Fri, Jul 11, 2025
[Archive]
Volume 21, Issue 5 (May 2023)
IJRM 2023, 21(5): 403-414 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Tarfdari A, Bandarian M, Hantoushzadeh S, Hadizadeh A, Shahsavari S, Razavi M A. Assessing the risk factors and management outcomes of ectopic pregnancy: A retrospective case-control study. IJRM 2023; 21 (5) :403-414
URL: http://ijrm.ir/article-1-2534-en.html
URL: http://ijrm.ir/article-1-2534-en.html
Azadeh Tarfdari1 

 , Mahin Bandarian2
, Mahin Bandarian2 

 , Sedigheh Hantoushzadeh1
, Sedigheh Hantoushzadeh1 

 , Alireza Hadizadeh *3
, Alireza Hadizadeh *3 

 , Saeedeh Shahsavari1
, Saeedeh Shahsavari1 

 , Maryam alsadat Razavi1
, Maryam alsadat Razavi1 




 , Mahin Bandarian2
, Mahin Bandarian2 

 , Sedigheh Hantoushzadeh1
, Sedigheh Hantoushzadeh1 

 , Alireza Hadizadeh *3
, Alireza Hadizadeh *3 

 , Saeedeh Shahsavari1
, Saeedeh Shahsavari1 

 , Maryam alsadat Razavi1
, Maryam alsadat Razavi1 


1- Department of Obstetrics and Gynecology, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran.
2- Department of Obstetrics and Gynecology, Ziaeian Hospital, Tehran University of Medical Sciences, Tehran, Iran.
3- Department of Obstetrics and Gynecology, Ziaeian Hospital, Tehran University of Medical Sciences, Tehran, Iran. ,Ali1375hadi@gmail.com
2- Department of Obstetrics and Gynecology, Ziaeian Hospital, Tehran University of Medical Sciences, Tehran, Iran.
3- Department of Obstetrics and Gynecology, Ziaeian Hospital, Tehran University of Medical Sciences, Tehran, Iran. ,
Full-Text [PDF 338 kb]
(901 Downloads)
| Abstract (HTML) (1043 Views)
1. Introduction
Ectopic pregnancy (EP) is the implantation of a fertilized egg outside the uterine cavity or in the scar resulting from a prior cesarean section. This complicated pregnancy has the potential to increase maternal morbidity and mortality. Even though the incidence of EP increased 6-fold between the 1970s and 1990s, recent research indicates that the statistics have remained relatively stable. EP still accounts for approximately 2% of all pregnancies and 10% of all pregnancy-related deaths (1-5). EP is diagnosed by a combination of symptoms, clinical examination, serial beta-human chorionic gonadotropin (β-hCG) measurements, and ultrasonographic imaging. Symptoms include abdominal pain and vaginal bleeding between the 6th and 10th wk of pregnancy (6).
Several factors have been linked to EP, with previous EP, pelvic surgery, pelvic inflammatory disease, Chlamydia trachomatis infection, and smoking is the most studied and well-known (7, 8). In recent years, however, it has been suggested that certain medications, specifically emergency contraceptive pills, are a major cause (9, 10). In addition, it has been hypothesized that ovulation induction and assisted reproductive technologies (ART) may also contribute to the development of EP (11-13).
The most common sites for the implantation of an ectopic gestational sac are the fallopian tubes, the cervix, the ovary, and the caesarian scar (14, 15). These pathologies necessitate a specific treatment; consequently, depending on the examinations, laboratory tests, and imaging, EP can be managed by observation, surgery, or medication (16-18). In cases of rupture, surgical removal of conception products is considered the definitive treatment for EP and is the method of choice. Surgical treatment includes salpingectomy, salpingostomy for tubal EP, and cesarean scar pregnancy curettage, among others (19-21).
Conservative therapies are recommended if the case meets the criteria for medical management, and the fallopian tubes are saved (1, 18, 22, 23). Medical methotrexate (MTX) regimens have a success rate of up to 93% for multidose protocols and 88% for single-dose therapy in tubal EP (1, 24). However, because single-dose MTX may fail, particularly at higher β-hCG concentrations (> 5000 IU/l), a double-dose regimen may lower the risk while making the case less susceptible to adverse effects. Because both treatment protocols are prone to failure, an adjuvant dose may help reduce failure rates (23, 25, 26).
Considering all management approaches and possible risk factors, this study was designed to evaluate risk factors and treatment outcomes in women with EP.
2. Materials and Methods
In this retrospective case-control study 191 women who were diagnosed with EP and were referred to the obstetrics and gynecology clinic or emergency department of Imam Khomeini hospital complex, Tehran, Iran between March 2020 and March 2021 were enrolled. For control groups 190 pregnant women along with 180 non-pregnant women were enrolled. EP diagnosis was made using physical examination, serial β-hCG, and ultrasonographic imaging. Pregnant control group included patients with intrauterine pregnancy and without any anatomic or uterine abnormalities. All patients with concurrent diseases such as cancers and autoimmune diseases were excluded from this study.
Data were collected using the medical records at the hospital. In addition to being interviewed, subjects were asked to complete a structured questionnaire assessing their past medical and surgical history, infertility and ART, and use of contraceptive pills and devices. The nonpregnant control subjects were women with regular sexual activity and no nonmedical causes of infertility. The choice of treatment according to the protocol was based on β-hCG concentration and if the patients were either hemodynamically unstable or showed signs of rupture or had any indications such as failure to medical treatment they were candidate for surgery of the patients (Table I).
Moreover, pregnant control subjects were selected from those without abnormalities or anatomical malformations that could confound the analysis.
This study was designed with 2 control groups, intrauterine and nonpregnant women, to assess the relationship between EP and risk factors, particularly contraceptives used during the most recent ovulation cycle. Both control groups consisted of sexually active women of reproductive age. The cases and controls were matched regarding age (± 5 yr), gravidity, and parity in a ratio of 1:1.

