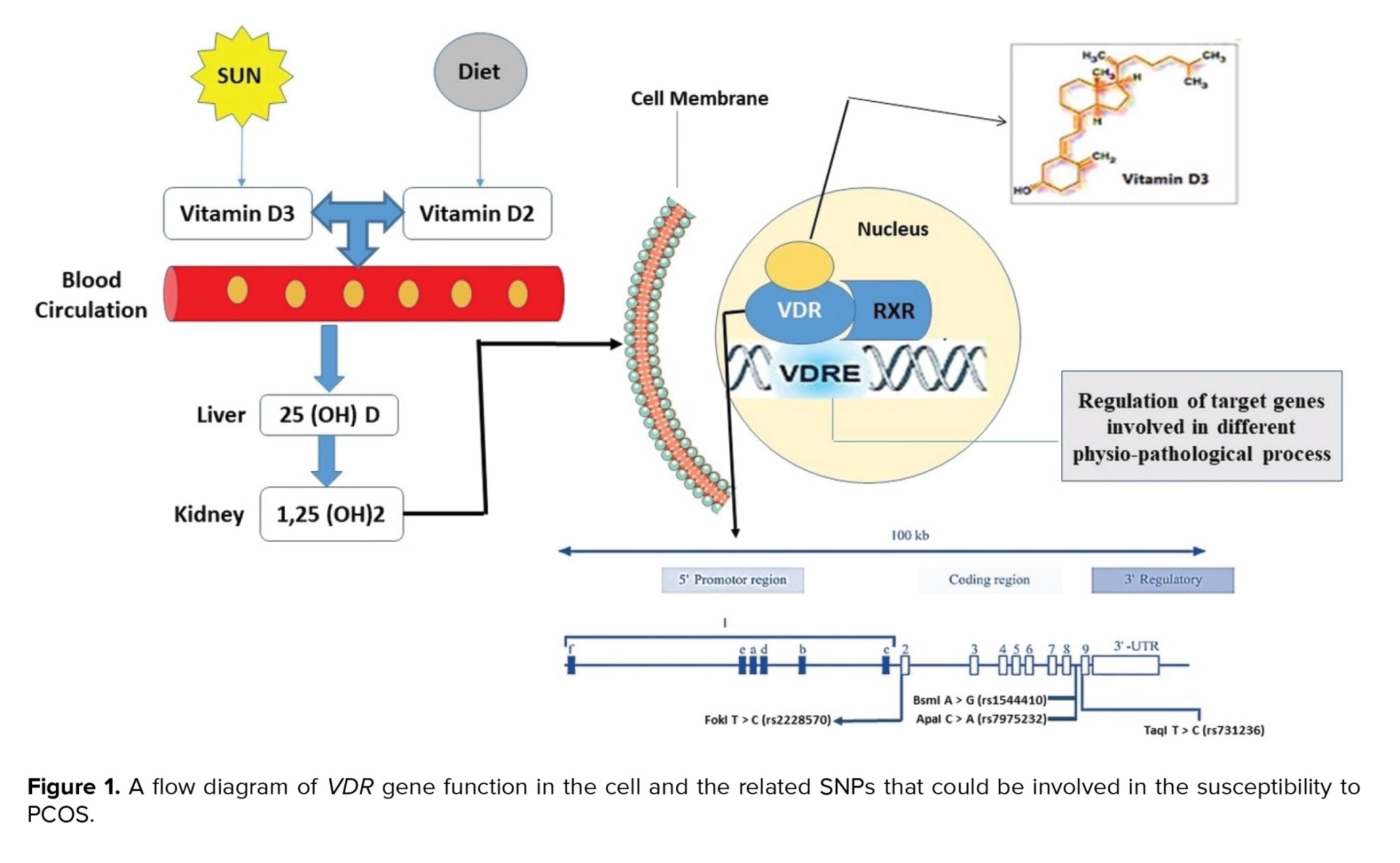
The vitamin D receptor (VDR), a ligand-dependent transcription factor belonging to the steroid/thyroid hormone receptor superfamily, is responsible for vitamin D's effect. VDR is commonly found in many tissues of the female reproductive system and regulates the biological effects of vitamin D (16, 17). As is illustrated in figure 1, VDR and unoccupied retinoid X receptor form a heterodimer transcription unit which regulates the transcription through binding to the vitamin D response element in the promoter region of target genes (18, 19). About 3% of the human genome, including genes essential to glucose metabolism, are regulated by VDR (13).
The VDR gene is located on chromosome 12q13.11and contains 14 exons that provide instruction for a 427-amino-acid protein (20). The VDR gene contain 4 mostly reported single nucleotide polymorphisms (SNPs) including FokI (rs10735810) in exon 2, ApaI (rs7975232) in intron 8, TaqI (rs731236) in exon 9, and BsmI (rs1544410) in intron 8 (21). The previous meta-analyses have reported the association of VDR gene polymorphisms with PCOS risk (14, 22). However, these analyses must be updated and performed on specific populations. It has been shown that the VDR polymorphisms may correspond to PCOS susceptibility in the Eastern Mediterranean Regional Office (EMRO) population, even though results are as yet uncertain and conflicting owing to the small sample size and limited statistical power (23-33). Appropriate and accurate data from developing countries in the EMRO are lacking; the majority of published data from these areas are outdated, heterogeneous, and suffering from limited sample sizes (34).
Consequently, the present study aimed to conduct an updated systematic review and meta-analysis to more reliably examine the association of VDR gene polymorphisms with susceptibility to PCOS in the EMRO population.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were applied to conduct the present meta-analysis (35). An online search was carried out in PubMed, Scopus, SCI, and Google Scholar to retrieve related reports up to the end of 2020. The search strategy was based on terms and keywords including: ‘polycystic ovary syndrome OR PCOS’, ‘vitamin D receptor OR VDR’, and ‘polymorphisms OR variants’. Manual searches in the references cited within retrieved articles were also performed to select the relevant publications. Other prospective studies not included in the database were manually sought in the references of the obtained papers.
The inclusion criteria for this meta-analysis required that the articles be written in English, and included case-control studies, research on the association of ApaI, BsmI, FokI, and TaqI polymorphisms in the VDR gene with PCOS, investigations on the accessibility of genotype distributions in cases and controls for calculating odds ratios (ORs) and their 95% confidence intervals (CI), as well as studies on PCOS diagnosis using Rotterdam and National Institute of Child Health and Human Development criteria. Case reports, publications without full text, reviews, editorial comments, repeat publications, those with unavailability of the genotypic data, reports on the association of other polymorphisms in the VDR gene with PCOS, and the current meta-analysis did not include research that used an animal model. In the case of multiple publications reported by the same team, the most recent or largest sample sizes were chosen.
2.2. Data extraction
The eligible studies were selected according to the following criteria by 2 independent authors (A.Az and A.SH), and any disagreements were decided by a third reviewer. The first author of each study, publication year, country of origin, the sample size of cases and controls, and data of the frequency genotype of distribution were recorded.
2.3. Ethical considerations
The study was performed under the approval of the Ethical Committee of Kurdistan University Medical Sciences, Sanandaj, Iran (Code: IR.MUK.REC.1398.216).
2.4. Statistical analysis
Using crude ORs and their 95% CI, the statistical power of the relationship between VDR gene variants and PCOS risk was determined. Allelic, recessive, and dominant genetic models were used in all analyses. A Chi-square test based on Q statistics was used to evaluate the inter-study heterogeneity, wherein the fixed model was applied when the p-value was > 0.10. Otherwise, a random-effects model was adopted. To examine which experiments had a substantial effect on the stability of the findings, one-way sensitivity tests were conducted. To evaluate possible publishing bias, we used the Begg funnel plot and Egger's linear regression test. STATA version 12.0 software (STATA Corporation, College Station, Texas, USA) was used to calculate the OR and 95% CI, and two-sided p-values < 0.05 were regarded as statistically significant.
