Sat, Jan 31, 2026
[Archive]
Volume 20, Issue 6 (June 2022)
IJRM 2022, 20(6): 483-490 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ebrahimi M, Akbari Asbagh F, Davari Tanha F, Pakniat H, Feizabad E, Rasouli Y. Co-treatment of gonadotropin and letrozole in infertile women with endometriosis: A double-blind randomized clinical trial. IJRM 2022; 20 (6) :483-490
URL: http://ijrm.ir/article-1-2271-en.html
URL: http://ijrm.ir/article-1-2271-en.html
Mahbod Ebrahimi1 

 , Firoozeh Akbari Asbagh1
, Firoozeh Akbari Asbagh1 

 , Fatemeh Davari Tanha1
, Fatemeh Davari Tanha1 

 , Hamideh Pakniat *2
, Hamideh Pakniat *2 

 , Elham Feizabad1
, Elham Feizabad1 

 , Yasin Rasouli3
, Yasin Rasouli3 




 , Firoozeh Akbari Asbagh1
, Firoozeh Akbari Asbagh1 

 , Fatemeh Davari Tanha1
, Fatemeh Davari Tanha1 

 , Hamideh Pakniat *2
, Hamideh Pakniat *2 

 , Elham Feizabad1
, Elham Feizabad1 

 , Yasin Rasouli3
, Yasin Rasouli3 


1- Department of Obstetrics and Gynecology, Yas Hospital, Tehran University of Medical Sciences, Tehran, Iran.
2- Department of Obstetrics and Gynecology, Yas Hospital, Tehran University of Medical Sciences, Tehran, Iran. Department of Obstetrics and Gynecology, Faculty of Medicine, Qazvin University of Medical Sciences, Qazvin, Iran. ,hpakniat@qums.ac.ir
3- Faculty of Pharmacy, University of Szeged, Hungary.
2- Department of Obstetrics and Gynecology, Yas Hospital, Tehran University of Medical Sciences, Tehran, Iran. Department of Obstetrics and Gynecology, Faculty of Medicine, Qazvin University of Medical Sciences, Qazvin, Iran. ,
3- Faculty of Pharmacy, University of Szeged, Hungary.
Full-Text [PDF 351 kb]
(1423 Downloads)
| Abstract (HTML) (2331 Views)
Full-Text: (537 Views)
1. Introduction
The prevalence of endometriosis, tissue and glands of endometrium placed outside the uterus, in infertile women is 25-50%, and it can cause the failure of in vitro fertilization (IVF) treatment (1, 2). The usual causes of infertility in women with endometriosis are folliculogenesis alternation, steroidogenesis and fertilization impairment, oocyte and embryo quality reduction, implantation defect, and pelvic adhesions (3). Endometriosis is an estrogen-dependent disease associated with increased aromatase enzyme expression and concentration and some pathologic mediator secretion, such as of estradiol and prostaglandin E2 (1). These pathologic mediators have an important role in promoting the growth and invasion of endometriotic tissue, pain, inflammation, and infertility (4).
Nowadays, assisted-reproductive technology can have a considerable role in resolving infertility problems in most couples. However, previous studies have shown significantly lower successful fertilization rates in endometriosis rather than other causes of infertility in IVF cycles (5-7). Letrozole is a selective aromatase inhibitor, that causes a decrease the estrogen concentration. The result of a decline in estrogen level is an increase in follicle-stimulating hormone secretion, ovarian Follicle-stimulating hormone receptor affinity, antral follicle growth, follicle phase enhancement, and follicle development. The other effect of letrozole is reducing estradiol and prostaglandin E2 production, which affects oocyte quality (8, 9). Letrozole has been recommended in some researches for improving fertility results in poor responder women, treatment of endometriosis-related pelvic pain, treatment of hormone receptor-positive breast cancer, and fertility preservation in women with breast cancer (10-12).
However, studies about the application of letrozole in the IVF cycles of women with endometriosis are rare and more studies are needed on this topic. This study was designed to compare IVF cycle success rates of women with endometriosis treated with letrozole + gonadotropin (LA) vs. placebo + gonadotropin (PA).
2. Materials and Methods
This double-blind, randomized clinical trial study with a parallel design was done with 94 infertile women with endometriosis referred to our IVF Unit at Yas hospital, Tehran, Iran from April-June 2021. The inclusion criteria included women with pelvic endometriosis and primary infertility, in their first IVF cycle, 18-35 yr old, body mass index < 30 kg/m2, serum anti-Mullerian hormone (AMH) > 1 ng/ml, and partner sperm motility of at least 20%.
Women who had undergone letrozole or clomiphene therapy with the aim of inducing ovulation, or who had deeply infiltrating endometriosis, or submucosal or intramural myoma, detected in transvaginal ultrasound (TVS), or with uterine diseases were excluded.
This study was conducted double-blind. The participants, because of placebo usage, did not know the type of their treatment. Also, the analyzer did not know about the treatment group codes in the analysis data sheet.
Using random allocation, participants were divided by the corresponding author into the 2 groups of LA and PA (n = 47/each). First, 47 letter (As) and 47 letter (Bs) were written on papers without other markings. All of the papers were placed in a bag, and for each woman, a paper was taken randomly and without replacement. In addition, interventions A and B were randomly assigned to the LA group and PA group, respectively.
For all participants, the long agonist protocol was applied. 300 mcg of gonadotropin-releasing hormone (GnRH) agonist (CinnaFact, CinnaGen Company, Iran) was prescribed in the mid-luteal stage (7 days before the anticipated menstruation). Then, on the third day of menstruation, the women were evaluated with TVS (4.5-7 MHz vaginal probe, Sono line G-40, Siemens, Germany) for endometrial thickness (ET) and antral follicle counts assessment in both ovaries.