2.1. Ethical considerations
This study was ethically approved by the Ethical Committee of Tehran University of Medical Sciences, Tehran, Iran (Code: IR.TUMS.IKHC.REC.1398.295). All participants signed informed consent forms to share data for scientific purposes.
2.2. Statistical analysis
We utilized SPSS software (v. 26, IBM Corp.) for statistical analysis. Pearson’s Chi-square test was used to determine the treatment outcomes’ differences. After using logistic regression models, we also calculated the odds ratio (OR) with a 95% confidence interval (CI) to analyze the possible association between risk factors and EP. Furthermore, we utilized t tests and Analysis of variance (ANOVA) to determine the significance of mean and variance differences. P < 0.05 were deemed statistically significant. To evaluate the risk factors, we utilized cross-tabulation, Chi-square tests, and logistic regression models that were adjusted and unadjusted for age, parity, gravidity, smoking, previous EP, pelvic inflammatory disease, and pelvic surgeries, including cesarean section.
3. Results
Among 234 patients who had a differential diagnosis of EP; 191 cases met the eligibility criteria and were included in the study. Furthermore, 190 pregnant women and 180 nonpregnant women were included in control groups. The patients in all groups were matched for age and demographic variables (Table II).
Among 191 cases, 126 patients had tubal EP, 46 had CSP and 8 had corneal EP (Figure 1). The average levels of β-hCG for expectant, medical, and surgical management were 1961, 7550, and 18,288 IU/ml, respectively. A total of 89 patients received MTX which resulted in 12 failures and 25 patients were only observed (Table III).
Result show that the average β-hCG levels prior to treatment, gestational age and parity were higher among corneal and cesarian scar in case group (Table IV).
Overall, 191 diagnosed EP cases were selected for the study. Based on our inclusion and exclusion criteria, we also selected 180 subjects for nonpregnant and 190 for intrauterine pregnancies after evaluation.
On average, our cases were 31.7 yr old; their demographic and gestational information is provided in table III. Out of the 191 cases, 90 (including failed cases) underwent surgery, 89 received MTX, and 25 underwent observation only. The demographics and characteristics are compared with various sites. Table II demonstrates that certain characteristics make subjects more susceptible to certain ectopic pregnancies. In addition, the levels of β-hCG, gestational age, and parity in corneal and scar pregnancies are significantly higher (Figure 1).
One of the primary objectives of this study was to determine the relationship between the proposed risk factors and EP. To this end, we compared the cases to 2 randomly selected control groups (intrauterine and nonpregnant). Only tubal EPs and scar pregnancies were analyzed because only these 2 categories had sufficient sample sizes for statistical analysis (Table V).