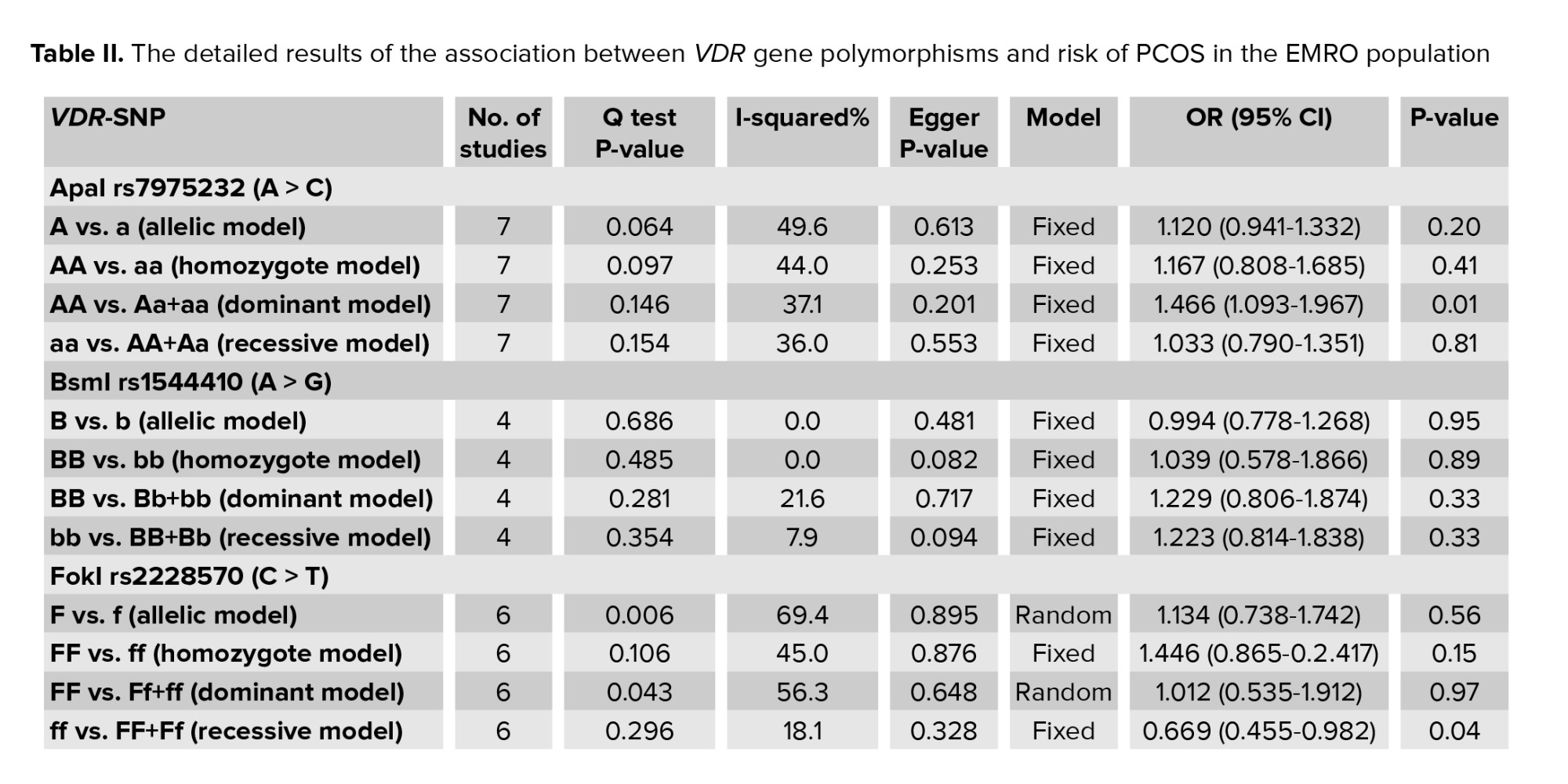
The studies used in this meta-analysis were selected using the preferred reporting items for systematic reviews, and meta-analyses flow diagram, as seen in figure 2. To begin, we searched the abovementioned databases and found a total of 1,516 reports. The 1,354 duplicates and ineligible records were removed before screening, and reviews, irrelevant reports, and studies not considering the VDR gene or PCOS were excluded from the extracted records, which consisted of 152 papers. Then, we excluded 3 studies because there was inadequate evidence to quantify ORs and 95% CIs, or it was not a case-control design. Finally, this meta-analysis contained 7 articles conducted on the association of ApaI, BsmI, FokI, and TaqI polymorphisms in the VDR gene with risk of PCOS in the EMRO population.
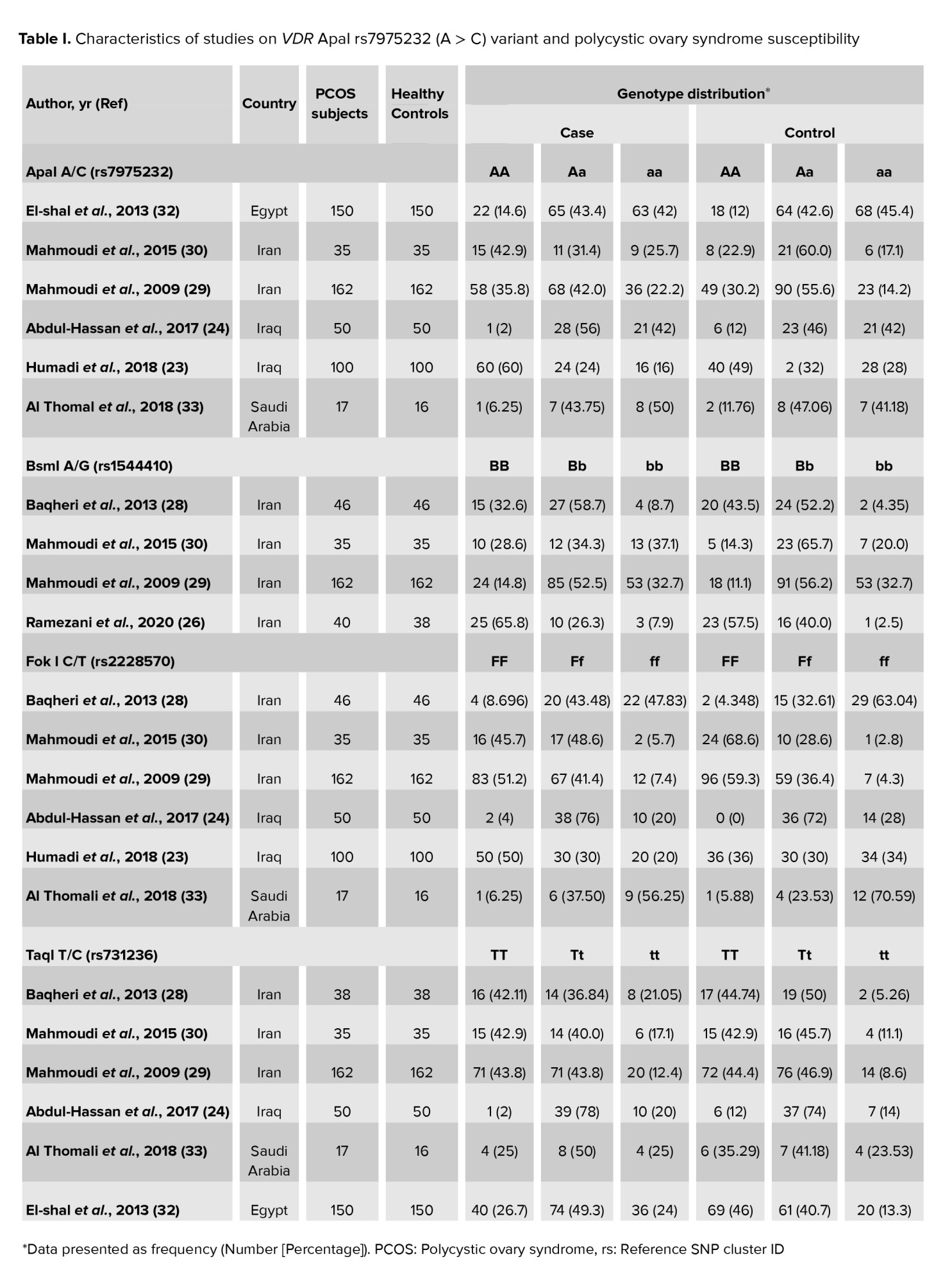
The association of the VDR gene ApaI rs7975232 (G > T) polymorphism, BsmI rs1544410 (A > G) variant, the FokI rs2228570 (C > T) polymorphism, and Taq1 rs731236 (T > C) variant was examined in 6 (23, 24, 29, 30, 32, 33), 4 (26, 29-31), 6 (23, 24, 29-31, 33), and 4 (24, 28-30) case-control studies, respectively. Table I shows the characteristics of studies on VDR ApaI, BsmI, FokI, and TaqI variants and PCOS susceptibility.