In both groups, from the third day of the cycle, and gonadotropin (CinnalF, CinnaGen Company, Iran) was started and its doses were regulated based on the patient’s age, serum AMH and follicle-stimulating hormone. From the third day of the menstrual cycle, 5 mg of letrozole daily for 5 days prescribed for LA group, while placebo prescribed for PA on the identical days and duration.
Repeated TVS examinations were done with aim of follicular maturation assessment. Human menopausal gonadotropin (Pooyesh Daru, Iran) was added whenever follicle(s) sizes were ≥ 10-12 mm. furthermore, CinnalF continued until the triggering day of ovulation.
Then, 250 µg of choriogonadotropin alfa (Ovitrelle, Merck Serono, Italy) was administrated subcutaneously if at least 2 follicles with ≥ 18 mm in diameter was reported and serum estradiol concentration (on trigger day) was ≥ 500 pg/mL. The cycle were cancelled when which of above criterias after 10-12 days following by stimulation did not detected.
After 34-36 hr following choriogonadotropin alfa initiation, oocyte retrieval with aid of TVS (Honda Company, Japan) was conducted under spinal anesthesia. Then, for all the cycles, intracytoplasmic sperm injection was carried out.
Fresh embryo transfer was done for all of the participants unless there were contraindications such as ovarian hyperstimulation syndrome, pelvic and abdominal pain due to endometriosis, endometrioma necessitating surgery, or at the woman's request. In these mentioned conditions, frozen embryo transfer was done.
In fresh embryo transfer, 100 mg of progesterone (Iran Hormone Company, Iran) was injected daily immediately after oocyte retrieval and for 2 wk. 3 days after puncture day, embryos (in cleavage form) were transferred. Serum β-human chorionic gonadotropin (β-hCG) was checked on the 14th day of embryo transfer, and if pregnancy was confirmed, 400 mg of suppository tablets of progesterone (Cyclogest, Actavis, Barnstaple, UK) was initiated and continued daily to the end of pregnancy.
In frozen embryo transfer, from the third day of the menstrual cycle, 6 mg of estradiol (Abu Reihan Pharmaceutical Company, Iran) was given daily as an oral pill. ET was assessed serially each 3-4 days, and when ET was > 8 mm, 100 mg of progesterone (Iran Hormone Company, Iran) was injected, and continued daily. After 4 days, progesterone-initiated embryos (in cleavage form) were transferred. Serum β-hCG was checked on the 14th day after embryo transfer, and if pregnancy occurred, 400 mg of suppository tablets of progesterone (Cyclogest, Actavis, Barnstaple, UK) were given daily with estradiol until the end of pregnancy.
The following data were recorded for both groups: age, marriage and infertility duration, body mass index, thyroid-stimulating hormone, prolactin, AMH, and Follicle-stimulating hormone.
The total prescribed dosage of gonadotropin (calculated on the trigger day), the serum estradiol level (measured on the trigger day), the oocyte number and quality (determined on the oocyte retrieval day according to the oocyte maturity grading), and the embryo quality (categorized based on the Gardner morphological assessment system, which grades expansion status, inner cell mass from A-C, trophectoderm from A-C, and blastocyst growth stage from 3-6) (13), were analyzed for all patients.
Serum β-hCG was measured on the 14th day after embryo transfer (to determine biochemical pregnancy), and pregnancy sac observation through TVS was assessed 6 wk after embryo transfer (to determine clinical pregnancy).
2.1. Ethical considerations
This study was approved by the Ethics Committee of Sina hospital, affiliated to Tehran University of Medical Sciences, Tehran, Iran (Code: IR.TUMS.SINAHOSPITAL.REC.1399.100). The study was registered in the Iranian randomized clinical trial registry and was done in compliance Declaration of Helsinki and all the participants signed an informed consent form.
2.2. Statistical analysis
All of the statistical analyses were done using the Statistical Package for the Social Sciences (SPSS) version 24.0. P-values < 0.05 were considered statistically significant. Independent t test and non-parametric Mann-Whitney U test were used to evaluate the differences in means. A Chi-square test and Fisher's exact test were applied to assess the differences in proportions.