Regarding medical treatment failure, out of the 89 medically treated cases, we observed 12 failures. The results show that the cesarean section scar site yielded the highest failure rate, with 46.7%, followed by the cornea (16.7%) and tubal EP (6.3%) (27).
In terms of the type of surgery, 41 cases of tubal pregnancy were treated with salpingectomy, while only 7 cases were treated with salpingostomy. We also evaluated the cases of CSPs who received potassium chloride injections; only one of 5 CSPs who received KCl injections failed and required a second injection. The failure of medical treatment may necessitate invasive surgery by the gynecologist. In our study, 12 cases did not respond to medical treatment, 4 underwent suction and curettage (cesarean scar pregnancy), 7 underwent salpingectomy, and 1 underwent fetal reduction (corneal pregnancy) (Table V).
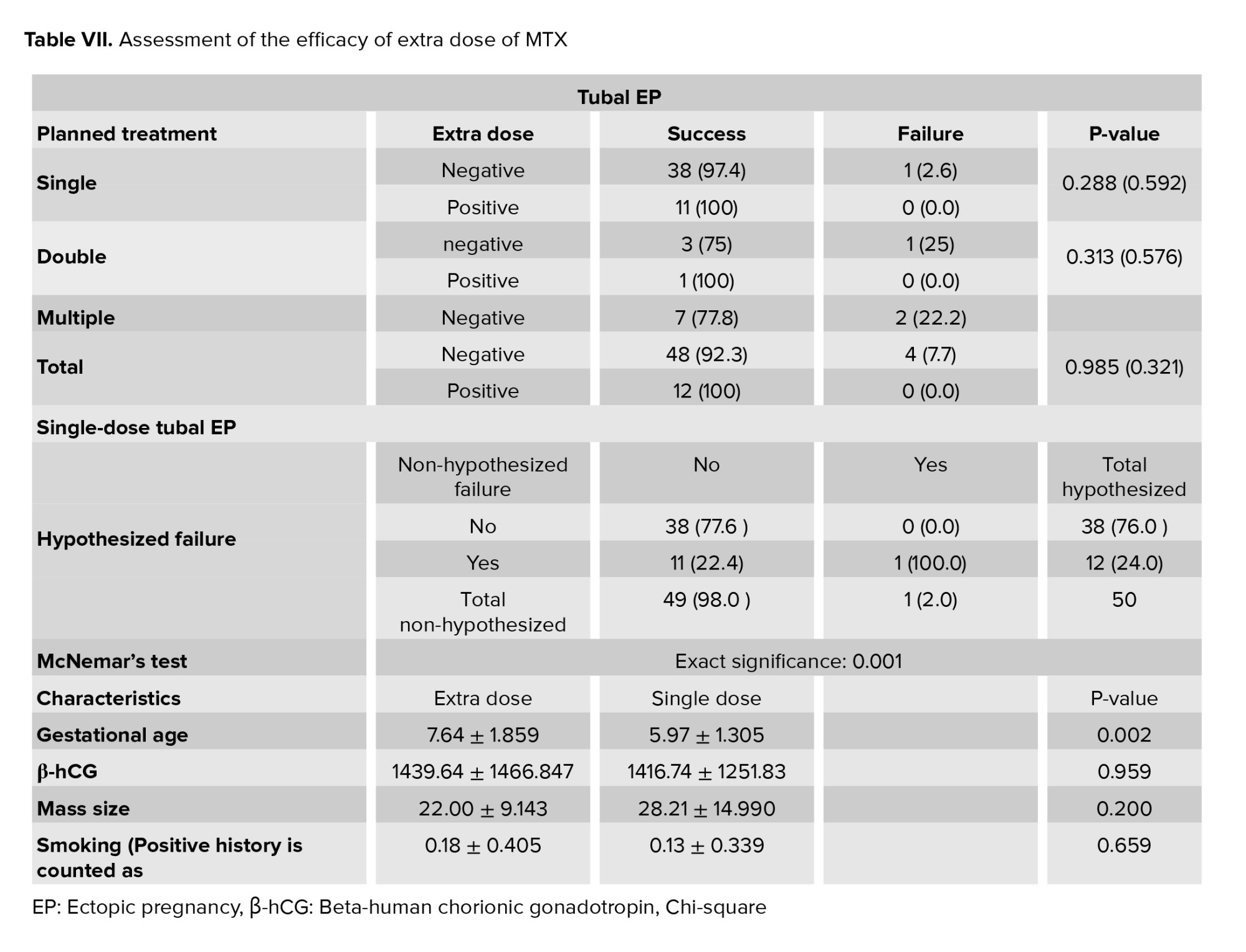
To evaluate the efficacy of our clinical protocol and the role of additional doses, we assessed the protocol using failure rates for various EP types. The overall analysis revealed that the additional dose could increase the success rate of both scar and tubal EPs. Since this data was a paired dependent sample and we only had sufficient samples in the tubal group to conduct a reliable test, we conducted McNemar’s test in a 2×2 contingency table in the tubal group to determine whether the hypothesized situation in which surgery was indicated for cases who received an extra dose could be avoided. The significance of this evaluation confirmed the null hypothesis. This indicates that a higher dosage could reduce the likelihood of medical treatment failure and the need for invasive procedures. We continued our examination using the student’s t test to determine if an additional dose was required. The results revealed that the primary difference between the 2 groups was in gestational age, which was almost 1.5 wk older in the group that required the additional dose (Tables VI and VII).
7 cases initially suspected of ovarian EP based on ultrasonographic evaluation were included in our study; one was successfully treated with MTX, 3 turned out to be tubal upon laparoscopic evaluation, 2 underwent salpingectomy, and one underwent salpingostomy. 3 cases had a gestational sac in their ovaries, resulting in the reduction of the fetus. Histopathological examination subsequently confirmed the diagnosis. Notably, 3 cases had a positive history of ART, but the number of cases was insufficient to conduct a reliable statistical analysis.
The corneal pregnancy cases included 8 subjects. 6 cases were administered MTX, one of which resulted in a fetal reduction, and one failed case underwent hysteroscopic contraceptive removal. 2 additional cases underwent laparoscopic surgery. Regarding risk factors, only one case had an EP and salpingectomy history.
3 cases of cervical pregnancy were managed during the course. One was only observed, while the other was treated medically and surgically.