3.2. Quantitative results
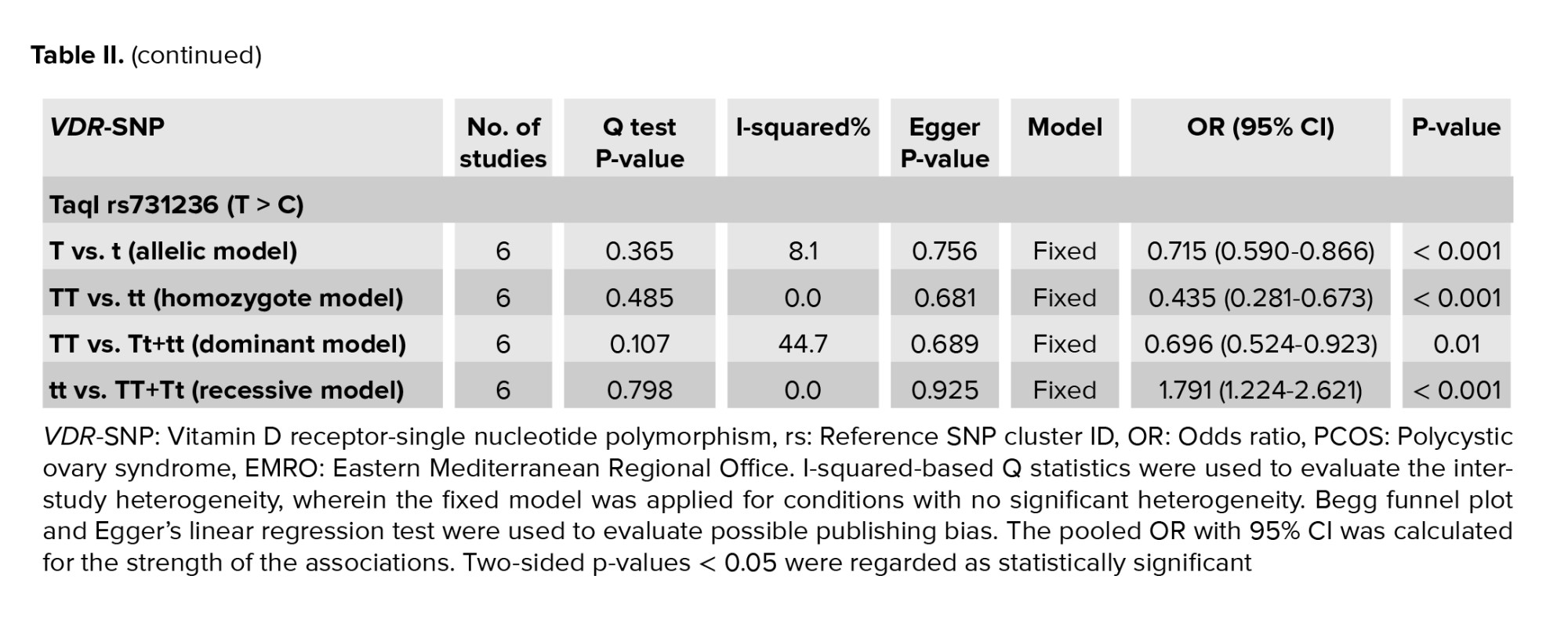
Table II shows the details of the association of VDR gene ApaI rs7975232 (A > C), BsmI rs1544410 (A > G), FokI rs2228570 (C > T), and TaqI rs731236 (T > C) polymorphisms with PCOS predisposition. ApaI (C > A) correlates to ApaI (A/a), BsmI (A/G) to BsmI (B/b), FokI (C/T) to FokI (F/f), and TaqI (T > C) to TaqI (T/t), where the minor vs major alleles were evaluated for their connection with the disease as a susceptible/protective factor.
3.2.1. VDR ApaI variant and PCOS risk
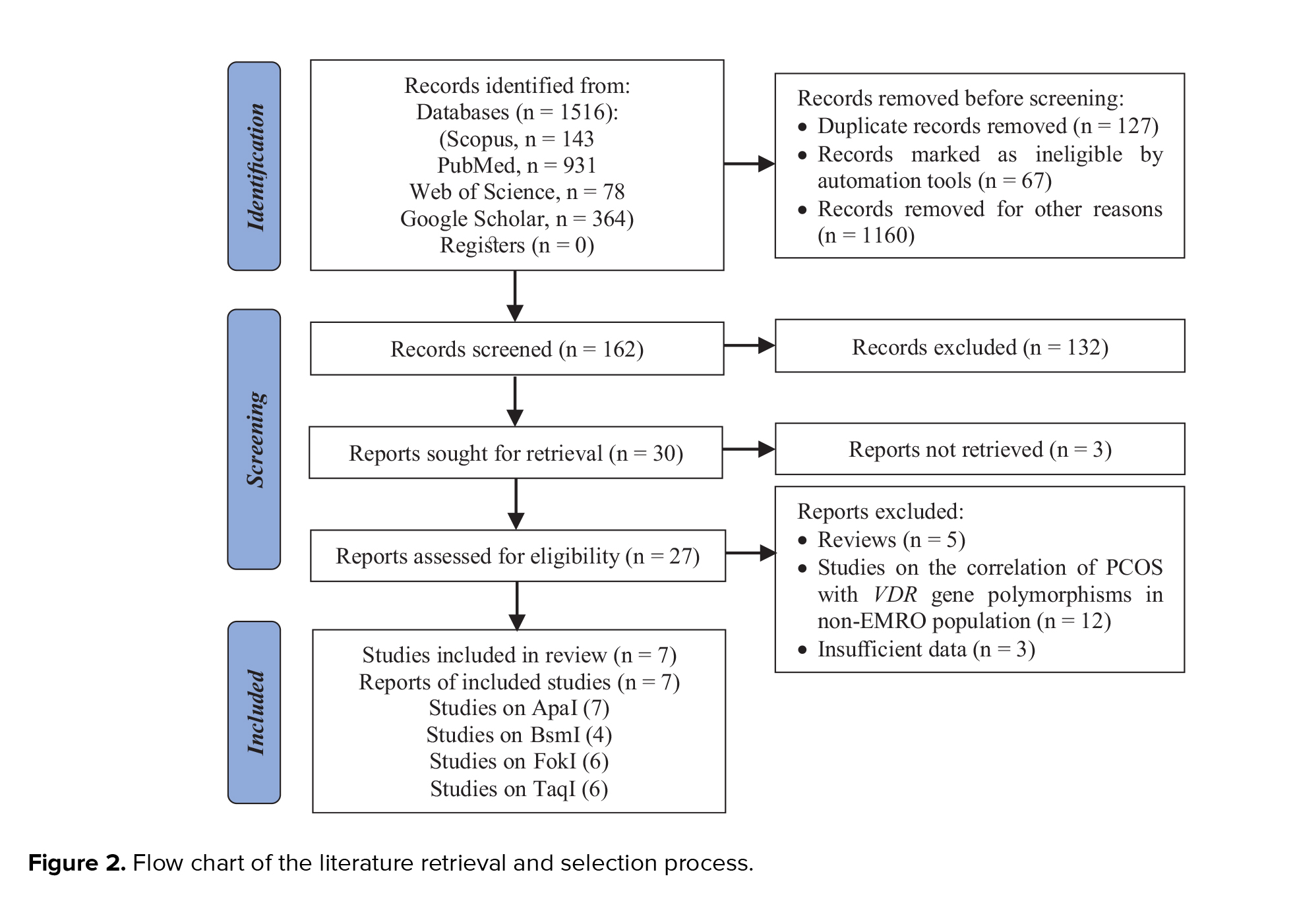
This review included 6 studies on the relationship between the VDR ApaI rs7975232 (A > C) polymorphism and risk of PCOS in the EMRO population. In all reports, the heterogeneity test showed no substantial heterogeneity. Therefore, the fixed effects model findings from the Mantel-Haenszel system were applied. As illustrated in figure 3, the correlation of ApaI SNP in the VDR gene with vulnerability to PCOS was statistically significant only in the dominant genetic model in the total populations (AA vs. Aa+aa: OR = 1.466, 95% CI = 1.093-1.9671, p = 0.01). No significant relationships of the VDR ApaI polymorphism with susceptibility to PCOS were observed in the other models (allelic, homozygote, and recessive genetic models, p > 0.05).

3.2.2. VDR BsmI variant and PCOS susceptibility
4 studies on the relationship between the VDR BsmI polymorphism and PCOS susceptibility were included in the meta-analysis (Table I). The heterogeneity test found that no substantial heterogeneity occurred in any of the included reports, and the fixed effects model results of the Mantel-Haenszel method were added to the analysis. There was no significant correlation between the VDR BsmI variant and PCOS susceptibility in the included EMRO population (p > 0.05).