3. Results
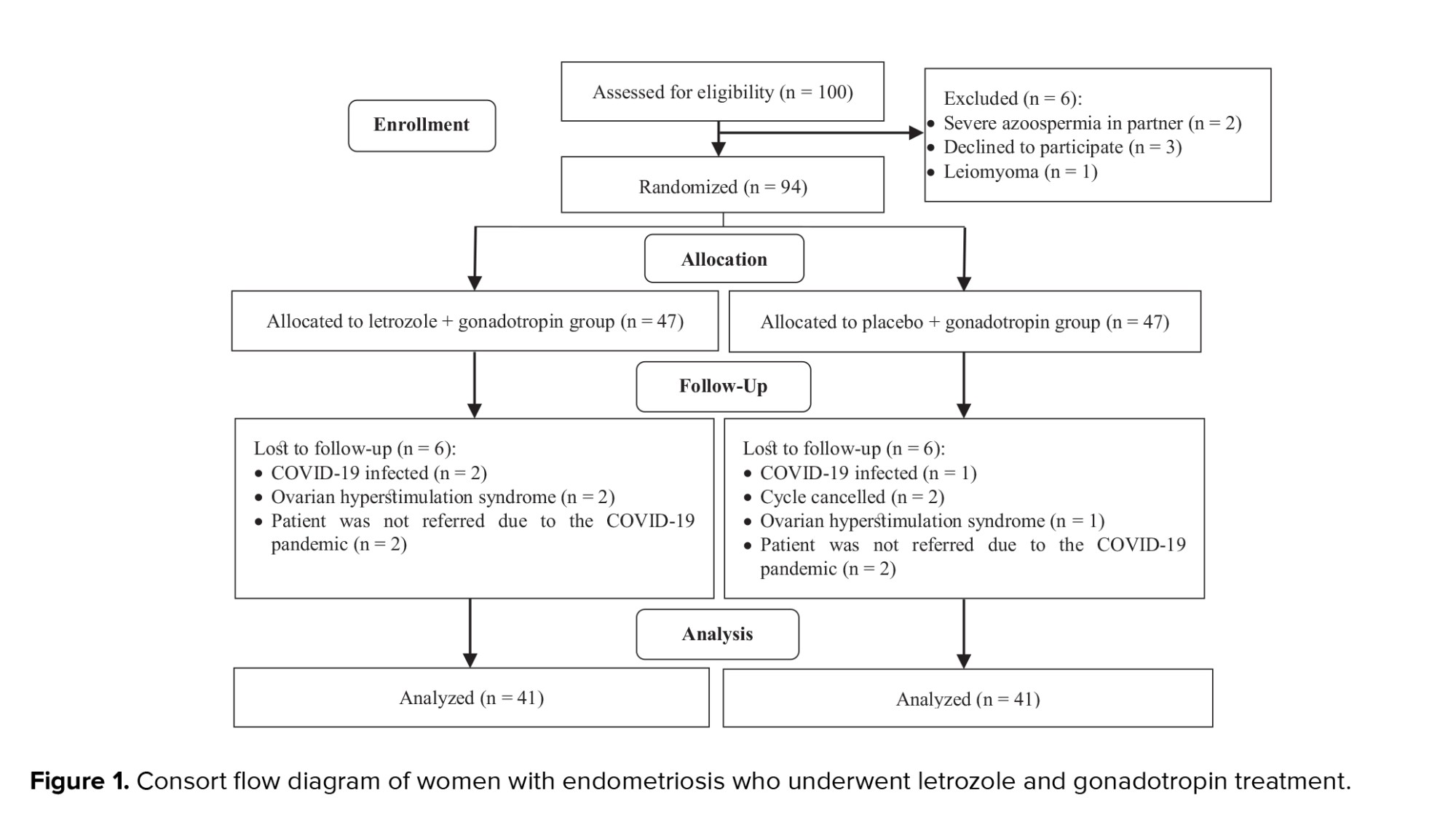
100 infertile women were assessed for eligibility; of them, 6 women were excluded due to: severe azoospermia in their partners (n = 2), leiomyoma (n = 1), and declined to participate (n = 3). In total, 94 women were randomized to the LA and PA groups. During the study, 12 participants (6 from each group) did not refer for follow-up and were considered lost to follow-up. Finally, data from 82 women with endometriosis were analyzed (Figure 1).
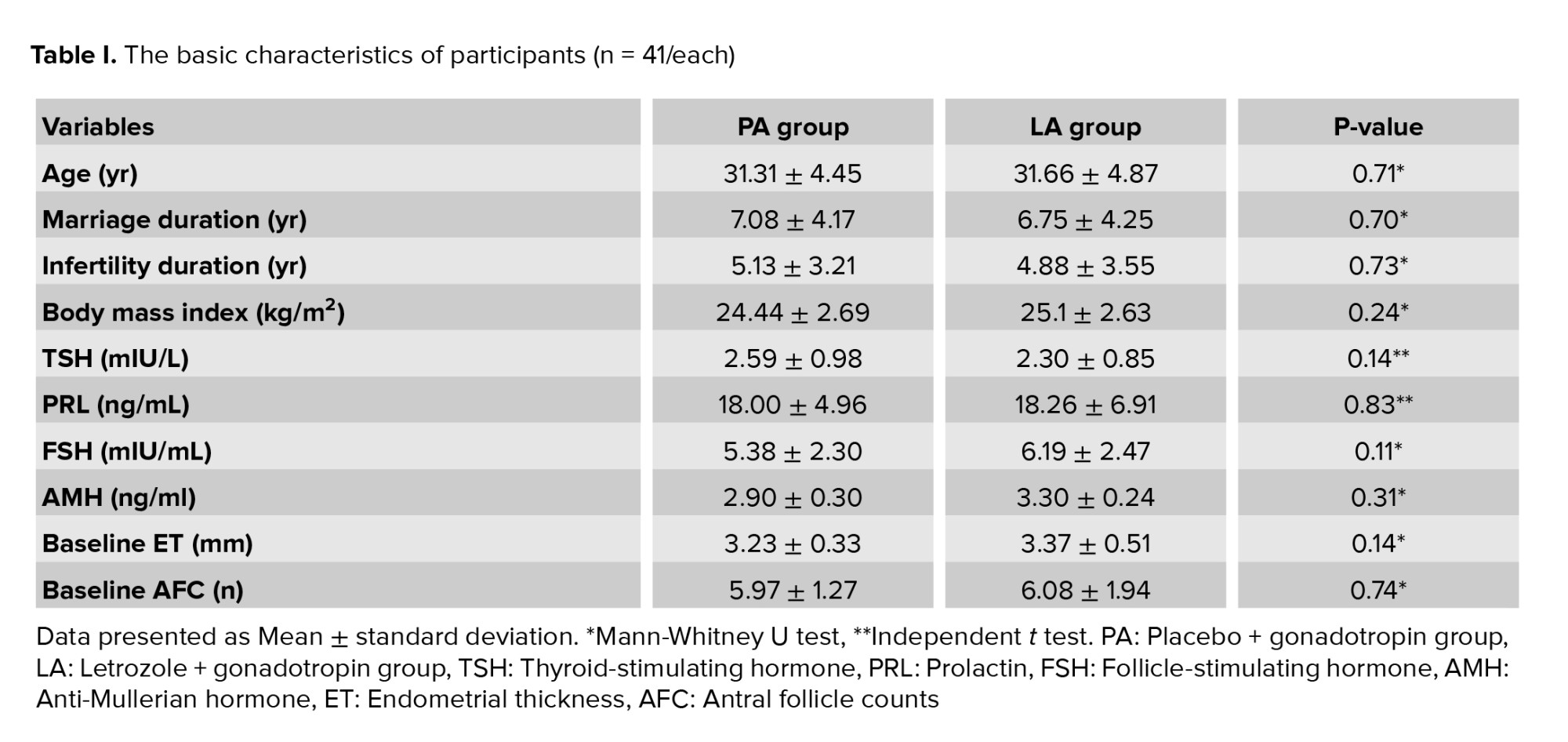
The mean age and infertility duration was 31.49 ± 4.64 yr and 5.01 ± 3.37 yr, respectively. The basic characteristics of participants did not differ significantly between the 2 study groups (Table I).