4. Discussion
This study aimed to assess risk factors and treatment outcomes for surgical and therapeutic approaches to EP treatment. In addition to assessing the correlation between proposed risk factors and EP, our results revealed that emergency contraceptives, oral contraceptives, and other risk factors increase the likelihood of ectopic pregnancies in the event of a failed pregnancy.
One of the primary goals of this study was to evaluate the efficacy of the medical treatment protocol and the significance of extra doses.
The findings indicate that this method may be an option for individuals who plan future pregnancies or wish to preserve their fallopian tubes and fertility. The results showed a significant improvement in the performance of single-dose treatment plans in tubal EPs. Since gestational age was shown to influence the need for the additional dose, this modification is considered for individuals over 7.5 wk into their pregnancies (11). In a 2017 study, a meta-analysis indicated that the success rate difference between single doses and multiple doses was not statistically significant. However, side effects were more prevalent in the multiple-dose case. Consequently, their analysis indicates that the double regimen is an effective and secure alternative to the multiple-dose and single-dose protocols (1, 28-30).
A 2003 meta-analysis concluded that no statistically significant differences exist between the outcomes of multiple-dose and single-dose protocols. Nonetheless, the multiple-dose regimen is more likely to cause adverse effects (29). Regarding β-hCG concentrations and failure rate, their outcomes are also consistent with the present study. Likewise, they concluded that higher β-hCG levels increase the failure rate (23). The subtle factor that may have influenced the results is that clinicians are not blind to the factors that may result in a poor prognosis for medical treatment; consequently, they tend to select multiple-dose protocols for cases with higher β-hCG levels and a relatively poor prognosis. Based on the β-hCG concentration, the clinicians in our facility determined the medical treatment protocol. A multiple-dose protocol was designed for cases with more than 10,000 IUs who did not wish to undergo surgical management, whereas double and single doses were chosen for cases with levels below 10,000 IUs and 5000 IUs, respectively. A prospective randomized study conducted in Turkey confirms the same findings and issues attributable to inconsistent results (4, 19, 24, 30, 31). In cases where the decline in β-hCG is neither satisfactory nor significantly low, our findings and previous research suggest that an additional dose may be necessary (24, 32-35).
Regarding our findings regarding risk factors, specifically pharmaceutical contraceptives, we hypothesize that using contraceptives reduces the likelihood of both IUP and EP. However, it significantly increases the chances of EP if it fails. This is crucial for emergency contraceptives because the correlation has not been extensively studied (12, 28, 36).
Other risk factors, including smoking, intrauterine device use, and previous surgery, were investigated, and a correlation was established (5). Furthermore, our research indicates that ovulation induction increases the likelihood of EP. In 2014, a study concluded that OCPs and LNG-EC increased the risk of EP by up to 4 times that of women who did not use contraception and by up to 7 times that of women whose contraception failed (10, 22).
Several additional cases of ectopic pregnancies with LNG-EC and other emergency contraceptives have been reported (3, 5, 16). This phenomenon indicates that individuals with a positive history of contraceptive use and other risk factors and symptoms should strongly suspect EP. As an alternative medical approach, this method not only reduces the rate of surgical procedures but also decreases the possibility of infertility.
5. Conclusion
In summary, we conclude that the recommended clinical approach can perform better if a higher dose is administered. Our data also indicates a strong correlation between the use of contraceptives, particularly emergency contraceptives, and EP.
Acknowledgments
This study received no funding.
Conflict of Interest
The authors have no conflict of interest to declare.
Full-Text: (368 Views)
1. Introduction
Ectopic pregnancy (EP) is the implantation of a fertilized egg outside the uterine cavity or in the scar resulting from a prior cesarean section. This complicated pregnancy has the potential to increase maternal morbidity and mortality. Even though the incidence of EP increased 6-fold between the 1970s and 1990s, recent research indicates that the statistics have remained relatively stable. EP still accounts for approximately 2% of all pregnancies and 10% of all pregnancy-related deaths (1-5). EP is diagnosed by a combination of symptoms, clinical examination, serial beta-human chorionic gonadotropin (β-hCG) measurements, and ultrasonographic imaging. Symptoms include abdominal pain and vaginal bleeding between the 6th and 10th wk of pregnancy (6).
Several factors have been linked to EP, with previous EP, pelvic surgery, pelvic inflammatory disease, Chlamydia trachomatis infection, and smoking is the most studied and well-known (7, 8). In recent years, however, it has been suggested that certain medications, specifically emergency contraceptive pills, are a major cause (9, 10). In addition, it has been hypothesized that ovulation induction and assisted reproductive technologies (ART) may also contribute to the development of EP (11-13).
The most common sites for the implantation of an ectopic gestational sac are the fallopian tubes, the cervix, the ovary, and the caesarian scar (14, 15). These pathologies necessitate a specific treatment; consequently, depending on the examinations, laboratory tests, and imaging, EP can be managed by observation, surgery, or medication (16-18). In cases of rupture, surgical removal of conception products is considered the definitive treatment for EP and is the method of choice. Surgical treatment includes salpingectomy, salpingostomy for tubal EP, and cesarean scar pregnancy curettage, among others (19-21).
Conservative therapies are recommended if the case meets the criteria for medical management, and the fallopian tubes are saved (1, 18, 22, 23). Medical methotrexate (MTX) regimens have a success rate of up to 93% for multidose protocols and 88% for single-dose therapy in tubal EP (1, 24). However, because single-dose MTX may fail, particularly at higher β-hCG concentrations (> 5000 IU/l), a double-dose regimen may lower the risk while making the case less susceptible to adverse effects. Because both treatment protocols are prone to failure, an adjuvant dose may help reduce failure rates (23, 25, 26).
Considering all management approaches and possible risk factors, this study was designed to evaluate risk factors and treatment outcomes in women with EP.
2. Materials and Methods
In this retrospective case-control study 191 women who were diagnosed with EP and were referred to the obstetrics and gynecology clinic or emergency department of Imam Khomeini hospital complex, Tehran, Iran between March 2020 and March 2021 were enrolled. For control groups 190 pregnant women along with 180 non-pregnant women were enrolled. EP diagnosis was made using physical examination, serial β-hCG, and ultrasonographic imaging. Pregnant control group included patients with intrauterine pregnancy and without any anatomic or uterine abnormalities. All patients with concurrent diseases such as cancers and autoimmune diseases were excluded from this study.
Data were collected using the medical records at the hospital. In addition to being interviewed, subjects were asked to complete a structured questionnaire assessing their past medical and surgical history, infertility and ART, and use of contraceptive pills and devices. The nonpregnant control subjects were women with regular sexual activity and no nonmedical causes of infertility. The choice of treatment according to the protocol was based on β-hCG concentration and if the patients were either hemodynamically unstable or showed signs of rupture or had any indications such as failure to medical treatment they were candidate for surgery of the patients (Table I).
Moreover, pregnant control subjects were selected from those without abnormalities or anatomical malformations that could confound the analysis.
This study was designed with 2 control groups, intrauterine and nonpregnant women, to assess the relationship between EP and risk factors, particularly contraceptives used during the most recent ovulation cycle. Both control groups consisted of sexually active women of reproductive age. The cases and controls were matched regarding age (± 5 yr), gravidity, and parity in a ratio of 1:1.