3.2.3. VDR FokI variant and PCOS susceptibility
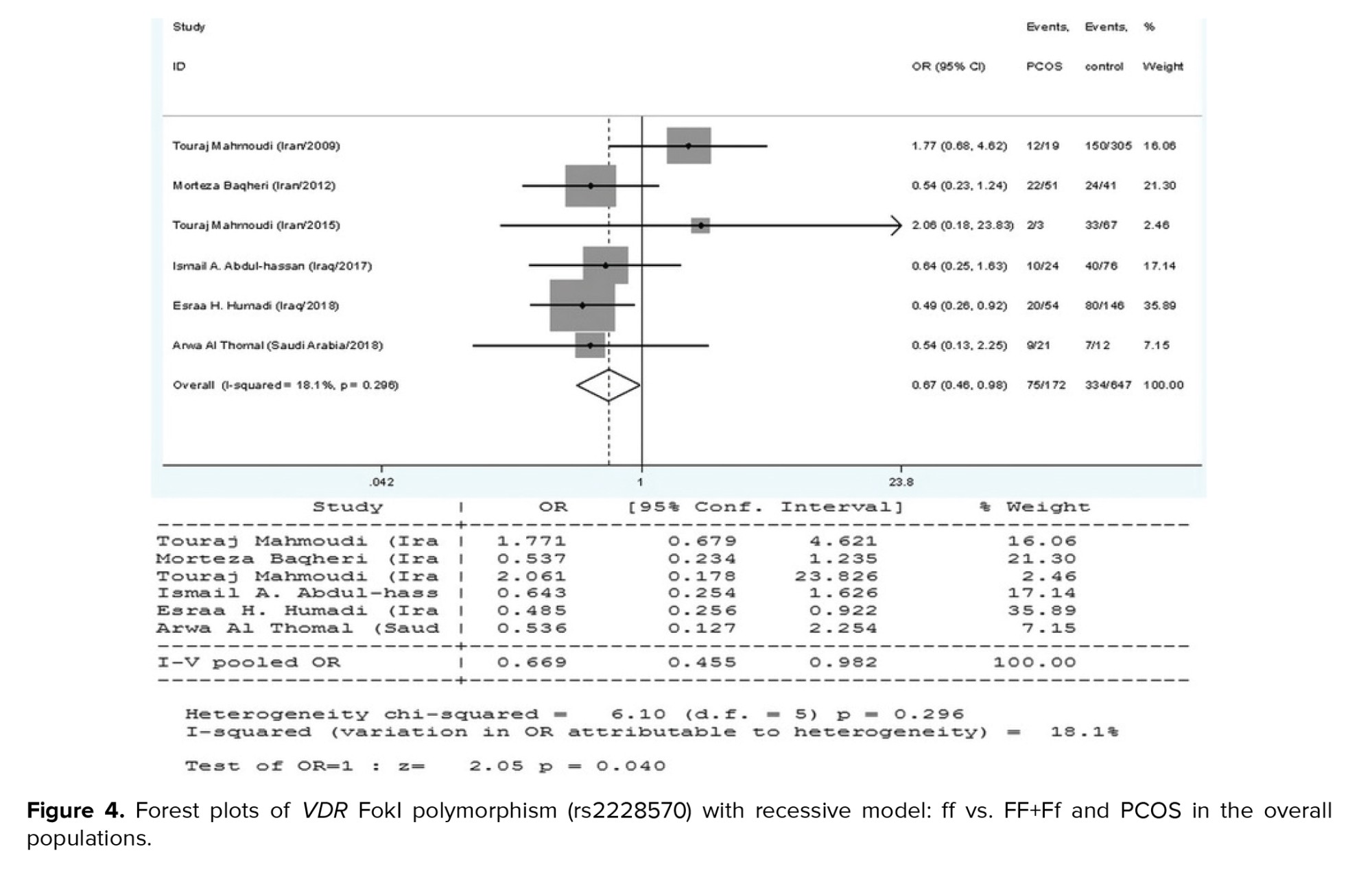
The current meta-analysis included a total of 8 reports on the relationship between the VDR FokI polymorphism and PCOS susceptibility, as seen in table I. We used the findings from the random-effects model on the grounds that heterogeneity was significant in certain model contrasts (allelic and homozygote models). A significant association of decreased risk was observed in the recessive genetic model (ff vs. FF+Ff: OR = 0.669, 95% CI = 0.455-0.982, p = 0.04) (Figure 4). In the allelic (F vs. f), homozygote (FF vs. ff), and dominant (FF vs. Ff+ff) genetic models, the pooled analysis found no significant association between this locus and PCOS risk (p > 0.05).
3.2.4. VDR TaqI variant and PCOS susceptibility
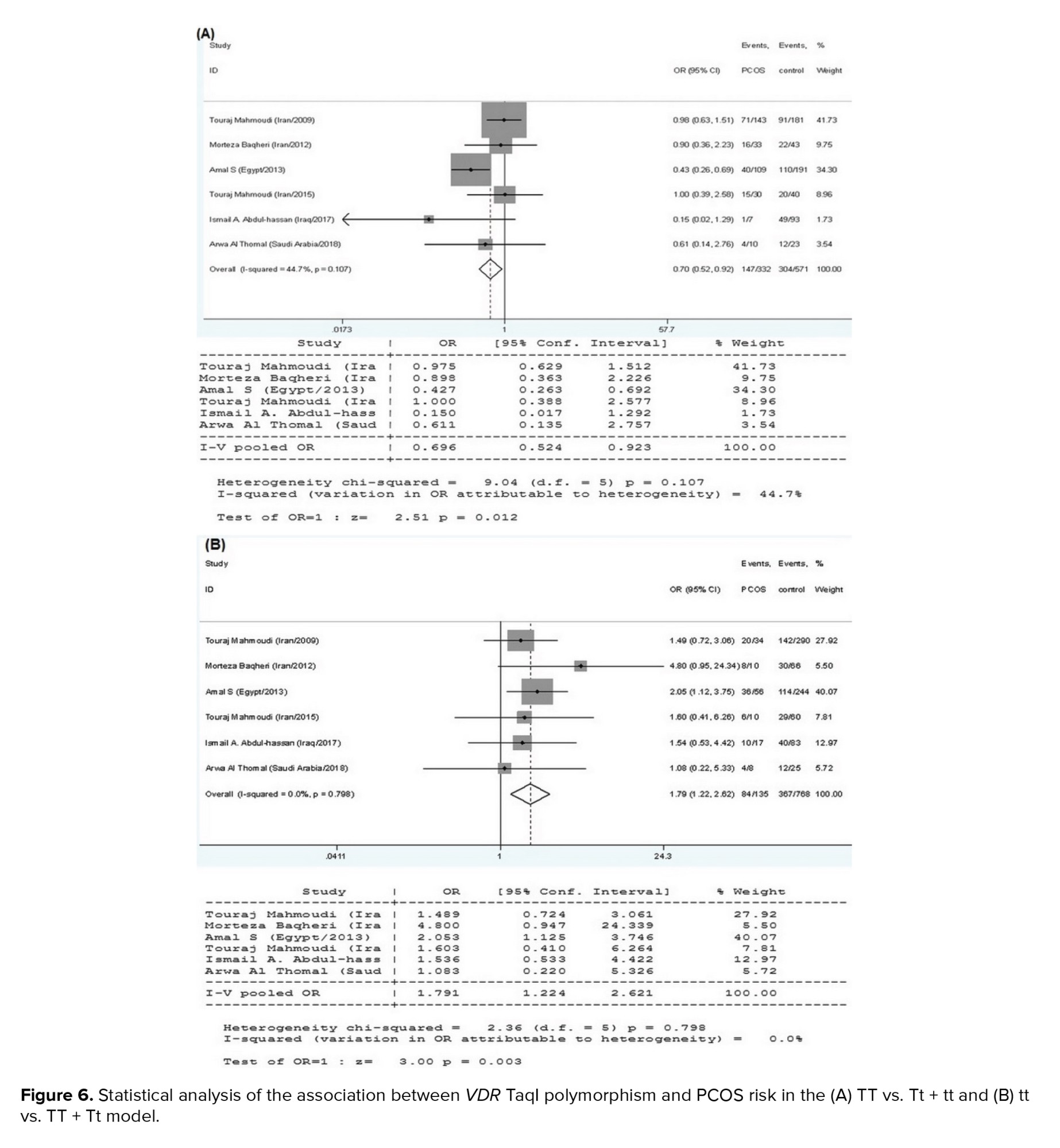
6 research papers on the association of the VDR TaqI polymorphisms with the risk of PCOS were found, as shown in table I. There was no significant heterogeneity in any of the comparisons, so the results of the random effects model were used in the analysis. In all 4 genetic models, there were significant associations of increased risk (T vs. t: OR = 0.715, 95% CI = 0.590-0.866, p < 0.001; TT vs. tt: OR = 0.435, 95% CI = 0.281-0.673, p < 0.001; TT vs. Tt+tt: OR = 0.696, 95% CI = 0.524-0.923, p = 0.01; tt vs. TT+Tt: OR = 1.791, 95% CI = 1.224-2.621, p < 0.001) in the included EMRO population (Table II, Figures 5 and 6).
3.3. Publication bias
As indicated in table II, the Begg and Egger trials and the Begg funnel plots were carried out in all comparisons. No significant asymmetrical evidence was found, indicating that there was no publication bias in the meta-analysis.

4. Discussion
In this meta-analysis, we presented the evidence on 4 VDR polymorphisms (ApaI, BsmI, FokI, and TaqI), and evaluated their association with PCOS risk in the EMRO population. The meta-analysis included a total of 7 articles based on 560 PCOS patients and 559 controls, which showed that the VDR TaqI polymorphism in all comparisons consisting of allelic, homozygote, dominant, or recessive genetic models was associated with PCOS susceptibility. Furthermore, the VDR ApaI variant in the dominant model and VDR FokI polymorphism in the recessive model were found to be significantly related to the risk of PCOS. However, the BsmI polymorphism was not significantly correlated with PCOS susceptibility in the included population.