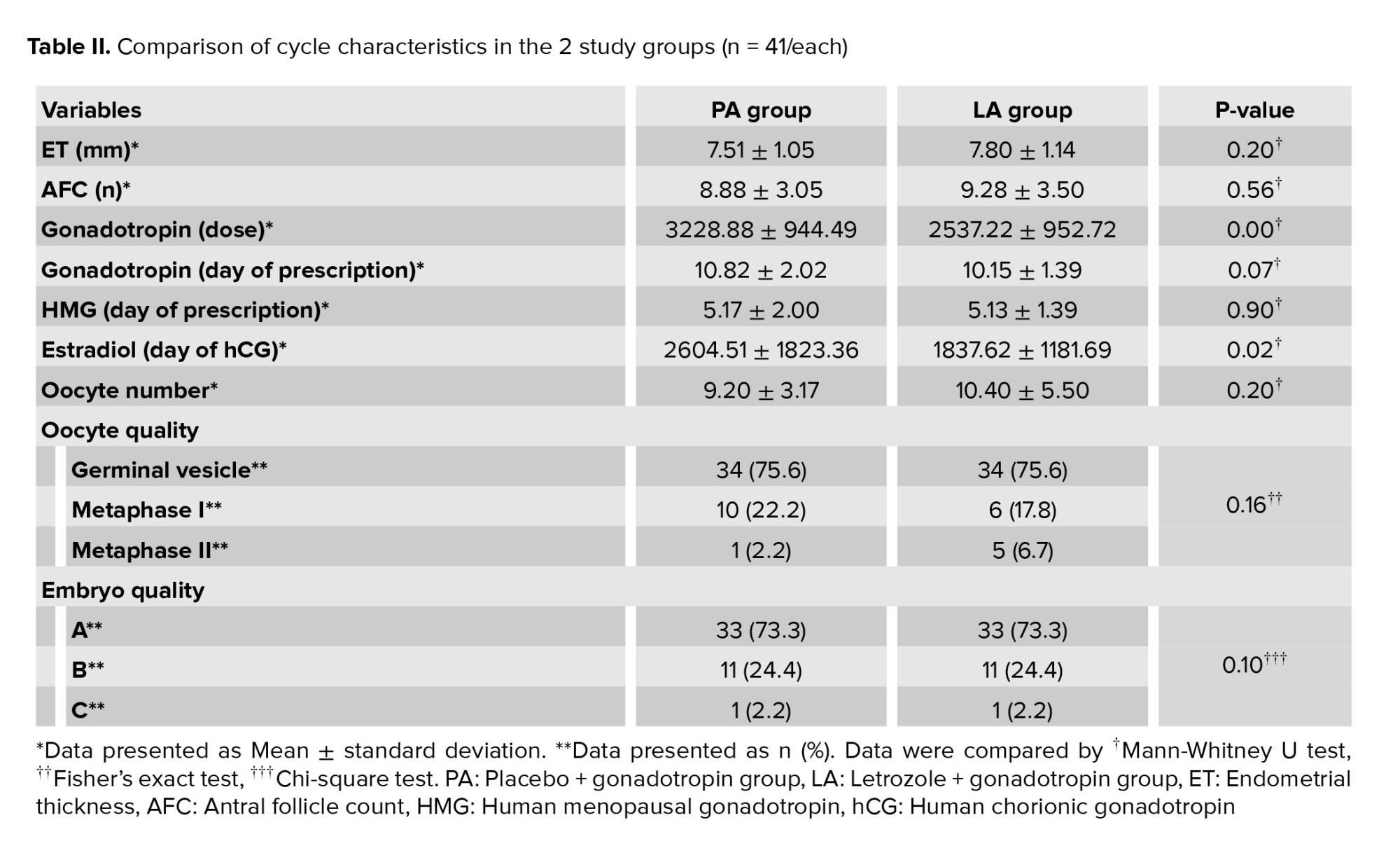
Gonadotropin dosage (p < 0.01) and estradiol level (p = 0.02) on the hCG administration day measurement were significantly lower in the LA group in comparison with the PA group. The other cycle characteristics did not differ significantly between the 2 study groups (Table II).