2.1. Ethical considerations
This study was ethically approved by the Ethical Committee of Tehran University of Medical Sciences, Tehran, Iran (Code: IR.TUMS.IKHC.REC.1398.295). All participants signed informed consent forms to share data for scientific purposes.
2.2. Statistical analysis
We utilized SPSS software (v. 26, IBM Corp.) for statistical analysis. Pearson’s Chi-square test was used to determine the treatment outcomes’ differences. After using logistic regression models, we also calculated the odds ratio (OR) with a 95% confidence interval (CI) to analyze the possible association between risk factors and EP. Furthermore, we utilized t tests and Analysis of variance (ANOVA) to determine the significance of mean and variance differences. P < 0.05 were deemed statistically significant. To evaluate the risk factors, we utilized cross-tabulation, Chi-square tests, and logistic regression models that were adjusted and unadjusted for age, parity, gravidity, smoking, previous EP, pelvic inflammatory disease, and pelvic surgeries, including cesarean section.
3. Results
Among 234 patients who had a differential diagnosis of EP; 191 cases met the eligibility criteria and were included in the study. Furthermore, 190 pregnant women and 180 nonpregnant women were included in control groups. The patients in all groups were matched for age and demographic variables (Table II).
Among 191 cases, 126 patients had tubal EP, 46 had CSP and 8 had corneal EP (Figure 1). The average levels of β-hCG for expectant, medical, and surgical management were 1961, 7550, and 18,288 IU/ml, respectively. A total of 89 patients received MTX which resulted in 12 failures and 25 patients were only observed (Table III).
Result show that the average β-hCG levels prior to treatment, gestational age and parity were higher among corneal and cesarian scar in case group (Table IV).
Overall, 191 diagnosed EP cases were selected for the study. Based on our inclusion and exclusion criteria, we also selected 180 subjects for nonpregnant and 190 for intrauterine pregnancies after evaluation.
On average, our cases were 31.7 yr old; their demographic and gestational information is provided in table III. Out of the 191 cases, 90 (including failed cases) underwent surgery, 89 received MTX, and 25 underwent observation only. The demographics and characteristics are compared with various sites. Table II demonstrates that certain characteristics make subjects more susceptible to certain ectopic pregnancies. In addition, the levels of β-hCG, gestational age, and parity in corneal and scar pregnancies are significantly higher (Figure 1).
One of the primary objectives of this study was to determine the relationship between the proposed risk factors and EP. To this end, we compared the cases to 2 randomly selected control groups (intrauterine and nonpregnant). Only tubal EPs and scar pregnancies were analyzed because only these 2 categories had sufficient sample sizes for statistical analysis (Table V).
Regarding medical treatment failure, out of the 89 medically treated cases, we observed 12 failures. The results show that the cesarean section scar site yielded the highest failure rate, with 46.7%, followed by the cornea (16.7%) and tubal EP (6.3%) (27).
In terms of the type of surgery, 41 cases of tubal pregnancy were treated with salpingectomy, while only 7 cases were treated with salpingostomy. We also evaluated the cases of CSPs who received potassium chloride injections; only one of 5 CSPs who received KCl injections failed and required a second injection. The failure of medical treatment may necessitate invasive surgery by the gynecologist. In our study, 12 cases did not respond to medical treatment, 4 underwent suction and curettage (cesarean scar pregnancy), 7 underwent salpingectomy, and 1 underwent fetal reduction (corneal pregnancy) (Table V).
To evaluate the efficacy of our clinical protocol and the role of additional doses, we assessed the protocol using failure rates for various EP types. The overall analysis revealed that the additional dose could increase the success rate of both scar and tubal EPs. Since this data was a paired dependent sample and we only had sufficient samples in the tubal group to conduct a reliable test, we conducted McNemar’s test in a 2×2 contingency table in the tubal group to determine whether the hypothesized situation in which surgery was indicated for cases who received an extra dose could be avoided. The significance of this evaluation confirmed the null hypothesis. This indicates that a higher dosage could reduce the likelihood of medical treatment failure and the need for invasive procedures. We continued our examination using the student’s t test to determine if an additional dose was required. The results revealed that the primary difference between the 2 groups was in gestational age, which was almost 1.5 wk older in the group that required the additional dose (Tables VI and VII).
7 cases initially suspected of ovarian EP based on ultrasonographic evaluation were included in our study; one was successfully treated with MTX, 3 turned out to be tubal upon laparoscopic evaluation, 2 underwent salpingectomy, and one underwent salpingostomy. 3 cases had a gestational sac in their ovaries, resulting in the reduction of the fetus. Histopathological examination subsequently confirmed the diagnosis. Notably, 3 cases had a positive history of ART, but the number of cases was insufficient to conduct a reliable statistical analysis.
The corneal pregnancy cases included 8 subjects. 6 cases were administered MTX, one of which resulted in a fetal reduction, and one failed case underwent hysteroscopic contraceptive removal. 2 additional cases underwent laparoscopic surgery. Regarding risk factors, only one case had an EP and salpingectomy history.
3 cases of cervical pregnancy were managed during the course. One was only observed, while the other was treated medically and surgically.