Complex gene-gene and gene-environment interactions have been identified as major risk factors for the development of PCOS. PCOS is often associated with several complications, including hyperandrogenism, oligoanovulation, insulin tolerance, and metabolic anomalies. According to various reports, vitamin D deficiency has been linked to several metabolic risks in women with PCOS (36, 37). The active form of vitamin D exerts its effect by binding to VDR, as seen in figure 1. VDR gene variations have been linked to serum levels of insulin in women with PCOS in previous investigations (38). Serum 25-hydroxyvitamin D [(25 [(OH]) D] has been demonstrated to have a negative influence on VDR-mediated insulin resistance via regulating the expression of target genes (30). The VDR gene, which is involved in the insulin signaling system, could be a candidate risk factor for PCOS (39).
The polymorphisms ApaI, BsmI (intron 8), and TaqI (exon 9) are adjacent to the 3'-untranslated portion of the VDR gene, but they do not affect the VDR protein. ApaI, BsmI, and TaqI variants have also been shown to be in deep linkage disequilibrium (40). Even though these SNPs may not affect structural or protein expression, they can impact VDR transcript stability by adding changes in miRNA binding sites or other regulatory factors and gene expression while they are in linkage disequilibrium with different variants in the VDR gene's 3-untranslated region (41). Nevertheless, VDR mRNA produced by the t allele is more stable in peripheral blood mononuclear cells with the Tt TaqI haplotype than those with the T allele (42). Consequently, these VDR gene polymorphisms could be involved in the development of PCOS; nevertheless, since these polymorphisms are mostly nonfunctional, linkage imbalances with another undisclosed functional variant of the VDR gene seem to be the most plausible reason for the observed association.
In allele comparisons, the pooled results of the current meta-analysis revealed a substantial connection between the TaqI polymorphism and PCOS susceptibility. Our findings revealed a 0.715-, 0.435- and 0.696-fold lower risk for PCOS in the allelic, homozygote, and dominant models, respectively. Accordingly, the recessive genetic model of the TaqI variant (tt vs. TT+Tt) showed a 1.791-fold higher risk for PCOS. We compared minor alleles vs. major alleles in all analysis models. Our data indicated that the minor allele of the TaqI polymorphism (T allele) provides a protective genetic background against PCOS and the major allele (t allele) is a predisposing factor. Furthermore, the present meta-analysis showed that the ApaI variant is significantly associated with PCOS in the dominant genetic model by increasing the disease risk by 1.466-fold. This highlights the predisposing effect of the minor allele of the ApaI polymorphism. Lack of association for the other genetic models could be due to the quality and power of the included studies and their low sample sizes. Besides, we did not find any significant protective/predisposing association between the VDR BsmI variant and PCOS risk. The association of the TaqI variant with PCOS has been assessed in several studies.
Consistent with our results, a meta-analysis disclosed that the T allele of the VDR TaqI SNP was significantly associated with PCOS risk. However, a correlation of VDR ApaI and BsmI with susceptibility to PCOS was not found in any of the included studies (43). In contrast to our findings, a meta-analysis published in 2019 found a link between ApaI and BsmI, and PCOS vulnerability in the Asian population. However, VDR TaqI did not show a connection (22). In a similar study, a group of researchers discovered an essential link between ApaI and PCOS vulnerability but no link between TaqI and risk of PCOS (14). However, they found only a small increase in PCOS risk associated with the BsmI A/G polymorphism in Asians based on ethnic diversity. Overall, this study’s results are aligned with some studies (24, 26, 28, 32) and are in contrast to others (44-46), and might shed some light on how to proceed henceforth in determining the etiology of PCOS. The fact that the meta-analysis contained different studies, with different sample sizes and different statistical abilities may explain the contradictory findings.
Another of the VDR variants is FokI, which is located on exon 2 and results in a protein of different sizes. While this SNP has not been shown to interact with ligand binding, heterodimer formation with retinoid X receptor, or DNA binding, most studies have indicated that the shorter type of the protein (424 aa) is somewhat more active than the longer form (427 aa). However, it tends to be a gene- and cell-type-specific phenomenon, indicating that specific genes and cell types could be more susceptible to the polymorphism's effect than others (40, 47). According to some research, this polymorphism controls mRNA expression and may lead to different diseases (48). Although our study found that VDR FokI in the recessive genetic model (ff vs. FF+Ff) can influence PCOS, the other genetic models did not reveal significant protective/susceptible effects. Similar to our findings, 2 other meta-analyses also showed that VDR FokI (rs2228570) did not reveal a relationship with PCOS susceptibility (14, 22). The association of the FokI polymorphism with PCOS susceptibility has been examined in several case-control studies with varied results (23, 29, 31). These inconsistencies may be due to different sample sizes, included reports and levels of statistical power.
Despite the usefulness of the present meta-analysis (with its amalgamation of the key findings from different reports to provide a more comprehensive oversight of the issue at hand), we are aware of several limitations that should be addressed in interpreting our results. These are: the potential language bias from the exclusive focus on English-language publications; the fact that most of the studies were conducted in Iranian populations and the comparatively small number of studies examining the other EMRO populations; the lack of consideration for other factors potentially impacting PCOS susceptibility (such as age, gender, genetic variation, environmental factors); and the relatively small sample size.
5. Conclusion
In summary, the current meta-analysis provided statistical evidence that VDR ApaI in the dominant model, VDR FokI in the recessive model, and VDR TaqI polymorphisms in all genetic models are associated with PCOS susceptibility in the EMRO population. The BsmI variant did not reveal a relationship with PCOS susceptibility. However, these observations may not extend to other ethnic groups. Further analysis with a larger sample size and consideration of other confounding variables are needed before a firm conclusion can be drawn.
Acknowledgments
The study was supported by the Kurdistan University of Medical Sciences, Sanandaj, Iran under grant number 1398.216.
Conflict of Interest
The authors declare that there is no conflict of interest.
Interactions between genetic and environmental factors are believed to play a significant role in the incidence and progression of PCOS (8, 9, 10). Insulin resistance and hyperinsulinemia are recurrent metabolic disturbances in PCOS. Research demonstrates that levels of vitamin D could be related to hormonal and metabolic conditions (11). Vitamin D is converted to 1, 25-dihydroxycholecalciferol in the kidneys and liver (12, 13). Lines of evidence suggest a significant relationship of vitamin D levels with the pathogenesis, signs, and symptoms of PCOS (14). A recent meta-analysis identified more considerable variation in serum 25-hydroxyvitamin D, total cholesterol, serum insulin, low-density lipoprotein cholesterol, and triglycerides in women with PCOS than in healthy subjects (15).
The vitamin D receptor (VDR), a ligand-dependent transcription factor belonging to the steroid/thyroid hormone receptor superfamily, is responsible for vitamin D's effect. VDR is commonly found in many tissues of the female reproductive system and regulates the biological effects of vitamin D (16, 17). As is illustrated in figure 1, VDR and unoccupied retinoid X receptor form a heterodimer transcription unit which regulates the transcription through binding to the vitamin D response element in the promoter region of target genes (18, 19). About 3% of the human genome, including genes essential to glucose metabolism, are regulated by VDR (13).
The VDR gene is located on chromosome 12q13.11and contains 14 exons that provide instruction for a 427-amino-acid protein (20). The VDR gene contain 4 mostly reported single nucleotide polymorphisms (SNPs) including FokI (rs10735810) in exon 2, ApaI (rs7975232) in intron 8, TaqI (rs731236) in exon 9, and BsmI (rs1544410) in intron 8 (21). The previous meta-analyses have reported the association of VDR gene polymorphisms with PCOS risk (14, 22). However, these analyses must be updated and performed on specific populations. It has been shown that the VDR polymorphisms may correspond to PCOS susceptibility in the Eastern Mediterranean Regional Office (EMRO) population, even though results are as yet uncertain and conflicting owing to the small sample size and limited statistical power (23-33). Appropriate and accurate data from developing countries in the EMRO are lacking; the majority of published data from these areas are outdated, heterogeneous, and suffering from limited sample sizes (34).
Consequently, the present study aimed to conduct an updated systematic review and meta-analysis to more reliably examine the association of VDR gene polymorphisms with susceptibility to PCOS in the EMRO population.

2. Materials and Methods
2.1. Identification of eligible studiesThe Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were applied to conduct the present meta-analysis (35). An online search was carried out in PubMed, Scopus, SCI, and Google Scholar to retrieve related reports up to the end of 2020. The search strategy was based on terms and keywords including: ‘polycystic ovary syndrome OR PCOS’, ‘vitamin D receptor OR VDR’, and ‘polymorphisms OR variants’. Manual searches in the references cited within retrieved articles were also performed to select the relevant publications. Other prospective studies not included in the database were manually sought in the references of the obtained papers.