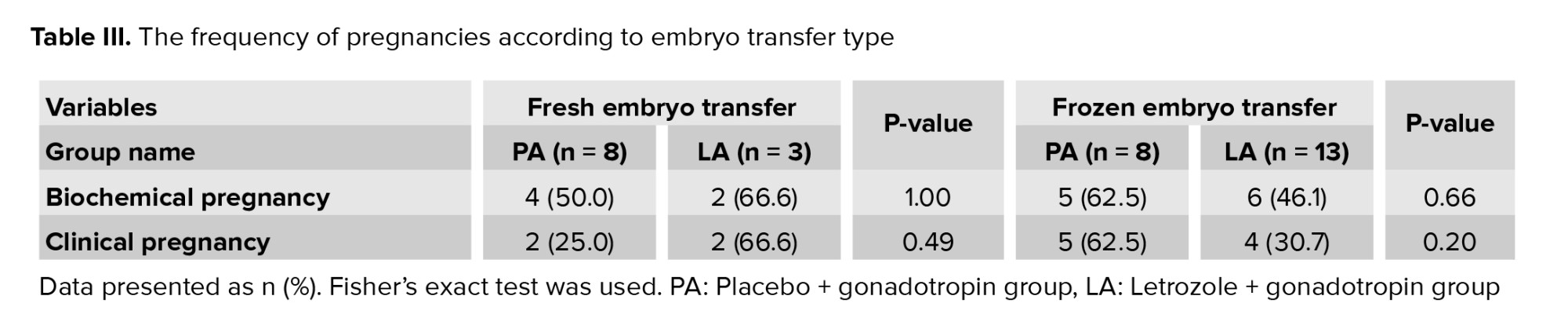
Embryo transfer was done for 32 women. Positive ß-hCG (indicating biochemical pregnancy) was observed in 17 women (53.1%), and clinical pregnancy in 13 (40.6%) women. No significant differences were detected between the study groups regarding biochemical or clinical pregnancy (p = 0.72 for both). The frequency of pregnancies according to embryo transfer type are summarized in table III.
4. Discussion
Our study showed that gonadotropin dosage was significantly lower in the LA group compared to the PA group. Furthermore, the estradiol level on the hCG administration day was significantly lower in the LA group in comparison with the PA group. However, no significant differences were detected between the study groups regarding biochemical or clinical pregnancy.
Infertile women with endometriosis have a poorer response to infertility treatment than women with other causes of infertility; the main reasons may be a reduction in embryo quality, endometrial reception, implantation rates, and enhancement in inflammation and aromatase synthesis (14-16).
Previous research has attempted to find solutions to counter the adverse impact of infertility in women with endometriosis, such as by evaluating the effect of a post-operative systematic GnRH agonist prescription (17, 18) and pre-treatment with GnRH agonist (19); however, this problem remains unresolved, and further studies are needed.
This study assessed the effect of a combination of letrozole and gonadotropin compared with gonadotropin alone on the outcomes of IVF treatment in infertile women with endometriosis. The results showed that this combination was associated with significantly lower estradiol levels and dose of Cinnal-f. However, it had no significant influence on oocyte number or quality, embryo quality, biochemical pregnancy, or clinical pregnancy rates.
Our results showing that letrozole application was associated with a significantly lower dose of gonadotropin and estradiol level, which are in line with studies conducted on normal responders (20), breast cancer women (21), and poor responders (22, 23). However, other studies found that the gonadotropin dose was similar in the letrozole vs. control groups that they examined (12, 24); this could be due to the lack of randomization in these studies and because they enrolled women with laparoscopic-approved endometriosis.
Some studies (2, 12, 20-22), similarly to this study, indicated that adding letrozole to the IVF regimen had no significant effect on stimulation duration, oocyte number, or embryo number or quality. In contrast, in other studies, letrozole was able to significantly affect the number and quality of oocytes (23, 24) and the length of stimulation (23, 25).
Although some previous studies (2, 20-24), in line with our study, indicated no significant variation in pregnancy proportions with letrozole application, Piedimonte et al. study (25) indicated that pregnancy and live-birth rates increased significantly following administration of letrozole and GnRH agonist combination. Given the varied findings of previous studies, future studies seem needed to assess the effect of varying doses of letrozole in infertile women with endometriosis, as well as compare the effect of a combination of letrozole with agonist vs. antagonist IVF protocol.
4.1. Limitation
The little sample size was a limitation of our study.
5. Conclusion
According to the study findings, using letrozole as a co-treatment drug in the IVF cycle of women with endometriosis can significantly reduce gonadotropin dosage and estradiol level with the same pregnancy rates.
Acknowledgments
This study was carried out as part of Dr. Hamideh Pakniat’s infertility fellowship thesis under the supervision of Dr. Mahbod Ebrahimi. The study was supported by the Tehran University of Medical Sciences, Tehran, Iran.
Conflict of Interest
The authors declare that there is no conflict of interest.
The prevalence of endometriosis, tissue and glands of endometrium placed outside the uterus, in infertile women is 25-50%, and it can cause the failure of in vitro fertilization (IVF) treatment (1, 2). The usual causes of infertility in women with endometriosis are folliculogenesis alternation, steroidogenesis and fertilization impairment, oocyte and embryo quality reduction, implantation defect, and pelvic adhesions (3). Endometriosis is an estrogen-dependent disease associated with increased aromatase enzyme expression and concentration and some pathologic mediator secretion, such as of estradiol and prostaglandin E2 (1). These pathologic mediators have an important role in promoting the growth and invasion of endometriotic tissue, pain, inflammation, and infertility (4).
Nowadays, assisted-reproductive technology can have a considerable role in resolving infertility problems in most couples. However, previous studies have shown significantly lower successful fertilization rates in endometriosis rather than other causes of infertility in IVF cycles (5-7). Letrozole is a selective aromatase inhibitor, that causes a decrease the estrogen concentration. The result of a decline in estrogen level is an increase in follicle-stimulating hormone secretion, ovarian Follicle-stimulating hormone receptor affinity, antral follicle growth, follicle phase enhancement, and follicle development. The other effect of letrozole is reducing estradiol and prostaglandin E2 production, which affects oocyte quality (8, 9). Letrozole has been recommended in some researches for improving fertility results in poor responder women, treatment of endometriosis-related pelvic pain, treatment of hormone receptor-positive breast cancer, and fertility preservation in women with breast cancer (10-12).
However, studies about the application of letrozole in the IVF cycles of women with endometriosis are rare and more studies are needed on this topic. This study was designed to compare IVF cycle success rates of women with endometriosis treated with letrozole + gonadotropin (LA) vs. placebo + gonadotropin (PA).