4. Discussion
This study aimed to assess risk factors and treatment outcomes for surgical and therapeutic approaches to EP treatment. In addition to assessing the correlation between proposed risk factors and EP, our results revealed that emergency contraceptives, oral contraceptives, and other risk factors increase the likelihood of ectopic pregnancies in the event of a failed pregnancy.
One of the primary goals of this study was to evaluate the efficacy of the medical treatment protocol and the significance of extra doses.
The findings indicate that this method may be an option for individuals who plan future pregnancies or wish to preserve their fallopian tubes and fertility. The results showed a significant improvement in the performance of single-dose treatment plans in tubal EPs. Since gestational age was shown to influence the need for the additional dose, this modification is considered for individuals over 7.5 wk into their pregnancies (11). In a 2017 study, a meta-analysis indicated that the success rate difference between single doses and multiple doses was not statistically significant. However, side effects were more prevalent in the multiple-dose case. Consequently, their analysis indicates that the double regimen is an effective and secure alternative to the multiple-dose and single-dose protocols (1, 28-30).
A 2003 meta-analysis concluded that no statistically significant differences exist between the outcomes of multiple-dose and single-dose protocols. Nonetheless, the multiple-dose regimen is more likely to cause adverse effects (29). Regarding β-hCG concentrations and failure rate, their outcomes are also consistent with the present study. Likewise, they concluded that higher β-hCG levels increase the failure rate (23). The subtle factor that may have influenced the results is that clinicians are not blind to the factors that may result in a poor prognosis for medical treatment; consequently, they tend to select multiple-dose protocols for cases with higher β-hCG levels and a relatively poor prognosis. Based on the β-hCG concentration, the clinicians in our facility determined the medical treatment protocol. A multiple-dose protocol was designed for cases with more than 10,000 IUs who did not wish to undergo surgical management, whereas double and single doses were chosen for cases with levels below 10,000 IUs and 5000 IUs, respectively. A prospective randomized study conducted in Turkey confirms the same findings and issues attributable to inconsistent results (4, 19, 24, 30, 31). In cases where the decline in β-hCG is neither satisfactory nor significantly low, our findings and previous research suggest that an additional dose may be necessary (24, 32-35).
Regarding our findings regarding risk factors, specifically pharmaceutical contraceptives, we hypothesize that using contraceptives reduces the likelihood of both IUP and EP. However, it significantly increases the chances of EP if it fails. This is crucial for emergency contraceptives because the correlation has not been extensively studied (12, 28, 36).
Other risk factors, including smoking, intrauterine device use, and previous surgery, were investigated, and a correlation was established (5). Furthermore, our research indicates that ovulation induction increases the likelihood of EP. In 2014, a study concluded that OCPs and LNG-EC increased the risk of EP by up to 4 times that of women who did not use contraception and by up to 7 times that of women whose contraception failed (10, 22).
Several additional cases of ectopic pregnancies with LNG-EC and other emergency contraceptives have been reported (3, 5, 16). This phenomenon indicates that individuals with a positive history of contraceptive use and other risk factors and symptoms should strongly suspect EP. As an alternative medical approach, this method not only reduces the rate of surgical procedures but also decreases the possibility of infertility.
5. Conclusion
In summary, we conclude that the recommended clinical approach can perform better if a higher dose is administered. Our data also indicates a strong correlation between the use of contraceptives, particularly emergency contraceptives, and EP.