The inclusion criteria for this meta-analysis required that the articles be written in English, and included case-control studies, research on the association of ApaI, BsmI, FokI, and TaqI polymorphisms in the VDR gene with PCOS, investigations on the accessibility of genotype distributions in cases and controls for calculating odds ratios (ORs) and their 95% confidence intervals (CI), as well as studies on PCOS diagnosis using Rotterdam and National Institute of Child Health and Human Development criteria. Case reports, publications without full text, reviews, editorial comments, repeat publications, those with unavailability of the genotypic data, reports on the association of other polymorphisms in the VDR gene with PCOS, and the current meta-analysis did not include research that used an animal model. In the case of multiple publications reported by the same team, the most recent or largest sample sizes were chosen.
2.2. Data extraction
The eligible studies were selected according to the following criteria by 2 independent authors (A.Az and A.SH), and any disagreements were decided by a third reviewer. The first author of each study, publication year, country of origin, the sample size of cases and controls, and data of the frequency genotype of distribution were recorded.
2.3. Ethical considerations
The study was performed under the approval of the Ethical Committee of Kurdistan University Medical Sciences, Sanandaj, Iran (Code: IR.MUK.REC.1398.216).
2.4. Statistical analysis
Using crude ORs and their 95% CI, the statistical power of the relationship between VDR gene variants and PCOS risk was determined. Allelic, recessive, and dominant genetic models were used in all analyses. A Chi-square test based on Q statistics was used to evaluate the inter-study heterogeneity, wherein the fixed model was applied when the p-value was > 0.10. Otherwise, a random-effects model was adopted. To examine which experiments had a substantial effect on the stability of the findings, one-way sensitivity tests were conducted. To evaluate possible publishing bias, we used the Begg funnel plot and Egger's linear regression test. STATA version 12.0 software (STATA Corporation, College Station, Texas, USA) was used to calculate the OR and 95% CI, and two-sided p-values < 0.05 were regarded as statistically significant.
3. Results
3.1. Characteristics of eligible studiesThe studies used in this meta-analysis were selected using the preferred reporting items for systematic reviews, and meta-analyses flow diagram, as seen in figure 2. To begin, we searched the abovementioned databases and found a total of 1,516 reports. The 1,354 duplicates and ineligible records were removed before screening, and reviews, irrelevant reports, and studies not considering the VDR gene or PCOS were excluded from the extracted records, which consisted of 152 papers. Then, we excluded 3 studies because there was inadequate evidence to quantify ORs and 95% CIs, or it was not a case-control design. Finally, this meta-analysis contained 7 articles conducted on the association of ApaI, BsmI, FokI, and TaqI polymorphisms in the VDR gene with risk of PCOS in the EMRO population.
The association of the VDR gene ApaI rs7975232 (G > T) polymorphism, BsmI rs1544410 (A > G) variant, the FokI rs2228570 (C > T) polymorphism, and Taq1 rs731236 (T > C) variant was examined in 6 (23, 24, 29, 30, 32, 33), 4 (26, 29-31), 6 (23, 24, 29-31, 33), and 4 (24, 28-30) case-control studies, respectively. Table I shows the characteristics of studies on VDR ApaI, BsmI, FokI, and TaqI variants and PCOS susceptibility.


3.2. Quantitative results
Table II shows the details of the association of VDR gene ApaI rs7975232 (A > C), BsmI rs1544410 (A > G), FokI rs2228570 (C > T), and TaqI rs731236 (T > C) polymorphisms with PCOS predisposition. ApaI (C > A) correlates to ApaI (A/a), BsmI (A/G) to BsmI (B/b), FokI (C/T) to FokI (F/f), and TaqI (T > C) to TaqI (T/t), where the minor vs major alleles were evaluated for their connection with the disease as a susceptible/protective factor.


3.2.1. VDR ApaI variant and PCOS risk
This review included 6 studies on the relationship between the VDR ApaI rs7975232 (A > C) polymorphism and risk of PCOS in the EMRO population. In all reports, the heterogeneity test showed no substantial heterogeneity. Therefore, the fixed effects model findings from the Mantel-Haenszel system were applied. As illustrated in figure 3, the correlation of ApaI SNP in the VDR gene with vulnerability to PCOS was statistically significant only in the dominant genetic model in the total populations (AA vs. Aa+aa: OR = 1.466, 95% CI = 1.093-1.9671, p = 0.01). No significant relationships of the VDR ApaI polymorphism with susceptibility to PCOS were observed in the other models (allelic, homozygote, and recessive genetic models, p > 0.05).

3.2.2. VDR BsmI variant and PCOS susceptibility
4 studies on the relationship between the VDR BsmI polymorphism and PCOS susceptibility were included in the meta-analysis (Table I). The heterogeneity test found that no substantial heterogeneity occurred in any of the included reports, and the fixed effects model results of the Mantel-Haenszel method were added to the analysis. There was no significant correlation between the VDR BsmI variant and PCOS susceptibility in the included EMRO population (p > 0.05).
3.2.3. VDR FokI variant and PCOS susceptibility
The current meta-analysis included a total of 8 reports on the relationship between the VDR FokI polymorphism and PCOS susceptibility, as seen in table I. We used the findings from the random-effects model on the grounds that heterogeneity was significant in certain model contrasts (allelic and homozygote models). A significant association of decreased risk was observed in the recessive genetic model (ff vs. FF+Ff: OR = 0.669, 95% CI = 0.455-0.982, p = 0.04) (Figure 4). In the allelic (F vs. f), homozygote (FF vs. ff), and dominant (FF vs. Ff+ff) genetic models, the pooled analysis found no significant association between this locus and PCOS risk (p > 0.05).

3.2.4. VDR TaqI variant and PCOS susceptibility
6 research papers on the association of the VDR TaqI polymorphisms with the risk of PCOS were found, as shown in table I. There was no significant heterogeneity in any of the comparisons, so the results of the random effects model were used in the analysis. In all 4 genetic models, there were significant associations of increased risk (T vs. t: OR = 0.715, 95% CI = 0.590-0.866, p < 0.001; TT vs. tt: OR = 0.435, 95% CI = 0.281-0.673, p < 0.001; TT vs. Tt+tt: OR = 0.696, 95% CI = 0.524-0.923, p = 0.01; tt vs. TT+Tt: OR = 1.791, 95% CI = 1.224-2.621, p < 0.001) in the included EMRO population (Table II, Figures 5 and 6).
3.3. Publication bias
As indicated in table II, the Begg and Egger trials and the Begg funnel plots were carried out in all comparisons. No significant asymmetrical evidence was found, indicating that there was no publication bias in the meta-analysis.


4. Discussion
In this meta-analysis, we presented the evidence on 4 VDR polymorphisms (ApaI, BsmI, FokI, and TaqI), and evaluated their association with PCOS risk in the EMRO population. The meta-analysis included a total of 7 articles based on 560 PCOS patients and 559 controls, which showed that the VDR TaqI polymorphism in all comparisons consisting of allelic, homozygote, dominant, or recessive genetic models was associated with PCOS susceptibility. Furthermore, the VDR ApaI variant in the dominant model and VDR FokI polymorphism in the recessive model were found to be significantly related to the risk of PCOS. However, the BsmI polymorphism was not significantly correlated with PCOS susceptibility in the included population.
Complex gene-gene and gene-environment interactions have been identified as major risk factors for the development of PCOS. PCOS is often associated with several complications, including hyperandrogenism, oligoanovulation, insulin tolerance, and metabolic anomalies. According to various reports, vitamin D deficiency has been linked to several metabolic risks in women with PCOS (36, 37). The active form of vitamin D exerts its effect by binding to VDR, as seen in figure 1. VDR gene variations have been linked to serum levels of insulin in women with PCOS in previous investigations (38). Serum 25-hydroxyvitamin D [(25 [(OH]) D] has been demonstrated to have a negative influence on VDR-mediated insulin resistance via regulating the expression of target genes (30). The VDR gene, which is involved in the insulin signaling system, could be a candidate risk factor for PCOS (39).
The polymorphisms ApaI, BsmI (intron 8), and TaqI (exon 9) are adjacent to the 3'-untranslated portion of the VDR gene, but they do not affect the VDR protein. ApaI, BsmI, and TaqI variants have also been shown to be in deep linkage disequilibrium (40). Even though these SNPs may not affect structural or protein expression, they can impact VDR transcript stability by adding changes in miRNA binding sites or other regulatory factors and gene expression while they are in linkage disequilibrium with different variants in the VDR gene's 3-untranslated region (41). Nevertheless, VDR mRNA produced by the t allele is more stable in peripheral blood mononuclear cells with the Tt TaqI haplotype than those with the T allele (42). Consequently, these VDR gene polymorphisms could be involved in the development of PCOS; nevertheless, since these polymorphisms are mostly nonfunctional, linkage imbalances with another undisclosed functional variant of the VDR gene seem to be the most plausible reason for the observed association.