2. Materials and Methods
This double-blind, randomized clinical trial study with a parallel design was done with 94 infertile women with endometriosis referred to our IVF Unit at Yas hospital, Tehran, Iran from April-June 2021. The inclusion criteria included women with pelvic endometriosis and primary infertility, in their first IVF cycle, 18-35 yr old, body mass index < 30 kg/m2, serum anti-Mullerian hormone (AMH) > 1 ng/ml, and partner sperm motility of at least 20%.
Women who had undergone letrozole or clomiphene therapy with the aim of inducing ovulation, or who had deeply infiltrating endometriosis, or submucosal or intramural myoma, detected in transvaginal ultrasound (TVS), or with uterine diseases were excluded.
This study was conducted double-blind. The participants, because of placebo usage, did not know the type of their treatment. Also, the analyzer did not know about the treatment group codes in the analysis data sheet.
Using random allocation, participants were divided by the corresponding author into the 2 groups of LA and PA (n = 47/each). First, 47 letter (As) and 47 letter (Bs) were written on papers without other markings. All of the papers were placed in a bag, and for each woman, a paper was taken randomly and without replacement. In addition, interventions A and B were randomly assigned to the LA group and PA group, respectively.
For all participants, the long agonist protocol was applied. 300 mcg of gonadotropin-releasing hormone (GnRH) agonist (CinnaFact, CinnaGen Company, Iran) was prescribed in the mid-luteal stage (7 days before the anticipated menstruation). Then, on the third day of menstruation, the women were evaluated with TVS (4.5-7 MHz vaginal probe, Sono line G-40, Siemens, Germany) for endometrial thickness (ET) and antral follicle counts assessment in both ovaries.
In both groups, from the third day of the cycle, and gonadotropin (CinnalF, CinnaGen Company, Iran) was started and its doses were regulated based on the patient’s age, serum AMH and follicle-stimulating hormone. From the third day of the menstrual cycle, 5 mg of letrozole daily for 5 days prescribed for LA group, while placebo prescribed for PA on the identical days and duration.
Repeated TVS examinations were done with aim of follicular maturation assessment. Human menopausal gonadotropin (Pooyesh Daru, Iran) was added whenever follicle(s) sizes were ≥ 10-12 mm. furthermore, CinnalF continued until the triggering day of ovulation.
Then, 250 µg of choriogonadotropin alfa (Ovitrelle, Merck Serono, Italy) was administrated subcutaneously if at least 2 follicles with ≥ 18 mm in diameter was reported and serum estradiol concentration (on trigger day) was ≥ 500 pg/mL. The cycle were cancelled when which of above criterias after 10-12 days following by stimulation did not detected.
After 34-36 hr following choriogonadotropin alfa initiation, oocyte retrieval with aid of TVS (Honda Company, Japan) was conducted under spinal anesthesia. Then, for all the cycles, intracytoplasmic sperm injection was carried out.
Fresh embryo transfer was done for all of the participants unless there were contraindications such as ovarian hyperstimulation syndrome, pelvic and abdominal pain due to endometriosis, endometrioma necessitating surgery, or at the woman's request. In these mentioned conditions, frozen embryo transfer was done.
In fresh embryo transfer, 100 mg of progesterone (Iran Hormone Company, Iran) was injected daily immediately after oocyte retrieval and for 2 wk. 3 days after puncture day, embryos (in cleavage form) were transferred. Serum β-human chorionic gonadotropin (β-hCG) was checked on the 14th day of embryo transfer, and if pregnancy was confirmed, 400 mg of suppository tablets of progesterone (Cyclogest, Actavis, Barnstaple, UK) was initiated and continued daily to the end of pregnancy.
In frozen embryo transfer, from the third day of the menstrual cycle, 6 mg of estradiol (Abu Reihan Pharmaceutical Company, Iran) was given daily as an oral pill. ET was assessed serially each 3-4 days, and when ET was > 8 mm, 100 mg of progesterone (Iran Hormone Company, Iran) was injected, and continued daily. After 4 days, progesterone-initiated embryos (in cleavage form) were transferred. Serum β-hCG was checked on the 14th day after embryo transfer, and if pregnancy occurred, 400 mg of suppository tablets of progesterone (Cyclogest, Actavis, Barnstaple, UK) were given daily with estradiol until the end of pregnancy.
The following data were recorded for both groups: age, marriage and infertility duration, body mass index, thyroid-stimulating hormone, prolactin, AMH, and Follicle-stimulating hormone.
The total prescribed dosage of gonadotropin (calculated on the trigger day), the serum estradiol level (measured on the trigger day), the oocyte number and quality (determined on the oocyte retrieval day according to the oocyte maturity grading), and the embryo quality (categorized based on the Gardner morphological assessment system, which grades expansion status, inner cell mass from A-C, trophectoderm from A-C, and blastocyst growth stage from 3-6) (13), were analyzed for all patients.
Serum β-hCG was measured on the 14th day after embryo transfer (to determine biochemical pregnancy), and pregnancy sac observation through TVS was assessed 6 wk after embryo transfer (to determine clinical pregnancy).
2.1. Ethical considerations
This study was approved by the Ethics Committee of Sina hospital, affiliated to Tehran University of Medical Sciences, Tehran, Iran (Code: IR.TUMS.SINAHOSPITAL.REC.1399.100). The study was registered in the Iranian randomized clinical trial registry and was done in compliance Declaration of Helsinki and all the participants signed an informed consent form.
2.2. Statistical analysis
All of the statistical analyses were done using the Statistical Package for the Social Sciences (SPSS) version 24.0. P-values < 0.05 were considered statistically significant. Independent t test and non-parametric Mann-Whitney U test were used to evaluate the differences in means. A Chi-square test and Fisher's exact test were applied to assess the differences in proportions.