Acknowledgments
This study received no funding.
Conflict of Interest
The authors have no conflict of interest to declare.
Type of Study: Original Article |
Subject:
Pregnancy Health
References
1. Yang C, Cai J, Geng Y, Gao Y. Multiple-dose and double-dose versus single-dose administration of methotrexate for the treatment of ectopic pregnancy: A systematic review and meta-analysis. Reprod Biomed Online 2017; 34: 383-391. [DOI:10.1016/j.rbmo.2017.01.004] [PMID]
2. Glenn TL, Bembry J, Findley AD, Yaklic JL, Bhagavath B, Gagneux P, et al. Cesarean scar ectopic pregnancy: Current management strategies. Obstet Gynecol Surv 2018; 73: 293-302. [DOI:10.1097/OGX.0000000000000561] [PMID]
3. Timor-Tritsch IE, Monteagudo A, Calì G, D'Antonio F, Agten AK. Cesarean scar pregnancy: Patient counseling and management. Obstet Gynecol Clin 2019; 46: 813-828.
https://doi.org/10.1016/j.ogc.2019.07.009 [DOI:10.1016/j.ogc.2019.07.010] [PMID]
4. Fowler ML, Wang D, Chia V, Handal-Orefice R, Latortue-Albino P, Mulekar S, et al. Management of cervical ectopic pregnancies: A scoping review. Obstet Gynecol 2021; 138: 33-41. [DOI:10.1097/AOG.0000000000004423] [PMID]
5. Timor-Tritsch I, Buca D, Di Mascio D, Cali G, D'Amico A, Monteagudo A, et al. Outcome of cesarean scar pregnancy according to gestational age at diagnosis: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 2021; 258: 53-59. [DOI:10.1016/j.ejogrb.2020.11.036] [PMID]
6. Liu D, Yang M, Wu Q. Application of ultrasonography in the diagnosis and treatment of cesarean scar pregnancy. Clin Chim Acta 2018; 486: 291-297. [DOI:10.1016/j.cca.2018.08.012] [PMID]
7. Parashi S, Moukhah S, Ashrafi M. Main risk factors for ectopic pregnancy: A case-control study in a sample of Iranian women. Int J Fertil Steril 2014; 8: 147-154.
8. Barnhart KT. Ectopic pregnancy. New Engl J Med 2009; 361: 379-387. [DOI:10.1056/NEJMcp0810384] [PMID]
9. Cleland K, Raymond E, Trussell J, Cheng L, Zhu H. Ectopic pregnancy and emergency contraceptive pills: A systematic review. Obstet Gynecol 2010; 115: 1263-1266. [DOI:10.1097/AOG.0b013e3181dd22ef] [PMID] [PMCID]
10. Li C, Zhao W-H, Meng C-X, Ping H, Qin G-J, Cao S-J, et al. Contraceptive use and the risk of ectopic pregnancy: A multi-center case-control study. PloS One 2014; 9: e115031. [DOI:10.1371/journal.pone.0115031] [PMID] [PMCID]
11. Zhou XY, Li H, Fu XD. Identifying possible risk factors for cesarean scar pregnancy based on a retrospective study of 291 cases. J Obstet Gynaecol Res 2020; 46: 272-278. [DOI:10.1111/jog.14163] [PMID] [PMCID]
12. Callahan R, Yacobson I, Halpern V, Nanda K. Ectopic pregnancy with use of progestin-only injectables and contraceptive implants: A systematic review. Contraception 2015; 92: 514-522. [DOI:10.1016/j.contraception.2015.08.016] [PMID]
13. Zhang J, Li C, Zhao W-H, Xi X, Cao S-J, Ping H, et al. Association between levonorgestrel emergency contraception and the risk of ectopic pregnancy: A multicenter case-control study. Sci Rep 2015; 5: 8487. [DOI:10.1038/srep08487] [PMID] [PMCID]
14. Harb HM, Knight M, Bottomley C, Overton C, Tobias A, Gallos I, et al. Caesarean scar pregnancy in the UK: A national cohort study. BJOG 2018; 125: 1663-1670.
https://doi.org/10.1111/1471-0528.15255 [DOI:10.1111/1471-0528.15325] [PMID]
15. Hasan M, Hasan FM, Zafar T. Ruptured scar pregnancy secondary to ingestion of misoprostol: A case report. Am J Sonography 2018; 1: 1. [DOI:10.25259/AJS-17-2018]
16. Webster K, Eadon H, Fishburn S, Kumar G. Ectopic pregnancy and miscarriage: Diagnosis and initial management: Summary of updated NICE guidance. BMJ 2019; 367: 6283. [DOI:10.1136/bmj.l6283] [PMID]
17. Elmokadem AH, Abdel-Wahab RM, El-Zayadi AA, Elrakhawy MM. Uterine artery embolization and methotrexate infusion as sole management for caesarean scar and cervical ectopic pregnancies: A single-center experience and literature review. Can Assoc Radiol J 2019; 70: 307-316. [DOI:10.1016/j.carj.2018.12.002] [PMID]
18. Pierce S, Bakker R, Myers DA, Edwards RK. Clinical insights for cervical ripening and labor induction using prostaglandins. AJP Rep 2018; 8: e307-e314. [DOI:10.1055/s-0038-1675351] [PMID] [PMCID]