In allele comparisons, the pooled results of the current meta-analysis revealed a substantial connection between the TaqI polymorphism and PCOS susceptibility. Our findings revealed a 0.715-, 0.435- and 0.696-fold lower risk for PCOS in the allelic, homozygote, and dominant models, respectively. Accordingly, the recessive genetic model of the TaqI variant (tt vs. TT+Tt) showed a 1.791-fold higher risk for PCOS. We compared minor alleles vs. major alleles in all analysis models. Our data indicated that the minor allele of the TaqI polymorphism (T allele) provides a protective genetic background against PCOS and the major allele (t allele) is a predisposing factor. Furthermore, the present meta-analysis showed that the ApaI variant is significantly associated with PCOS in the dominant genetic model by increasing the disease risk by 1.466-fold. This highlights the predisposing effect of the minor allele of the ApaI polymorphism. Lack of association for the other genetic models could be due to the quality and power of the included studies and their low sample sizes. Besides, we did not find any significant protective/predisposing association between the VDR BsmI variant and PCOS risk. The association of the TaqI variant with PCOS has been assessed in several studies.
Consistent with our results, a meta-analysis disclosed that the T allele of the VDR TaqI SNP was significantly associated with PCOS risk. However, a correlation of VDR ApaI and BsmI with susceptibility to PCOS was not found in any of the included studies (43). In contrast to our findings, a meta-analysis published in 2019 found a link between ApaI and BsmI, and PCOS vulnerability in the Asian population. However, VDR TaqI did not show a connection (22). In a similar study, a group of researchers discovered an essential link between ApaI and PCOS vulnerability but no link between TaqI and risk of PCOS (14). However, they found only a small increase in PCOS risk associated with the BsmI A/G polymorphism in Asians based on ethnic diversity. Overall, this study’s results are aligned with some studies (24, 26, 28, 32) and are in contrast to others (44-46), and might shed some light on how to proceed henceforth in determining the etiology of PCOS. The fact that the meta-analysis contained different studies, with different sample sizes and different statistical abilities may explain the contradictory findings.
Another of the VDR variants is FokI, which is located on exon 2 and results in a protein of different sizes. While this SNP has not been shown to interact with ligand binding, heterodimer formation with retinoid X receptor, or DNA binding, most studies have indicated that the shorter type of the protein (424 aa) is somewhat more active than the longer form (427 aa). However, it tends to be a gene- and cell-type-specific phenomenon, indicating that specific genes and cell types could be more susceptible to the polymorphism's effect than others (40, 47). According to some research, this polymorphism controls mRNA expression and may lead to different diseases (48). Although our study found that VDR FokI in the recessive genetic model (ff vs. FF+Ff) can influence PCOS, the other genetic models did not reveal significant protective/susceptible effects. Similar to our findings, 2 other meta-analyses also showed that VDR FokI (rs2228570) did not reveal a relationship with PCOS susceptibility (14, 22). The association of the FokI polymorphism with PCOS susceptibility has been examined in several case-control studies with varied results (23, 29, 31). These inconsistencies may be due to different sample sizes, included reports and levels of statistical power.
Despite the usefulness of the present meta-analysis (with its amalgamation of the key findings from different reports to provide a more comprehensive oversight of the issue at hand), we are aware of several limitations that should be addressed in interpreting our results. These are: the potential language bias from the exclusive focus on English-language publications; the fact that most of the studies were conducted in Iranian populations and the comparatively small number of studies examining the other EMRO populations; the lack of consideration for other factors potentially impacting PCOS susceptibility (such as age, gender, genetic variation, environmental factors); and the relatively small sample size.
5. Conclusion
In summary, the current meta-analysis provided statistical evidence that VDR ApaI in the dominant model, VDR FokI in the recessive model, and VDR TaqI polymorphisms in all genetic models are associated with PCOS susceptibility in the EMRO population. The BsmI variant did not reveal a relationship with PCOS susceptibility. However, these observations may not extend to other ethnic groups. Further analysis with a larger sample size and consideration of other confounding variables are needed before a firm conclusion can be drawn.
Acknowledgments
The study was supported by the Kurdistan University of Medical Sciences, Sanandaj, Iran under grant number 1398.216.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Review Article |
Subject:
Reproductive Genetics
References
1. Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol 2013; 6: 1-13. [DOI:10.2147/CLEP.S37559] [PMID] [PMCID]
2. Jalilian A, Kiani F, Sayehmiri F, Sayehmiri K, Khodaee Z, Akbari M. Prevalence of polycystic ovary syndrome and its associated complications in Iranian women: A meta-analysis. Iran J Reprod Med 2015; 13: 591-604.
3. Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004; 89: 2745-2749.
https://doi.org/10.1210/jcem.89.9.9990 [DOI:10.1210/jc.2003-032046] [PMID]
4. Repaci A, Gambineri A, Pasquali R. The role of low-grade inflammation in the polycystic ovary syndrome. Mol Cell Endocrinol 2011; 335: 30-41. [DOI:10.1016/j.mce.2010.08.002] [PMID]
5. Ollila MM, West S, Keinänen-Kiukaanniemi S, Jokelainen J, Auvinen J, Puukka K, et al. Overweight and obese but not normal weight women with PCOS are at increased risk of Type 2 diabetes mellitus: A prospective, population-based cohort study. Hum Reprod 2017; 32: 423-431.
https://doi.org/10.1093/humrep/dex030 [DOI:10.1093/humrep/dew329]
6. Maitra A, Pingle RR, Menon PS, Naik V, Gokral JS, Meherji PK. Dyslipidemia with particular regard to apolipoprotein profile in association with polycystic ovary syndrome: A study among Indian women. Int J Fertil Womens Med 2001; 46: 271-277.
7. Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: A controlled study. J Clin Endocrinol Metab 2005; 90: 3236-3242. [DOI:10.1210/jc.2004-1843] [PMID]
8. Diamanti-Kandarakis E, Kandarakis H, Legro RS. The role of genes and environment in the etiology of PCOS. Endocrine 2006; 30: 19-26. [DOI:10.1385/ENDO:30:1:19]
9. Sheikhha MH, Kalantar SM, Ghasemi N. Genetics of polycystic ovary syndrome. Iran J Reprod Med 2007; 5: 1-5.
10. Mehdizadeh A, Kalantar SM, Sheikhha MH, Aali BSh, Ghanei A. Association of SNP rs. 2414096 CYP19 gene with polycystic ovarian syndrome in Iranian women. Int J Reprod BioMed 2017; 15: 491-496. [DOI:10.29252/ijrm.15.8.491] [PMCID]
11. Wehr E, Möller R, Horejsi R, Giuliani A, Kopera D, Schweighofer N, et al. Subcutaneous adipose tissue topography and metabolic disturbances in polycystic ovary syndrome. Wien Klin Wochenschr 2009; 121: 262-269. [DOI:10.1007/s00508-009-1162-2] [PMID]
12. Thys-Jacobs S, Donovan D, Papadopoulos A, Sarrel P, Bilezikian JP. Vitamin D and calcium dysregulation in the polycystic ovarian syndrome. Steroids 1999; 64: 430-435. [DOI:10.1016/S0039-128X(99)00012-4]
13. Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes: A systematic review and meta-analysis. J Clin Endocrinol Metab 2007; 92: 2017-2029. [DOI:10.1210/jc.2007-0298] [PMID] [PMCID]
14. Niu YM, Wang YD, Jiang GB, Bai G, Chai HB, Li XF, et al. Association between vitamin D receptor gene polymorphisms and polycystic ovary syndrome risk: A meta-analysis. Front Physiol 2019; 9: 1902. [DOI:10.3389/fphys.2018.01902] [PMID] [PMCID]
15. Bacopoulou F, Kolias E, Efthymiou V, Antonopoulos CN, Charmandari E. Vitamin D predictors in polycystic ovary syndrome: A meta‐analysis. Eur J Clin Invest 2017; 47: 746-755. [DOI:10.1111/eci.12800] [PMID]
16. Gyorffy B, Vásárhelyi B, Krikovszky D, Madácsy L, Tordai A, Tulassay T, et al. Gender-specific association of vitamin D receptor polymorphism combinations with type 1 diabetes mellitus. Eur J Endocrinol 2002; 147: 803-808. [DOI:10.1530/eje.0.1470803] [PMID]
17. Bid HK, Konwar R, Aggarwal C, Gautam S, Saxena M, Nayak VL, et al. Vitamin D receptor (FokI, BsmI and TaqI) gene polymorphisms and type 2 diabetes mellitus: A North Indian study. Indian J Med Sci 2009; 63: 187-194. [DOI:10.4103/0019-5359.53164] [PMID]
18. Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1α, 25 (OH) 2vitamin D3: Genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab 2011; 25: 543-559. [DOI:10.1016/j.beem.2011.05.010] [PMID]
19. Shahmoradi A, Ghaderi K, Aghaei A, Azarnezhad A. Associations of vitamin D receptor rs1544410 polymorphism with type 1 diabetes mellitus risk: Systematic review and meta-analysis. Meta Gene 2021; 30: 100973. [DOI:10.1016/j.mgene.2021.100973]
20. Baker AR, McDonnell DP, Hughes M, Crisp TM, Mangelsdorf DJ, Haussler MR, et al. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci 1988; 85: 3294-3298. [DOI:10.1073/pnas.85.10.3294] [PMID] [PMCID]
21. Abdollahzadeh R, Sobhani Fard M, Rahmani F, Moloudi K, Sadeghi Kalani B, Azarnezhad A. Predisposing role of vitamin D receptor (VDR) polymorphisms in the development of multiple sclerosis: A case-control study. J Neurol Sci 2016; 367: 148-151. [DOI:10.1016/j.jns.2016.05.053] [PMID]
22. Shi XY, Huang AP, Xie DW, Yu XL. Association of vitamin D receptor gene variants with polycystic ovary syndrome: A meta-analysis. BMC Med Genet 2019; 20: 32. [DOI:10.1186/s12881-019-0763-5] [PMID] [PMCID]
23. Humadi EH, Showman HA, Al-azzawie HF. Association of vitamin-D receptor gene polymorphisms (FokI and ApaI) and vitamin D serum levels in a sample of Iraqi women with polycystic ovary syndrome. Indian J Public Health Res Dev 2018; 9: 401-408. [DOI:10.5958/0976-5506.2018.01249.4]
24. Abdul-Hassan IA, Badr AH, Kadhim SJ. Association of vitamin d receptor gene polymorphism at three snps and their haplotypes with polycystic ovary syndrome risk in iraqi women. Pak J Biotechnol 2017; 14: 615-622.