3. Results
100 infertile women were assessed for eligibility; of them, 6 women were excluded due to: severe azoospermia in their partners (n = 2), leiomyoma (n = 1), and declined to participate (n = 3). In total, 94 women were randomized to the LA and PA groups. During the study, 12 participants (6 from each group) did not refer for follow-up and were considered lost to follow-up. Finally, data from 82 women with endometriosis were analyzed (Figure 1).
The mean age and infertility duration was 31.49 ± 4.64 yr and 5.01 ± 3.37 yr, respectively. The basic characteristics of participants did not differ significantly between the 2 study groups (Table I).
Gonadotropin dosage (p < 0.01) and estradiol level (p = 0.02) on the hCG administration day measurement were significantly lower in the LA group in comparison with the PA group. The other cycle characteristics did not differ significantly between the 2 study groups (Table II).
Embryo transfer was done for 32 women. Positive ß-hCG (indicating biochemical pregnancy) was observed in 17 women (53.1%), and clinical pregnancy in 13 (40.6%) women. No significant differences were detected between the study groups regarding biochemical or clinical pregnancy (p = 0.72 for both). The frequency of pregnancies according to embryo transfer type are summarized in table III.




4. Discussion
Our study showed that gonadotropin dosage was significantly lower in the LA group compared to the PA group. Furthermore, the estradiol level on the hCG administration day was significantly lower in the LA group in comparison with the PA group. However, no significant differences were detected between the study groups regarding biochemical or clinical pregnancy.
Infertile women with endometriosis have a poorer response to infertility treatment than women with other causes of infertility; the main reasons may be a reduction in embryo quality, endometrial reception, implantation rates, and enhancement in inflammation and aromatase synthesis (14-16).
Previous research has attempted to find solutions to counter the adverse impact of infertility in women with endometriosis, such as by evaluating the effect of a post-operative systematic GnRH agonist prescription (17, 18) and pre-treatment with GnRH agonist (19); however, this problem remains unresolved, and further studies are needed.
This study assessed the effect of a combination of letrozole and gonadotropin compared with gonadotropin alone on the outcomes of IVF treatment in infertile women with endometriosis. The results showed that this combination was associated with significantly lower estradiol levels and dose of Cinnal-f. However, it had no significant influence on oocyte number or quality, embryo quality, biochemical pregnancy, or clinical pregnancy rates.
Our results showing that letrozole application was associated with a significantly lower dose of gonadotropin and estradiol level, which are in line with studies conducted on normal responders (20), breast cancer women (21), and poor responders (22, 23). However, other studies found that the gonadotropin dose was similar in the letrozole vs. control groups that they examined (12, 24); this could be due to the lack of randomization in these studies and because they enrolled women with laparoscopic-approved endometriosis.
Some studies (2, 12, 20-22), similarly to this study, indicated that adding letrozole to the IVF regimen had no significant effect on stimulation duration, oocyte number, or embryo number or quality. In contrast, in other studies, letrozole was able to significantly affect the number and quality of oocytes (23, 24) and the length of stimulation (23, 25).
Although some previous studies (2, 20-24), in line with our study, indicated no significant variation in pregnancy proportions with letrozole application, Piedimonte et al. study (25) indicated that pregnancy and live-birth rates increased significantly following administration of letrozole and GnRH agonist combination. Given the varied findings of previous studies, future studies seem needed to assess the effect of varying doses of letrozole in infertile women with endometriosis, as well as compare the effect of a combination of letrozole with agonist vs. antagonist IVF protocol.
4.1. Limitation
The little sample size was a limitation of our study.
5. Conclusion
According to the study findings, using letrozole as a co-treatment drug in the IVF cycle of women with endometriosis can significantly reduce gonadotropin dosage and estradiol level with the same pregnancy rates.
Acknowledgments
This study was carried out as part of Dr. Hamideh Pakniat’s infertility fellowship thesis under the supervision of Dr. Mahbod Ebrahimi. The study was supported by the Tehran University of Medical Sciences, Tehran, Iran.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Fertility & Infertility
References
1. Ozkan S, Murk W, Arici A. Endometriosis and infertility: Epidemiology and evidence‐based treatments. Ann N Y Acad Sci 2008; 1127: 92-100. [DOI:10.1196/annals.1434.007] [PMID]
2. Abd Rabbo MS, Elmaghraby HA, Mashali NA, Moneim MEA. Effect of aromatase inhibitor (letrozole) with long agonist protocol on the results of ICSI/ET in females with minimal and mild endometriosis. Alexandria J Med 2012; 48: 303-307. [DOI:10.1016/j.ajme.2012.04.001]
3. Abu Hashim H. Aromatase inhibitors for endometriosis-associated infertility; Do we have sufficient evidence? Int J Fertil Steril 2016; 10: 270-277.
4. Pavone ME, Bulun SE. Aromatase inhibitors for the treatment of endometriosis. Fertil Steril 2012; 98: 1370-1379. [DOI:10.1016/j.fertnstert.2012.08.053] [PMID] [PMCID]
5. Akbari Asbagh F, Davari Tanha F, Rezaei Z, Ebrahimi M, Hemmati T, Talebbidokhti M, et al. Evaluation of the in vitro fertilization success rate in transfer of top-quality embryo versus poor-quality embryos: A cohort study. Int J Women's Health Reprod Sci 2021; 9: 1-5.