19. Sindiani AM, Alshdaifat E, Obeidat B, Obeidat R, Rawashdeh H, Yaseen H. The use of single dose methotrexate in the management of ectopic pregnancy and pregnancy of unknown location: 10 years' experience in a tertiary center. Int J Women's Health 2020; 12: 1233-1239. [DOI:10.2147/IJWH.S279426] [PMID] [PMCID]
20. Hoover KW, Tao G, Kent CK. Trends in the diagnosis and treatment of ectopic pregnancy in the United States. Obstet Gynecol 2010; 115: 495-502. [DOI:10.1097/AOG.0b013e3181d0c328] [PMID]
21. Sivalingam VN, Duncan WC, Kirk E, Shephard LA, Horne AW. Diagnosis and management of ectopic pregnancy. J Fam Plann Reprod Health Care 2011; 37: 231-240. [DOI:10.1136/jfprhc-2011-0073] [PMID] [PMCID]
22. Farren J, Jalmbrant M, Falconieri N, Mitchell-Jones N, Bobdiwala S, Al-Memar M, et al. Posttraumatic stress, anxiety and depression following miscarriage and ectopic pregnancy: A multicenter, prospective, cohort study. Am J Obstet Gynecol 2020; 222: 367.e1-e22. [DOI:10.1016/j.ajog.2019.10.102] [PMID]
23. Barnhart KT, Gosman G, Ashby R, Sammel M. The medical management of ectopic pregnancy: A meta-analysis comparing "single dose" and "multidose" regimens. Obstet Gynecol 2003; 101: 778-784.
https://doi.org/10.1097/00006250-200304000-00028 [DOI:10.1016/S0029-7844(02)03158-7] [PMID]
24. Morlando M, Buca D, Timor‐Tritsch I, Cali G, Palacios‐Jaraquemada J, Monteagudo A, et al. Reproductive outcome after cesarean scar pregnancy: A systematic review and meta‐analysis. Acta Obstet Gynecol Scand 2020; 99: 1278-1289. [DOI:10.1111/aogs.13918] [PMID]
25. Dior UP, Palma-Dias R, Reidy KL, Cheng C, Healey M. Cesarean scar pregnancies: Incidence and factors associated with conversion to surgery from medical management. J Minim Invasive Gynecol 2019; 26: 919-927. [DOI:10.1016/j.jmig.2018.09.771] [PMID]
26. Dziedzic JM, Patel PV. Cervical ectopic pregnancy: A rare site of implantation. J Emerg Med 2019; 56: e123-e125. [DOI:10.1016/j.jemermed.2019.03.024] [PMID]
27. Ashtari Kh, Nazari H, Ko H, Tebon P, Akhshik M, Akbari M, et al. Electrically conductive nanomaterials for cardiac tissue engineering. Adv Drug Deliv Rev 2019; 144: 162-179. [DOI:10.1016/j.addr.2019.06.001] [PMID] [PMCID]
28. Levine Y, Yahav L, Schwarzman P, Yohai D, Hershkovitz R, Weintraub AY. The correlation between endometrial thickness and the criteria for MTX treatment for ectopic pregnancy. J Obstet Gynaecol 2021; 41: 1230-1233. [DOI:10.1080/01443615.2020.1849068] [PMID]
29. Jauniaux E, Mavrelos D, De Braud LV, Dooley W, Knez J, Jurkovic D. Impact of location on placentation in live tubal and cesarean scar ectopic pregnancies. Placenta 2021; 108: 109-113. [DOI:10.1016/j.placenta.2021.03.019] [PMID]
30. Hüner B, Lato K, Reister F, Janni W, Deniz M. A scary complication: Single-center study on management and outcome of cesarean scar pregnancy. Rev Bras Ginecol Obstet 2021; 43: 311-316. [DOI:10.1055/s-0041-1728781] [PMID]
31. Zhang J, Zhang Y, Gan L, Liu X-Y, Du S-P. Predictors and clinical features of methotrexate (MTX) therapy for ectopic pregnancy. BMC Pregnancy Childbirth 2020; 20: 654. [DOI:10.1186/s12884-020-03350-8] [PMID] [PMCID]
32. Li H, Liu Y, Wen S, Jia H, Du Y. Evaluation of serum biomarkers and efficacy of MTX in women with ectopic pregnancy. Mol Med Rep 2019; 20: 2902-2908. [DOI:10.3892/mmr.2019.10533] [PMID]
33. Orhan A, Kasapoğlu I, Demir BÇ, Özerkan K, Duzok N, Uncu G. Different treatment modalities and outcomes in cesarean scar pregnancy: A retrospective analysis of 31 cases in a unıversity hospital. Ginekol Pol 2019; 90: 291-307. [DOI:10.5603/GP.2019.0053] [PMID]
34. Hendriks E, Rosenberg R, Prine L. Ectopic pregnancy: Diagnosis and management. Am Fam Physician 2020; 101: 599-606.
35. Guvendag Guven ES, Dilbaz S, Dilbaz B, Aykan Yildirim B, Akdag D, Haberal A. Comparison of single and multiple dose methotrexate therapy for unruptured tubal ectopic pregnancy: A prospective randomized study. Acta Obstet Gynecol Scand 2010; 89: 889-895. [DOI:10.3109/00016349.2010.486825] [PMID]
36. Tarafdari A, Malek M, Pahlevan Falahy E, Hadizadeh A. IUD perforation and embedment within omentum: A rare and perplexing incidence. Clin Case Rep 2022; 10: e05732. [DOI:10.1002/ccr3.5732] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