25. Hamdi RA, Abdul-Qahar ZH, Ahmed SJ, Tawfeeq RK. Assessment of vitamin D receptor gene polymorphism in Iraqi women with polycystic ovary syndrome. J Clin Diagn Res 2018; 12: BC27-BC30. [DOI:10.7860/JCDR/2018/37349.12084]
26. Ramezani N, Ostadsharif M, Nayeri H. Association of BsmI variant of vitamin D receptor gene with polycystic ovary syndrome: A case-control study. Int J Reprod BioMed 2020; 18: 877-884. [DOI:10.18502/ijrm.v13i10.7772] [PMID] [PMCID]
27. Zadeh-Vakili A, Ramezani Tehrani F, Daneshpour MS, Zarkesh M, Saadat N, Azizi F. Genetic polymorphism of vitamin D receptor gene affects the phenotype of PCOS. Gene 2013; 515: 193-196. [DOI:10.1016/j.gene.2012.11.049] [PMID]
28. Bagheri M, Rad IA, Jazani NH, Nanbakhsh F. Vitamin D receptor TaqI gene variant in exon 9 and polycystic ovary syndrome risk. Int J Fertil Steril 2013; 7: 116-121.
29. Mahmoudi T. Genetic variation in the vitamin D receptor and polycystic ovary syndrome risk. Fertil Steril 2009; 92: 1381-1383. [DOI:10.1016/j.fertnstert.2009.05.002] [PMID]
30. Mahmoudi T, Majidzadeh-A K, Farahani H, Mirakhorli M, Dabiri R, Nobakht H, et al. Association of vitamin D receptor gene variants with polycystic ovary syndrome: A case control study. Int J Reprod BioMed 2015; 13: 793-800. [DOI:10.29252/ijrm.13.12.793] [PMID] [PMCID]
31. Bagheri M, Abdi Rad I, Hosseini Jazani N, Nanbakhsh F. Lack of association of vitamin D receptor FokI (rs10735810)(C/T) and BsmI (rs1544410)(A/G) genetic variations with polycystic ovary syndrome risk: A case-control study from Iranian Azeri Turkish women. Maedica 2012; 7: 303-308.
32. El-shal AS, Shalaby SM, Aly NM, Rashad NM, Abdelaziz AM. Genetic variation in the vitamin D receptor gene and vitamin D serum levels in Egyptian women with polycystic ovary syndrome. Mol Biol Rep 2013; 40: 6063-6073. [DOI:10.1007/s11033-013-2716-y] [PMID]
33. Al Thomali A, Daghestani MH, Daghestani MH, Kaya N, Warsy A. Polymorphic variations in VDR gene in Saudi women with and without polycystic ovary syndrome (PCOS) and significant influence of seven polymorphic sites on anthropometric and hormonal parameters. J Med Biochem 2018; 37: 415-425. [DOI:10.2478/jomb-2018-0007] [PMID] [PMCID]
34. Afzal M. Health research in the Word Health Organization Regional Office for the Eastern Mediterranean. East Mediterr Health J 2008; 14 (Suppl.): 67-73.
35. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg 2010; 8: 336-341. [DOI:10.1016/j.ijsu.2010.02.007] [PMID]
36. Asemi Z, Foroozanfard F, Hashemi T, Bahmani F, Jamilian M, Esmaillzadeh A. Calcium plus vitamin D supplementation affects glucose metabolism and lipid concentrations in overweight and obese vitamin D deficient women with polycystic ovary syndrome. Clin Nutr 2015; 34: 586-592. [DOI:10.1016/j.clnu.2014.09.015] [PMID]
37. Foroozanfard F, Talebi M, Samimi M, Mehrabi S, Badehnoosh B, Jamilian M, et al. Effect of two different doses of vitamin D supplementation on metabolic profiles of insulin-resistant patients with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Horm Metab Res 2017; 49: 612-617. [DOI:10.1055/s-0043-112346] [PMID]
38. Nandi A, Sinha N, Ong E, Sonmez H, Poretsky L. Is there a role for vitamin D in human reproduction? Horm Mol Biol Clin Investig 2016; 25: 15-28. [DOI:10.1515/hmbci-2015-0051] [PMID]
39. Shahrokhi SZ, Ghaffari F, Kazerouni F. Role of vitamin D in female reproduction. Clin Chim Acta 2016; 455: 33-38. [DOI:10.1016/j.cca.2015.12.040] [PMID]
40. Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene 2004; 338: 143-156. [DOI:10.1016/j.gene.2004.05.014] [PMID]
41. Abdollahzadeh R, Moradi Pordanjani P, Rahmani F, Mashayekhi F, Azarnezhad A, Mansoori Y. Association of VDR gene polymorphisms with risk of relapsing-remitting multiple sclerosis in an Iranian Kurdish population. Int J Neurosci 2018; 128: 505-511. [DOI:10.1080/00207454.2017.1398158] [PMID]
42. Goulart LR, Ferreira FR, Goulart IMB. Interaction of Taq I polymorphism at exon 9 of the vitamin D receptor gene with the negative lepromin response may favor the occurrence of leprosy. FEMS Immunol Med Microbiol 2006; 48: 91-98. [DOI:10.1111/j.1574-695X.2006.00128.x] [PMID]
43. Han FF, Lv YL, Gong LL, Liu H, Wan ZR, Liu LH. VDR gene variation and insulin resistance related diseases. Lipids Health Dis 2017; 16: 157. [DOI:10.1186/s12944-017-0477-7] [PMID] [PMCID]
44. Dasgupta S, Dutta J, Annamaneni S, Kudugunti N, Battini MR. Association of vitamin D receptor gene polymorphisms with polycystic ovary syndrome among Indian women. Indian J Med Res 2015; 142: 276-285. [DOI:10.4103/0971-5916.166587] [PMID] [PMCID]
45. Wehr E, Trummer O, Giuliani A, Gruber HJ, Pieber TR, Obermayer-Pietsch B. Vitamin D-associated polymorphisms are related to insulin resistance and vitamin D deficiency in polycystic ovary syndrome. Eur J Endocrinol 2011; 164: 741-749. [DOI:10.1530/EJE-11-0134] [PMID]
46. Liang F, Ren N, Zhang H, Zhang J, Wu Q, Song R, et al. A meta-analysis of the relationship between vitamin D receptor gene ApaI polymorphisms and polycystic ovary syndrome. Adv Clin Exp Med 2019; 28: 255-262. [DOI:10.17219/acem/85882] [PMID]
47. Jurutka PW, Remus LS, Whitfield GK, Thompson PD, Hsieh JC, Zitzer H, et al. The polymorphic N terminus in human vitamin D receptor isoforms influences transcriptional activity by modulating interaction with transcription factor IIB. Mol Endocrinol 2000; 14: 401-420. [DOI:10.1210/mend.14.3.0435] [PMID]
48. Ahmadi S, Mirzaei K, Hossein-Nezhad A, Shariati G. Vitamin D receptor FokI genotype may modify the susceptibility to schizophrenia and bipolar mood disorder by regulation of dopamine D1 receptor gene expression. Minerva Med 2012; 103: 383-391.
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