6. Suzuki T, Izumi ShI, Matsubayashi H, Awaji H, Yoshikata K, Makino T. Impact of ovarian endometrioma on oocytes and pregnancy outcome in in vitro fertilization. Fertil Steril 2005; 83: 908-913. [DOI:10.1016/j.fertnstert.2004.11.028] [PMID]
7. Tavmergen E, Ulukus M, Goker EN. Long-term use of gonadotropin-releasing hormone analogues before IVF in women with endometriosis. Curr Opin Obstet Gynecol 2007; 19: 284-288. [DOI:10.1097/GCO.0b013e3281053a52] [PMID]
8. Lee KH, Kim ChH, Suk HJ, Lee YJ, Kwon SK, Kim SH, et al. The effect of aromatase inhibitor letrozole incorporated in gonadotrophin-releasing hormone antagonist multiple dose protocol in poor responders undergoing in vitro fertilization. Obstet Gynecol Sci 2014; 57: 216-222. [DOI:10.5468/ogs.2014.57.3.216] [PMID] [PMCID]
9. Yarali H, Esinler I, Polat M, Bozdag G, Tiras B. Antagonist/letrozole protocol in poor ovarian responders for intracytoplasmic sperm injection: A comparative study with the microdose flare-up protocol. Fertil Steril 2009; 92: 231-235. [DOI:10.1016/j.fertnstert.2008.04.057] [PMID]
10. Goswami SK, Das T, Chattopadhyay R, Sawhney V, Kumar J, Chaudhury K, et al. A randomized single-blind controlled trial of letrozole as a low-cost IVF protocol in women with poor ovarian response: A preliminary report. Hum Reprod 2004; 19: 2031-2035. [DOI:10.1093/humrep/deh359] [PMID]
11. Mitwally MFM, Casper RF. Aromatase inhibition improves ovarian response to follicle-stimulating hormone in poor responders. Fertil Steril 2002; 77: 776-780. [DOI:10.1016/S0015-0282(01)03280-0]
12. Schwartz K, Llarena NC, Rehmer JM, Richards EG, Falcone T. The role of pharmacotherapy in the treatment of endometriosis across the lifespan. Expert Opin Pharmacother 2020; 21: 893-903. [DOI:10.1080/14656566.2020.1738386] [PMID]
13. Inoue T, Ono Y, Yonezawa Y, Kishi J, Emi N. Improvement of live birth rate following frozen-thawed blastocyst transfer by combination of prednisolone administration and stimulation of endometrium embryo transfer. Open Journal of Obstetrics and Gynecology 2014; 4: 745-750. [DOI:10.4236/ojog.2014.413103]
14. Chen TJ, Zheng WL, Liu CH, Huang I, Lai HH, Liu M. Using deep learning with large dataset of microscope images to develop an automated embryo grading system. Fertil Reprod 2019; 1: 51-56. [DOI:10.1142/S2661318219500051]
15. Harb HM, Gallos ID, Chu J, Harb M, Coomarasamy A. The effect of endometriosis on in vitro fertilisation outcome: A systematic review and meta-analysis. BJOG 2013; 120: 1308-1320. [DOI:10.1111/1471-0528.12366] [PMID]
16. Opøien HK, Fedorcsak P, Omland AK, Abyholm Th, Bjercke S, Ertzeid G, et al. In vitro fertilization is a successful treatment in endometriosis-associated infertility. Fertil Steril 2012; 97: 912-918. [DOI:10.1016/j.fertnstert.2012.01.112] [PMID]
17. Brosens J, Verhoeven H, Campo R, Gianaroli L, Gordts S, Hazekamp J, et al. High endometrial aromatase P450 mRNA expression is associated with poor IVF outcome. Hum Reprod 2004; 19: 352-356. [DOI:10.1093/humrep/deh075] [PMID]
18. Dunselman GAJ, Vermeulen N, Becker C, Calhaz-Jorge C, D'Hooghe T, De Bie B, et al. ESHRE guideline: Management of women with endometriosis. Hum Reprod 2014; 29: 400-412. [DOI:10.1093/humrep/det457] [PMID]
19. Yap C, Furness S, Farquhar C. Pre and post operative medical therapy for endometriosis surgery. Cochrane Database Syst Rev 2004; 2004: Cd003678. [DOI:10.1002/14651858.CD003678.pub2] [PMID] [PMCID]
20. Sallam HN, Garcia-Velasco JA, Dias S, Arici A. Long-term pituitary down-regulation before in vitro fertilization (IVF) for women with endometriosis. Cochrane Database Syst Rev 2006; 2006: Cd004635. [DOI:10.1002/14651858.CD004635.pub2] [PMID] [PMCID]
21. Eftekhar M, Saeed L. Effect of adding letrozole to gonadotropin on in vitro fertilization outcomes: An RCT. Int J Reprod Biomed 2020; 18: 287-294. [DOI:10.18502/ijrm.v13i4.6891] [PMID] [PMCID]
22. Oktay K, Hourvitz A, Sahin G, Oktem O, Safro B, Cil A, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab 2006; 91: 3885-3890. [DOI:10.1210/jc.2006-0962] [PMID]
23. ElSharkawy SS, Abd Raboo MSAD, Al Abd MM, Khamis Mohamed SM. Study of the effect of adding letrozole to gonadotropin-releasing hormone antagonist protocol in poor responders undergoing intracytoplasmic sperm injection. Evidence Based Women's Health Journal 2021; 11: 211-218.
24. Moini A, Lavasani Z, Kashani L, Mojtahedi MF, Yamini N. Letrozole as co-treatment agent in ovarian stimulation antagonist protocol in poor responders: A double-blind randomized clinical trial. Int J Reprod Biomed 2019; 17: 653-660. [DOI:10.18502/ijrm.v17i9.5101] [PMID] [PMCID]
25. Piedimonte S, Volodarsky-Perel A, Tannus S, Tan SL, Dahan MH. Pretreatment with a gonadotropin-releasing hormone agonist and an aromatase inhibitor may improve outcomes in in vitro fertilization cycles of women with stage I-II endometriosis. F&S Science 2020; 1: 98-103. [DOI:10.1016/j.xfss.2020.06.005] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





